Back to Journals » Cancer Management and Research » Volume 12
Bone Metastases of Gastrointestinal Stromal Tumor: A Review of Published Literature
Authors Yang J, Yan J, Zeng M, Wan W, Liu T, Xiao JR
Received 30 September 2019
Accepted for publication 20 January 2020
Published 26 February 2020 Volume 2020:12 Pages 1411—1417
DOI https://doi.org/10.2147/CMAR.S232936
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Chien-Feng Li
Jian Yang,1,* Jijie Yan,2,* Meihui Zeng,3,* Wei Wan,1 Tielong Liu,1 Jian-Ru Xiao1
1Department of Orthopedic Oncology, Changzheng Hospital, Second Military Medical University, Shanghai, People’s Republic of China; 2Ningbo University, Ningbo, Zhejiang, People’s Republic of China; 3Department of Neurosurgery, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tielong Liu; Jian-Ru Xiao
Tel +8602181885634
Fax +8602163720099
Email [email protected]; [email protected]
Background: With the occurrence and improvement of immunohistochemistry and other pathological diagnostic techniques, gastrointestinal stromal tumor (GIST) has been gradually recognized. With the prolonged survival of patients with GISTs, reports about the bone metastasis of GIST increased. However, the knowledge of GISTs is rather limited due to its very low incidence.
Methods: Cochrane and Medline database (via PubMed) were searched in July 2019 with related keywords to acquire the literature related to the bone metastasis of GIST. Then, the literature was reviewed and references were also scanned to identify the possible related reports. Study data comprising age, sex, primary location, metastasis interval time, immunohistochemistry index, management and prognosis were recorded and analyzed.
Results: Forty-five patients with bone metastases of GIST, with a mean age of 61.09 years, were included. The small intestine and stomach were the most common primary sites, followed by the rectum. Patients with small intestine primary sites had bone metastases that occurred earlier than the bone metastases stomach and rectum primary sites. The spine was the most common site of bony metastases. The mean survival time after GIST diagnosis was more than 64.02 months. Patients younger than 60 years old had a worse prognosis than those older than 60 years old. Furthermore, patients with spinal involvement had a worse prognosis than those without spinal involvement. Surgical interventions combined with targeted therapies guaranteed a better prognosis.
Conclusion: Bone metastasis of GIST, which mainly occurs in the spine, is rather rare. Patients with GISTs of the small intestine and stomach suffered from bone metastasis more frequently and earlier than patients with GISTs in other primary sites. Age, sex, primary tumor location, treatment mode for the primary lesions and metastases, and spine involvement may be potential factors that affect the prognosis of GIST patients with bone metastases.
Keywords: gastrointestinal stromal tumor, bone metastasis, tyrosine kinase inhibitors, prognosis
Introduction
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal neoplasm of the gastrointestinal tract and is considered to have a potential malignant tendency.1–3 The term GIST, which was purposed by Mazur and Clark in 1983, covered a heterogeneous group of nonepithelial neoplasms with spindle or epithelioid cells.4 Previously, GIST was considered to be a type of sarcoma and was usually misdiagnosed as either smooth muscle cell-derived or neurogenic malignancy.5 GIST is now thought to originate from interstitial cells of Cajal (ICC), with an incidence of 10–30 cases per million people.6–9 The molecular pathogenesis of GIST is thought to be closely related to mutation of KIT and platelet-derived growth factor receptor alpha gene (PDGFRA).10 The stomach is the most common primary site of GIST (60–70%), followed by small intestine (20–30%), colon and rectum (5%), esophagus (<5%), and occasionally the omentum and mesentery.6,11–13
Surgical resection is the mainstay of treatment for primary GISTs.1,14 However, the 5-year survival rate is approximately 50% though complete resection. With imatinib and sunitinib approved for the treatment of GIST in 2002 and 2006, the median survival of patients was significantly prolonged, but metastasis of GISTs increased as well. Usually, metastasis of GIST occurs in the abdominal cavity, while bone involvement is rather rare.15,16 It is of great importance to recognize bone metastases of GIST since they may subsequently develop skeleton-related events associated with severe results. As there are only single case reports or small series focusing on bone metastasis of GIST, the clinicopathological characteristics of these tumors and their metastases have not yet been well characterized. Therefore, this study was conducted to create a systematic review that will enrich our knowledge of the bone metastases of GIST.
Materials and Methods
Search Strategy
The Cochrane and Medline database (via PubMed) were searched for relevant English literature using the keywords of “gastrointestinal stromal tumor” and “bone metastasis.” The search was conducted in July 2019 and no time limit was imposed on publication dates. Articles were selected if they were written in English with abstract available online, while others were excluded.
Case Selection
Titles and Abstracts were selected by two of the authors according to the study inclusion and exclusion criteria. Full-text articles were then selected when titles and abstracts appeared relevant. Full-text reading for inclusion was performed by the two authors independently, with discussion to achieve consensus in case of disagreement. Furthermore, the references were also scanned to identify possible related reports.
Statistical Analysis
The included articles were studied to record the essential data for analysis. Study data included age, sex, primary location, metastasis interval time (MIT, from primary locations to bone metastases), immunohistochemical staining of CD117 and CD34, management and outcome. Considering the differences in management and the limited number of cases found, descriptive analyses were mainly adopted and the data were presented as the mean or median.
Results
With the above search strategy, 27 articles1–3,5–7,14–34 with 45 bony metastases of GISTs were acquired and showed in Table 1. Of the 45 patients, 31 were male and 14 were female, with a mean age of 61.09 years (37~85 years). Regarding the primary locations of GIST, 14 cases were from the stomach and small intestine, respectively (31.11%), 11 cases were from the colorectal (24.44%), 2 cases were from the esophagus (4.44%), 2 cases were of unknown origination (4.44%), and 1 case was from the ovary and liver, respectively (2.22%). Bone metastases mainly occurred in the spine (21), followed by the rib (15), femur (8), humerus (8), pelvis (8) and other bones.
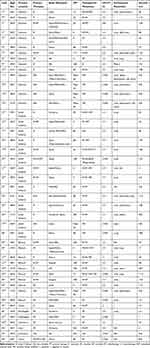 |
Table 1 Characteristics of the Published Cases with Bone Metastases of the GIST |
The mean time to bone metastases in small intestine, stomach and colorectal GIST was 49.50 months, 61.86 months and 67.64 months, respectively, after the initial diagnosis, with an average metastasis interval time (MIT, from primary locations to metastases) of 55.57 months. The most obvious pathological characteristic, CD 117 expression, occurred in 43 cases (95.56%), and the positive expression of CD34 was observed in 29 cases (64.44%). The primary tumors were mainly treated with surgical resection (30, 66.67%), with or without tyrosine kinase inhibitor (TKI) treatment. For bone metastases, therapies predominantly include a TKI, combined with or without surgical resections. Radiotherapy was also chosen as an adjuvant therapy. The average survival of the 45 patients was more than 64.02 months (the prognosis of nine patients was unclear): 65.10 months for males and 61.55 months for females. Patients younger than 60 years old had a shorter survival than those older than 60 (55.65 and 75.47 months, respectively). Patients treated with surgery for primary GISTs had an average survival of 84.98 months compared to 24.88 months in the conservative group (non-resection). For bone metastases, prognosis of the combined group (resection and TKI) was about 77.78 months, while that of the TKI only group was approximately 43.09 months. In addition, whether bone metastases involved the spine also affected the prognosis of patients. The patients with spinal involvement (prognosis of 2 patients was unavailable) had an average prognosis of 41.78 months, while those without spinal involvement occupied a two-fold prognosis of 82.49 months.
Discussions
GIST has previously been considered as a smooth muscle-derived tumor, and diagnosed as leiomyomas, leiomyoblastomas, or leiomyosarcomas.5 GIST frequently occurs in middle-aged patients (55–65 years), similar to the observations made in the studies reviewed here (61.09 years). The diagnosis of GIST mainly includes the following three aspects: First, the tumor is rich in epithelioid cells (20%) or spindle cells (70%) or a mix of the two; Second, CD117 (C-KIT protein) and CD34 are highly expressed (usually about 95% and 60–70%, respectively); Third, part of the patients have mutations in KIT or PDGFRA (usually 90% and 3%, respectively).5,6,10 According to our statistical analysis, CD 117 expression occurred in 95.56% (n=43), while CD34 expression was found in 64.44% (n=29), similar to the data in the literature. Nevertheless, most of the reviewed literature had not showed the mutations of KIT or PDGFRA. According to the reported ones and literature about GIST, the most frequent gene mutations are those in exon 9 and 11 of the KIT gene, occasionally in exon 13 and 17.10,14,16,23,27 And, the relationship between gene mutation and prognosis of GIST needs to be studied furthermore.
Overall, approximately 20–30% of GISTs show malignant behaviors, mainly depending on the tumor size, mitotic activity and necrosis within the tumors.12 The liver and peritoneum are reported to be the most common metastatic sites of GISTs. Extra-abdominal metastases are less common and typically involve the lung, scar tissue, pleura and bone.14,28 In this study, liver metastasis occurred in 31 cases, and peritoneum and lung metastasis occurred in 7 cases, respectively. Other metastases included subcutaneous tissue, pleura, iliac fossa and hip. The bony metastases were found mainly due to the occurrence of local pain, pathological fracture, spinal cord compression symptoms, or accidental discovery when receiving advanced imaging including CT, MRI and especially PET/CT examinations. In a recent study of GIST metastases, the incidence of bone metastases in GIST was limited to only 0.47%,9 much less than that in previous reports of 3.2–6%.2,15 Not unexpectedly, GIST metastasis to the bone may become more prevalent due to extensive use of imaging techniques and increased patient life expectancy resulting from the improvement in therapeutic modalities. Nevertheless, only 45 cases of bone metastases were included in this study after searching the whole database.
Among them, a slight male bias with a ratio of 2.21:1 to female was found (31:14). Compared with that 60–70% primary GISTs occurred in the stomach, only 31.11% (n=14) of bone metastases originated from the stomach GISTs. The same percentage of bone metastases originated from the small intestine, while 24.44% from the colon and rectum (n=11), with other parts of the gastrointestinal tract next in frequency. This data suggests that the stomach GISTs showed weak aggressiveness. Spine is the most common site for bony metastases of malignant tumors, and GIST is no exception. In this series, spine involvement occurred in 21 patients, followed by the rib, femur, humerus and pelvis. This is largely because of the proximity of spine to other organ systems, the presence of abundant valveless vascular channels, and the red marrow composition.28,29 The most common symptoms of bone metastases in GIST were local pain and limb weakness, followed by pathological fractures or no symptoms.1,5–7,14 The median postoperative time for distant metastases was about 24 months.35 In this study of bone metastases, that time was about 56.83 months. This difference may be caused by the early abdominal cavity metastases that occurred at about 19 months.4 The MIT also differed according to different primary locations. GISTs of the small intestine metastasized earlier (49.50 months) than those in other locations, while colorectal GISTs seem to be the latest (67.64 months), with stomach GISTs in the second place (61.86 months). The high prevalence and early occurrence of bone metastases of small intestine GISTs may indicate its aggressiveness and poor outcome, which was previously mentioned in Yang’s study.9
Surgical resection is currently recognized as the main treatment for primary GISTs, as in this study. However, there is no consensus about the treatments for patients with bone metastases of GIST. Currently, in addition to surgical resection, imatinib and sunitinib are recommended as the first and the second-line drugs for GIST by American national comprehensive cancer network (NCCN) guidelines. Before the introduction of imatinib mesylate, patients with irresectable GISTs had limited therapeutic options. TKIs can specifically bind to the tyrosinase receptor on the surface of tumor cells and block signal transmission, thus inhibiting the tumor growth. Nearly all bone metastases in this series were treated with imatinib or sunitinib (sorafenib and regorafenib in 1 case). Concretely, 22 cases received operation plus TKI, and other 14 received TKI only. Generally, surgical resection has become the dominant option for both primary and bone metastatic GISTs when possible. TKI is recommended for patients who can tolerate it and those whose tumors are not resistant to it. Thus, a TKI combined with surgical resection can be the ideal choice for GISTs with bony metastases. The vast majority of patients received targeted therapy of imatinib 400 mg/d or sunitinib 37.5 ~ 50 mg/d, which could be adjusted according to the curative effect and patients’ tolerance. However, there are no clear data on the duration of targeted therapy for intraperitoneal recurrence or bone metastasis. It is now widely believed that for metastatic GIST, it is necessary to administer imatinib for a long time until the patient develops disease progression, drug resistance or intolerable side effects. Then, the second-line drugs such as sunitinib or others should be considered.
Bisphosphonate is the current standard therapy for osteoporosis and bone metastases from malignant tumors.14 However, few data can be found in the reviewed literature on the treatment with bisphosphonate in bone metastases of GISTs.14,20,33 In all, the effect of bisphosphonate administration is unknown yet.20
At present, it is believed that the main factors affecting the prognosis of GIST include the tumor size, grade, necrosis degree, resection degree, mitotic activity of tumor cells, whether the metastatic tumors can be surgically removed, and the sensitivity of targeted therapy and mutation of KIT and PDGFRA.6,12,36 According to previous reports, patients with bone metastasis had a poorer prognosis (median overall survival: 18 months) than patients with metastasis in other sites or without metastasis.2,9 Due to the limited obtained from case reports, we can only know that the mean survival of GIST patients with bone metastases was more than 64.02 months. According to our statistics, the male patients may have a longer survival than the female (65.10 and 61.55 months, respectively). Guy et al reported that a poor prognosis was conferred by advanced patient age (>65 years).4 We divided the 45 cases into two groups (≤60 and >60 years old) and found that the younger group had a worse survival (55.65 vs 75.47 months).
Whether surgical resection was employed for primary GISTs also influenced the prognosis of patients with bone metastases. Compared with 24.88 months of the non-resection group of primary GISTs, the operation group occupied a better survival of 84.98 months. This may should be attributed to the effect of surgical interventions on GIST. Regarding the therapeutic efficiency for bony metastases, survival of the combined group (resection and TKI) was about 77.78 months, while that of the TKI only group was about 43.09 months, highlighting the predominance of surgical resection as the top treatment option. Based on previous studies, spine involvement or not was of great significance to the prognosis of malignant tumors.37,38 In accordance with the literature, spine involvement also decreased the prognosis of patients with bone metastases. Patients with spinal involvement (prognosis of 2 patients was unavailable) had an average survival of 41.78 months, while those without spinal involvement occupied a two-fold increase survival time of 82.49 months. Since spine involvement may result in serious pain, paralysis, motor dysfunctions, impaired function of sense and excretion, and polysystemic infections, affecting the patients’ survival negatively. Obviously, surgical interventions are necessary with the aims of pain control, preservation of spinal stability, maintenance of sphincter control and mobility.
Studies have suggested that the mitotic activity of tumor cells and the mutation of KIT and PDGFRA also affect the response to imatinib and thus the prognosis of GIST.6,12 With the limited available information, their effects on bone metastatic GISTs were not available. In conclusion, the age, sex, primary tumor location, the treatment mode of the primary lesions and metastases, and spine involvement may be potential factors that affect the prognosis of patients with GIST bone metastases. If GIST bone metastasis occurs, surgical resection should be recommended if possible, then targeted therapies with imatinib or sunitinib must be subsequently adopted. Only in this way, can the quality of life and survival of such patients be improved.
However, there are also some limitations to our study. First, the number of patients was limited due to the low incidence. Second, the present study is a kind of retrospective study that might have selection bias because not all GIST patients had routine tests for bone metastases. Finally, the therapies differed in different centers. Anyway, regardless of these limitations, this is the first study focusing on the clinical features, therapeutic options and potential prognostic factors of bony metastases of GIST. Further studies with larger sample sizes are needed and we hope our study can be helpful for improving the clinical knowledge of GIST as well as its bone metastases.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Ozan E, Oztekin O, Alacacioğlu A, et al. Esophageal gastrointestinal stromal tumor with pulmonary and bone metastases. Diagn Interv Radiol. 2010;16:217. doi:10.4261/1305-3825.DIR.1861-08.2
2. Kosemehmetoglu K, Kaygusuz G, Fritchie K, et al. Clinical and pathological characteristics of gastrointestinal stromal tumor (GIST) metastatic to bone. Virchows Arch. 2017;471(2):77–90. doi:10.1007/s00428-017-2138-7
3. Nannini M, Biasco G, Di Scioscio V, et al. Clinical, radiological and biological features of lung metastases in gastrointestinal stromal tumors (Case reports). Oncol Rep. 2011;25(1):113–120.
4. Burkill GJ, Badran M, Al-Muderis O, et al. Malignant gastrointestinal stromal tumor: distribution, imaging features, and pattern of metastatic spread. Radiology. 2003;226(2):527–532. doi:10.1148/radiol.2262011880
5. BorRen H, HsienChih C, Tai-Ngar L, et al. Epidural metastases from a gastrointestinal stromal tumor. J Clin Neurosci. 2008;15(1):82–84. doi:10.1016/j.jocn.2006.05.023
6. Gil-Arnaiz I, Martínez-Trufero J, Pazo-Cid RA, et al. Skull metastasis from rectal gastrointestinal stromal tumours. Clinical and Translational Oncology. 2009;11(9):625–627. doi:10.1007/s12094-009-0415-x
7. Barriere J, Thariat J, Vandenbos F, et al. Diplopia as the first symptom of an aggressive metastatic rectal stromal tumor. Onkologie. 2009;32:345–347. doi:10.1159/000215712
8. Kitamura Y. Gastrointestinal stromal tumors: past, present, and future. J Gastroenterol. 2008;43(7):499–508. doi:10.1007/s00535-008-2200-y
9. Yang DY, Wang X, Yuan WJ, et al. Metastatic pattern and prognosis of gastrointestinal stromal tumor (GIST): a SEER-based analysis. Clin Transl Oncol. 2019;21:1654–1662. doi:10.1007/s12094-019-02094-y
10. Christopher LC, Jonathan AF, Michael CH. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22(18):3813–3825. doi:10.1200/JCO.2004.05.140
11. Miettinen M, Makhlouf H, Sobin LH, et al. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30(4):477–489. doi:10.1097/00000478-200604000-00008
12. Nowain A, Bhakta H, Pais S, et al. Gastrointestinal stromal tumors: clinical profile, pathogenesis, treatment strategies and prognosis. J Gastroenterol Hepatol. 2005;20(6):818–824. doi:10.1111/j.1440-1746.2005.03720.x
13. Joensuu H, Kindblom LG. Gastrointestinal stromal tumors-a review. Acta Orthop. 2004;75(311):432–438.
14. Di Scioscio V, Greco L, Pallotti MC, et al. Three cases of bone metastases in patients with gastrointestinal stromal tumors. Rare Tumors. 2011;3(2):51–53. doi:10.4081/rt.2011.e17
15. Kanda H, Furuta N, Takazawa Y, et al. Cytological findings of gastrointestinal stromal tumor-derived bone metastasis. Acta Cytol. 2018;62:430–435. doi:10.1159/000492709
16. Abuzakhm SM, Acre-Lara CE, Zhao W, et al. Unusual metastases of gastrointestinal stromal tumor and genotypic correlates: case report and review of the literature. J Gastrointest Oncol. 2011;2(1):45–49. doi:10.3978/j.issn.2078-6891.2011.006
17. Tariq Z, Ghose A, Rafiq E, et al. Gastrointestinal stromal tumor with primary resistance to imatinib and extensive bone metastases. Am J Ther. 2011;18(5):e188–e190. doi:10.1097/mjt.0b013e3181d41eef
18. Selcukbiricik F, Tural D, Ozturk MA, et al. Gastrointestinal stromal tumor of the rectum with scapular metastasis: a case report. J Med Case Rep. 2012;6(1):145. doi:10.1186/1752-1947-6-145
19. Feki J, Bouzguenda R, Ayedi L, et al. Bone metastases from gastrointestinal stromal tumor: a case report. Case Rep Oncol Med. 2012;2012:1–3. doi:10.1155/2012/509845
20. Sahin E, Yetişyiğit T, Oznur M, et al. Gastric Gastrointestinal Stromal Tumor with Bone Metastases – case Report and Review of the Literature. Klin Onkol. 2014;27(1):56–59. doi:10.14735/amko201456
21. Ishi Y, Nakayama N, Kobayashi H, et al. Successful removal of a metastatic gastrointestinal stromal tumor in the craniovertebral junction using an occipital artery to posterior inferior cerebellar artery bypass. Case Rep Neurol. 2014;6:139–143. doi:10.1159/000362867
22. Aktan M, Koc M, Yavuz BB, et al. Two cases of gastrointestinal stromal tumor of the small intestine with liver and bone metastasis. AnnTransl Med. 2015;3(17):259. doi:10.3978/j.issn.2305–5839.2015pg.09.46
23. Gupta S, Bi WL, Dunn IF, et al. Metastatic gastrointestinal stromal tumor to the skull. World Neurosurg. 2016;89(725):e11–16. doi:10.1016/j.wneu.2016.01.019
24. Park I, Chung DH, Yoo CJ, et al. Skull metastasis of gastric gastrointestinal stromal tumor successfully managed by surgery. J Korean Neurosurg Soc. 2017;60(1):94–97. doi:10.3340/jkns.2014.0506.007
25. Jati A, Tatlı S, Morgan JA, et al. Imaging features of bone metastases in patients with gastrointestinal stromal tumors. Diagn Interv Radiol. 2012;18(4):391–396. doi:10.4261/1305-3825.DIR.5179-11.1
26. Suzuki K, Yasuda T, Nagao K, et al. Bone metastasis of a gastrointestinal stromal tumor: a report of two cases. Oncol Lett. 2015;9(4):1814–1818. doi:10.3892/ol.2015.2976
27. Kroep JR, Bovée VMG, Molen AJ, et al. Extra-abdominal subcutaneous metastasis of a gastrointestinal stromal tumor: report of a case and a review of the literature. J Cutan Pathol. 2009;36(5):565–569. doi:10.1111/j.1600-0560.2008.01067.x
28. Slimack NP, Liu JC, Koski T, et al. Metastatic gastrointestinal stromal tumor to the thoracic and lumbar spine: first reported case and surgical treatment. Spine J. 2012;12(1):e7–12. doi:10.1016/j.spinee.2011.10.037
29. Waterman BR, Kusnezov N, Dunn JC, et al. Aggressive gastrointestinal stromal tumor with spinal metastases: a case report. Mil Med. 2015;180(5):e618–e621. doi:10.7205/MILMED-D–14–00552
30. Bajel A, Simpson I, Longano A, et al. Bone marrow metastasis in gastrointestinal stromal tumor. Br J Haematol. 2010;147(1):2. doi:10.1111/j.1365-2141.2009.07735.x
31. Baeg MK, Bae SH, Lee KH, et al. Diplopia as a presenting symptom in a gastric gastrointestinal stromal tumor. Jpn J Clin Oncol. 2011;41(2):265–268. doi:10.1093/jjco/hyq176
32. Zheng CK, Kan WS, Li P, Case A. Report of a metastatic gastrointestinal stromal tumor occurring in femur. Case Rep Gastrointestinal Med. 2011;2011:1–3. doi:10.1155/2011/926179
33. Tezcan Y, Koc M. Gastrointestinal stromal tumor of the rectum with bone and liver metastasis: a case study. Med Oncol. 2011;28(1 supplement):S204–S206. doi:10.1007/s12032-010-9697-7
34. Wejih DM, Leila GE, Ali CM, et al. Rectal stromal tumor with an exceptional liver and bone metastatic locations. Pan Afr Med J. 2019;32. doi:doi:10.11604/pamj.2019.32.133.17985
35. Rammohan A, Sathyanesan J, Rajendran K. A gist of gastrointestinal stromal tumors: a review. World J Gastrointest Oncol. 2013;5(6):102–l12. doi:10.4251/wjgo.v5.i6.102
36. DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231(1):51–58. doi:10.1097/00000658-200001000-00008
37. Kumar N, Tan JJ, Zaw AS, et al. Evaluation of scoring systems and prognostic factors in patients with spinal metastases from nasopharyngeal carcinoma. Spine J. 2014;14(12):2946–2953. doi:10.1016/j.spinee.2014.06.001
38. Shen L, Dong J, Li S, et al. M1 stage subdivision and treatment outcome of patients with bone-only metastasis of nasopharyngeal carcinoma. Oncologist. 2015;20(3):291–298. doi:10.1634/theoncologist.2014-0206
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
