Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Body Mass Index z Scores Correlate with Epidermal Function in Chinese Children
Authors Yang B, Lai Q, Chen A, Ye L, Wang X, Lai Y, Liu D, Man MQ
Received 15 August 2023
Accepted for publication 15 October 2023
Published 30 October 2023 Volume 2023:16 Pages 3393—3401
DOI https://doi.org/10.2147/DMSO.S435512
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Bin Yang,1,* Qingsong Lai,2,* Aiqi Chen,2 Li Ye,1 Xiaohua Wang,1 Yulin Lai,2 Dan Liu,3 Mao-Qiang Man3
1Department of Dermatology, Dermatology Hospital, Southern Medical University, Guangzhou City, Guangdong, 510091, People’s Republic of China; 2Department of Dermatology, Medical Center for Public Health of Puning, Puning City, Guangdong, 515300, People’s Republic of China; 3Department of Product Development, Dermatology Hospital, Southern Medical University, Guangzhou City, Guangdong, 510091, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mao-Qiang Man, Department of Dermatology, Dermatology Hospital, Southern Medical University, 2 Lujing Road, Guangzhou City, 510091, Guangdong, People’s Republic of China, Tel +86 20 8725 7353, Email [email protected]
Background/Objective: Epidermal function is altered in a number of cutaneous and extracutaneous disorders. To determine whether epidermal function is also altered in children with obesity, we assessed the correlation between the body mass index (BMI) z score and epidermal function in children.
Participants and Methods: Participants were enrolled from outpatient clinic, schools and kindergartens. Epidermal biophysical properties, including transepidermal water loss rate, stratum corneum hydration and skin surface pH, were measured on the flexor forearm and shin. Correlations between epidermal biophysical properties and BMI were analyzed. In addition, the association of epidermal biophysical properties with BMI z score was also determined.
Results: Overall, BMI did not differ significantly between boys and girls among the age groups. BMI z scores correlated negatively with stratum corneum hydration levels and positively with skin surface pH in boys, but not in girls. The negative correlation between TEWL and BMI z score was not significant. Moreover, stratum corneum hydration levels were lower in boys with a BMI z score of ≥ 2 than in those with a BMI z score of − 2 to 0.99.
Conclusion: Both stratum corneum hydration levels and skin surface pH are significantly correlated with BMI z scores in boys, but not in girls. Whether epidermal function influences BMI or vice versa remains to be determined.
Keywords: transepidermal water loss, stratum corneum hydration, skin surface pH, body mass index, BMI z score
Introduction
Obesity is defined as a body mass index (BMI) ≥30 in adults, and ≥the 95th percentile of body weight in children. The prevalence of obesity is 38% in USA and 3.7% in Japan.1 In China, the prevalence of obesity was 12.8% in children in 20202 and 14.4% in adults in 2021.3 The prevalence of obesity is higher in females than in males with a tendency to increase over the years.1,4 Several risk factors contribute to the development of obesity. In comparison to nonalcoholic drinkers, occasional light or moderate alcohol consumption increases the risk of obesity in males, while heavy alcohol consumption decreases the risk of obesity in females.4 In adults, the prevalence of obesity is higher in females than in males (32.7% vs 16%).3 But in children, the prevalence of obesity is higher in boys than in girls (18.6% vs 6.3%).2 Low physical activity and high energy intake are risk factors for obesity in both males and females.2,5 Healthy foods, such as green vegetables, vitamin A-enriched vegetables, fruits and whole grains, can decrease, while eating animal products increases the risk of obesity.6 The risk of obesity increases with the amount of smoking.7 Air pollution, shorter sleep time and longer time viewing television or other devices are also risk factors of obesity.8,9 Thus, the etiopathogenesis of obesity is multifactorial.
Obesity can be associated with other health-related conditions. In addition to type 2 diabetes and cardiovascular disease, the prevalence of psychological disorders such as depression and anxiety is higher in individuals with obesity than in normal weight controls.10,11 Moreover, obesity negatively affects the health-related quality of life in both adults and children.11,12 Furthermore, individuals with obesity exhibit lower work productivity, particularly in the fields of construction and hospitality, compared to normal weight controls.11,13 Additionally, obesity increases annual medical costs by 100% in comparison to individuals with normal weight. Collectively, this line of evidence indicates the negative impacts of obesity on personal life and economy.
Obesity has been linked to several cutaneous conditions. For example, prevalence of obesity is higher in individuals with inflammatory disorders such as atopic dermatitis, psoriasis and prurigo nodularis.14–16 The link between body mass index (BMI), an indicator of body fatness, and epidermal function has also been reported.17 BMI positively correlates with skin surface pH, and negatively correlates with stratum corneum hydration in adult females.18 Because of the differences in skin physiology and metabolism between children and adults, correlation between BMI and epidermal function in children may be different from that in adults. Therefore, we assessed here the correlation between BMI z score and epidermal function in Chinese children.
Participants and Methods
This study was carried out in Guangdong (South China) between October and June. All participants were enrolled from the outpatient clinic of the Medical Center for Public Health of Puning, child daycare centers, kindergartens and schools. The inclusion criteria were subjects aged ≤18 years, without current or a history of atopic dermatitis or psoriasis, or diseases with abnormal keratinization. Except for normal bathing or showering, no topical products were used on the forearm and the shin within 24 hours prior to the measurements of epidermal biophysical properties. Epidermal biophysical properties, including TEWL and stratum corneum hydration were measured with GPskin Barrier® (GPower Inc., Seoul, South Korea), while skin surface pH was measured with a portable skin pH meter (Nate Instrument, Suzhou, Jiangsu, China) on the flexor of the left forearm and the right shin. The measurement sites were 5 cm above the wrist and 5 cm below the knee in children ≤5 years old, and 10 cm above the wrist and 10 cm below the knee in children >5 years old. This study was approved by the institutional review board of the Dermatology Hospital of Southern Medical University (GDDHLS2021025) and carried out in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from the participants’ parent/legal guardians prior to the study.
Because BMI varies with age and sex in children,19 BMI z-score is more suitable than BMI to assess the body fatness in children. BMI z-score was calculated separately in the following age groups: 2 to 3, 4 to 5, 6 to 12, and 13 to 18 years old. The correlation of BMI z score with epidermal function was determined. According to BMI z score, body fatness is classified into underweight/normal weight (z score: −2 to +0.99), overweight (z score: 1 to 1.99), obese (z score: 2 to 2.99), and very obese (z score: ≥3). All body weight and height measurements included clothes, but not shoes.
Statistics
GraphPad Prism 8.3.0 software was used for all statistical analyses. The Mann Whitney test was used to determine the significance between males and females, while Pearson correlation analysis was used to determine significances of the association between BMI z-score and epidermal biophysical properties. Data are expressed as mean ± SEM. p values are indicated in the tables, figures or figure legends.
Results
Demographic Characteristics of Participants
A total of 1221 children, including 673 boys and 548 girls, aged 2 to 18 years, were enrolled in this study (Table 1).
 |
Table 1 Demographic Characteristics of Participants |
Changes of BMI and BMI z Score with Age
We first determined the pattern of changes in BMI and BMI z-scores with age. As shown in Figure 1a, BMI decreased from 2 to 5 years of age, followed by a gradual increase with age in both boys and girls (Table 1). However, BMI z-scores did not change remarkably from 2 to 10 years of age, followed by a gradual increase in boys (Figure 1b). In boys, 579 (86%) children were underweight/normal weight, while 48 (7%) children were overweight. Children who were obese and very obese accounted for 5.6% (38 cases) and 1% (8 cases), respectively. In girls, BMI z-score was < −2 in two girls, and 478 (87%) girls were underweight/normal weight. Girls with overweight and obesity accounted for 8% (45 cases) and 1.8% (10 cases), respectively, while girls who were very obese accounted for 2.4% (13 cases). BMI z-score in girls declined from 2 to ≈7 years of age, followed by an age-associated increase (Figure 1b). These results demonstrate sex-related differences in the changes in BMI z-scores with age.
 |
Figure 1 Changes in BMI and BMI z Score with Age in Children. (a) Changes in BMI with age; (b) Changes in BMI z score with age. Number of subjects are indicated in Table 1. |
Correlation of BMI z Score and BMI with Epidermal Function
Next, we assessed the correlation of BMI z-scores with epidermal function. Epidermal permeability barrier function did not significantly correlate with BMI z-scores (Figure 2a). However, stratum corneum hydration levels were negatively correlated with BMI z-scores in boys (Figure 2b) (p<0.0001). In contrast, skin surface pH was positively correlated with BMI z-score in boys (p<0.05), but not in girls (Figure 2c). Moreover, BMI did not correlate significantly with transepidermal water loss rates (Figure 3a), but negatively correlated with stratum corneum hydration levels in both boys and girls (Figure 3b, p<0.05 in girls and p<0.0001 in boys). Stratum corneum hydration levels were significantly lower in boys with BMI z-score ≥2.0 than in those with BMI z-scores −2.0 to 0.99 (Table 2). In addition, skin surface pH was positively correlated with BMI in boys (p<0.01), but not in girls (Figure 3c). These results indicate that the correlation of epidermal function with body fatness varies with sex in children.
 |
Table 2 Comparison of Epidermal Biophysical Properties Among Children with Different BMI z-Score |
 |
Figure 2 Correlation of BMI z Score with Epidermal Function. (a) depicts correlation of BMI z score with TEWL, while (b) shows correlation of BMI z score with stratum corneum hydration levels; (c) illustrates correlation between BMI z score and skin surface pH. Number of subjects are indicated in Table 1. Significances are indicated in the Figures. |
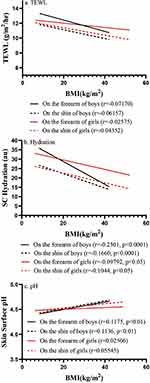 |
Figure 3 Correlation of BMI with Epidermal Function. (a and b) show correlation of BMI with TEWL and stratum corneum hydration levels, respectively; (c) depicts correlation between BMI and skin surface pH. Number of subjects are indicated in Table 1. Significances are indicated in the Figures. |
Discussion
Epidermal function is dominated by keratinocyte function, which is regulated by various external and internal factors, including air pollution, UV irradiation, smoking, diet, psychological stress, sex and age. Epidermal function can be assessed by measuring epidermal biophysical properties such as TEWL, stratum corneum hydration levels and skin surface pH. Some cutaneous conditions, such as atopic dermatitis and psoriasis, display increased TEWL and skin surface pH, and reduced stratum corneum hydration levels.20 Likewise, reduced stratum corneum hydration levels have been observed in individuals with extracutaneous disorders, including type 2 diabetes and obesity.21 Age-associated changes in epidermal function have also been well documented.22–24 We demonstrate here that epidermal function was also associated with the body fatness. TEWL tended to be negatively correlated with BMI z score in both boys and girls, which is contrary to the changes in adults, showing significantly positive correlation of TEWL with BMI.25 However, other studies have shown higher TEWL in children with obesity and lower TEWL in adults with obesity than in normal weight controls. However, the underlying mechanisms accounting for such discrepancies in these studies are unknown. This could be due to differences in the severity of obesity among those studies because individuals with severe obesity display higher TEWL, while subjects with overweight, or class I/ II exhibit lower TEWL.26 However, further studies are needed to elucidate the mechanisms that contribute to the differences in the correlation between BMI and TEWL between children and adults.
Consistent with the results in adults, the present study also demonstrated a negative correlation of BMI with stratum corneum hydration levels in children. However, the correlation between BMI z-scores and stratum corneum hydration levels was not significant in girls. The underlying mechanisms by which BMI z scores are negatively correlated with stratum corneum hydration levels are likely attributable to the reductions in natural moisturizers in the stratum corneum of individuals with obesity. In comparison to normal weight individuals, individuals with obesity have lower levels of ceramide NP and aquaporin 3, a glycerol transporter, in the epidermis, while both ceramide and glycerol are natural moisturizers in the stratum corneum.27 Topical applications of either ceramide NP or glycerol increase stratum corneum hydration.27,28 Thus, reductions in stratum corneum lipid content and epidermal aquaporin 3 expression can contribute to obesity-related decrease in stratum corneum hydration levels, resulting in a negative correlation of BMI z score with stratum corneum hydration levels.
A previous study demonstrated that BMI positively correlates with skin surface pH in adult females, but not in males.18 In contrast, we showed here that both BMI and BMI z-scores were positively correlated with skin surface pH in boys, but not in girls. The positive correlation of BMI with skin surface pH in boys is probably because boys are more physically active than girls. High physical activity increases sweating, whereas sweat rate correlates positively with BMI. The major components of sweat are Na+, K+, CI− and urea. The sweat pH is ≈6.3,29 which is higher than the normal skin surface pH (pH≈5.0).30 Thus, boys display a positive correlation between BMI z score and skin surface pH. In adult females, the significantly positive correlation of BMI with skin surface pH can be ascribed to the estrogen-regulated sweating. The binding of estrogen to its receptor activates endothelial nitric oxide synthase, resulting in an increase in nitric oxide production, consequently leading to increased cutaneous vasodilation and sweating.31 High estrogen decreases threshold for sweating.32 Thus, females sweat more than males do. Thus, correlation of BMI with skin surface pH differs between adults and children.
Conclusions
Both BMI and BMI z score correlate negatively with stratum corneum hydration levels and positively with skin surface pH in boys, but not in girls. Either low stratum corneum hydration or elevated stratum corneum pH can provoke and/exacerbate cutaneous inflammation,33,34 while inflammation is linked to the pathogenesis of obesity.35 Therefore, improvements in stratum corneum hydration and stratum corneum pH are of great importance for mitigation of cutaneous inflammation in individuals with obesity. However, whether improvement in stratum corneum hydration or stratum corneum pH could benefit obesity remains to be explored.
Data Sharing Statement
Data supporting this report are available from corresponding author.
Ethics Approval
The study protocol was reviewed and approved by the Institutional Review Board of Dermatology Hospital (Approval #GDDHLS-2021025).
Consent to Participate Statement
Written informed consent was obtained from the participants’ parent/legal guardians prior to the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported in part by the Guangdong Administration Bureau of Chinese Medicine (20201237 & 20212158) and the Research Foundation of the Joint Laboratory of Dermatology Hospital of Southern Medical University-China Sanjiu Medical & Pharmaceutical Co., Ltd. (HR202107).
Disclosure
All authors declare no conflicts of interest.
References
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–298. doi:10.1038/s41574-019-0176-8
2. Hu J, Liu J, Wang J, et al. Unfavorable progression of obesity in children and adolescents due to COVID-19 pandemic: a school-based survey in China. Obesity. 2021;29(11):1907–1915. doi:10.1002/oby.23276
3. Mu L, Liu J, Zhou G, et al. Obesity Prevalence and Risks Among Chinese Adults: findings From the China PEACE Million Persons Project, 2014-2018. Circ Cardiovasc Qual Outcomes. 2021;14(6):e007292. doi:10.1161/CIRCOUTCOMES.120.007292
4. Kruger J, Ham SA, Prohaska TR. Behavioral risk factors associated with overweight and obesity among older adults: the 2005 National Health Interview Survey. Prev Chronic Dis. 2009;6:A14.
5. Wiklund P. The role of physical activity and exercise in obesity and weight management: time for critical appraisal. J Sport Health Sci. 2016;5(2):151–154. doi:10.1016/j.jshs.2016.04.001
6. Paulo HA, Mosha D, Mwanyika-Sando M, et al. Role of dietary quality and diversity on overweight and obesity among women of reproductive age in Tanzania. PLoS One. 2022;17(4):e0266344. doi:10.1371/journal.pone.0266344
7. Dare S, Mackay DF, Pell JP. Relationship between smoking and obesity: a cross-sectional study of 499,504 middle-aged adults in the UK general population. PLoS One. 2015;10:
8. Yang Z, Song Q, Li J, et al. Air Pollution as a Cause of Obesity: micro-Level Evidence from Chinese Cities. Int J Environ Res Public Health. 2019;16(21):4296. doi:10.3390/ijerph16214296
9. Malihi Z, Portch R, Hashemi L, et al. Modifiable Early Childhood Risk Factors for Obesity at Age Four Years. Child Obes. 2021;17(3):196–208. doi:10.1089/chi.2020.0174
10. Sarwer DB, Polonsky HM. The Psychosocial Burden of Obesity. Endocrinol Metab Clin North Am. 2016;45(3):677–688. doi:10.1016/j.ecl.2016.04.016
11. Rozjabek H, Fastenau J, LaPrade A, et al. Adult Obesity and Health-Related Quality of Life, Patient Activation, Work Productivity, and Weight Loss Behaviors in the United States. Diabetes Metab Syndr Obes. 2020;13:2049–2055. doi:10.2147/DMSO.S245486
12. Tsiros MD, Buckley JD, Howe PR, et al. Day-to-day physical functioning and disability in obese 10- to 13-year-olds. Pediatr Obes. 2013;8(1):31–41. doi:10.1111/j.2047-6310.2012.00083.x
13. Kudel I, Huang JC, Ganguly R. Impact of Obesity on Work Productivity in Different US Occupations: analysis of the National Health and Wellness Survey 2014 to 2015. J Occup Environ Med. 2018;60(1):6–11. doi:10.1097/JOM.0000000000001144
14. Ali Z, Suppli Ulrik C, Agner T, et al. Is atopic dermatitis associated with obesity? A systematic review of observational studies. J Eur Acad Dermatol Venereol. 2018;32:1246–1255. doi:10.1111/jdv.14879
15. Barros G, Duran P, Vera I, et al. Exploring the Links between Obesity and Psoriasis: a Comprehensive Review. Int J Mol Sci. 2022;23:
16. Huang AH, Roh YS, Sutaria N, et al. Real-world disease burden and comorbidities of pediatric prurigo nodularis. J Am Acad Dermatol. 2022;86(3):655–657. doi:10.1016/j.jaad.2021.02.030
17. Monteiro Rodrigues LM, Palma L, Santos O, et al. Excessive Weight Favours Skin Physiology - Up to a Point: another Expression of the Obesity Paradox. Skin Pharmacol Physiol. 2017;30(2):94–101. doi:10.1159/000464338
18. Ye L, Lai Q, Wen S, Wang X, Yang B, Man MQ. Correlation of Body Mass Index with Epidermal Biophysical Properties Varies with Gender in Chinese. Skin Pharmacol Physiol. 2022;35(4):215–223. doi:10.1159/000524295
19. Must A, Anderson SE. Body mass index in children and adolescents: considerations for population-based applications. Int J Obes. 2006;30(4):590–594. doi:10.1038/sj.ijo.0803300
20. Montero-Vilchez T, Segura-Fernández-Nogueras MV, Pérez-Rodríguez I, et al. Skin Barrier Function in Psoriasis and Atopic Dermatitis: transepidermal Water Loss and Temperature as Useful Tools to Assess Disease Severity. J Clin Med. 2021;10:
21. Man MQ, Wakefield JS, Mauro TM, et al. Alterations in epidermal function in type 2 diabetes: implications for the management of this disease. J Diabetes. 2022;14:586–595. doi:10.1111/1753-0407.13303
22. Man MQ, Xin SJ, Song SP, et al. Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large Chinese population. Skin Pharmacol Physiol. 2009;22(4):190–199. doi:10.1159/000231524
23. Mehta HH, Nikam VV, Jaiswal CR, Mehta HB. A cross-sectional study of variations in the biophysical parameters of skin among healthy volunteers. Indian J Dermatol Venereol Leprol. 2018;84(4):521. doi:10.4103/ijdvl.IJDVL_1151_15
24. Zhao C, Wang X, Mao Y, et al. Variation of biophysical parameters of the skin with age, gender, and lifestyles. J Cosmet Dermatol. 2021;20(1):249–255. doi:10.1111/jocd.13453
25. Yudhistira MY, Kusumawardani A, Widhiati S, et al. The relationship between increased body mass index with transepidermal water loss: a comparative study. J Gen Proced Dermatol Venereol Indones. 2022;6:25–30. doi:10.19100/jdvi.v6i1.351
26. Tavares L, Palma L, Santos O, et al. Impact of overweight on the normal physiology of human in vivo skin. Biomed Biopharm Res. 2013;10:55–63.
27. Fluhr JW, Mao-Qiang M, Brown BE, et al. Glycerol regulates stratum corneum hydration in sebaceous gland deficient (asebia) mice. J Invest Dermatol. 2003;120(5):728–737. doi:10.1046/j.1523-1747.2003.12134.x
28. Lim SH, Kim EJ, Lee CH, et al. A Lipid Mixture Enriched by Ceramide NP with Fatty Acids of Diverse Chain Lengths Contributes to Restore the Skin Barrier Function Impaired by Topical Corticosteroid. Skin Pharmacol Physiol. 2022;35(2):112–123. doi:10.1159/000518517
29. Sato K, Kang WH, Saga K, et al. Biology of sweat glands and their disorders. I Normal Sweat Gland Function J Am Acad Dermatol. 1989;20:537–563. doi:10.1016/s0190-9622(89)70063-3
30. Lambers H, Piessens S, Bloem A, Pronk H, Finkel P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci. 2006;28(5):359–370. doi:10.1111/j.1467-2494.2006.00344.x
31. Charkoudian N, Stachenfeld N. Sex hormone effects on autonomic mechanisms of thermoregulation in humans. Auton Neurosci. 2016;196:75–80. doi:10.1016/j.autneu.2015.11.004
32. Stephenson LA, Kolka MA. Esophageal temperature threshold for sweating decreases before ovulation in premenopausal women. J Appl Physiol. 1985;86:22–28. doi:10.1152/jappl.1999.86.1.22
33. Xu W, Jia S, Xie P, et al. The expression of proinflammatory genes in epidermal keratinocytes is regulated by hydration status. J Invest Dermatol. 2014;134(4):1044–1055. doi:10.1038/jid.2013.425
34. Jang H, Matsuda A, Jung K, et al. Skin pH Is the Master Switch of Kallikrein 5-Mediated Skin Barrier Destruction in a Murine Atopic Dermatitis Model. J Invest Dermatol. 2016;136(1):127–135. doi:10.1038/JID.2015.363
35. Yang S, Zhu T, Wakefield JS, Mauro TM, Elias PM, Man MQ. Link between obesity and atopic dermatitis: does obesity predispose to atopic dermatitis, or vice versa? Exp Dermatol. 2023;32(7):975–985. doi:10.1111/exd.14801
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
