Back to Journals » Journal of Pain Research » Volume 13
Blue Mussel (Mytilus edulis) Water Extract Ameliorates Inflammatory Responses and Oxidative Stress on Osteoarthritis in Obese Rats
Authors Chang HW, Sudirman S , Yen YW , Mao CF , Ong AD, Kong ZL
Received 31 December 2019
Accepted for publication 6 March 2020
Published 21 May 2020 Volume 2020:13 Pages 1109—1119
DOI https://doi.org/10.2147/JPR.S244372
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Robert B. Raffa
Heng-Wei Chang,1 Sabri Sudirman,2 Yu-Wen Yen,1 Chien-Feng Mao,1 Alan Darmasaputra Ong,1 Zwe-Ling Kong1
1Department of Food Science, National Taiwan Ocean University, Keelung City 20224, Taiwan; 2Fisheries Product Technology, Faculty of Agriculture, Universitas Sriwijaya, Palembang, Ogan Ilir Regency 30862, Indonesia
Correspondence: Zwe-Ling Kong
Department of Food Science, National Taiwan Ocean University, Keelung City 20224, Taiwan
Tel/Fax +886-24622192 #5130
Email [email protected]
Purpose: To investigate the effects of Mytilus edulis water extract (MWE) on an anterior cruciate ligament transection and a partial medial meniscectomy surgery to induced osteoarthritis (OA) with the high-fat diet (HFD)-induced obese rats.
Methods: The male Sprague-Dawley rats were fed with HFD for 4 weeks before surgery. The OA rats were orally administered with MWE (108.5, 217.0, and 542.5 mg/kg) for 6 weeks.
Results: The administration of MWE affected weight loss, triglycerides content, and total cholesterol level. MWE also enhanced the activity of superoxide dismutase and decreased lipid peroxidation degree. Moreover, MWE reduced proinflammatory cytokines level, alleviated inflammation and swelling of the osteoarthritic knee, and reduced loss of proteoglycan in articular cartilage tissue.
Conclusion: MWE suppressed proinflammatory mediators and attenuated the cartilage degradation and pain in osteoarthritis rats under obesity condition. Therefore, MWE has the potential to act as an alternative for osteoarthritis treatment.
Keywords: medial meniscectomy, Mytilus edulis, obesity, osteoarthritis
Introduction
Osteoarthritis (OA) is one of the age-related diseases which often occurs in the older population and has high prevalence on the knees, hips, and hands.1 OA causes joint pain, stiffness, and insufficient bending angle bring to inconvenience life.2 Obesity is considered as one of the OA risk factors, especially for knee OA.3 The proportion of obese patients with arthritis was significantly higher than normal-weight patients.4 Excessive body weight causes increasing of the mechanical loading in the knee joint and stimulates the chondrocytes of articular cartilage to release proinflammatory cytokines.5 Additionally, adipocytes under the obese condition secrete cytokines and adipokines, and leads to OA progression.6
Post-traumatic arthritis is known as an animal model for OA studies described by a previous study.7 According this model, anterior cruciate ligament transection (ACLT) can result in the instability of the knee joint.8,9 Trauma sustained in joint tissues, mainly tears of the meniscus or anterior cruciate ligament caused injuries to articular cartilage. Total or partial meniscectomy can disturb the natural loading mechanism of the knee joint, which in turn its increases the amount of strain on the articular cartilage associated with OA development.10–12 The recent study reported that obesity from a high-fat diet was an independent risk factor for the onset of OA in the rat with an ACLT knee.13 The OA treatments such as oral drugs therapy and artificial joint replacement surgery can ameliorate signs and symptoms of OA, but it may be accompanied with some side effects and seems not effective for long-term application.14 Therefore, we would like to prevent or alleviate the pain and OA progression through functional foods or natural products. Marine organisms were recognized by their biological functions such as antioxidant, anti-inflammatory, and anticancer activity.15–17
The blue mussel (Mytilus edulis) is a commercially valuable aquatic resource. This mussel has some important nutrient contents, such as polysaccharides, protein, essential fatty acids, riboflavin, and carotenoids.18,19 Additionally, Mytilus edulis protein hydrolysates show antioxidant activity and anti-inflammatory properties.18 Carbohydrate is known as the major bioactive component of the mussel and primarily represented by polysaccharides. In some cases, sugar components can covalently be linked to the polypeptide side chain of the cell membrane proteins and form glycoprotein.20–22 Glycoprotein is an essential molecule involved in the immune response.23 According to the previous studies, carbohydrate and glycoprotein can be extracted by using a hot-water.20,24 A previous study also reported that Mytilus edulis contains gluconic polysaccharides and possesses scavenging effects to suppresses oxidative stress.25 The Mytilus edulis water extract suppressed adipogenesis in vitro.26 The most recent study reported that supplementation of M. edulis reduces the pain and fatigue in patients with rheumatoid arthritis.27 A previous study also reported that water extract from Mytilus edulis possesses anti-obesity effects by inhibited lipid accumulation and also improves the male reproduction dysfunction in high-fat diet-induced obese rats. It also reduced leptin levels and enhanced some enzymatic antioxidant activities such as superoxide dismutase and glutathione peroxidase.28 An increasing level of leptin and reducing activities of enzymatic antioxidants were positively associated with osteoarthritis development.29,30 According to these findings, we hypothesized that water extract from Mytilus edulis also can ameliorates the cartilage degradation in this experimental model. However, the effects of this mussel extract on inflammatory cytokines, oxidative stress, and proteoglycans loss, as well as some mechanical evaluation in post-traumatic osteoarthritis with obesity model, has not been reported. Therefore, this study was aimed to investigate the ameliorative effects of Mytilus edulis water extract (MWE) on an anterior cruciate ligamentous and a medial meniscal surgery (ACLT+MMx) with a high-fat diet (HFD)-induced osteoarthritis in rat model.
Materials and Methods
Mytilus Edulis Extraction
The blue mussel (Mytilus edulis) was harvested in Matsu Island (Lienchiang, Taiwan). The mussel was extracted by boiling water according to a previous method.26 Briefly, the whole blue mussel was extracted by the boiling water (1:2, w/v) for 1 h and the filtrate and residue were separated. The extraction was performed two times with the same condition. Finally, the Mytilus edulis water extract (MWE) powder was obtained by a freeze-drying process of the filtrate. According to a previous study, the major compounds of this extract was composed by carbohydrate (34.71%) and protein (30.20%).28
Animal Experiment
Forty-two of 5-weeks old male Sprague-Dawley (SD) rats were purchased from the BioLASCO Taiwan Co., Ltd. (Yilan, Taiwan) and housed individually in a 12 h dark/light cycle. All of the procedures were carried out according to the Animal Protection Act (Act/APC) and the Experimental Animal Ethics Committee of the Council of Agriculture (CoA) of the Executive Yuan and the standard of Institutional Animal Care and Use Committee (IACUC Approval No. 105,030) of National Taiwan Ocean University, Taiwan. Briefly, rats were fed with standard rodent chow (chow-fed diet, LabDiet 5001 Rodent Diet) for 1-week acclimatization phase, and then it divided into 2 main groups. One group (n = 7) was continued to fed with a chow-fed diet (CFD) and the other groups (n = 35) were fed with the high-fat diet (HFD) for 4 weeks for developing obesity. The CFD and HFD diet compositions were shown in Table 1. The body weight of obesity was approximately more than 20% of normal or sham body weight.31
 |
Table 1 Chow-Fed and High-Fat Diet Compositions |
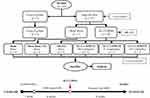
After 4 weeks, the obese group were divided into 5 subgroups: Obese sham group, the rats were fed with HFD and without any surgeries on the right knee (OB Sham, oral gavage by water); Obese rats with an anterior cruciate ligament transection and a partial medial meniscectomy (ACLT+MMx), but without MWE-treated (OB+OA group, oral gavage by water); and OB+OA groups were daily oral gavage with three different doses of MWE (108.5 mg/kg of body weight/day, OB+OA+MWE1X; 217.0 mg/kg/day, OB+OA+MWE2X, and 542.5 mg/kg/day, OB+OA+MWE5X) and were treated for 6 weeks (total fed with HFD about 10 weeks) (Figure 1). The rats were fasted for 12 h before sacrifice and euthanatized by exposure CO2 in the empty chamber. The blood sample, organs, and the operated knee were collected for future analysis.
Knee Surgery
After the phase of diet-induced obesity, the ACLT+MMx surgery was performed to induce the post-traumatic OA model. Rats were anesthetized with 25 mg/kg of Zoletil 50 (Virbac, France) by intraperitoneal injection. After, the hairs on right knee were removed and disinfected with iodine, cut off the skin vertically on the knee; an anterior cruciate ligament and a partial medial meniscus were transacted and removed to induce OA (OB+OA group). A surgery was conducted in sham group by entering the joint without any damages to ligament and meniscus (OB sham group). After the surgery, the wound was washed with sterile saline and was sutured by 4–0 chromic catgut (Unik, Taiwan) and braided silk (Unik, Taiwan). Cephalosporin antibiotic (10 mg/kg) was injected intraperitoneally to avoids postoperative infection for 3 days.33
Sample Collection
Blood samples were collected by using heparinized-syringe into collection tubes and centrifuged at 1000× g at 4°C for 15 min to get the plasma. The plasma was stored for future analyses at −80°C. The removed-knees were fixed in 4% formaldehyde solution and sent to Rapid Science Co., Ltd. (Taichung, Taiwan) for making slices.
Knee Width and Pain Behavior Measurement
The width of the knee joints of rats before the ACLT+MMx surgery was measured by using an electronic digital caliper. The width joints were measured every week after the surgery.34 The pain behavior of the rat’s joints after the surgery was evaluated by using Incapacitance tester.35 Briefly, the rats were trained to stand in the box located on the Incapacitance tester to evaluate the bipedal balance. Under normal situations, the weight of the rat hindlimb will be distributed equally. However, one side of the knee injury contributed to the bipedal imbalance. The measurement was tested 3 times during analysis.
Plasma Biochemical Assay
Plasma triglycerides (TG) and total cholesterol (TC) concentration were detected by commercial kits (Randox Laboratories, UK). The low-density lipoprotein-cholesterol (LDL-C) and high-density lipoprotein-cholesterol (HDL-C) were determined by commercial kits (Fortress Diagnostics, UK). Superoxide dismutase (SOD) activity was measured by kit (Randox Laboratories, UK). Tumor necrosis factor (TNF)-α (Abcam, UK) and interleukin (IL)-1β (R&D Systems, USA) in plasma were detected by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocols. Plasma malonaldehyde (MDA) was estimated by following the previous method.36 Plasma (100 μL) was mixed with 200 μL of a reactive solution (15% (w/v) trichloroacetic acid in 0.25 N HCl and 0.375% (w/v) thiobarbituric acid in 0.25 N HCl) and was placed at 100°C in a water bath for 15 min. After the cooling, 300 μL of n-butanol was added and centrifuged at 1500× g for 10 min. The supernatant was collected and measured at the 532 nm absorbance.
Knee Histopathology Analysis
Safranin-O/Fast green staining method was adopted according to the previous method.37 The slices were deparaffinized, hydrated with distilled water, and immersed in Wiegert’s iron hematoxylin (ferric chloride, aqueous: 1% hematoxylin 1%, alcoholic = 1:1) for 5 min. Then, it was differentiated by 1% acid alcohol and washed the slices gently by distilled water. The slices were soaked in 0.1% fast green, 1% acetic acid and 1% Safranin-O for 5 min and rinsed in 95% alcohol in the end. The histopathology of OA on knee joints was scored by according to the Osteoarthritis Research Society International (OARSI) as described by previous methods as shown in Table 2.38,39
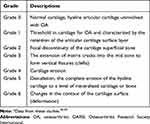 |
Table 2 The Knee Histopathology Scoring According to OARSI Grading* |
Statistical Analysis
The SPSS 22.0 software was used to analyze the experimental data. All data were expressed as mean ± standard error of the mean (SEM.). The comparison of specific the group was based on a single factor difference analysis, and the statistical difference was analyzed by a unique t-test (independent sample). Multiple comparisons of the different groups were analyzed by Duncan’s test at a value of p < 0.05 significant level.
Results
Effects of MWE on Weight and Body Compositions
The weight of all the OB groups significantly higher than the Sham group and it was about 27% (Figure 2). The data showed that the obesity was induced in OB groups. We also observed the weight of the OB+OA+MWE5X group was lower than the OB+OA groups. The adipose tissue weight of the OB group significantly higher than the Sham group. Treated with high doses of MWE (MWE5X) significantly decreased the abdominal adipose tissue weight (Table 3). Therefore, we found that the MWE has beneficial effects on inhibiting weight gain.
 |
Table 3 Effects of Mytilus Edulis Water Extract Supplementation on Body Weight, Organ Weight, and Adipose Tissue Weight of Rats After Treatment for 6 Weeks |
Effects of MWE on Plasma Lipid
The total plasma cholesterol (TC) increased significantly in the OB group when compared to the Sham group. After treatment with MWEs for 6 weeks, the TC decreased significantly as compared to the OB group (Table 4). The LDL-C/HDL-C also significantly decreased after treated with high-dose of MWE especially in the high-dose of MWE (MWE5X).
 |
Table 4 Effects of Mytilus Edulis Water Extract on Plasma Triglycerides, Total Cholesterol, and LDL-Cholesterol/HDL-Cholesterol of Rats After Treatment for 6 Weeks |
Effects of MWE on Proinflammatory Cytokines Level
To investigate the inflammation level under OB and OA conditions, we used the ELISA kits to detect the plasma TNF-α and IL-1β. The results indicated that the concentration of TNF-α increased in the OB+OA group and declined after fed by MWE (Figure 3A). However, there were no differences in the plasma IL-1β level in all groups (Figure 3B).
Effects of MWE on SOD Activity and MDA Level
The result showed that the MWE enhanced superoxide dismutase (SOD) activities especially in the high-dose of the MWE group, however no significant when compared to the OB+OA group (Figure 4A). Additionally, we also measured the concentration of the plasma malondialdehyde (MDA) to evaluate lipid peroxidation and the results indicated that the plasma MDA in the OB+OA+MWE2X group and the OB+OA+MWE5X group was significantly reduced when compared to untreated OB+OA group. Therefore, MWE might have the effect of attenuating oxidative stress (Figure 4B).
MWE Improved Articular Cartilage Degradation
As shown in Figure 5A, hematoxylin and eosin (H&E) staining was used to evaluate the distribution of chondrocyte and histology of articular cartilage. We found that there was no tears and abnormal chondrocytes on the articular cartilage surface on the Sham group and OB group. The abnormal proliferation (clusters) and hypertrophy of chondrocytes were observed in the OB+OA group. Furthermore, we used the Safranin-O/fast green stain to examine the level of proteoglycan loss on the cartilage surface. The results indicated that MWE2X or MWE5X could reduce proteoglycan loss (Figure 5B). The sham group showed the healthy (normal) joint tissue with the normal architecture matrix (Grade 0). However, the OB+OA group showed the degradation of articular cartilage, as indicated by the loss of staining on the slice (Grade 2). Whereas, treatment with high-dose of MWE suppresses loss of cartilage (Grade 1).
Effects of MWE on Knee Joint
After the ACLT+MMx surgery, the bipedal balance has broken down in all the OB+OA groups. However, the bipedal imbalance improved after treated by MWEs (Figure 6A). Additionally, we found that the rats were fed with MWE2X and MWE5X could significantly alleviated the level of swelling (Figure 6B).
Discussion
This study demonstrated the oral administration of Mytilus edulis water extracts on post-traumatic osteoarthritis (OA) with obesity in the rat model. An anterior cruciate ligament transection induced post-traumatic OA with a partial medial meniscectomy in the right knee was the key of experimental design. Whereas, a high-fat diet-induced the obesity condition for 4 weeks before induced OA. The Mytilus edulis water extract (MWE) was orally administrated to rat for 6 weeks (Figure 1). A previous study reported that the major compounds of Mytilus edulis water extract was carbohydrates and protein.28 In some cases, sugar components can covalently be linked to the polypeptide side chain of the cell membrane proteins and form glycoprotein.22 Glycoprotein is an essential molecule involved in the immune response.23 In this study, the glycoprotein compound was extracted by using boiling water according to the previously studies.26 Additionally, a previous study also reported that the glycoprotein could be extracted by using boiling water and the glycoprotein form was verified by using high-performance liquid chromatography (HPLC) and Sepharose CL-6B column chromatography.28,40 According to these studies, glycoprotein compounds could exhibit their biological activities, although it extracted by boiling water. In the clinical applications, M. edulis extract possesses anti-obesity protein on a high-fat diet-induced obese rat model.28 The most recent study related to arthritis disease, it reduced the pain and fatigue in a patient with rheumatoid arthritis.27
Obesity condition is characterized by increasing body weight and it results from the excessive accumulation of fat in the adipose tissues. Previous studies reported that the joint loading on the knee was increased with increasing body weight.41,42 The present study showed that the body weight was increased in high-fat diet group when it compared to chow-fed diet group (Figure 2) and high-dose of MWE treatment could reduce the body weight. Reducing the body weight could decrease the joint loading or mechanical force of the knee. Therefore, reducing body weight is one of the non-pharmacological treatment for OA management.43 Additionally, reduction of the body weight also accompanied by a reduction of the epididymal and retroperitoneal fat (Table 3). Adipose tissues are mainly composed of adipocytes. Its source of some proinflammatory cytokines, such as TNF-α, IL-1β, and leptin.44 Therefore, by reducing adipose weight, it also can reduce these proinflammatory expressions. The extract also reduced the plasma lipid properties, such as triglycerides, total cholesterol, and LDL-cholesterol (Table 4). Previous studies reported that high expressions of TG and TC were associated with OA progression.45,46 These results supported by the earlier study that the in-vitro study of Mytilus edulis water extract possessed anti-adipogenesis activity and inhibited the lipid accumulation on 3T3-L1 cells.26 A previous study also reported that Mytilus edulis water extract showed the anti-obesity properties by inhibited lipid accumulation.28 Additionally, MWE increased the HDL-cholesterol level. These results show that MWE possessed an anti-obesity and might be promoting as anti-arthritic.
Accumulation of adipose tissue can induce the production of some proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6.47 These cytokines have been reported it is associated with the development of OA by induction some degradation enzymes include matrix metalloproteinases (MMPs), such as MMP-1, MMP-3, and MMP-13.48,49 Our results showed that treated with MWE could reduce TNF-α expression, however no effect on IL-1β expression (Figure 3A). This condition, due to different types of cytokines, also shown a different response or interaction mechanism to bioactive compounds.50 A previous study also reported that similar conditions with the present study, whereas fucoidan extract could be significantly reduced TNF-α, but no significant effect on IL-1β level.39
Blocking the production of TNF-α is one of targeting for prevents or reduces the OA progression.51 Additionally, overexpression of proinflammatory has a strong correlation with the raising of oxidative stress. We found that obesity with osteoarthritis (OB+OA) has a high level of MDA (lipid peroxidation marker) production and low level of superoxide dismutase (SOD) antioxidant (Figure 4). The main function of antioxidant enzymes is to eliminate free radicals and prevent the cells and tissues. The previous study reported that oxidative stress positively correlated with OA development.52,53 Therefore, reducing the oxidative stress level is a typical method to reduce the OA progression by increasing the antioxidant activity.54 Oral administration of MWE for 6 weeks could reduce oxidative stress by enhancing the SOD activity and reducing the level of lipid peroxidation product such as MDA (Figure 4).
Based on the Safranin-O/fast green staining, Sham or normal group showed the normal cartilage matrix without loss of proteoglycan structure (Grade 0). Grade 0 is characterized by hyaline articular cartilage uninvolved with OA and smooth of the cartilage surface. On another hand, the OA condition leads to the loss of proteoglycan and focal discontinuity of the superficial cartilage zone (Grade 2). After treatment with MWE for 6 weeks, the high-dose of MWE suppressed the proteoglycan loss (Grade 1) (Figure 5B). Grade 1 is characterized by the retention of the articular surface layer.38 The structure of the cartilage proteoglycan changed and it was considered the most common event during OA.55 The overexpression of some degradation enzymes (e.g., MMPs) lead to cartilage degeneration, whereas MMPs can be induced by some proinflammatory cytokines such as TNF and IL-1 family.56,57 We found that after ACLT+MMx surgery, the weight-bearing difference and knee swelling were increased in all surgery groups (Figure 6A) and it was evaluated by taking incapacitance tester measurement (Figure 6B). Treatment with MWE for 6 weeks reduced the weight-bearing difference and knee swelling. In most cases, OA causes joint swelling, pain, and disability to the patient.58
In the present study, we also found that low-dose of MWE treatment (OB+OA+MWE1x) show more proteoglycan loss than untreated-OA group (OB+OA). However, the weight-bearing and knee swelling cases, low-dose treatment more effective effects. We hypothesized that even if low-dose treatment of MWE, it could be reducing the prostaglandin (PG)-E2 expression. However, the dose is not enough to reduce enzymes related to cartilage degradation, such as MMPs. Additionally, the PG-E2 expression of is correlated with the sign of inflammation such as pain and swelling.59 Through the transcriptional factor NF-κB pathway, the expression of some chondrolytic mediators, such as PG-E2 and MMPs can induce by IL-1β and TNF-α.56
Conclusion
Overall, oral supplementation of Mytilus edulis water extract (MWE) showed the ameliorative effects on osteoarthritis (OA) progression in the obese rat model. The MWE reduced total cholesterol level and ameliorated oxidative stress by enhancing the activity of superoxide dismutase and decreasing the degree of lipid peroxidation. Moreover, MWE also reduced the level of the proinflammatory cytokines and thereby alleviated the inflammation and knee swelling. Oral administration of MWE also reduced the loss of proteoglycan in articular cartilage tissue of the knee OA. Therefore, MWE has the potential to be an alternative for osteoarthritis treatment.
Acknowledgments
The sponsor had no role in the design and conduct of the study, in the collection, analysis, and in the preparation, review or approval of the manuscript and the decision to submit the manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shane Anderson A, Loeser RF. Why is osteoarthritis an age-related disease? Best Pract Res Clin Rheumatol. 2010;24(1):15–26. doi:10.1016/j.berh.2009.08.006
2. Bijlsma JWJ, Berenbaum F, Lafeber FPJG. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–2126. doi:10.1016/S0140-6736(11)60243-2
3. Felson DT. Osteoarthritis: new insights. part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. doi:10.7326/0003-4819-133-8-200010170-00016
4. Koonce RC, Bravman JT. Obesity and osteoarthritis: more than just wear and tear. J Am Acad Orthop Surg. 2013;21(3):161–169. doi:10.5435/JAAOS-21-03-161
5. Coggon D, Reading I, Croft P, McLaren M, Barrett D, Cooper C. Knee osteoarthritis and obesity. Int J Obes. 2001;25(5):622–627. doi:10.1038/sj.ijo.0801585
6. Berenbaum F, Eymard F, Houard X. Osteoarthritis, inflammation and obesity. Curr Opin Rheumatol. 2013;25(1):114–118. doi:10.1097/BOR.0b013e32835a9414
7. Castrogiovanni P, Di Rosa M, Ravalli S, et al. Moderate physical activity as a prevention method for knee osteoarthritis and the role of synoviocytes as biological key. Int J Mol Sci. 2019;20(3):3. doi:10.3390/ijms20030511
8. Anderson DD, Chubinskaya S, Guilak F, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29(6):802–809. doi:10.1002/jor.v29.6
9. Szychlinska M, Trovato F, Di Rosa M, et al. Co-expression and co-localization of cartilage glycoproteins CHI3L1 and lubricin in osteoarthritic cartilage: morphological, immunohistochemical and gene expression profiles. Int J Mol Sci. 2016;17(3):359. doi:10.3390/ijms17030359
10. Song Y, Greve JM, Carter DR, Giori NJ. Meniscectomy alters the dynamic deformational behavior and cumulative strain of tibial articular cartilage in knee joints subjected to cyclic loads. Osteoarthr Cartilage. 2008;16(12):1545–1554. doi:10.1016/j.joca.2008.04.011
11. Little CB, Fosang AJ. Is cartilage matrix breakdown an appropriate therapeutic target in osteoarthritis – insights from studies of aggrecan and collagen proteolysis? Curr Drug Targets. 2010;11(5):561–575. doi:10.2174/138945010791011956
12. Roemer FW, Frobell R, Hunter DJ, et al. MRI-detected subchondral bone marrow signal alterations of the knee joint: terminology, imaging appearance, relevance and radiological differential diagnosis. Osteoarthr Cartilage. 2009;17(9):1115–1131. doi:10.1016/j.joca.2009.03.012
13. Collins KH, Reimer RA, Seerattan RA, Leonard TR, Herzog W. Using diet-induced obesity to understand a metabolic subtype of osteoarthritis in rats. Osteoarthr Cartilage. 2015;23(6):957–965. doi:10.1016/j.joca.2015.01.015
14. Fibel KH, Howard JH, Brian CH. State-of-the-art management of knee osteoarthritis. World J Clin Cases. 2015;3(2):89–101. doi:10.12998/wjcc.v3.i2.89
15. Li G, Fu Y, Zheng J, Li D. Anti-inflammatory activity and mechanism of a lipid extract from hard-shelled mussel (Mytilus coruscus) on chronic arthritis in rats. Mar Drugs. 2014;12(2):568–588. doi:10.3390/md12020568
16. Kim E-K. Purification of a novel anticancer peptide from enzymatic hydrolysate of Mytilus coruscus. J Microbiol Biotechnol. 2012;22(10):1381–1387. doi:10.4014/jmb
17. Gorinstein S, Moncheva S, Katrich E, et al. Antioxidants in the black mussel (Mytilus galloprovincialis) as an indicator of black sea coastal pollution. Mar Pollut Bull. 2003;46(10):1317–1325. doi:10.1016/S0025-326X(03)00239-X
18. Park SY, Ahn C-B, Je J-Y. Antioxidant and anti-inflammatory activities of protein hydrolysates frommytilus edulisand ultrafiltration membrane fractions. J Food Biochem. 2014;38(5):460–468. doi:10.1111/jfbc.12070
19. McPhee S, Hodges LD, Wright PFA, Wynne PM, Kalafatis N, Macrides TA. Prophylactic and therapeutic effects of Mytilus edulis fatty acids on adjuvant-induced arthritis in male wistar rats. Prostaglandins Leukot Essent Fatty Acids. 2010;82(2–3):97–103. doi:10.1016/j.plefa.2009.12.003
20. Grienke U, Silke J, Tasdemir D. Bioactive compounds from marine mussels and their effects on human health. Food Chem. 2014;142:48–60. doi:10.1016/j.foodchem.2013.07.027
21. Debray H, Michalski J-C, Spik G. Glycoprotein analysis: general methods. In: Encyclopedia of Analytical Chemistry. 2006.
22. Smital T, Kurelec B. The chemosensitizers of multixenobiotic resistance mechanism in aquatic invertebrates: a new class of pollutants. Mutat Res. 1998;399(1):43–53. doi:10.1016/S0027-5107(97)00265-0
23. Rudd PM. Glycosylation and the immune system. Science. 2001;291(5512):2370–2376. doi:10.1126/science.291.5512.2370
24. Asada M, Fukumori Y, Inoue M, et al. Glycoprotein derived from the hot water extract of mint plant, perilla frutescensBritton. J Agric Food Chem. 1999;47(2):468–472. doi:10.1021/jf9802777
25. Cheng S, Yu X, Zhang Y. Extraction of polysaccharides from Mytilus edulis and their antioxidant activity in vitro. Shipin Gongye Keji. 2010;31:132–134.
26. Shon M-S, Kim S-K, Song J-H, Lee S-C, Kim G-N. Anti-adipogenic activity of blue mussel (Mytilus edulis) extract by regulation of 3T3-L1 adipogenesis through Wnt/β-catenin signaling pathway. Food Sci Biotechnol. 2015;24(1):315–321. doi:10.1007/s10068-015-0042-y
27. Lindqvist H, Gjertsson I, Eneljung T, Winkvist A. Influence of blue mussel (Mytilus edulis) intake on disease activity in female patients with rheumatoid arthritis: the MIRA randomized cross-over dietary intervention. Nutrients. 2018;10(4):481. doi:10.3390/nu10040481
28. Lu Y-C, Sudirman S, Mao C-F, Kong Z-L. Glycoprotein from Mytilus edulis extract inhibits lipid accumulation and improves male reproductive dysfunction in high-fat diet-induced obese rats. Biomed Pharmacother. 2019;109:369–376. doi:10.1016/j.biopha.2018.10.180
29. Vuolteenaho K, Koskinen A, Kukkonen M, et al. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage—mediator role of NO in leptin-inducedPGE2, IL-6, and IL-8 production. Mediators Inflamm. 2009;2009:1–10. doi:10.1155/2009/345838
30. Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta Mol Basis Dis. 2016;1862(4):576–591. doi:10.1016/j.bbadis.2016.01.003
31. Komaroff M. For researchers on obesity: historical review of extra body weight definitions. J Obes. 2016;2016:1–9.
32. Woods SC, Seeley RJ, Rushing PA, D’Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133(4):1081–1087. doi:10.1093/jn/133.4.1081
33. Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong LT. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38(2):234–243. doi:10.1016/j.bone.2005.08.007
34. Liu -C-C, Su L-J, Tsai W-Y, et al. 20 attenuates posttraumatic osteoarthritis progression: association with upregulated expression of the circadian gene NPAS2. Life Sci. 2015;141:20–24. doi:10.1016/j.lfs.2015.09.007
35. Sagar DR, Ashraf S, Xu L, et al. Osteoprotegerin reduces the development of pain behaviour and joint pathology in a model of osteoarthritis. Ann Rheum Dis. 2014;73(8):1558–1565. doi:10.1136/annrheumdis-2013-203260
36. Placer ZA, Cushman LL, Johnson BC. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem. 1966;16(2):359–364. doi:10.1016/0003-2697(66)90167-9
37. Schmitz N, Laverty S, Kraus VB, Aigner T. Basic methods in histopathology of joint tissues. Osteoarthr Cartilage. 2010;18:S113–S116. doi:10.1016/j.joca.2010.05.026
38. Pritzker KPH, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartilage. 2006;14(1):13–29. doi:10.1016/j.joca.2005.07.014
39. Sudirman S, Ong AD, Chang HW, Kong ZL. Effect of fucoidan on anterior cruciate ligament transection and medial meniscectomy induced osteoarthritis in high-fat diet-induced obese rats. Nutrients. 2018;10(6):686. doi:10.3390/nu10060686
40. Chen N, Zhao J, Li Y, et al. Chemical composition of glycoprotein from boiling water extract of sea cucumber. J Food Sci. 2015;36(8):125–128.
41. Pottie P, Presle N, Terlain B, Netter P, Mainard D, Berenbaum F. Obesity and osteoarthritis: more complex than predicted. Ann Rheum Dis. 2006;65(11):1403–1405. doi:10.1136/ard.2006.061994
42. Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52(7):2026–2032. doi:10.1002/(ISSN)1529-0131
43. Puett DW, Griffin MR. Published trials of nonmedicinal and noninvasive therapies for hip and knee osteoarthritis. Ann Intern Med. 1994;121(2):133–140. doi:10.7326/0003-4819-121-2-199407150-00010
44. Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:1–12. doi:10.1155/2013/139239
45. Davies-Tuck ML, Hanna F, Davis SR, et al. Total cholesterol and triglycerides are associated with the development of new bone marrow lesions in asymptomatic middle-aged women - a prospective cohort study. Arthritis Res Ther. 2009;11(6):6. doi:10.1186/ar2873
46. Garcia-Gil M, Reyes C, Ramos R, et al. Serum lipid levels and risk of hand osteoarthritis: the chingford prospective cohort study. Sci Rep. 2017;7(1). doi:10.1038/s41598-017-03317-4
47. Chandel NS, Schumacker PT, Arch RH. Reactive oxygen species are downstream products of TRAF-mediated signal transduction. J Biol Chem. 2001;276(46):42728–42736. doi:10.1074/jbc.M103074200
48. Ahn S-J, Rhim E-M, Kim J-Y, et al. Tumor necrosis factor-α induces matrix metalloproteinases-3, −10, and −13 in human periodontal ligament cells. J Periodontol. 2014;85(3):490–497. doi:10.1902/jop.2013.130063
49. Rigoglou S, Papavassiliou AG. The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013;45(11):2580–2584. doi:10.1016/j.biocel.2013.08.018
50. Pan M-H, Lai C-S, Dushenkov S, Ho C-T. Modulation of inflammatory genes by natural dietary bioactive compounds. J Agric Food Chem. 2009;57(11):4467–4477. doi:10.1021/jf900612n
51. Maksymowych WP, Russell AS, Chiu P, et al. Targeting tumour necrosis factor alleviates signs and symptoms of inflammatory osteoarthritis of the knee. Arthritis Res Ther. 2012;14(5):5. doi:10.1186/ar4044
52. Maneesh M, Jayalekshmi H, Suma T, Chatterjee S, Chakrabarti A, Singh TA. Evidence for oxidative stress in osteoarthritis. Indian J Clin Biochem. 2005;20(1):129–130. doi:10.1007/BF02893057
53. Srivastava S, Saksena AK, Khattri S, Kumar S, Dagur RS. Status of oxidative stress biomarkers in osteoarthritis patients in North Indian population. Osteoarthr Cartilage. 2015;23:A84–A85. doi:10.1016/j.joca.2015.02.785
54. Poulet B, Beier F. Targeting oxidative stress to reduce osteoarthritis. Arthritis Res Ther. 2016;18(1):1. doi:10.1186/s13075-015-0908-7
55. Hardingham T, Bayliss M. Proteoglycans of articular cartilage: changes in aging and in joint disease. Semin Arthritis Rheum. 1990;20(3):12–33. doi:10.1016/0049-0172(90)90044-G
56. Robinson WH, Lepus CM, Wang Q, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(10):580–592. doi:10.1038/nrrheum.2016.136
57. Burrage PS. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11(1):1. doi:10.2741/1817
58. Krasnokutsky S, Attur M, Palmer G, Samuels J, Abramson SB. Current concepts in the pathogenesis of osteoarthritis. Osteoarthr Cartilage. 2008;16:S1–S3. doi:10.1016/j.joca.2008.06.025
59. Ricciotti E, FitzGerald GA. Prostaglandins and Inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986–1000. doi:10.1161/ATVBAHA.110.207449
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.