Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Blood Eosinophil Count and Hospital Readmission in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease
Authors Hegewald MJ , Horne BD , Trudo F, Kreindler JL, Chung Y , Rea S, Blagev DP
Received 12 June 2020
Accepted for publication 7 October 2020
Published 23 October 2020 Volume 2020:15 Pages 2629—2641
DOI https://doi.org/10.2147/COPD.S251115
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Matthew J Hegewald,1,2 Benjamin D Horne,3,4 Frank Trudo,5 James L Kreindler,5 Yen Chung,5 Susan Rea,6 Denitza P Blagev1,2
1Pulmonary and Critical Care Medicine Division, Intermountain Medical Center, Murray, UT, USA; 2Pulmonary and Critical Care Medicine, University of Utah, Salt Lake City, UT, USA; 3Intermountain Heart Institute at Intermountain Healthcare, Salt Lake City, UT, USA; 4Division of Cardiovascular Medicine, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA; 5Health Economics Outcomes Research, AstraZeneca, Wilmington, DE, USA; 6Enterprise Analytics, Intermountain Healthcare, Salt Lake City, UT, USA
Correspondence: Matthew J Hegewald
Pulmonary and Critical Care Medicine Division, Intermountain Medical Center, 5121 S Cottonwood Street, Murray, UT 84107, USA
Tel +1-801-507-4870
Fax +1-801-507-4792
Email [email protected]
Purpose: This retrospective, observational cohort study investigated the association of blood eosinophil counts within 1 week of hospitalization for acute exacerbation of COPD (AECOPD) with subsequent risk of all-cause and COPD-related readmission from a large integrated health system.
Patients and Methods: Electronic medical records were extracted for index hospitalization for AECOPD at all Intermountain Healthcare hospitals. The primary outcome was the relationship of blood eosinophil count to 30-day all-cause readmission; secondary outcomes were 60-day, 90-day, and 12-month all-cause readmission, COPD-related readmission, and empiric derivation of the eosinophil count with the highest area under the curve (AUC) for predicting 30-day all-cause readmission.
Results: Of 2445 included patients, 1935 (79%) had a blood eosinophil count < 300 cells/μL and 510 (21%) had a count ≥ 300 cells/μL. Using a 300-cells/μL threshold, there was no significant difference between high and low eosinophil groups in 30-day (odds ratio [OR]=1.05, 95% confidence interval [CI]=0.75– 1.47) or 60-day (OR=1.15, 95% CI=0.88– 1.51) all-cause readmissions. However, patients with greater (versus lesser) eosinophil counts had increased 90-day and 12-month all-cause readmissions (OR=1.35, 95% CI=1.06– 1.72, and OR=1.32, 95% CI=1.07– 1.62). COPD-related readmission rates were significantly greater for patients with greater (versus lesser) eosinophil counts at 30, 60, and 90 days and 12 months (OR range=1.52– 1.97). A total of 70 cells/μL had the most discriminatory power to predict 30-day all-cause readmission (highest AUC).
Conclusion: Eosinophil counts in patients with COPD were not associated with a difference in 30-day all-cause readmissions. However, greater eosinophil counts were associated with increased risk of all-cause readmission at 90 days and 12 months and COPD-related readmission at 30, 60, and 90 days and 12 months. Patients with eosinophils < 70 cells/μL had the lowest risk for 30-day all-cause readmission. Blood eosinophils in patients hospitalized with AECOPD may be a useful biomarker for the risk of hospital readmission.
Keywords: AECOPD, phenotype, biomarkers, eosinophils, electronic medical records
Plain Language Summary
- The eosinophilic phenotype in patients with chronic obstructive pulmonary disease (COPD) is common and may have clinical implications.
- Reports vary on whether blood eosinophil counts measured at hospital admission for acute exacerbation of COPD (AECOPD) predict the risk of hospital readmission between 30 days and 12 months.
- We extracted electronic medical records from all Intermountain Healthcare hospitals and evaluated the relationship of blood eosinophil counts to the risk of subsequent hospital readmission.
- Using 300 cells/μL as an eosinophil count threshold, there was no difference in all-cause hospital readmission at 30 days (primary outcome) or 60 days. However, there was a greater risk of hospital readmission for those with greater versus lesser eosinophil counts at 90 days and 12 months. Furthermore, there was a greater risk of COPD-related hospital readmissions at all time points (30, 60, and 90 days, and 12 months) for those with greater (versus lesser) blood eosinophil counts on admission.
- We further found that an eosinophil threshold of 70 cells/μL had the most discriminatory power to predict all-cause hospital readmission at 30 days. Namely, patients admitted with AECOPD who have peripheral blood eosinophilia lower than that threshold have a lower risk of all-cause hospital readmission at 30 days.
- All-cause mortality at 12 months was significantly lower for patients in the high blood eosinophil group compared with patients in the low blood eosinophil group.
- Our study shows that blood eosinophils may be a useful biomarker for the risk of hospital readmission in patients hospitalized with AECOPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is heterogeneous with different phenotypes and varied prognoses.1,2 The eosinophilic phenotype of COPD is common, ranging from 20–64%,3–8 although there is variation among studies in the absolute eosinophil count that defines the phenotype.
Some studies have found the eosinophilic phenotype to be associated with an increased risk of acute exacerbations of COPD (AECOPD).5,9–13 There are conflicting reports on whether high blood eosinophil counts at hospital admission for AECOPD predict increased risk for all-cause readmission, with studies suggesting an increased risk,14 no effect,15,16 or decreased risk.17 Furthermore, there is no consensus on the method that should be used to determine the eosinophilic phenotype in patients with COPD. Studies have used absolute blood eosinophil counts, blood eosinophil counts as a percentage of the total white blood cell (WBC) count, and eosinophil counts in sputum or bronchoalveolar lavage fluid.3,5–8,18 For practical reasons, peripheral blood eosinophil counts are more commonly used, and there is a fair agreement between sputum and blood eosinophil counts in COPD.7,19
To address these uncertainties, we investigated the association of blood eosinophil counts at the time of AECOPD hospitalization with subsequent risk of all-cause and COPD-related readmission in real-world patient health care utilization data from a large US medical care delivery network. We also determined the blood eosinophil count that best predicted the 30-day all-cause readmission risk.
Patients and Methods
Study Design and Data Collection
This was a retrospective, observational cohort study to examine the association between blood eosinophil counts in COPD patients at the time of AECOPD hospitalization and the risk of subsequent all-cause and COPD-related hospital readmission at an Intermountain Healthcare facility between January 1, 2011, and December 31, 2016. Intermountain is a large Integrated Delivery Network based in Utah, serving patients in Utah, Idaho, and Wyoming, that provides medical services to over 1 million patients annually. The data source was the electronic medical records (EMRs) in the Enterprise Data Warehouse, which includes data from both inpatient and outpatient settings.
The exposure variable was the highest blood eosinophil count measured 7 days prior to the index admission through 24-hour post-index admission and before administration of systemic (oral or intravenous) corticosteroids. Laboratory data are available and integrated across the health care system in the inpatient and outpatient settings. Other descriptive and covariate data were included in the analyses. Medication data were identified through medication reconciliation at the date of admission, nursing intake documentation, discharge records, and some outpatient prescription data. Associated comorbidity data, defined by Charlson comorbidity index (CCI) and billing codes, within 1 year prior to the index hospitalization were collected. Self-reported smoking status was classified as never, former, or current smoker. Pulmonary function test (PFT) data were available for the subset of patients who performed spirometry at a hospital PFT laboratory but were not available if tests were performed at an outpatient clinic. The Intermountain Institutional Review Board approved the study and did not require informed consent for this de-identified data analysis due to the large number of enrolled subjects and designation as a less than minimal risk study that did not adversely affect the rights and welfare of the subjects. This waiver of informed consent was in compliance with the Declaration of Helsinki.
Study Patients
The study population included patients with COPD aged ≥40 years with ≥1 AECOPD hospitalization between January 1, 2011, and December 31, 2016, and a blood eosinophil count within 7 days prior to the index admission through 24-hour post-index admission. AECOPD-related hospitalization was defined as hospitalization for AECOPD, or COPD as the primary diagnosis, or acute respiratory failure as a primary diagnosis with COPD as the secondary diagnosis. Diagnoses for COPD and AECOPD were identified using International Classification of Diseases, Ninth (ICD-9) and Tenth (ICD-10) Revision diagnosis codes 491.xx, 492.xx, 496.xx, and J41.x, J42.x, J43.x, J44.x, respectively. Patients with a secondary diagnosis code related to asthma were included. The first AECOPD-related hospitalization that had an eligible blood eosinophil count on admission in the study period was selected as the index admission. Data from eligible patients were analyzed from the date of discharge until 12-month post-index discharge date or death of the patient, whichever occurred first. Patients were excluded from the study if they had a known malignancy, were pregnant during the study period, or died during the index admission.
Study Outcomes and Statistical Analyses
The primary outcome was the relationship of blood eosinophil count to 30-day all-cause readmission, excluding planned hospitalizations. Secondary outcomes were 60-day, 90-day, and 12-month all-cause readmission and 30-day, 60-day, 90-day, and 12-month COPD-related readmission. The association of blood eosinophil count to all-cause mortality at 12 months was also assessed.
For the primary outcome, a blood eosinophil count ≥300 cells/µL was used to stratify patient groups; other thresholds defined a priori were ≥220 cells/µL, ≥400 cells/µL, and ≥500 cells/µL. A validation analysis of the prespecified eosinophil thresholds ≥220 vs <220 cells/µL, ≥300 vs <300 cells/µL, ≥400 vs <400 cells/µL, and ≥500 vs <500 cells/µL was also conducted, with correction for multiple testing requiring p≤0.0125 (corrected) for significance.
Hospital readmission rates were compared between patients with eosinophil counts ≥300 cells/µL and <300 cells/µL. Multivariable logistic regression analyses adjusted for demographic factors (age, sex, ethnicity), smoking history, CCI and constituent comorbidities, complete blood count–derived laboratory results (red blood cell distribution width, absolute neutrophil, hemoglobin, mean corpuscular volume), inhaled corticosteroids (ICS) at discharge, and oral corticosteroid (OCS) use were performed. Because only 17.6% of the patient population had spirometry tests available for analysis, these were not included in analyses. P-values among eosinophil threshold groups were calculated using a chi-squared test for categorical variables and t-test for continuous variables. Statistical tests were 2 sided with an α level of 0.05 for statistical significance.
Subgroup analysis was conducted to evaluate the association between blood eosinophilia and hospital readmission among patients with COPD and comorbid asthma, those receiving OCS in the 2 weeks prior to admission, and those prescribed ICS at discharge from the index admission.
To determine the optimal threshold associated with readmission risk, absolute eosinophil count was treated as a continuous variable, and specificity and sensitivity for predicting 30-day and 12-month all-cause readmission were assessed using receiver operating curve (ROC) analysis and the area under the curve (AUC). This analysis was repeated for predicting 30-day and 12-month COPD-related readmission. Positive predictive value, negative predictive value, and accuracy for 30-day and 12-month all-cause readmission were calculated for the new threshold.
Kaplan–Meier curves for readmission and mortality up to 12 months after discharge from the index hospitalization were constructed using the determined optimal eosinophil threshold derived above and for the predetermined ≥300 vs <300 cells/µL threshold. In this study, patients who died were not censored, which may account for a lower admission rate.
Results
Overall, 3853 hospitalizations for AECOPD during the time interval were identified; after excluding those hospitalizations without blood eosinophil counts, hospitalizations occurring after the patients’ index hospitalization, early deaths, pregnancies, and malignancies, the final sample size was 2445 unique patients hospitalized with AECOPD with blood eosinophil counts (Figure 1). The mean (standard deviation) blood eosinophil count on admission for all patients was 215 (363) cells/µL. There were 1935 (79%) patients with blood eosinophil counts <300 cells/µL and 510 (21%) with counts ≥300 cells/µL.
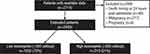 |
Figure 1 CONSORT flow diagram. |
Baseline characteristics are listed in Table 1. Twenty-six percent of patients had asthma, with no significant difference between the high and low eosinophil groups (28.2% vs 25.4%; p=0.19). There was a greater percentage of never-smokers in the high eosinophil group than in the low eosinophil group (11.6% vs 8.8%; p=0.026), and the neutrophil count was greater in the low eosinophil group compared with the high eosinophil group (7.54 K/µL vs 6.72 K/µL; p<0.001). Outpatient OCS were used by 4.7% of patients in the 2 weeks prior to eosinophil testing with no significant difference between groups. ICS use was reported in 33.6% of all patients in the year prior to admission, and 54.4% of all patients were prescribed ICS at discharge with no difference between the high and low eosinophil groups for either measure. In addition, ICS prescription at discharge was significantly associated with increased all-cause readmission at 12 months (OR=1.37; p<0.001).
 |
Table 1 Baseline Characteristics of the Study Population |
Blood Eosinophil Count and Readmission
There was no significant difference in the primary outcome of 30-day all-cause readmissions between the high eosinophil and low eosinophil groups using a threshold of 300 cells/μL (10.4% vs 10.5%, p=0.76, odds ratio [OR]=1.05, 95% confidence interval [CI]=0.75–1.47) (Table 2), nor was there a difference between the groups for all-cause readmission at 60 days (p=0.30). However, patients with high eosinophil counts had increased 90-day and 12-month all-cause readmissions compared with patients with low eosinophil counts (23.9% vs 20.1%, p=0.016, OR=1.35, CI=1.06–1.72, and 44.5% vs 39.2%, p=0.009, OR=1.32, CI=1.07–1.62). For COPD-related readmissions, there were significantly greater readmission rates for patients with high eosinophil counts compared with low eosinophil counts at all time points evaluated (30, 60, and 90 days, and 12 months).
 |
Table 2 Association of Blood Eosinophil Counts with All-Cause Readmission from Multivariable Logistic Regressiona |
Tables 2 and 3 present all-cause and COPD-related readmission results for the various eosinophil thresholds. Using blood eosinophil limits of ≥220 and ≥400 cells/µL, there was significantly increased all-cause readmission at 12 months and COPD-related readmissions at 60 days, 90 days, and 12 months (p≤0.0125). With a threshold of ≥500 cells/µL, there was no significant difference in all-cause readmission at any time point (p>0.0125), but there were significantly increased COPD-related readmission risks at 60 days, 90 days, and 12 months (p<0.0125) compared with those with eosinophil counts <500 cells/μL. Of 2445 patients, 247 (10%) had eosinophils that were elevated in the ≥500-cells/μL group.
 |
Table 3 Association of Blood Eosinophil Counts with COPD-Related Readmission from Multivariable Logistic Regressiona |
Potential Confounding Factors
We explored the effects of potential confounding factors, such as a secondary diagnosis of asthma, OCS use prior to eosinophil measurement, and ICS use at discharge on the association between eosinophil count and readmissions. In the subgroup of 635 patients with comorbid asthma, the association between eosinophil count and all-cause readmission in the univariable analysis was not significant at any time points between 30 days and 12 months (p=0.17–0.85). There were 115 patients (4.7% of study population) who had received OCS in the 14 days prior to eosinophil testing, including 4.9% of the low eosinophil group and 4.1% of the high eosinophil group (p=0.48). Excluding these patients had no significant effect on the association between eosinophil count and all-cause readmission. ICS were prescribed at discharge in 54.1% of the low eosinophil group and 55.5% of the high eosinophil group (p=0.58). ICS prescription was associated with a trend toward lower all-cause readmission in multivariable analysis at 30 days (p=0.18, OR=0.83, 95% CI=0.64–1.09) but was significantly associated with a greater risk of readmission at 12 months (p<0.001, OR=1.36, 95% CI=1.15–1.62). Even with adjusting for ICS at discharge, high eosinophils remained a significant predictor of 12-month readmission (OR=1.32, 95% CI=1.07–1.62, p=0.009).
Optimal Blood Eosinophil Count Associated with Readmission
To determine the optimal eosinophil threshold associated with increased readmission risk, blood eosinophil count was treated as a continuous variable and the specificity and sensitivity for predicting 30-day and 12-month all-cause and COPD-related readmission were assessed using ROC curves and the AUC. A blood eosinophil count of >70 cells/µL was optimal for discriminating all-cause readmission risk at 30 days and 12 months, although the association was moderate (areas under ROC curve of 0.532 and 0.551, respectively). Sixty-two percent of patients had a blood eosinophil count >70 cells/µL. There was a significant association with all-cause readmission and a blood eosinophil count of >70 cells/µL at all time points evaluated between 30 days and 12 months (OR=1.57–1.95; p<0.001) (Table 2); a significant association was also noted for COPD-related readmission (OR=1.79–3.10; p<0.001) (Table 3). The predictive value of a blood eosinophil threshold of >70 cells/µL for all-cause readmission at 30 days had a sensitivity of 73%, specificity of 40%, and negative predictive value of 93%; the corresponding values at 12 months were 68%, 43%, and 66%, respectively. The negative predictive value for a blood eosinophil threshold of ≤70 cells/µL for COPD-related readmission at 30 days was 97%, and at 12 months, was 87%. Our findings suggest that a blood eosinophil count ≤70 cells/µL identifies a patient group that is unlikely to require readmission.
For comparison, the predictive value of a blood eosinophil threshold of either <300 cells/µL or ≥300 cells/µL for all-cause readmission at 30 days had a sensitivity of 21%, specificity of 79%, and negative predictive value of 90%; the corresponding values at 12 months were 23%, 81%, and 61%, respectively. The negative predictive value for a blood eosinophil threshold of either <300 cells/µL or ≥300 cells/µL for COPD-related readmission at 30 days was 95% and at 12 months was 83%. The negative predictive values for the thresholds of 70 cells/μL and 300 cells/μL were therefore similar.
Kaplan-Meier survival curves, utilizing time to the event and censoring mortality as a nonevent, accentuating the association of eosinophil counts with all-cause readmission and COPD-related readmission at 1 year are shown in Figures 2 and 3.
 |
Figure 2 Association of blood eosinophil counts ≥300 cells/µL and <300 cells/µL with (A) all-cause readmission and (B) COPD-related readmission. |
 |
Figure 3 Association of blood eosinophil counts >70 cells/µL and ≤70 cells/µL with (A) all-cause readmission and (B) COPD-related readmission. |
Blood Eosinophil Count and Mortality
Mortality at 12 months for the entire population was 18.2% (445 of 2445 patients). Mortality was significantly lower in the high blood eosinophil group (14.9%, 76 of 510 patients) than the low blood eosinophil group (19.1%, 369 of 1935 patients; p=0.027). Kaplan-Meier survival curves showing the association of eosinophil threshold of ≥300 cells/µL with survival free from all-cause mortality at 12 months is shown in Supplemental Figure E1. For eosinophils >70 cells/µL compared to ≤70 cells/µL, mortality at 12 months was similar in the group with eosinophils >70 cells/µL (18.3%, 276 of 1510 patients) as in the lower blood eosinophil group (18.1%, 169 of 935 patients; p=0.89).
Discussion
There is increasing evidence that the eosinophilic phenotype, common in COPD, is also clinically important.3–8 However, methodologies and thresholds used to define the eosinophilic phenotype are not yet standardized. We found that blood eosinophil counts of ≥300 cells/µL are common, occurring in 21% of patients, and although not associated with increased all-cause readmission at 30 days (primary outcome), they were significantly associated with increased all-cause readmission at 90 days and 12 months. Moreover, such counts were significantly associated with COPD-related readmission rates at all evaluated time points between 30 days and 12 months. The association of high blood eosinophil counts with all-cause readmission were similar for all of the blood eosinophil thresholds >70 cells/µL for the time points between 30 days and 12 months; there did not appear to be a “dose-response” relationship between blood eosinophil level and risk of all-cause readmission for blood eosinophil levels >70 cells/µL. We found that a blood eosinophil count ≤70 cells/μL identifies patients unlikely to be readmitted within 30 days or throughout the entire 12 months.
Several studies have evaluated the association between blood eosinophils and AECOPD. There is evidence that the eosinophilic phenotype is associated with an increased risk of exacerbations, although studies have used varying thresholds to define this phenotype.5,9–11 There is conflicting evidence regarding the association of increased eosinophils (generally defined as a blood eosinophil count ≥200 cells/µL and/or ≥2% of total WBCs) and increased readmissions following admission for AECOPD. Studies have shown an increased risk of readmission with increased eosinophils.14,16 Couillard14 et al evaluated 167 patients admitted for an AECOPD, of whom 33% presented with an eosinophilic phenotype (defined as ≥200 cells/µL and/or ≥2% of total WBCs). The high eosinophil group was associated with increased risk of 12-month all-cause readmission (OR=2.32; p=0.0277) and COPD-related readmission (OR=3.59; p=0.0013).14 Other studies have found no association,15 or even decreased readmission risk17 for patients with increased blood eosinophils. There has been speculation that recent OCS use prior to eosinophil measurement in some studies15 may have contributed to the disparate results.14 The current study included a larger sample size than these previous studies, employed multiple thresholds of eosinophil counts, and explored the relationships with readmission over more time points.
The biological explanation for the association between readmissions and increased eosinophils is not clearly understood. Eosinophils regulate the type 2 immune response pathway, and exposure to allergens results in mediator release that contributes to airway inflammation. Eosinophilia is also associated with a wide variety of allergic, rheumatologic, infectious, and rare idiopathic disorders.20 Comorbidities may partly explain the association between elevated blood eosinophils and all-cause readmission in patients with COPD.
The use of ICS prior to and following admission for AECOPD was also evaluated. ICS use, combined with a long-acting bronchodilator, has been associated with decreased AECOPD, most prominently in patients with a history of frequent exacerbations and elevated eosinophil counts.6,21,22 In this study, the prescription of ICS at discharge was not significantly associated with decreased readmission at 30 days, and, unexpectedly, ICS prescription at discharge was significantly associated with increased all-cause readmission at 12 months. This increased risk could be caused by clinicians being more likely to prescribe controller ICS medications for sicker patients. Alternatively, ICS use has been associated with increased risk of pneumonia,23 and thus long-term ICS use in these patients may contribute to a greater readmission risk. These data should be interpreted with caution because ICS prescription data may not accurately reflect persistent ICS usage; not all prescribers use the Intermountain EMR prescription database, suggesting that prescriptions may be under-reported. Also, patient compliance with prescribed ICS-containing medications and the possible role of selection bias were unknown.
We found that high blood eosinophil counts are associated with significantly lower 12-month mortality. This observation is in agreement with previous studies,12,13 although potential confounders influencing the observed lower mortality rate cannot be ruled out. Among patients with COPD and persistently elevated eosinophils over a 2-year period (thresholds of 250, 300, and 350 cells/μL),12 there was a consistent significantly lower mortality rate compared with patients with eosinophil counts below these thresholds. In a separate study showing similar results (threshold of 150 cells/μL), the authors hypothesized that some beneficial factors may be present in patients with high eosinophil counts,13 such as better forced expiratory volume in the 1 second (FEV1), fewer symptoms, less dyspnea, less emphysema, fewer comorbidities, and a lower incidence of pneumonia.13 However, this association has not been consistently reported.16,24,25 In addition, having elevated eosinophil counts predicts improved response to ICS in patients with COPD,7,21,26 which may influence mortality. Because a greater percentage of deaths occurred in patients with eosinophil counts <300 cells/μL, this may have influenced the readmission risk.
Our study provides further evidence that increased blood eosinophil counts are associated with greater all-cause readmission at 90 days and 12 months across multiple thresholds, and greater COPD-related readmissions as early as 30 days for thresholds of 220, 300, and 400 cells/μL. The lack of a significant association between high blood eosinophils and all-cause readmission at 30 and 60 days may be because only 30–60% of all-cause readmissions are attributable to COPD, with other causes being due to comorbidities or socioeconomic factors that may not be associated with blood eosinophils.27–29
Limitations
The current study has several limitations, including OCS administration, the inclusion of patients with a secondary diagnosis of asthma, and COPD diagnostic uncertainty. The association between blood eosinophil counts and readmission may be affected by OCS administration prior to blood eosinophil count measurements because OCS use decreases both eosinophil counts and function.14,30–32 In our patient population, ~5% of patients received OCS in the 2 weeks prior to eosinophil testing; this percentage was similar between the high and low eosinophil groups. Limiting the analysis to patients free of OCS use prior to eosinophil testing resulted in no change in the association of high eosinophils with all-cause readmission.
Another potential confounding factor in the current study was the inclusion of patients with a secondary diagnosis of asthma. Asthma is more commonly associated with eosinophilic inflammation than COPD, and increased blood eosinophil counts in patients with asthma are associated with increased exacerbation frequency.33,34 A recent historical cohort study investigated the relationship between high blood eosinophil counts (≥350 cells/µL) and hospital readmission in 2613 patients with asthma hospitalized for acute exacerbation.35 Results showed that a high blood eosinophil count in the year before asthma-related hospitalization is associated with an increased risk of readmission within the following year.35 A secondary diagnosis of asthma was present in 26% of the patient population with no difference between the high and low eosinophil groups. Among the subgroup of patients with COPD who had a secondary diagnosis of asthma, the association of blood eosinophil count with all-cause readmission in the univariable analysis was not significant, and patients with a secondary diagnosis of asthma had similar readmission rates compared to those without asthma. Therefore, the inclusion of patients with a secondary diagnosis of asthma likely was not responsible for the association between high blood eosinophils and readmission.
An additional limitation of this work is uncertainty regarding the diagnosis of COPD. This limitation is inherent in clinical data sets in which spirometry data, which are central to the diagnosis of COPD,36 are unavailable for analysis; spirometry results were available for only 17.6% of the patients in this analysis. We used ICD-9 and ICD-10 codes to identify patients with COPD and AECOPD; algorithms based on such codes for identifying hospitalized patients with AECOPD have high specificity and low sensitivity.37 In addition, we likely missed patients who were readmitted to non-Intermountain hospitals, and this may explain why the observed 30-day all-cause readmission rate in the current study is approximately half the reported 30-day all-cause readmission rate for Medicare patients.38 However, Intermountain Healthcare is a large integrated system that has relative population stability, and the system generally has lower readmission rates than the national population. Finally, our study population is racially homogeneous (94% white) and our results may not apply to more diverse populations.
Conclusion
Blood eosinophilia is not associated with 30-day all-cause readmission risk in patients with AECOPD. However, the eosinophilic phenotype in COPD is associated with an increased risk of all-cause readmission at 90 days and 12 months and COPD-related readmission at all time points assessed between 30 days and 12 months. Measurement of blood eosinophils in hospitalized patients with COPD may be a useful biomarker to help determine the risk of hospital readmission.
Abbreviations
AECOPD, acute exacerbations of chronic obstructive pulmonary disease; AUC, area under the curve; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; FEV1, forced expiratory volume in the 1 second; GERD, gastroesophageal reflux disease; ICD-9, International Classification of Diseases, Ninth Revision; ICD-10, International Classification of Diseases, Tenth Revision; ICS, inhaled corticosteroids; OCS, oral corticosteroid; OR, odds ratio; PVD, peripheral vascular disease; ROC, receiver operating curve; SD, standard deviation; WBC, white blood cell; EMR, electronic medical record; CCI, Charlson comorbidity index; PFT, pulmonary function test.
Acknowledgments
The authors thank Kathleen M. Fox, MHS, PhD, for her contributions to the study protocol and initial data analyses. Editing of the manuscript was provided by Katie Gersh, PhD, of MedErgy (Yardley, PA, USA), which was in accordance with Good Publication Practice (GPP3) guidelines and funded by AstraZeneca (Wilmington, DE, USA). Selected data from this manuscript were presented at the 2018 CHEST annual meeting (6–10 October 2018; San Antonio, Texas) as a poster presentation and discussion with interim findings and the poster’s abstract was published in CHEST 2018; 154 (4): Suppl. Pg 728A. DOI: https://doi.org/10.1016/j.chest.2018.08.661.
Author Contributions
MJH, BDH, FT, DPB contributed to the conception and design, analysis and interpretation of the data. SR identified data in multiple Intermountain EMRs, resolved provenance and quality issues with the study team, and extracted, transformed, and rolled up the data to create the analytic data set. YC and JK contributed to data analysis. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was funded by AstraZeneca (Wilmington, DE, USA).
Disclosure
MJH has performed consulting for GlaxoSmithKline and has been on the speakers bureau for Boehringer Ingelheim. MJH reports grants from AstraZeneca during the conduct of the study. BDH is a PI of grants from GlaxoSmithKline, CareCentra, AstraZeneca, the Intermountain Foundry innovation program, and the Intermountain Research and Medical Foundation that are not related to this work, and a co-investigator on the grant supporting this study. BDH is also an inventor of clinical decision tools that are licensed to CareCentra. FT is an employee and shareholder of AstraZeneca. JLK is an employee and stockholder of AstraZeneca. YC is an employee of AstraZeneca. SR reports grants from AstraZeneca during the conduct of the study.DPB reports grants from ProLung, AstraZeneca, and Zebra Medical, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Segal LN, Martinez FJ. Chronic obstructive pulmonary disease subpopulations and phenotyping. J Allergy Clin Immunol. 2018;141(6):1961–1971. doi:10.1016/j.jaci.2018.02.035
2. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604.
3. Bafadhel M, Pavord ID, Russell REK. Eosinophils in COPD: just another biomarker? Lancet Respir Med. 2017;5(9):747–759. doi:10.1016/S2213-2600(17)30217-5
4. Tashkin DP, Wechsler ME. Role of eosinophils in airway inflammation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2018;13:335–349.
5. Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The copenhagen general population study. Am J Respir Crit Care Med. 2016;193(9):965–974. doi:10.1164/rccm.201509-1869OC
6. Watz H, Tetzlaff K, Wouters EF, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4(5):390–398. doi:10.1016/S2213-2600(16)00100-4
7. Singh D, Kolsum U, Brightling CE, et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697–1700. doi:10.1183/09031936.00162414
8. Oshagbemi OA, Burden AM, Braeken DCW, et al. Stability of blood eosinophils in patients with chronic obstructive pulmonary disease and in control subjects, and the impact of sex, age, smoking, and baseline counts. Am J Respir Crit Care Med. 2017;195(10):1402–1404. doi:10.1164/rccm.201701-0009LE
9. Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi:10.1164/rccm.201104-0597OC
10. Siddiqui SH, Guasconi A, Vestbo J, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(4):523–525.
11. Yun JH, Lamb A, Chase R, et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141(6):2037–2047 e2010. doi:10.1016/j.jaci.2018.04.010
12. Casanova C, Celli BR, de-Torres JP, et al. Prevalence of persistent blood eosinophilia: relation to outcomes in patients with COPD. Eur Respir J. 2017;50:5. doi:10.1183/13993003.01162-2017
13. Turato G, Semenzato U, Bazzan E, et al. Blood eosinophilia neither reflects tissue eosinophils nor worsens clinical outcomes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(9):1216–1219. doi:10.1164/rccm.201708-1684LE
14. Couillard S, Larivee P, Courteau J, Vanasse A. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest. 2017;151(2):366–373. doi:10.1016/j.chest.2016.10.003
15. Bafadhel M, Greening NJ, Harvey-Dunstan TC, et al. Blood eosinophils and outcomes in severe hospitalized exacerbations of COPD. Chest. 2016;150(2):320–328.
16. Belanger M, Couillard S, Courteau J, et al. Eosinophil counts in first COPD hospitalizations: a comparison of health service utilization. Int J Chron Obstruct Pulmon Dis. 2018;13:3045–3054. doi:10.2147/COPD.S170743
17. Duman D, Aksoy E, Agca MC, et al. The utility of inflammatory markers to predict readmissions and mortality in COPD cases with or without eosinophilia. Int J Chron Obstruct Pulmon Dis. 2015;10:2469–2478.
18. Rutgers SR, Timens W, Kaufmann HF, van der Mark TW, Koeter GH, Postma DS. Comparison of induced sputum with bronchial wash, bronchoalveolar lavage and bronchial biopsies in COPD. Eur Respir J. 2000;15(1):109–115. doi:10.1183/09031936.00.15110900
19. Negewo NA, McDonald VM, Baines KJ, et al. Peripheral blood eosinophils: a surrogate marker for airway eosinophilia in stable COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1495–1504. doi:10.2147/COPD.S100338
20. Klion AD. Eosinophilia: a pragmatic approach to diagnosis and treatment. Hematology Am Soc Hematol Educ Program. 2015;2015:92–97. doi:10.1182/asheducation-2015.1.92
21. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. doi:10.1016/S2213-2600(15)00106-X
22. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
23. Pavord ID, Lettis S, Anzueto A, Barnes N. Blood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient-level meta-analysis. Lancet Respir Med. 2016;4(9):731–741. doi:10.1016/S2213-2600(16)30148-5
24. Zysman M, Deslee G, Caillaud D, et al. Relationship between blood eosinophils, clinical characteristics, and mortality in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1819–1824.
25. DiSantostefano RL, Hinds D, Le HV, Barnes NC. Relationship between blood eosinophils and clinical characteristics in a cross-sectional study of a US population-based COPD cohort. Respir Med. 2016;112:88–96. doi:10.1016/j.rmed.2016.01.013
26. Pavord ID, Lettis S, Locantore N, et al. Blood eosinophils and inhaled corticosteroid/long-acting beta-2 agonist efficacy in COPD. Thorax. 2016;71(2):118–125. doi:10.1136/thoraxjnl-2015-207021
27. Hakim MA, Garden FL, Jennings MD, Dobler CC. Performance of the LACE index to predict 30-day hospital readmissions in patients with chronic obstructive pulmonary disease. Clin Epidemiol. 2018;10:51–59. doi:10.2147/CLEP.S149574
28. Goto T, Faridi MK, Camargo CA
29. Sharif R, Parekh TM, Pierson KS, Kuo YF, Sharma G. Predictors of early readmission among patients 40 to 64 years of age hospitalized for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(5):685–694.
30. Wardlaw AJ, Moqbel R, Kay AB. Eosinophils: biology and role in disease. Adv Immunol. 1995;60:151–266.
31. Essellier AF, Jeanneret RL, Morandi L. The mechanism of glucocorticoid eosinopenia; contribution to the physiology of eosinophile granulocytes. Blood. 1954;9(5):531–549. doi:10.1182/blood.V9.5.531.531
32. Wallen N, Kita H, Weiler D, Gleich GJ. Glucocorticoids inhibit cytokine-mediated eosinophil survival. J Immunol. 1991;147(10):3490–3495.
33. Gibson PG. Inflammatory phenotypes in adult asthma: clinical applications. Clin Respir J. 2009;3(4):198–206. doi:10.1111/j.1752-699X.2009.00162.x
34. Denlinger LC, Phillips BR, Ramratnam S, et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195(3):302–313. doi:10.1164/rccm.201602-0419OC
35. Kerkhof M, Tran TN, van den Berge M, et al. Association between blood eosinophil count and risk of readmission for patients with asthma: historical cohort study. PLoS One. 2018;13(7):e0201143. doi:10.1371/journal.pone.0201143
36. Watt MJ, Howlett KF, Febbraio MA, Spriet LL, Hargreaves M. Adrenaline increases skeletal muscle glycogenolysis, pyruvate dehydrogenase activation and carbohydrate oxidation during moderate exercise in humans. J Physiol. 2001;534(Pt 1):269–278. doi:10.1111/j.1469-7793.2001.t01-1-00269.x
37. Stein BD, Bautista A, Schumock GT, et al. The validity of International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest. 2012;141(1):87–93. doi:10.1378/chest.11-0024
38. Dorsey K. Centers medicare medicaid services. 2015 condition-specific measures updates and specifications report hospital-level 30-day risk-standardized readmission measures. Available from: https://www.qualitynet.org/dcs/BlobServer?blobkey=id&blobnocache=true&blobwhere=1228890435217&blobheader=multipart%2Foctetstream&blobheadername1=Content-Disposition&blobheadervalue1=attachment%3Bfilename%3DRdmn_AMIHFPNCOPDSTK_Msr_UpdtRpt.pdf&blobcol=urldata&blobtable=MungoBlobs.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
