Back to Journals » Drug Design, Development and Therapy » Volume 16
Blockade of the P2Y2 Receptor Attenuates Alcoholic Liver Inflammation by Targeting the EGFR-ERK1/2 Signaling Pathway
Authors Liu ZN, Su QQ, Wang YH, Wu X, Lv XW
Received 26 October 2021
Accepted for publication 1 April 2022
Published 13 April 2022 Volume 2022:16 Pages 1107—1120
DOI https://doi.org/10.2147/DDDT.S346376
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Zhen-Ni Liu,1– 3,* Qian-Qian Su,4,* Yu-Hui Wang,1– 3,* Xue Wu,1– 3 Xiong-Wen Lv1– 3
1Inflammation and Immune Mediated Diseases Laboratory of Anhui Province, Anhui Institute of Innovative Drugs, School of Pharmacy, Anhui Medical University, Hefei, People’s Republic of China; 2The Key Laboratory of Anti-Inflammatory and Immune Medicines, Ministry of Education, Hefei, People’s Republic of China; 3Institute for Liver Diseases of Anhui Medical University, Hefei, People’s Republic of China; 4Department of Pharmacy, the First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiong-Wen Lv, School of Pharmacy, Anhui Medical University, 81 Mei Shan Road, Hefei, Anhui Province, 230032, People’s Republic of China, Email [email protected]
Purpose: It is well known that inflammation plays a key role in complex pathological progressions of alcohol-associated liver disease (ALD). To date, effective therapy for ALD is lacking. P2Y2 receptor (P2Y2R), a G protein-coupled P2Y purinergic receptor, represents a novel pharmacological target in many inflammations.
Methods: The alcohol-associated liver injury and inflammation mouse model was established. The effect of P2Y2R on alcohol-induced liver injury and inflammation was evaluated using quantitative real-time PCR, Western blot and immunohistochemical assay. An alcohol-stimulated (100 mmol/L, for 24 h) AML-12 cell model was established. Different agonists, antagonists and P2Y2R siRNA were used to explore the possible mechanisms of P2Y2R.
Results: In vivo, results showed that the hepatoprotective effect of P2Y2R blockade by significantly suppressed liver structural abnormalities and lipid infiltration, and decreased levels of ALT/AST and TNF-α/IL-1β in the high dosage group of suramin (20 mg/kg) compared to control diet (CD)-fed mice. At the same time, we found that alcohol feeding promoted the phosphorylation of EGFR and ERK1/2, both of which were effectively inhibited by suramin (20 mg/kg). In vitro, suramin or P2Y2R silencing effectively inhibited the phosphorylation of EGFR and ERK1/2, similar to the down-regulated effects of their corresponding inhibitors (EGFR inhibitor AG1478 and ERK1/2 inhibitor U0126) accompanied by reduced levels of TNF-α and IL-1β compared to alcohol-induced AML-12 cell. In addition, we found that silencing P2Y2R attenuated the apoptosis of hepatocyte.
Conclusion: Our findings suggest that P2Y2R regulates alcoholic liver inflammation by targeting the EGFR-ERK1/2 signaling pathway and plays an important role in hepatocyte apoptosis, which may provide new ideas for the development of methods to treat ALD.
Keywords: P2Y2 receptor, alcoholic liver inflammation, EGFR, ERK1/2
Introduction
As the most common chronic liver disease in the world, alcohol-associated liver disease (ALD) causes great harm, but there is still no effective treatment method.1 ALD is an intricate pathological process containing a wide spectrum from steatosis, and alcohol-associated hepatitis (AH) to cirrhosis. ALD has become a public health problem with high incidence and mortality.2,3 Because of the limitations in the therapeutic options, it is no doubt that the newer and more effective pharmacological agents for ALD patients are imperative. Previous studies revealed that alcohol and enterogenous endotoxin can both promote macrophage activation, followed by a series of inflammatory reactions and eventually lead to liver damage.4,5 High levels of inflammatory factors such as TNF-α and IL-1β have been documented among ALD patients, probably because of elevated circulating lipopolysaccharides (LPS).6 Notably, inflammatory responses are particularly critical in the progression of ALD.7 It appears to be significant to identify an inflammatory target for the treatment of ALD. P2Y2 receptor (P2Y2R), a member of the G protein-coupled P2Y purinergic receptors, represents a novel pharmacological target in many inflammations such as radiotherapy-resistant breast cancer, diabetic nephropathy vascular inflammation and atherosclerosis.8–11 It was reported that extracellular ATP and purinergic P2Y2R signaling promoted liver tumorigenesis in both mice and humans.12,13 Our group has previously shown that blockade of extracellular ATP-P2Y2R signaling represents a potential therapeutic effect on alcohol-induced steatohepatitis, but the specific mechanism is still unclear.14 Furthermore, recent findings have suggested that the P2Y2R-specific agonist, UTP, increases ERK phosphorylation and thus cell proliferation via P2Y2R in hepatocytes from CCl4-induced fibrotic mice.15 In esophageal cancer, P2Y2 receptors can also promote cell proliferation via the ERK1/2 pathway.16 Importantly, another study demonstrated that early activation of p42/44 ERK and subsequent hepatocyte proliferation in response to 70% partial hepatectomy were impaired in P2Y2R knockout mice.17 These studies highlight the importance of purinergic P2Y2R signaling in liver diseases. Epidermal growth factor receptor (EGFR) plays an important role in liver regeneration.18 Extracellular signal-regulated kinase 1/2 (ERK1/2), downstream of EGFR-mediated signaling, regulates the formation of various inflammatory cytokines.19,20 The EGFR-ERK1/2 pathway plays a crucial role in hepatocellular carcinoma (HCC).21 Another study indicated that blocking the P2Y2R receptor attenuates tumor cell proliferation and the tumorigenesis is related to EGRF-ERK1/2.22 Accumulating evidence implies a close relationship between P2Y2R and EGFR-ERK1/2, but their roles in ALD are still unclear. Here, we examined the roles of P2Y2R and EGFR-ERK1/2 in alcoholic liver inflammation using a mouse model of chronic plus binge alcohol feeding and an alcohol-stimulated AML-12 cell model.
Materials and Methods
Antibodies and Reagents
The selective P2Y2R agonist UTP was purchased from TOCRIS (USA). The competitive P2Y2R antagonist suramin was purchased from Sigma (USA). The selective EGFR antagonist AG1478 was provided by Selleck (USA). The ERK1/2 antagonist U0126 was supplied by MCE (USA). Antibodies against β-actin, P2Y2R and tumor necrosis factor-α (TNF-α) were purchased from Bioss (Beijing, China). Antibodies against Interleukin-1β (IL-1β) were purchased from Proteintech (Chicago, USA). Antibodies against total ERK, phospho-ERK1/2 (p-ERK1/2), total EGFR and phospho-EGFR (p-EGFR) were obtained from Elabscience (Wuhan, China). Goat anti-rabbit immunoglobulin G (IgG) horseradish peroxidase (HRP) and goat anti-mouse IgG HRP were supplied by ZSGB-BIO (Beijing, China). TRIzol reagent was obtained from Invitrogen (California, USA). PrimeScript RT Master Mix (Perfect Real Time) and Maxima SYBR Green qPCR Master were purchased from TaKaRa (Dalian, China). Alanine aminotransferase (ALT) assay kit and aspartate aminotransferase (AST) assay kit, Oil Red O were from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Ethyl alcohol (ETOH) was provided by Shanghai Zhen Qi Chemical Reagent (China).
Cell Culture and Cell Treatment with Alcohol
AML-12 cells, immortalized mouse hepatocytes, were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s modified Eagle’s medium/Ham’s nutrient mixture F-12 (DME/F-12, HyClone, USA) containing 8% fetal bovine serum (Clark, Australia) and incubated at 37°C in an atmosphere with 5% CO2. An in vitro model of ALD was induced by treating AML-12 cells with ethanol (100 mmol/L) for 24 h (Kim et al 2015). UTP (1.89 μM), suramin (200 μM), AG1478 (3 nM) or U0126 (65 nM) was administrated one hour before alcohol exposure.
RNA Interference
Sequences of small interfering RNA (siRNA) oligonucleotides against the P2Y2R gene and negative control were designed and synthesized by GenePharma (Shanghai, China) as follows: P2Y2R siRNA (forward: 5′-GCU CUC UAU AUC UUC CUA UTT-3′ and reverse: 5′-AUA GGA AGA UAU AGA GAG CTT-3′); negative control (forward: 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and reverse: 5′-ACG UGA CAC GUU CGG AGA ATT-3′). Cells were transfected with siRNA using siRNA-Mate (GenePharma, China) according to the manufacturer’s instruction manual.
Animals and Treatment
C57BL/6 male mice, weighing 18–22 g, were purchased from the Laboratory Animal Center of Anhui Medical University and acclimated for 3 days prior to the initiation of experiments. Animals and protocols used in experiments were approved by the institutional subcommittees on animal care. Animals were serviced following the Guides of Center for Developmental Biology, Anhui Medical University for the Care and Use of Laboratory. A total of 70 mice were randomly divided into five groups. Control mice, Alcohol mice, and suramin-treated mice at doses of 5, 10 and 20 mg/kg/day (Liu et al 2019). A mouse model of chronic plus binge ethanol feeding was established as described (Bertola et al 2013). During the first 5 days, all mice were fed a liquid control diet supplemented with vitamins and choline. Then, the EtOH-fed mice were fed a liquid diet containing 5% (v/v) ethanol for 10 days according to the feeding protocol, while the CD-fed mice were fed with a liquid diet containing isocaloric maltose-dextrin. From the ninth day, all suramin-treated mice were administrated by intraperitoneal injections daily. At day 16, the EtOH-fed animals and drug-treated animals were gavaged with a single binge of ethanol (5 g/kg body weight, 20% ethanol), while the CD-fed mice were given an isocaloric dose of dextrin maltose gavage. Nine hours after the last gavage, the mice were anesthetized with pentobar-bital sodium. Blood was obtained and stored at room temperature for serum collection. Liver samples and serum were preserved at −80°C for further analysis.
Histopathological Assessment
Hematoxylin/eosin (HE) staining: Liver tissues were embedded in wax after fixation in 4% paraformaldehyde solution and then sliced (3–5 μm). The sections were dewaxed in gradient xylene (xylene I and xylene II) and gradient ethanol (100% ethanol, 95% ethanol, 80% ethanol), and were stained using hematoxylin and eosin dye.
Immunohistochemistry (IHC): After dewaxing, the repair of tissue antigen was proceeded, and then the sections were incubated with primary antibody against P2Y2R and second antibody following sealing with bovine serum albumin. P2Y2R was visualized by 3,3′-diaminobenzidine tetrahydrochloride (DAB) staining.
Oil Red O staining: Fresh liver tissues were immersed in freezing medium and stored at −80°C. The liver tissues were sliced and stained with Oil Red O according to the instructions from the manufacturer.
Biochemical Analysis
After 3 h of storage at room temperature, mouse serum was obtained by separating blood (3000 rpm, 30 min at 4°C) and then was saved at −80°C until further processing. The serum levels of ALT and AST were detected by the Reitman-Frankel method using commercial kits.
Real-Time Quantitative PCR (RT-qPCR)
Total RNA was extracted from AML-12 cells or liver tissues using TRIzol reagent in accordance with the manufacturer’s protocol, and the concentrations and purity of RNA were measured using a Nanodrop 2000 (Thermo Scientific, USA). Single-strand cDNA was generated utilizing a Transcripter Synthesis Kit. The relative levels of objective mRNAs (P2Y2R, TNF-α, IL-1β) were determined through qPCR analysis with a PikoRreal 96 Real-Time PCR detection system (Thermo Scientific, USA) using Maxima SYBR Green qPCR Master. The primer sequences used in the study were designed and synthesized via Sangon Biotech (Shanghai, China) as follows: P2Y2R (forward: 5′-ATA TCA GCC CCT TTA ACA AGC-3′ and reverse: 5′-CAG TCA GCA GTG ACG ACT CAA-3′); TNF-α (forward: 5′-CAG GTC ACT GTC CCA GCA TCT-3′ and reverse: 5′-GAG TCC GGG CAG GTC TAC TTT-3′); IL-1β (forward: 5′-GGT AAG TGG TTG CCC ATC AGA-3′ and reverse: 5′-GTC GCT CAG GGT CAC AAG AAA-3′).
Western Blot
Total proteins were extracted from AML-12 cells or liver tissues using enhanced RIPA lysis buffer containing phenylmethanesulfonyl fluoride (PMSF, 100:1) (Beyotime, China). Protein concentrations were measured with a BCA protein assay kit (Beyotime, China). Protein separation was performed through SDS-PAGE followed by transfer onto PVDF membranes (Millipore, USA), which were blocked with 5% skimmed milk for three hours at room temperature. Then, the transferred PVDF membranes were incubated in primary antibody solution overnight at 4°C. After three washes using TBS/T (Tris buffered saline + 0.05% Tween 20), the membranes were incubated with goat anti-mouse or goat anti-rabbit horseradish peroxidase (HRP) conjugate antibody for 1 h. After three washes, protein bands were visualized using an ECL-chemiluminescent kit (Thermo Scientific, USA).
Flow Cytometry
The Annexin-V-FITC/PI Apoptosis Detection Kit (Best bio, China) was used to detect AML-12 cell apoptosis. AML-12 cells were collected in 1.5 mL centrifuge tubes. In dark conditions, Annexin V binding solution was added to resuspend cells, Annexin V-FITC staining solution was added to react for 15 min at 4°C, PI staining solution was added, and the cells were filtered with mesh cloth and transferred to a flow tube, followed by detection of the stained cells using flow cytometry.
Statistical Analysis
The data are representative of at least three independent experiments and all values are expressed as the mean ± SD using GraphPad Prism (San Diego, CA). Statistical analysis of the results was determined by one-way ANOVA. P < 0.05 was considered to be statistically significant.
Results
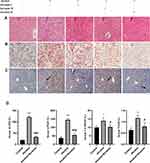
Suramin Mitigated Alcohol-Induced Liver Injury
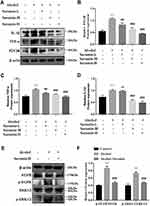
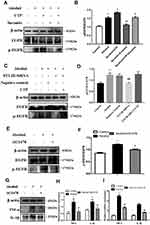
First, we further confirmed that there was a protective effect of suramin on chronic plus binge alcohol feeding-induced liver injury. As shown by the results of HE staining and Oil Red O staining (Figure 1A and B), the liver tissues of Alcohol group displayed marked hepatic cord disorders and inflammatory cell infiltration as well as abundant fat vacuoles compared to the control group. Meanwhile, Western Blot results showed that alcohol feeding induced the expression of P2Y2R (P < 0.01, compared to control group). However, P2Y2R expression was suppressed by suramin dose-dependently, especially in the highest dose group (20 mg/kg) (Figure 2A and B). As shown in Figure 1C, IHC further confirmed the inhibitory effect of suramin on P2Y2R (negative control is shown in Supplementary Figure 1), implying that P2Y2R may play a key role in ALD. Moreover, alcohol-induced liver damage mentioned above was also reduced by suramin in a dose-dependent manner (Figure 1A and B). The serum parameter evaluation showed that ALT (P < 0.01, compared to control group) and AST (P < 0.01, compared to control group) levels were significantly increased by alcohol feeding (Figure 1D), which is consistent with HE staining. Similarly, suramin (20 mg/kg) significantly decreased ALT (P < 0.01, compared to Alcohol group) and AST (P < 0.01, compared to Alcohol group) levels. We also measured the effect of suramin on the alcohol-induced inflammatory response in mice. Western Blot data showed that alcohol feeding caused elevated protein levels of TNF-α (P < 0.01, compared to control group) and IL-1β (P < 0.01, compared to control group), which were significantly suppressed by suramin (20 mg/kg, P < 0.01, compared to Alcohol group, Figure 2A, C and D). Given that suramin (20 mg/kg) sufficiently blocked liver injury, this dose was used to analyze the possible mechanisms in subsequent in vivo trials.
Hepatoprotective Effect of Suramin Was Associated with Inhibiting EGFR-ERK1/2 Signaling Pathway
Western Blot data of liver tissues revealed that the phosphorylation levels of EGFR (P < 0.01, compared to control group) and ERK1/2 (P < 0.01, compared to Alcohol group) were significantly promoted in Alcohol group, both of which were effectively inhibited by suramin (20 mg/kg, P < 0.01, compared to Alcohol group, Figure 2E and F). Current findings suggest that suramin mitigated alcohol-induced liver injury, which may be associated with EGFR-ERK1/2 signaling inhibition.
Inhibition or Silencing of P2Y2R Mitigated Alcohol-Induced AML-12 Cell Inflammation
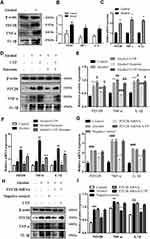
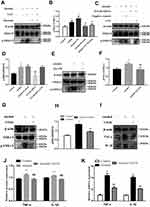
To further evaluate the role of P2Y2R in ALD at the cellular level, we interfered with P2Y2R using agonists, antagonists and siRNA in alcohol-stimulated AML-12 cells. The results of Western Blot and RT-qPCR consistently showed that TNF-α (P < 0.01, compared to control group) and IL-1β (P < 0.01, compared to the control group) expression was obviously increased after alcohol exposure (Figure 3A–C), suggesting that alcohol exposure induced AML-12 cell inflammation. Meanwhile, there was a significant upregulation of P2Y2R expression at the protein (P < 0.05) and mRNA (P < 0.01) levels compared to the control group (Figure 3A–C), suggesting that P2Y2R may play a key role in alcohol-induced AML-12 cell inflammation. Moreover, we found that compared to alcohol group, UTP (1.89 μM) treatment, a P2Y2R agonist, further upregulated the expression of P2Y2R (P < 0.05), TNF-α (P < 0.05) and IL-1β (P < 0.05) (Figure 3D–F). Conversely, suramin (200 μM) significantly downregulated the levels of P2Y2R (P < 0.05), TNF-α (P < 0.05) and IL-1β (P < 0.05) in alcohol plus suramin-treated cells (Figure 3D–F) compared to the alcohol group. Moreover, to a certain extent, suramin reversed the high expression of P2Y2R (P < 0.05), TNF-α (P < 0.01) and IL-1β (P < 0.05) stimulated by alcohol plus UTP compared to the alcohol plus UTP group (Figure 3D–F). Then, P2Y2R siRNA was applied to verify the protective effect of P2Y2R blockade on alcohol-stimulated cell inflammation. As shown in Figure 3G–I, P2Y2R knockdown reduced P2Y2R (P < 0.01), TNF-α (P < 0.01) and IL-1β (P < 0.05) expression at the protein and mRNA levels compared to the alcohol group. We also used P2Y2R-siRNA+UTP as a negative control, and it can be seen from the results that there was no significant difference between this group and the alcohol group (Figure 3G–I).
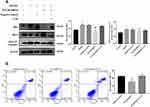
Silencing P2Y2R inhibited apoptosis in AML-12 cell primed with alcohol.
To explore whether P2Y2R has an effect on the apoptosis of AML-12 cell, P2Y2R siRNA was used. The levels of the cell apoptosis in AML-12 cell were assessed with methods based on Western Bolt and flow cytometry. Western blot results showed that the ratio of Bax/Bcl-2 and the expression of cleaved-caspase 3 increased because of EtOH stimulation, whereas silencing of P2Y2R decreased the protein levels of them. These illustrated that silencing P2Y2R could inhibit the apoptosis of AML-12 cell. There was no significant difference in apoptosis between the P2Y2R-siRNA+UTP group and the Alcohol group. The flow cytometry results also proved this view (Figure 4A and B).
EGFR Inhibition Mitigated Alcohol-Induced AML-12 Cell Inflammation
We blocked EGFR to further evaluate the role of EGFR in the protective effect of P2Y2R inhibition or silencing in alcohol-induced AML-12 cells. Analysis of Western Blot showed that alcohol exposure markedly promoted EGFR phosphorylation (P < 0.01, compared to the control group), which was further augmented by UTP treatment (P < 0.05, compared to the alcohol group, Figure 5A and B). However, suramin (P < 0.01, compared to alcohol group) or P2Y2R siRNA (P < 0.01, compared to alcohol group) significantly inhibited EGFR phosphorylation (Figure 5A–D) just as the downregulated effect of EGFR inhibitor AG1478 (3 nM, P < 0.05, compared to alcohol group, Figure 5E and F). Moreover, suramin decreased the p-EGFR expression (P < 0.05, compared to alcohol plus UTP group) caused by alcohol plus UTP exposure (Figure 5A and B). However, there was no significant difference between the P2Y2R-siRNA+UTP group and the Alcohol group (Figure 5C and D). In addition, AG1478 significantly decreased the levels of inflammatory cytokines such as TNF-α (P < 0.01) and IL-1β (P < 0.01, Figure 5G–I) compared to the alcohol group, suggesting that EGFR inhibition alleviates alcohol-induced AML-12 cell inflammation.
ERK1/2 Inhibition Mitigated Alcohol-Induced AML-12 Cell Inflammation
We blocked ERK1/2 or EGFR to further evaluate the role of EGFR-ERK1/2 in the protective effect of P2Y2R inhibition or silencing on alcohol-induced AML-12 cell inflammation. The results revealed that alcohol stimulation promoted phosphorylation of ERK1/2 (P < 0.01, compared to the control group) in AML-12 cell. The phosphorylation level of ERK1/2 was further augmented by UTP (P < 0.05, compared to the alcohol group) but was substantially decreased by suramin (P < 0.01, compared to the alcohol group) or P2Y2R siRNA (P < 0.01, compared to the alcohol group, Figure 6A–D). Moreover, suramin decreased alcohol plus UTP-induced ERK1/2 phosphorylation (P < 0.01, compared to the alcohol plus UTP group, Figure 6A and B). And there was no significant difference between the P2Y2R-siRNA+UTP group and the alcohol group (Figure 6C and D). These findings suggested that ERK1/2 activity was regulated by P2Y2R. What is noteworthy is that alcohol-promoted ERK1/2 phosphorylation was also effectively restrained by AG1478 compared to the alcohol group (P < 0.05, Figure 6E and F), similar to the role of U0126 (P < 0.05, compared to the alcohol group, Figure 6G and H), identifying the EGFR-ERK1/2 pathway participated in alcohol-induced AML-12 cell inflammation. Furthermore, U0126 significantly downregulated the levels of TNF-α (P < 0.05) and IL-1β (P < 0.05, Figure 6I–K) compared to the alcohol group, suggesting that ERK1/2 inhibition alleviated alcohol-induced AML-12 cell inflammation.
Discussion
It has been reported that approximately 3.3 million deaths occurring in adults are related to addictive alcohol consumption worldwide.23 ALD in individuals with heavy alcohol consumption begins with steatosis and progresses into more severe alcohol-associated hepatitis and/or cirrhosis, but the early stage of ALD is considered to be reversible.2,24 Frank BurrMallory’s landmark observation in 1911 first identified a link between inflammation and ALD by histopathology analysis of ALD. Accumulating evidence shows that multiple factors result in the liver inflammatory response including alcohol metabolites, enriched free fatty acids (FFAs), necrotic cell products and the well-known gut flora-derived LPS. Importantly, in addition to their important roles in inflammation, many of these proinflammatory factors and their downstream intermediates are known to be common causes of cell/tissue injury.7,25 Elevated levels of inflammatory factors such as TNF-α and IL-1β have been documented in the circulation and liver of ALD patients.6 These close links between alcoholic inflammation and liver injury strongly support that cytokines play a key role in the development of ALD. Following liver injury, several cell types including hepatocytes secrete inflammatory cytokines. Chronic alcohol consumption also sensitizes hepatocytes to inflammatory signals and impairs their ability to respond to protective signals.
Some studies have shown that purinergic P2Y2R represents a novel pharmacological target in many inflammatory reactions.10,26 Accumulating studies have demonstrated that P2Y2R plays a critical role in various liver disorders.12,13 It was claimed that P2Y2R inhibition protected mice against acute ethanol-induced liver injury by reducing oxidative stress and lipid accumulation.27,28 These studies highlight the importance of purinergic P2Y2R signaling in liver diseases including ALD. Then, the data from our work team suggested that inhibition of ATP-P2Y2R signaling with suramin effectively alleviated liver steatosis and inflammation induced by chronic-binge alcohol feeding.14 In the present study, we used an alcohol-stimulated AML-12 cell model and a mouse model of chronic plus binge alcohol feeding to perform experiments. We confirmed that there was a protective effect of suramin on chronic plus binge alcohol feeding-induced liver injury, as evidenced by significantly suppressed liver structural abnormalities and lipid infiltration and decreased levels of ALT/AST and TNF-α/IL-1β in the high dosage group of suramin (20 mg/kg) compared to CD-fed mice (Figures 1 and 2). Meanwhile, the conclusion of in vitro experiments revealed by Figure 3 were consistent with the mouse experiments.
On the one hand, Liu et al demonstrated that CD39 attenuated alcohol-induced steatohepatitis by scavenging extracellular ATP to indirectly regulate the expression of P2Y2R. On the other hand, there may be more potential mechanisms by which P2Y2R blockade exerts hepatoprotective effects that need to be explored. Recent findings have suggested that the P2Y2R-specific agonist, UTP, increases ERK phosphorylation and thus cell proliferation via P2Y2R in hepatocytes from CCl4-induced fibrotic mice.15 Importantly, another study demonstrated that early activation of p42/44 ERK and subsequent hepatocyte proliferation in response to 70% partial hepatectomy were impaired in P2Y2R knockout mice.17 ERK1/2, an important member of the MAPK signaling pathway, plays a major regulatory role in various cellular processes and inflammation.29 Emerging evidence has clarified that regulating MAPK signaling pathways exerts hepatoprotective and anti-inflammatory effects in nonalcoholic fatty liver disease.30 EGFR is a transmembrane tyrosine kinase receptor belonging to the ErbB family and has been identified as a key factor in multiple phases of liver injury from early inflammation, and fibrogenesis to tumor transformation.31,32 Evidence has revealed that EGFR and P2Y2R are both involved in the physiology and pathophysiology of the liver.33 Fuchs et al found that EGFR inhibition protected against hepatocyte lesions.34 Therefore, the possible roles of P2Y2R/EGFR/ERK1/2 in ALD aroused our interest.
In vivo, we found that alcohol feeding promoted the phosphorylation of EGFR and ERK1/2, both of which were effectively inhibited by suramin (20 mg/kg), implying that EGFR-ERK1/2 signaling may play a role in ALD. In vitro, silencing of P2Y2R inhibited apoptosis in AML-12 cell and suramin or P2Y2R silencing effectively inhibited the phosphorylation levels of EGFR induced by alcohol exposure just as the down-regulated effect of EGFR inhibitor AG1478 (3 nM). Similarly, suramin or P2Y2R silencing effectively inhibited the phosphorylation levels of ERK1/2 induced by alcohol exposure. Notably, alcohol-promoted ERK1/2 phosphorylation was also effectively restrained with AG1478, similar to the role of ERK1/2 inhibitor U0126. Furthermore, treatment with AG1478 or U0126 significantly downregulated the levels of TNF-α and IL-1β caused by alcohol treatment. P2Y2R has two Src-homology-3 (SH3) binding domains in the intracellular COOH-terminus facilitating transactivation of growth factor receptors such as EGFR.35,36 ERK is a major downstream factor of the EGFR-RAS-RAF signaling pathway in the liver. Accordingly, we suggest that P2Y2R blockade attenuates alcoholic liver inflammation via inhibiting EGFR- ERK1/2 signaling (Figure 7). However, Tackett et al found that cytokines such as TNF-α and IL-6 and cytokine-mediated signaling were intact in P2Y2R knockout remnant livers.17 We will explore whether there is a hepatoprotective effect in mice with P2Y2R deletion in the future. More potential purinergic mechanisms promoting ALD still need to be found.
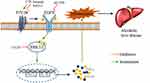 |
Figure 7 Blockade of the P2Y2 receptor attenuates alcoholic liver inflammation by targeting the EGFR-ERK1/2 signaling pathway. |
Conclusion
Collectively, our data distinctly suggest that P2Y2R plays a crucial role in alcohol-induced liver inflammation and injury. We demonstrated the hepatoprotective effect of P2Y2R blockade on alcohol-associated liver damage by targeting the EGFR-ERK1/2 signaling pathway in vivo and in vitro. This study provides insight into the roles of purinergic receptors in the pathogenesis of ALD and may help to develop a reasonable use of P2Y2R inhibitor-based therapy.
Acknowledgments
This project was supported by the National Science Foundation of China (No. 81270498), and the 2016 Annual Leading Talent Introduction and Cultivation Project in Universities (No. gxbjZD2016032). Zhen-Ni Liu, Qian-Qian Su, and Yu-Hui Wang are co-first authors for this study.
Disclosure
The first three authors equally contributed to this work. Z-n L, Q-q S, and Y-h W both performed the experiments, analyzed the data, and drafted the manuscript. X W helped to analyze the data. X-w L conceived the study and revised the manuscript. All authors declare that there are no conflicts of interest associated with this work.
References
1. Seitz HK, Mueller S, Dancygier H. Alcoholic Liver Disease. Springer Berlin Heidelberg; 2018.
2. Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastro Hepat. 2015;12(4):231–242. doi:10.1038/nrgastro.2015.35
3. Saberi B, Dadabhai AS, Jang YY, Gurakar A, Mezey E. Current management of alcoholic hepatitis and future therapies. J Clin Transl Hepatol. 2016;4(2):113–122. doi:10.14218/JCTH.2016.00006
4. Lin HZ, Yang SQ, Zeldin G, Diehl AM. Chronic ethanol consumption induces the production of tumor necrosis factor- and related cytokines in liver and adipose tissue. Alcohol Clin Exp Res. 2010;22(s5):231S–237S. doi:10.1111/j.1530-0277.1998.tb04008.x
5. Kamimura S, Tsukamoto DH, Tsukamoto H. Cytokine gene expression by Kupffer cells in experimental alcoholic liver disease. Hepatology. 2010;22(4):1304–1309.
6. Mcclain C, Barve S, Deaciuc I, Kugelmas M, Hill D. Cytokines in alcoholic liver disease. Semin Liver Dis. 1999;19(02):205–219. doi:10.1055/s-2007-1007110
7. Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32(1):343–368. doi:10.1146/annurev-nutr-072610-145138
8. Dusabimana T, Kim SR, Park EJ, Je J, Sang WP. P2Y2R contributes to the development of diabetic nephropathy by inhibiting autophagy response. Mol Metab. 2020;42:101089. doi:10.1016/j.molmet.2020.101089
9. Choi R, Chu G, Siow NL, et al. Activation of UTP-sensitive P2Y2 receptor induces the expression of cholinergic genes in cultured cortical neurons: a signaling cascade triggered by Ca2+ mobilization and extracellular regulated kinase phosphorylation. Mol Pharmacol. 2013;84(1):50–61. doi:10.1124/mol.112.084160
10. Stachon P, Geis S, Peikert A, Heidenreich A, Zirlik A. Extracellular ATP induces vascular inflammation and atherosclerosis via P2Y2 in Mice. Arterioscler Thromb Vasc Biol. 2016;36(8):1577–1586. doi:10.1161/ATVBAHA.115.307397
11. Jin H, Ko YS, Kim HJ. P2Y2R-mediated inflammasome activation is involved in tumor progression in breast cancer cells and in radiotherapy-resistant breast cancer. Int J Oncol. 2018;53:1953–1966.
12. Xie R, Xu J, Wen G, et al. The P2Y2Nucleotide receptor mediates the proliferation and migration of human hepatocellular carcinoma cells induced by ATP. J Biol Chem. 2014;289(27):19137–19149. doi:10.1074/jbc.M113.540047
13. Schulien I, Hockenjos B, Marck VV, Ayata CK, Hasselblatt P. Extracellular ATP and purinergic P2Y2 receptor signaling promote liver tumorigenesis in Mice by exacerbating DNA damage. Cancer Res. 2019;80(4):1909–2019.
14. Liu ZN, Jia WQ, Jiang T, Dai JW, Lv XW, Lv X-W. Regulation of CD39 expression in ATP-P2Y2R-mediated alcoholic liver steatosis and inflammation. Int Immunopharmacol. 2019;77:105915. doi:10.1016/j.intimp.2019.105915
15. Velázquez-Miranda E, Molina-Aguilar C, González-Gallardo A, Vázquez-Martínez O, Vázquez-Cuevas FG, Vázquez-Cuevas FG. Increased purinergic responses dependent on P2Y2 receptors in hepatocytes from CCl4-treated fibrotic mice. Int J Mol Sci. 2020;21(7):2305. doi:10.3390/ijms21072305
16. Zaparte A, Cappellari AR, Brando CA, Souza J, Morrone FB. P2Y2 receptor activation promotes esophageal cancer cells proliferation via ERK1/2 pathway. Eur J Pharmacol. 2020;891(3):173687. doi:10.1016/j.ejphar.2020.173687
17. Tackett BC, Sun H, Mei Y, et al. P2Y2 purinergic receptor activation is essential for efficient hepatocyte proliferation in response to partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2014;307(11):G1073–G1087. doi:10.1152/ajpgi.00092.2014
18. Zhang H, Shi JH, Jiang H, et al. ZBTB20 regulates EGFR expression and hepatocyte proliferation in mouse liver regeneration. Cell Death Dis. 2018;9(5):462. doi:10.1038/s41419-018-0514-0
19. Arany I, Megyesi JK, Nelkin BD, Safirstein RL. STAT3 attenuates EGFR-mediated ERK activation and cell survival during oxidant stress in mouse proximal tubular cells. Kidney Int. 2006;70(4):669. doi:10.1038/sj.ki.5001604
20. Sun X, Liang J, Yao X, Lu C, Tang J. The activation of EGFR promotes myocardial tumor necrosis factor-α production and cardiac failure in endotoxemia. Oncotarget. 2015;6(34):35478–35495. doi:10.18632/oncotarget.6071
21. Ding H, Jiang Y, Zhu L, et al. Reticulocalbin-2 enhances hepatocellular carcinoma proliferation via modulating the EGFR-ERK pathway. Oncogene. 2017;36(48):6691–6700.
22. Ltwa B, Kjja B, Kmfa B, et al. P2Y 2 receptors mediate nucleotide-induced EGFR phosphorylation and stimulate proliferation and tumorigenesis of head and neck squamous cell carcinoma cell lines. Oral Oncol. 2020;109:104808.
23. Gowing LR, Ali RL, Allsop S, Marsden J, Witton J. Global statistics on addictive behaviours: 2014 status report. Addiction. 2015;110(6):904–919. doi:10.1111/add.12899
24. Siegmund SV, Brenner DA. Molecular pathogenesis of alcohol‐induced hepatic fibrosis. Alcoholism. 2006;29:102S–109S.
25. Malhi H, Gores G. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease ©. Semin Liver Dis. 2008;28(04):360–369. doi:10.1055/s-0028-1091980
26. Mueller T, Vieira RDP, Grimm M, et al. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65(12):1545–1553. doi:10.1111/j.1398-9995.2010.02426.x
27. He S, Rehman H, Shi Y, Krishnasamy Y, Zhi Z. Suramin decreases injury and improves regeneration of ethanol-induced steatotic partial liver grafts. J Pharmacol Exp Ther. 2012;344(2):417–425. doi:10.1124/jpet.112.199919
28. Eichhorst ST, Krueger A, Ster SM, et al. Suramin inhibits death receptor-induced apoptosis in vitro and fulminant apoptotic liver damage in mice. Nat Med. 2004;10(6):602–609. doi:10.1038/nm1049
29. Fabio M, Maria C, Arrighi M, et al. Extracellular signal-regulated kinase activation differentially regulates platelet-derived growth factor’s actions in hepatic stellate cells, and is induced by in vivo liver injury in the rat. Hepatology. 1999;30(4):951–958. doi:10.1002/hep.510300406
30. Jiang J, Yan LA, Shi ZA, Wang LD, Shan LA, Te E. Hepatoprotective and anti-inflammatory effects of total flavonoids of Qu Zhi Ke (peel of Citrus changshan-huyou) on non-alcoholic fatty liver disease in rats via modulation of NF-κB and MAPKs. Phytomedicine. 2019;64:153082. doi:10.1016/j.phymed.2019.153082
31. Natarajan A, Wagner B, Sibilia M. The EGF receptor is required for efficient liver regeneration. P Natl Acad Sci Usa. 2007;104(43):17081–17086. doi:10.1073/pnas.0704126104
32. Perugorria MJ, Latasa MU, Nicou A, Cartagena-Lirola H, Avila MA. The epidermal growth factor receptor ligand amphiregulin participates in the development of mouse liver fibrosis. Hepatology. 2010;48(4):1251–1261. doi:10.1002/hep.22437
33. Brygida A, Marzena ZZ, Strzelczyk JK, et al. Hepatocyte growth factor and epidermal growth factor activity during later stages of rat liver regeneration upon interferon α-2b influence. Clin Exp Hepatol. 2017;3:9–15.
34. Bryan C, Fuchs YHTF, Fujii T. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59(4):1577–1590. doi:10.1002/hep.26898
35. Seye CI, Yu N, Gonzalez FA, Erb L, Weisman GA. The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1). J Biol Chem. 2004;279(34):35679–35686. doi:10.1074/jbc.M401799200
36. Liu J, Liao Z, Camden J, et al. Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem. 2004;279(9):8212–8218. doi:10.1074/jbc.M312230200
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.