Back to Journals » Integrated Pharmacy Research and Practice » Volume 13
Biosimilars Would Reduce Health Care Costs But Are Yet Poorly Known – Patient Survey Study Among Biological Medicine Users
Received 18 September 2023
Accepted for publication 29 December 2023
Published 3 February 2024 Volume 2024:13 Pages 9—16
DOI https://doi.org/10.2147/IPRP.S440888
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Walid Al-Qerem
Mari Pölkki, Tuire Prami
Oriola, Expert Services, Espoo, Finland
Correspondence: Tuire Prami, Oriola Finland Oy, Orionintie 5, 02200 Espoo, Finland, Tel +358505468459, Email [email protected]
Background: From the beginning of the year 2024, gradually implemented amendment to the Medicines Act will enable interchange of biological medicines in pharmacies in Finland. The legislative change aims to reduce health care costs.
Methods: Opinions of the biological medicine users regarding substitution in pharmacies and knowledge about biological medicines were determined by a patient survey in community pharmacies and via patient organizations in Finland.
Results: In total, 199 users of biological medicines responded to the survey. The respondents did not always know which product they were using, an originator or a biosimilar. This was more prominent among patients with biosimilars determined according to brand names. The more recently the biological medicine had been prescribed, the more likely a biosimilar was in use. Only about 40% of the respondents would enable pharmacies to substitute their biological medicine to a lower cost product. The most common obstacle to the idea of interchange in pharmacies was that the respondents wanted to keep the product the doctor had prescribed for them. In general, biosimilar users were more accepting towards possible interchange than originator users.
Conclusion: Although the most recent treatments appear to be initiated with biosimilars, interchange in pharmacies could enable an efficient way to lower health care costs. However, guidance and awareness regarding biosimilars and biological medicines in general would improve patients’ willingness towards the change, but also help pharmacists and prescribing doctors in their meaningful role.
Plain Language Summary: The use of biological medicines and the costs thereafter are rapidly growing.In Finland, switching between an originator and a biosimilar currently requires doctor’s prescription, but interchange in pharmacies will be possible in the beginning of year 2024.Due to notable differences in prices between biological medicines, active switching to lower cost products in pharmacies would restrain health care costs remarkably.Oriola Expert Services ran a survey with an electronic questionnaire among patients using biological medicines.Survey data were collected in pharmacies and via patient organizations.Oriola research team found out that patients do not generally know the differences between biological medicines, and if they are using an originator or a biosimilar.Despite the upcoming pharmacy substitution, future cost savings rely on decisions made by doctors at the time of prescribing biological medication.To ensure patient compliance, sufficient patient information about biosimilars is required.
Keywords: biological medicine, originator, biosimilar, reference product, interchange
Introduction
Biological medicines are a rapidly growing type of therapy available to treat for example cancers and immune-mediated inflammatory conditions such as rheumatic diseases, inflammatory bowel diseases (IBD), and psoriasis. Adalimumab and etanercept were among the most reimbursement costs causing biological medicines in Finland in 2022.1 Introduction of biosimilars has resulted in considerable cost savings in public funded health care regimes in many countries.2,3 Biosimilars are in structure and function similar to originator biological medicines and can therefore be extrapolated for use in same indications. In 2017, experts representing medicines agencies from four European countries have concluded that switching between comparable biological substances approved in the European Union (EU) is not expected to trigger or enhance immunogenicity, which has been the main concern in discussion about changes between different biological medicines.4 Biosimilars are expected to increase patients' access to in every case high cost biological medicines, and gradually, more patients will be switched to biosimilars either by doctors’ prescriptions or mandatory national indications.5 Economic impact of these switches is expected to be remarkable in the coming years.
Heads of Medicines Agencies (HMA) and European Medicines Agency (EMA) have recently considered that once a biosimilar is approved in the EU it is interchangeable, which means that the biosimilar can be used instead of its reference product (or vice versa) or one biosimilar can be replaced with another biosimilar of the same reference product.6 Already before that, since 2015, the position of the local authority of this EU country, Finnish Medicines Agency Fimea, has been that biosimilars are interchangeable under supervision of a health care professional.7 Similarly, in another Nordic country, since 2017, the position of the Norwegian Medicines Agency is that switching between reference products and biosimilars during ongoing treatment is safe.8
Currently, interchange between the originator and its biosimilar requires a doctor’s prescription in Finland, but next year, according to amendment in Medicines Act, pharmacies can substitute biological medicines that Fimea has assessed interchangeable.9 The new legislation will be implemented gradually starting on January 01, 2024, and it will first concern small molecular heparins, continuing later to cover rest of the biological medicines, excluding short-acting insulins.
Introduction of biosimilars has been modest, and interchange in pharmacies has been suggested to increase their use, but the practice is not yet allowed or implemented.10 It has been discussed, that safe and efficient implementation of interchange in pharmacies requires new research.11 Current evidence on interchange of biological medicines is scarce reflecting low knowledge and attitude towards biosimilars.10 Even though the upcoming legislation will lower technical threshold of substitution by enabling pharmacies to offer the lower price products to patients, the final decision to adhere to the offered option depends on patients themselves. The aim of this study was to investigate patients’ awareness regarding biosimilars in general, and their opinion about lower-cost alternatives for the biological medicine they use, as well as their willingness for interchange.
Materials and Methods
This was a patient survey based on an electronic questionnaire in which adult patients using continuous, self-injectable, biological medicines with biosimilars on the market could participate. Data collection was conducted in 88 community pharmacies around Finland as well as via Finnish patient organizations, the Finnish Rheumatism Association, and the IBD and Other Intestinal Diseases Association, between May and October 2022. In pharmacies, the survey was addressed to customers purchasing self-administrated biological medicines containing the following active substances: adalimumab, etanercept, pegfilgrastim, or filgrastim. The patient organizations informed their members about the survey via social media platforms.
The original semi-structured questionnaire consisted of 25 questions (with several sub-questions) specifically tailored for the purposes of this study (Supplementary Material). The questions covered the following topics: 1) demographics, 2) information about current biological medication, 3) knowledge regarding biosimilars, and opinions about possible interchange in pharmacies. Part of the results originating from the large questionnaire were reported here.
The data collection was conducted anonymously, and the participation was voluntary for the respondents. The respondents were able to skip questions, stop answering, and delete answers at any point during the participation, and they were informed about this possibility. Privacy statement, based on the EU General Data Protection Regulation (GDPR), was available for the participants during the study in Finnish, Swedish, and English. However, due to the anonymous nature of the data collection, the GDPR-specified right to correct or delete given answers afterwards was not applicable. The participation was based on consent, and the survey was conducted following the guidelines of the Finnish National Advisory Board on Research Integrity.12
The data were analyzed using the statistical software R Studio version 4.2.2 for Windows. Categorical variables were reported as frequencies of received responses and percentage distributions. Continuous variables were reported as means, medians with interquartile ranges and ranges of minimum and maximum. Due to the voluntary nature of the data collection, some answers were missing. In the analyses, the number of given responses were divided with the number of respondents. If the number of responses in certain answer category was <5, results were presented as “<5” to ensure individual privacy.
Results
In total, 199 respondents participated to the survey, and 69% were females (Table 1). Females were more commonly biosimilar users, as of originator users 58% and of biosimilar users 70% were females. Median age of the respondents was 50 years (mean=49.8; range=20–83; Q1=40; Q3=61). Originator users were somewhat older than biosimilar users, their median ages were 54 (mean: 53.3; Q1=45; Q3=63) and 48 years (mean: 48.7; Q1=38; Q3=60), respectively. For almost half of the respondents (45%), the highest education level was secondary education (Table 1). Of the originator users 72% and of the biosimilar users 58% were using the biological medicine originally prescribed and had not used any other biological medicine for the same condition.
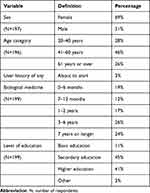 |
Table 1 Demographic Characteristics of the Respondents |
There are five university hospitals and their catchment areas in Finland. Most of the responses were collected in Helsinki, Oulu, and Tampere (27%, 27%, and 26%, respectively), and less in Kuopio (12%) and Turku (8%) specific catchment areas. Originators were used more commonly in Helsinki and Turku, whereas in Oulu and Tampere biosimilars were more commonly in use. In Kuopio, the use of originators and biosimilars was equally common.
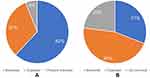
Half of the respondents (50%) had used a biological medicine longer than three years (Table 1), and 31% had used the same product longer than 3 years. Based on reported product names, 62% of the respondents currently used a biosimilar, and 6% could not name the product they currently used (Figure 1A). On the other hand, based on respondents’ general knowledge, the majority (46%) of the participants reported using an originator, and up to 23% did not know the nature of their biological medication (Figure 1B).
Of originator users, 72% had been using the current biological medicine longer than three years (Figure 2). In contrast, of biosimilar users, 89% had been using the current biological medicine less than three years.
 |
Figure 2 Time for how long the current biological medicine had been in use (N=188) with current medication based on self-reported product name as a background variable. |
In total, 47% of biosimilar users who were able to name the brand of the biological medicine they used, were aware that the product was a biosimilar, whereas 31% thought that the biological medicine was an originator (Figure 3). Of originator users, 80% knew they used an originator, and none of them miss-thought they were using a biosimilar. From 20% to 23% of originator and biosimilar users, respectively, did not know which one they were using.
 |
Figure 3 Awareness of the respondents regarding to the biological medicine type they currently used (N=188) with current medication based on self-reported product name as a background variable. |
When the question was more about the costs, 40% of all respondents wished pharmacies could suggest a lower-cost alternative for the biological medicine, but the most common (41%) answer was “I do not know/not important”. In this category, the youngest respondents were the most prominent (59%) compared to the other age groups (33% and 35% in 41–60-year-old and 61-year-old and older, respectively). Discretized age categories have been presented in Table 1. Almost one-fifth (19%) of all respondents answered that they would not wish for the interchange to take place in pharmacies.
For 76% of these, the main reason was the wish to use the medicine the doctor had prescribed for them. The other three most common reasons for unwillingness against possible interchange were that the new product would be unsuitable or less effective and desire to use a familiar product.
Respondents who were current biosimilar users were more willing (45%) towards the interchange in pharmacies than the originator users (31%; Figure 4). On the other hand, originator users were more against the interchange versus the biosimilar users (30% versus 15%, respectively), and in total up to 79% did not really have an opinion.
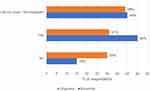 |
Figure 4 Respondents’ willingness regarding the interchange of lower-cost alternative biosimilars (N=188) with current medication based on self-reported product name as a background variable. |
Discussion
The first results of this large patient survey study show that the longer the biologic medicine had been in use, the more likely it was about an originator and not switched to a biosimilar. Of those respondents who had used a biological medicine longer than three years, less than a quarter were on a biosimilar suggesting that interchange has not yet been actively conducted by doctors. At the same time, the findings indicate that in recent years, biological therapy has more likely than earlier been initiated with a biosimilar, but there is still demand towards this trend in order to restrain health care costs.
Since 2017, Social Insurance Institution of Finland has actively sent guidance containing feedback on the prescriptions for doctors prescribing biological medicines.13 In 2020, a “Dear Doctor” letter acknowledged those doctors who had been prescribing biosimilars. This positive feedback did not increase the use of biosimilars, and in 2022, Social Insurance Institution of Finland sent a comment to those doctors who had been prescribing the most expensive biological medicines despite less expensive products on the market. Again, very recently, there have been efforts to provide doctors with more up-to-date information about the prices of biological products encouraging them to choose the most reasonably priced product available.14
Our findings indicate that patients’ knowledge regarding biosimilars and biological medicines in general could be notably improved. In addition, despite high level general education in Finland and among the respondents of this study, the patients’ own awareness of their currently used biosimilar or originator was surprisingly weak. Of the originator users, 80% knew that they were using an originator and none miss-thought they were using a biosimilar. Of the biosimilar users, one-third thought that they were using an originator, and only half of them were sure that they were using a biosimilar. On the other hand, only one-third of the originator users knew the meaning of biosimilars (data not shown). This type of unawareness might set barriers to discussions when planning a substitution.
This was a cross-sectional survey in 2022. Respondents were guided to answer to the survey only once. We managed to capture targeted number of respondents: about 200. The most common biological medicine among the respondents was adalimumab originator followed by its biosimilars. These results are in line with the unit sales data from Finland, according to which use of the biosimilars of these medicines are not yet dominating the market. At the time of the data collection, switching between originators and biosimilars was only possible with a doctor’s prescription.
Comparing with other European countries, Finland is ahead in culture of biosimilar use, and direct interchange of biological medicines in pharmacies starts in the beginning of next year. In Ireland, for example, biosimilar uptake has been reported to be very slow when estimating the use of best-value biological products of adalimumab or etanercept.15 Increasing use of biosimilars would create financial savings that further could support invest in new innovative therapies beneficial for even more patients. This is possible through the introduction of biosimilars that, when placed on the market, reduce the price of the original medication.16 Last year, it was reported how prices and market shares of outpatient biosimilars had been developed in Finland during 2009–2020.3 Public reimbursement scheme related to the market entry of biosimilars and changes in pricing policy were the main reasons for the decrease in the prices of reference products.
The new Finnish legislation about interchange of biological medicines in pharmacies will be implemented gradually.9 The parliament has required strengthening guidance and advice on interchange, especially in use of administration devices, for that patient safety will not be jeopardized. Currently, also the Norwegian legislation allows biosimilar change in pharmacies, and the Norwegian Medicines Agency assesses whether and how it would be safe to introduce this procedure.17 In addition to device use counselling, other risk themes related to interchange of biological medicines, such as patients lacking advise from pharmacies and distracting support material, were identified in semi-structured qualitative interviews in Finland.11 This study identified potentially a new role for community pharmacists to facilitate safe and effective substitution of biological medicines. Further analyses with the data collected in this study project will concentrate on this important angle of view.
According to the present results, 40% of the respondents felt positive about future substitution in pharmacies. They saw that this would decrease costs for themselves and for the society (57% and 33% of the respondents with positive opinion, respectively). Further, it seemed that biosimilar users were more willing towards substitution than originator users, but in both groups equally many were uncertain or did not consider this topic important (Figure 4). The main reason to oppose substitution was willingness to stick to the product that the doctor had prescribed. This suggests that even if interchange to a less expensive product in pharmacies would be possible, the doctors’ role in choosing the more affordable option remains important. In addition, it is important to understand that the price is not the only key influencing factor in prescribing of a specific product, as stated before.2
Use of biosimilars instead of originators saves costs, that is widely understood, but according to our knowledge there is only very little published information about effects and importance of interchange in pharmacies. This will be an interesting research topic in the future. In addition to the interchange operation itself, pharmacies will have an important role in guiding patients, especially among those who currently use originators, towards more affordable alternatives and further in decreasing health care costs. The patients must feel comfortable with the substitution when offered to them, and they need to understand the similarity of efficacy and safety of different products to guarantee good compliance. Furthermore, pharmacists need clear instructions how to proceed with this guidance and with what type of material. Future studies will show how the patients’ attitudes about interchange have changed compared to the results of today.
Conclusions
The most recent treatments were initiated with biosimilars, and patients with originators were more often against possible interchange than biosimilar users. It is important that costs of biological medicines are considered at the time of prescription, especially at the initiation of medication. Relevant guidance for patients and pharmacy staff but also for doctors who prescribe biological medicines is needed.
Abbreviations
EMA, European Medicines Agency; EU, European Union; GDPR, General Data Protection Regulation; HMA, Heads of Medicines Agencies; IBD, Inflammatory Bowel Diseases; Q, quarter.
Ethics Approval and Informed Consent
The study was carried out in accordance with national and EU requirements for ensuring the well-being and rights of the participants. In addition, the survey was conducted following the guidelines of the Finnish National Advisory Board on Research Integrity.12 Data collection was based on individual consent of each respondent; thus the legitimate purposes of the data collection and use were both informed consent and scientific research. Before data collection, Oriola provided an EU GDPR-based data protection impact assessment with risk assessment about the study database. According to the local legislation, an external ethical review was not needed in case of a volunteer anonymous survey.
Consent for Publication
The authors state that the details of any images can be published as they are prepared by authors themselves.
Data Sharing Statement
The survey data were collected for this research project only. The data are not shared.
Acknowledgments
We are deeply grateful to all respondents for participating the survey. Oriola Research Pharmacies, the Finnish Rheumatism Association, and the IBD and Other Intestinal Diseases Association as well as other parties collaborating in the data collection are acknowledged for their valuable help. BSc Pharm Iiro Mytty, BSc Pharm Mia Nevalainen, and Study Nurse Merja Pihlaja from Oriola are acknowledged for their professional assistance in study coordination. Further acknowledgements are addressed to MSc Pharm Ilona Iso-Mustajärvi and MSc Pharm Jarno Ruotsalainen from Oriola for their helpful and supportive advice. BSc Pharm Emily Laajanen and BHC Nina Uurtamo from Viatris Oy are acknowledged for their kind participation in study meetings and active role as the sponsor contacts throughout the project. (At the time of revision, Mia Nevalainen’s current post is at Wellpharma Oy and Nina Uurtamo’s at Biocon Biologics Finland.)
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was financially supported by Viatris Oy. The sponsor was not involved in any of the stages from study design to submission of the paper for publication. After the original submission, during the review round, Viatris completed biosimilars transaction with Biocon Biologics in Europe.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Lääkekorvaukset kasvoivat vuonna 2022 – lääkkeiden uudet käyttötarkoitukset kasvattavat kustannuksia, mutta rinnakkaislääkkeiden käyttöönotto voisi tuoda säästöjä [Drug reimbursements increased in 2022 – new indications of drugs increase costs, but the introduction of synonymous preparations could bring savings] [website on the Internet]. Helsinki: Kela [Social Insurance Institution of Finland]; 2023. Finnish. Available from: https://www.kela.fi/yhteistyokumppanit-laakekorvaukset-ajankohtaista-apteekkitiedotteet/5198559/laakekorvaukset-kasvoivat-vuonna-2022-laakkeiden-uudet-kayttotarkoitukset-kasvattavat-kustannuksia-mutta-rinnakkaislaakkeiden-kayttoonotto-voisi-tuoda-saastoja.
2. Aladul MI, Fitzpatrick RW, Chapman SR. Impact of infliximab and etanercept biosimilars on biological disease-modifying antirheumatic drugs utilisation and NHS budget in the UK. BioDrugs. 2017;31(6):533–544. doi:10.1007/s40259-017-0252-3
3. Luukkanen SV, Tolonen HM, Airaksinen M, Saarukka LSM. The price and market share evolution of the original biologics and their biosimilars in Finland. BioDrugs. 2022;36(4):537–547. doi:10.1007/s40259-022-00540-y
4. Kurki P, van Aerts L, Wolff-Holz E, Giezen T, Skibeli V, Weise M. Interchangeability of biosimilars: a European perspective. BioDrugs. 2017;31(2):83–91. doi:10.1007/s40259-017-0210-0
5. Scheinberg M, Azevedo V. The future landscape of biosimilars in rheumatology: where we are where we are going. Autoimmun Rev. 2019;18(2):203–208. doi:10.1016/j.autrev.2018.09.005
6. Statement on the scientific rationale supporting interchangeability of biosimilar medicines in the EU [website on the Internet]. Amsterdam: European Medicines Agency, Heads of Medicines Agencies; 2023. Available from: https://www.ema.europa.eu/en/documents/public-statement/statement-scientific-rationale-supporting-interchangeability-biosimilar-medicines-eu_en.pdf.
7. Interchangeability of biosimilars – position of Finnish Medicines Agency Fimea [website on the Internet]. Helsinki: Finnish Medicines Agency Fimea; 2015. Available from: https://web.archive.org/web/20220331024125/https://fimea.fi/documents/542809/838272/29197_Biosimilaarien_vaihtokelpoisuus_EN.pdf.
8. Switching between a reference product and a biosimilar [website on the Internet]. Oslo: The Norwegian Medicines Agency; 2017. Available from: https://www.legemiddelverket.no/en/news/switching-between-a-reference-product-and-a-biosimilar.
9. Eduskunnan vastaus hallituksen esitykseen STM/2022/219 [Parliament’s response to the government’s motion STM/2022/219] [website on the Internet]. Helsinki: Valtioneuvosto [Finnish Government]; 2022. Finnish. Available from: https://valtioneuvosto.fi/paatokset/paatos?decisionId=0900908f80813f78.
10. Tolonen H, Falck J, Kurki P, et al. Is there any research evidence beyond surveys and opinion polls on automatic substitution of biological medicines? A systematic review. BioDrugs. 2021;35(5):547–561. doi:10.1007/s40259-021-00493-8
11. Tolonen H, Airaksinen M, Ruokoniemi P, Hämeen-Anttila K, Shermock K, Kurki P. Medication safety risks to be managed in national implementation of automatic substitution of biological medicines: a qualitative study. BMJ Open. 2019;9(10):e032892. doi:10.1136/bmjopen-2019-032892
12. The ethical principles of research with human participants and ethical review in the human sciences in Finland - Finnish National Board on Research Integrity TENK guidelines 2019 [website on the Internet]. Helsinki: Finnish National Board On Research Integrity Tenk; 2019:3. Available from: https://tenk.fi/sites/default/files/2021-01/Ethical_review_in_human_sciences_2020.pdf.
13. Klintrup K, Kastarinen H, Helminen S, Leinonen J, Saastamoinen L. Kela kehottaa: suosi biosimilaareja, kun määräät biologisia lääkkeitä [Social Insurance Institution of Finland urges: favor biosimilars when prescribing biological medicines]. Suom Laakaril. 2022;77(21–22):1012–1014. Finnish.
14. Kela muistuttaa lääkäreitä edullisempien biologisten lääkkeiden määräämisestä [Social Insurance Institution of Finland reminds doctors to prescribe more affordable biological medicines] [website on the Internet]. Helsinki: Kela [Social Insurance Institution of Finland]; 2023. Finnish. Available from: https://tietotarjotin.fi/uutinen/694823/kela-muistuttaa-laakareita-edullisempien-biologisten-laakkeiden-maaraamisesta.
15. Duggan B, Smith A, Barry M. Uptake of biosimilars for TNF-α inhibitors adalimumab and etanercept following the best-value biological medicine initiative in Ireland. Int J Clin Pharm. 2021;43(5):1251–1256. doi:10.1007/s11096-021-01243-0
16. Kowalik K, Węgierska M, Barczyńska T, Jeka S. Pharmacoeconomic evaluation of costs of rheumatoid arthritis therapy with selected biological treatment. Reumatologia. 2018;56(6):340–345. doi:10.5114/reum.2018.80710
17. Biotilsvarende legemidler kan byttes i apotek [Biosimilar medicines can be exchanged in pharmacies] [website on the Internet]. Oslo: Statens legemiddelverk [Norwegian Medicines Agency]; 2021. Norwegian. Available from: https://www.legemiddelverket.no/nyheter/biotilsvarende-legemidler-kan-byttes-i-apotek.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.