Back to Journals » Medical Devices: Evidence and Research » Volume 9
Bioresorbable vascular scaffolds technology: current use and future developments
Authors Giacchi G, Ortega-Paz L, Brugaletta S, Ishida K, Sabaté M
Received 25 March 2016
Accepted for publication 11 June 2016
Published 11 July 2016 Volume 2016:9 Pages 185—198
DOI https://doi.org/10.2147/MDER.S90461
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Giuseppe Giacchi, Luis Ortega-Paz, Salvatore Brugaletta, Kohki Ishida, Manel Sabaté
Cardiology Department, Clinic Cardiovascular Institute, Hospital Clinic, August Pi and Sunyer Biomedical Research Institute (IDIBAPS), University of Barcelona, Barcelona, Spain
Abstract: Coronary bioresorbable vascular scaffolds are a new appealing therapeutic option in interventional cardiology. The most used and studied is currently the Absorb BVS™. Its backbone is made of poly-l-lactide and coated by a thin layer of poly-d,l-lactide, it releases everolimus and is fully degraded to H2O and CO2 in 2–3 years. Absorb BVS™ seems to offer several theoretical advantages over metallic stent, as it gives temporary mechanical support to vessel wall without permanently caging it. Therefore, long-term endothelial function and structure are not affected. A possible future surgical revascularization is not compromised. Natural vasomotion in response to external stimuli is also recovered. Several observational and randomized trials have been published about BVS clinical outcomes. The main aim of this review is to carry out a systematic analysis about Absorb BVS™ studies, evaluating also the technical improvements of the Absorb GT1 BVS™.
Keywords: Absorb GT1, Absorb BVS™, bioresorbable vascular scaffold, BRS, coronary scaffold
Introduction
Percutaneous coronary intervention (PCI) is commonly performed by implantation of metallic stents.1 However, stent implantation is affected by a substantial burden of complications as, for example, in-stent restenosis and stent thrombosis.2–5
In this scenario, a new valuable therapeutic option may be represented by bioresorbable vascular scaffolds, which give temporary post-PCI support to the vessel wall and then are biodegraded. Several scaffolds are currently under development, but currently only two have the certificate Conformité Européenne mark approval for coronary angioplasty: the Absorb BVS™ (Abbott Laboratories, Abbott Park, IL, USA) and the DESolve™ (Elixir Medical Corporation, Sunnyvale, CA, USA). The best studied and the most used is the former, with several registries/trials published and >100,000 patients treated.6,7
This review aims to perform a systematic literature analysis about clinical outcomes of Absorb BVS™ in coronary artery disease (CAD), evaluating also technical improvements of the Absorb GT1 BVS™.
Absorb BVS™ design and technology
The Absorb BVS™ has a bioresorbable polymeric structure made of poly-l-lactide, coated by a thin polymer of poly-d,l-lactide, which controls the release of the antiproliferative drug everolimus. Poly-l-lactide and poly-d,l-lactide are hydrolyzed and fully metabolized to lactic acid. It is degraded via the Krebs cycle to H2O and CO2.8 The Absorb BVS™ has two platinum radio-opaque markers at each edge that allow angiographic visualization. Average strut thickness is 150 μm and crossing profile ∼1.2 mm. First-generation backbone (version 1.0) presented circumferential out-of-phase zigzag circles linked together by longitudinal struts. Conversely, the second-generation (Absorb BVS™ 1.1) has in-phase zigzag rings linked by bridges, with better mechanical integrity and higher support to vessel wall.9,10 Absorb BVS™ 1.0 clinical performance was evaluated in the ABSORB A study.11 This is a prospective, multicenter, single-arm study. It enrolled 30 patients suffering from stable/unstable angina or silent ischemia and de novo coronary lesions treated by BVS 1.0 delivery. Five-year clinical outcome was satisfactory, with an ischemia-driven major adverse cardiac event (MACE) rate of 3.4%. No scaffold thrombosis (ST) was reported.
After coronary delivery, the Absorb lifecycle has three phases: revascularization, restoration, and reabsorption. In the first phase (lasting ∼3 months), the scaffold performs similarly to a drug-eluting stent (DES) in terms of deliverability, radial strength, acute recoil, and neointimal thickening. In the restoration phase, the BVS is degraded and starts losing its radial strength. Natural vasomotion is theoretically restored at the end of the second phase. Finally, in the last phase, the polymeric backbone is totally degraded into lactic acid monomers and oligomers, rapidly metabolized by the body.12
The Absorb scaffold offers several theoretical advantages over permanent metallic caging of the vessels.13,14 Temporary scaffolding allows long-term restoration of endothelial structure and function. It does not affect a possible future surgical coronary revascularization. Scaffolded vessels show late lumen gain, as well as recovery of natural vasomotion, in response to external stimuli.
Absorb BVS™ in literature: state of the art
A lot of original articles, case reports/case series, abstracts, reviews, and editorials have been published about Absorb scaffold, accounting for >400 records.15,16
We herein considered all published studies reporting clinical outcomes of subjects treated with second-generation BVS, excluding case reports/series (accounting for less than ten patients), studies assessing only angiographic outcomes, preclinical studies, reviews, and articles evaluating BVS performance in non-CAD (Table 1).
Studies are classified into three arms, according to study design: registries and single-arm studies, propensity-score matching comparison, and randomized trials. Registries account both for single- and multicenter registries.
Meta-analyses are also reported (Table 2).
Registries and single-arm studies
Several single- and multicenter registries on the Absorb BVS™ have been published. We herein divided them and single-arm studies into four groups, according to clinical follow-up: short-term follow-up, mid-term follow-up (also accounting for registries focusing on specific lesions subsets), 1-year follow-up, and long-term follow-up.
Short-term follow-up
The BVS ST-segment elevation myocardial infarction (STEMI) first study and Kajiya et al registry reported good early outcomes of BVS in STEMI. The former reported a 1-month MACE rate of 2.6% and the latter 9.1%.17,18
The Polish National Registry reported good acute clinical outcomes of BVS delivery in an all-comers population, with no peri-procedural deaths.19
The ABSORB FIRST is a prospective, all-comers registry, currently ongoing but not recruiting (NCT01759290).20 Target lesion failure (TLF), defined as the composite of cardiac death, target vessel myocardial infarction (MI), and target lesion revascularization (TLR), will be evaluated at 1 year. ST, device success (successful BVS delivery with residual stenosis of <50%), and procedure success (device delivery with no TLF within 3 days of the index procedure) will also be investigated. This registry is intended to provide ongoing postmarketing surveillance on safety and performance of the Absorb BVS™. It will enroll a minimum of 1,800 patients with de novo coronary lesions treated with at least one scaffold Absorb. An interim analysis about a 30-day outcome of the first 1,200 patients has been reported, with good device and procedure success rate (98.4% and 97.9%, respectively).21
Mid-term follow-up and specific lesion subsets
Six-month BVS performance in acute coronary syndrome (ACS) has been evaluated in several registries.22–25 MACE rate was satisfactory, ranging between 4.9% and 10.7%. In BVS-RAI registry, Prague 19, and Gori et al series, BVS patients were also compared with a control group of subjects treated with metallic stent, with no difference in clinical endpoint.22–24
BVS 6-month outcome has also been investigated in specific lesions subsets.
Capranzano et al evaluated BVS performance in bifurcation lesion subset, with good results (one TLR at day 227).26 Grundeken et al also investigated BVS outcome in bifurcations.27 Indeed, they studied 6-month outcomes in bifurcation lesions treated by combined Tryton dedicated coronary bifurcation stent and Absorb scaffold delivery. Results were satisfactory with a TLR rate of 20% (n=2) and no deaths, MIs, or stent thrombosis.
Some series investigated BVS outcomes in coronary chronic total occlusions.28–31 Results were good, with a MACE rate at short–mid-term ranging between 0 and 4.8%. Besides, in GHOST-CTO registry, BVS subjects were also compared with a historical group of DES treated patients. Although there was no in-hospital clinical event in the BVS arm, the DES group had a higher rate of technical success (successful scaffold/stent delivery and implantation, postprocedural residual diameter stenosis <30% within the treated segment, and restoration of thrombolysis in MI grade 3 flow: BVS 78.1% vs DES 96.3%; P=0.012) and procedural success (technical success with no in-hospital MACE: BVS 78.1% vs DES 94.4%; P=0.035).
Ielasi et al and Moscarella et al in their multicenter registries investigated the outcomes with BVS implantation in in-stent restenosis.32,33 In both series, 7-month results were satisfactory: Ielasi et al reported a MACE incidence of 8.0%; meanwhile, Moscarella et al reported a major adverse cardiac or cerebrovascular event rate of 12%.
BVS performance in complex anatomical and/or clinical conditions was investigated by Jaguszewski et al.34 They assessed a patient-oriented composite endpoint (POCE) (defined as a composite of death, MI, and any revascularization) and a device-oriented composite endpoint (DOCE) (composite of cardiac death, target vessel MI, and ischemia-driven TLR) at mid-term. Results were satisfactory, with POCE and DOCE rates of 6.1% and 2.0%, respectively.
Mattesini et al studied MACE rate with BVS delivery in complex coronary lesions.35 BVS was compared with a DES control group. In both registry arms, device implantation was guided by optical coherence tomography. There was no difference in the primary outcome measure between the BVS and DES groups (BVS 5.7% vs DES 5.3%, P>0.05).
The AMC PCI registry and the GHOST-EU registry investigated mid-term BVS outcomes in an all-comers population.36,37 At 6-month follow-up, they reported a TLF rate of 8.5% and 4.4%, respectively. Of note, the cumulative incidence of definite/probable ST was rather high (3.0% in AMC PCI registry and 2.1% in GHOST-EU registry). GHOST-EU 1-year follow-up data have been presented at EuroPCR Congress 2015.38 The TLF rate was satisfactory, with a cumulative incidence of 5.2%. Definite/probable ST incidence was 2.0%, with a very low rate after 6 months (0.1%). Thrombotic events were mostly clustered in the first month. Tamburino et al recently matched 1-year outcome of GHOST-EU patients with Xience DES USA registry subjects by applying propensity score.39 The primary endpoint was DOCE rate (composite of cardiac death, target vessel MI, and ischemia-driven TLR). Definite/probable device thrombosis was also investigated. No significant difference was detected for DOCE (BVS 5.8% vs DES 7.6%; P=0.12) and ST/stent thrombosis (1.8% vs 1.1%; P=0.23). In the BVS group, cardiac death was less frequent (0.7% vs 1.9%; P=0.03) and a trend toward a reduction in MI rate was also present (2.4% vs 4.0%; P=0.07).
One-year follow-up
Several series studied 1-year BVS outcome in an all-comers population.40–42 They reported a MACE rate of 4.5%–8%, thus confirming good BVS performance in routine clinical practice.
Gil et al studied 1-year BVS clinical performance in stable CAD.43 They performed a subanalysis for patients treated with the combination of BVS and DES (hybrid revascularization). Results were satisfactory with a MACE rate of 7.2% and 4.5% in whole population and hybrid revascularization subgroup, respectively. There was no device thrombosis in hybrid revascularization subgroup; meanwhile, two cases were reported in the whole population (1.4%), one subacute (0.7%) and one late (0.7%).
Kawamoto et al compared 1-year clinical outcome between BVS patients with “full-plastic jacket” (FPJ) (BVS total length ≥60 mm) and those without FPJ (BVS total length <60 mm).44 Patients were all affected by stable CAD or unstable angina. No difference between the two groups was found in MACE rate (FPJ 19.2% vs NO-FPJ 13.0%; P=0.14). One late ST was reported in the FPJ group (5.3%) in a patient who had stopped double antiplatelet therapy.
Several registries assessed long-term BVS performance in ACS.
Kochman et al in their small series reported a satisfactory 1-year BVS clinical performance in STEMI, with only one nontarget vessel revascularization (5.3%).45 No thrombotic event at follow-up was reported.
Gori et al and POLAR ACS study evaluated the clinical outcomes of BVS in ACS.46,47 At 1-year, MACE incidence was 13.5% and 2%, respectively. Definite/probable ST rate was 3.0% and 1.0%, respectively. STs were all clustered in the first 6 months in both the series.
Long-term follow-up
The BVS EXPAND evaluated BVS performance in subjects suffering from silent ischemia, stable/unstable angina, or non-STEMI.48 Inclusion angiographic criteria were lesion length ≤28 mm and reference vessel diameter in a range between 2.0 and 3.8 mm. The primary outcome was a composite of cardiac death, all MI, and TLR. Median follow-up period was 559 days (interquartile range 371–733 days). BVS performance was satisfactory, with a primary outcome rate of 5.5% at 1-year follow-up. Definite 12-month ST incidence was 1.4%.
The ABSORB EXTEND is a prospective, multicenter registry (NCT01023789).49 It recruited patients with silent ischemia or stable/unstable angina (final planned enrollment 800 subjects). Abizaid et al in their preliminary report of 512 patients reported satisfactory BVS outcome at 1-year follow-up.50 The composite endpoints of ischemia-driven MACE, ischemia-driven TLF, and ischemia-driven target vessel failure were 4.3%, 4.3%, and 4.9%, respectively. Definite/probable ST was also investigated, with a 1-year rate of 0.8%. There was no acute ST case; meanwhile, subacute and late ST rates were 0.4% for both. Three-year follow-up data of the first 250 recruited subjects have been reported at the Transcatheter Cardiovascular Therapeutics meeting 2014 by Pieter Smits.51 The rates of ischemia-driven MACE, ischemia-driven TLF, and ischemia-driven target vessel failure were 9.3%, 8.9%, and 10.1%, respectively. The overall rate of definite/probable ST was 1.2%. Research compared ABSORB EXTEND subjects with a control group of subjects selected from other trials and treated with DES Xience (Abbott Laboratories, Abbott Park, IL, USA) by propensity score matching. There was no difference in 3-year MACE (hazard ratio 0.73; 95% confidence interval [CI] 0.38–1.41), definite/probable device thrombosis (hazard ratio 0.83; 95% CI 0.08–9.15), and MI rate (hazard ratio 1.06; 95% CI 0.41–2.73). Target vessel failure rate was significantly lower in the ABSORB EXTEND group (BVS 8.1% vs DES 14.2%; P=0.0488).
The ABSORB B is a multicenter, prospective, single-arm study. It included patients with de novo coronary lesions and silent ischemia or stable/unstable angina.52 The authors performed a multimodality imaging analysis and the primary clinical outcome measure was a composite of cardiac death, MI, and ischemia-driven TLR. It is the registry with the longest follow-up available for all the subjects (5 years). BVS performance was good, with a 5-year MACE rate of 11.0%. No ST was reported.
Propensity score matching comparisons
Costopoulos et al compared BVS and everolimus-eluting stent 6-month outcome in a real-world population, with the majority being B2/C class lesions according to the classification of the American College of Cardiology/American Heart Association (BVS 83.9% vs DES 77.4%; P=0.19).53 There was no difference between the two groups with respect to MACE (3.3% vs 7.6%; P=0.19) and TLR (3.3% vs 5.4%, P=0.41). No definite/probable device thrombosis was reported.
Sato et al also compared BVS and DES performance in a real-world population.54 Procedure time, total contrast medium adminstered, and fluoroscopy time were higher in the BVS group (P<0.001, P=0.02, and P<0.001, respectively). BVS delivery was also an independent predictor of long (>2 hours) procedure time in multivariable analysis (odds ratio =7.83%; 95% CI 2.81–25.78; P<0.001). There was no difference in 1-year MACE (BVS 10.2% vs DES 10.5%; P=0.82) and stent thrombosis/ST (1.0% vs 2.1%; P=0.58) rates between the two groups.
Muramatsu et al in their retrospective analysis compared BVS performance in diabetic patients and non-diabetic patients.55 Diabetic BVS patients were also matched and compared with diabetic DES patients of the SPIRIT trials,56 by applying propensity score matching. The primary outcome measure was 1-year DOCE rate, including cardiac death, target vessel MI, and TLR. Definite/probable device thrombosis rate was also studied. There was no significant difference in the primary outcome measure, both between diabetic BVS and non-diabetic BVS patients (diabetic BVS 3.7% vs non-diabetic BVS 5.1%; P=0.64) and between diabetic BVS and diabetic DES (diabetic BVS 3.9% vs diabetic DES; P=0.38). Definite/probable device thrombosis incidence did not differ too (diabetic BVS 0.7% vs non-diabetic BVS 0.7%; P=1.00) (diabetic BVS 1.0% vs diabetic DES 1.7%; P=1.00)
In the BVS-EXAMINATION study, we compared clinical outcomes of BVS with the ones of DES and bare metal stent (BMS) in STEMI patients (control groups from EXAMINATION trial).57 The primary endpoint studied was DOCE rate. There was no difference in the 1-year primary outcome measure both between BVS and DES (BVS 4.1% vs DES 4.1%, hazard ratio 0.99, 95% CI 0.23–4.32; P=0.994) and between BVS and BMS (BVS 4.1% vs BMS 5.9%, hazard ratio 0.50, 95% CI 0.13–1.88; P=0.306). Definite/probable stent thrombosis/ST was numerically higher in the BVS group both at 30 days (BVS 2.1% vs DES 0.3%, P=0.059; BVS 2.1% vs BMS 1.0%, P=0.324) and 1 year (BVS 2.4% vs DES 1.4%, P=0.948; BVS 2.4% vs BMS 1.7%, P=0.825, respectively), but it was not significant.
Randomized trials
The EVERBIO II is a single-center randomized trial, comparing BVS with everolimus- and biolimus-eluting stent (ratio 1:1:1) in an all-comers population.58 The primary endpoint was late lumen loss at 9 months, but patient-oriented MACE (POCE), DOCE, and ST were also studied. There was no significant difference in primary angiographic endpoint (P=0.30). POCE (BVS 27% vs DES 26%; P=0.30) and DOCE (12% vs 9%; P=0.60) rates were also similar between the two study groups. Only one case of possible device thrombosis was reported in the BVS arm, with no difference with the DES group (1% vs 0%; P=0.33).
The ABSORB-STEMI TROFI II is a noninferiority, multicenter trial.59 It recruited STEMI patients who were randomized to Absorb scaffold or Xience DES in a 1:1 ratio. The primary endpoint was the 6-month healing score evaluated at optical coherence tomography as surrogate for safety and efficacy of the treatment. DOCE rate was assessed as clinical outcome. BVS proved to be noninferior to Xience DES for the first imaging outcome (P<0.001 for noninferiority). DOCE (composite of cardiac death, target vessel MI, or clinically driven TLR) rate was also comparable between the two study arms (BVS 1.1% vs DES 0.0%; P>0.05). No stent thrombosis was reported; meanwhile, one definite subacute case was described in the BVS arm (1.1% vs 0.0%; P>0.05).
The ABSORB II, ABSORB JAPAN, and ABSORB III trials are three randomized clinical studies, enrolling subjects with silent ischemia or stable/unstable angina.60–62 In all trials, patients were randomized to Absorb or DES Xience in a 2:1 ratio.
In ABSORB II, the coprimary endpoints were vasomotion (change in mean lumen diameter before and after nitrate administration at 3 years) and difference between minimum lumen diameter (after nitrate administration) after the index procedure and at 3 years. Clinical endpoints were also investigated. Serruys et al recently published a 1-year interim analysis, reporting similar DOCE and MACE rates between the two study arms (BVS 5% vs DES 3%; P=0.35 for both), mainly driven by MI (4% vs 1%; P=0.06) and TLR (1% vs 2%; P=0.69).60 Definite/probable stent thrombosis/ST incidence did not differ between the two groups (0.9% vs 0.0%; P=0.55).
The ABSORB JAPAN is single blind, multicenter trial designed to enable approval of the Absorb BVS™ in Japan.61 The primary outcome was TLF at 1 year. Definite/probable device thrombosis was also studied. Scaffold proved to be noninferior to DES, both for TLF (BVS 4.2% vs DES 3.8%; P<0.0001 for noninferiority) and device thrombosis (1.5% vs 1.5%; P=1.0).
The ABSORB III is to date the biggest randomized BVS trial published. It investigated as a primary clinical outcome the 1-year TLF rate, investigated for both noninferiority and superiority.62 Device thrombosis was also studied. The Absorb BVS™ proved to be noninferior to DES Xience, but not superior for the primary endpoint (BVS 7.8% vs DES 6.1%, P=0.007 for noninferiority, P=0.16 for superiority). Stent thrombosis/ST frequency did not differ between the two study arms (1.5% vs 0.7%; P=0.13), although events were numerically higher in the BVS group.
The ABSORB CHINA has been designed to support regulatory approval of the Absorb BVS™ in People’s Republic of China.63 Patients with silent ischemia or stable/unstable angina were randomized to Absorb BVS™ or DES Xience in a 1:1 ratio and stratified according to diabetes and number of lesions treated. The primary endpoint was in-segment late loss at 1-year. Clinical outcomes were also studied. BVS proved to be noninferior to DES for the angiographic primary outcome (P=0.01). POCE (BVS 8.0% vs DES 9.7%; P=0.51) and DOCE (3.4% vs 4.2%; P=0.62) rates at 1 year were also similar. Device thrombosis did not significantly differ between the two study arms (0.4% vs 0.0%; P=1.0).
Meta-analysis
Three BVS meta-analyses have been published.16,64,65
Stone et al pooled data of ABSORB II, ABSORB III, ABSORB CHINA, and ABSORB JAPAN trials.64 They carried out a patient level, intention-to-treat analysis and outcomes analyzed were relative at 1-year POCE (composite of all-cause mortality, all MI, and all revascularization) rate and relative 1-year DOCE (cardiac death, target vessel MI, and ischemia-driven TLR) rate. Primary outcome did not differ between the two groups, both in patient-oriented (BVS 11.9% vs DES 10.6%, relative risk 1.09, 95% CI: 0.89–0.34; P=0.38) and in device-oriented (6.6% vs 5.2%, relative risk 1.22, 95% CI: 0.91–1.64; P=0.17) analyses. Target vessel MI was more frequent in BVS group (5.1% vs 3.3%, relative risk 1.45, 95% CI: 1.02–2.07; P=0.04) and there was a trend in definite/probable device thrombosis incidence (1.3% vs 0.6%, relative risk 2.09, 95% CI: 0.92–4.75; P=0.08) (Figure 1). Even if clinical outcomes did not differ between the two groups, technical BVS performance was inferior to the DES one. Actually, post-percutaneous in-device coronary intervention quantitative analysis proved that BVS lesions had lower acute gain (1.41±0.45 vs 1.58±0.45 mm; P<0.0001), lower minimal luminal diameter (2.37±0.39 vs 2.53±0.40 mm; P<0.0001), and higher diameter stenosis (12.4% vs 7.5%; P<0.0001). This could be due to drawbacks that still affect BVS technology. Indeed, even if BVS is clinically noninferior to DES, some technical matters still affect it and its technical performance.
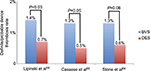  | Figure 1 Definite and probable device thrombosis frequency in BVS and DES patients. Notes: Definite/probable device thrombosis rate in BVS and DES population at mean ± SD 6.4±5.1 months, 1-year (interquartile range 9–12 months), and 1-year follow-up for Lipinski et al, Cassese et al, and Stone et al, respectively. Data from references.16,64,65 Abbrevations: BVS, bioresorbable vascular scaffold; DES, drug-eluting stent. |
Cassese et al pooled data of trials analyzed by Stone et al, also adding EVERBIO II and TROFI II trials.65 The primary efficacy endpoint was TLR and the primary safety outcome was definite/probable device thrombosis. Median follow-up was 12 months (interquartile range 9–12). TLR rate did not differ between the two study groups (odds ratio 0.97, 95% CI: 0.66–1.43; P=0.87), even though BVS subjects had a greater risk of device thrombosis (odds ratio 1.99, 95% CI: 1.00–3.98; P=0.05), especially in the first month (odds ratio 3.11, 95% CI: 1.24–7.82; P=0.02) (Figures 1 and 2).
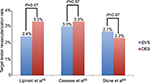  | Figure 2 Target lesion revascularization rate in BVS and DES populations. Notes: It shows BVS noninferiority to DES in terms of target lesion revascularization rate in three published meta-analyses. Follow-up was performed at mean ± SD 6.4±5.1 months, 1 year (interquartile range 9–12 months), and 1 year for Lipinski et al, Cassese et al, and Stone et al, respectively. Data from references.16,64,65 Abbrevations: BVS, bioresorbable vascular scaffold; DES, drug-eluting stent. |
Lipinski et al pooled data of published randomized trials and also registries, accounting for a total of 10,510 patients treated with BVS (n=8,351) and DES (n=2,159).16 They studied relative risk of device thrombosis at the longest follow-up. Other clinical outcomes, such as death, MI, and MACE rate, were also evaluated. BVS patients proved to have a higher risk of device thrombosis (odds ratio: 2.06, 95% CI: 1.07–3.98; P=0.03) and MI (odds ratio: 2.06, 95% CI: 1.31–3.22; P=0.002) (Figure 1). MACE (odds ratio: 0.87, 95% CI: 0.66–1.16; P=0.35) and cardiovascular death (odds ratio: 0.81, 95% CI: 0.42–1.58; P=0.54) rates did not differ between the two groups, even though there was a trend toward decreased all-cause mortality in the BVS arm (odds ratio: 0.40, 95% CI: 0.15–1.06; P=0.06).
Technical issues and scaffold pitfalls
Published data suggest that implantation of Absorb BVS™ is noninferior to second-generation DES in terms of clinical outcomes (Figure 2). However, the outcomes so far reported are varying. Potential concerns of this technology include limitations in acute performance and the occurrence of ST.
Efforts should be made to perform an accurate patient/lesion selection and implement an optimal implantation technique to minimize these risks.
First of all, a correct evaluation of patient/lesion suitability for BVS implantation is of great importance.66 The great benefit with BVS delivery is expected for young patients with long lesions. Due to the bulky structure of the BVS (strut thickness ≈150 μm), vessels with extreme angulation of the segment proximal to the lesion should be avoided.
Everaert et al gave five simple rules in BVS implantation that could improve scaffold performance.67 They are summarized in the five golden P for BVS implantation: prepare the lesion, properly size the vessel, pay attention to the expansions limits of the scaffold, postdilate the BVS with a properly sized noncompliant balloon, and pay attention to dual antiplatelet therapy patient compliance.
Adequate lesion preparation is mandatory. Predilation should be performed with increasing balloon size and the final balloon should have a diameter equal or only minimally undersized compared to the diameter of the selected BVS. Noncompliant balloon should be preferred. Full opening of the last balloon with no waist image on balloon profile is the goal that has to be reached.
Correct scaffold sizing is of upmost importance due to limits in scaffold postdilatation. BVS implantation in large vessel (with diameter >4.0 mm) is not allowed. The use of intravascular imaging is greatly encouraged. Optical coherence tomography, with its high resolution, could give important information about lesion characteristics, optimal scaffold dimensions, scaffold implantation results, and scaffold–vessel interactions.
Scaffold implantation has to be performed gradually, pressurizing the system in two atmosphere increments every 5 seconds. Target pressure should be maintained for at least 30 seconds. Due to polymeric backbone of the device, the final scaffold caliber cannot exceed the nominal diameter above 0.5 mm.
Routine postdilatation with noncompliant balloon is greatly encouraged, especially if some waist image is present on device profile. As for scaffold implantation, it is of great importance that the postdilatation balloon caliber does not exceed nominal scaffold diameter above 0.5 mm, in contrast to DESolve™, which has a wide expansion range.68
Dual antiplatelet therapy for at least 12 months is suggested in all BVS subjects, both treated for ACS and stable CAD. However, longer dual antiplatelet therapy duration (18–24 months) and/or more potent agents (ticagrelor or prasugrel) can be explored, especially in patients at high risk for thrombotic events.
Major concerns arise from the ST rate (Figure 1). Consistently in randomized trials and registries, ST rate is higher than expected. Moreover, some cases of very late ST have also been reported.69 ST may be linked to several factors: patient-, lesion-, device-, and procedure-related factors.70
Patient-related factors include all patient conditions associated with a higher prothrombotic status (ie, smoking, diabetes mellitus, chronic kidney disease, ACS clinical presentation, etc).
Challenging lesion subsets (ie, small vessels, thrombotic lesions, long lesions, bifurcations, etc) are known to be associated with DES thrombosis and similarly could have a pivotal role in ST.
Absorb BVS™ has greater strut dimensions than second-generation DES. This could create flow disturbances and delay scaffold re-endothelialization. Other factors that can be implied in ST are late-acquired incomplete strut apposition and neo-atherosclerosis.
Procedure-related factors (ie, fracture, incomplete apposition, under-expansion, flow limiting dissection) seem to have an important role in ST, especially in acute ST. The influence of these predisposing factors may be minimized by systematically applying the BVS delivery recommendations. Indeed, Puricel et al recently proved that ST rate can be reduced by the use of a “BVS-specific implantation protocol.”71
Future perspective and scaffold evolution: the Absorb GT1 BVS™
BVS is routinely used in clinical practice. The kind of patient who most could benefit from a BVS is a young subject admitted for ACS. Indeed, Absorb BVS™ could perform the best in soft and ruptured plaque and in patients with a high expectancy of life.
Conversely, in calcified and chronic coronary lesions, BVS performance is suboptimal, due to its technologic pitfalls.
The main drawback of BVS backbone is its bulky structure. The considerable thickness of its struts limits the use in bending and calcified vessels. A possible resolution could be to reduce strut thickness so as to improve the navigability and deliverability of the device. However, a good solution could also be the new Absorb GT1 BVS™ (Figure 3).72
New Absorb GT1 BVS™ scaffold has the same features of the previous bioresorbable coronary device regarding the scaffold. All the modifications concern the catheter delivery system.
The Absorb BVS™ delivery system presents three parts: a proximal high supportive jacketed hypotube, a mid shaft (with the skive transition), and the flexible distal shaft, with its distal hydrophilic coating (Figure 3A). The hypotube ends at the hypotube seal, where the outer member starts. The guide wire notch is located at the proximal end of the distal shaft and is ∼25.5 cm from the tip of the catheter. The distal shaft presents three seals: from proximal to distal the mid lap seal, the proximal seal and the distal seal. Distally to the mid lap seal, the catheter presents the distal part of the outer member. Between the proximal and the distal seals, we have the scaffold with the balloon, and just distally the distal seal we have the distal balloon shaft with the soft catheter tip.
Compared with the old version of the device, in the Absorb GT1 BVS™, designers made the hypotube more robust, integrated the skive transition, and optimized the distal catheter (Figure 3B).
The Absorb GT1 BVS™ hypotube has a stronger proximal member and is unjacketed. It has a higher cross-sectional area of the fluid part and wall that allows a faster deflation of the balloon and an increased pushability, respectively.
The skive joint has been simplified and integrated so as to improve push transmission across the material junction and reduce the guide wire notch profile.
The outer member of the distal catheter is now constituted by a single piece with an increase in the outer member diameter. The outer member extends from the hypotube to the proximal balloon seal. The mid shaft and the mid lap seal have been eliminated, improving scaffold control. There has been an increase in the cross-sectional area of the outer member and fluid path, thus allowing a faster deflation and improving the pushability of the system.
The only study evaluating Absorb GT1 BVS™ outcome is the SUGAR-EVE, not recruiting yet. This is a randomized Phase IV clinical trial, comparing the Absorb GT1 BVS™ versus an everolimus-eluting stent in 224 diabetic patients with de novo coronary stenosis. The primary endpoint is in-device late lumen loss at 9-month follow-up. Clinical outcomes will also be evaluated.73
Another important pitfall of the device is the reduced expansion range. This severely limits the use of the device in case of vasoconstriction or wrong vessel diameter evaluation. A greater expansibility could also reduce early ST burden.
The DESolve™ seems to be a valuable alternative. It has a great range of expansion (a 3.0 mm can be postdilated until 4.5 mm) and the property of “self-correction” acute recoil.68,74
However, several coronary scaffolds are currently under clinical development with the main aim to reduce strut thickness and improve scaffold distensibility. The Fortitude (Amaranth Medical, Inc., Mountain View, CA, USA) has a poly-l-lactic acid platform, a strut thickness of 120 μm, and can be postdilated 1 mm above nominal caliber. Another poly-l-lactic acid scaffold, the MeRes (Meril Life Science, Vapi, India), has a strut thickness of 100 μm. The Reva Fantom (REVA Medical, Inc., San Diego, CA, USA), with its tyrosine polycarbonate alloy, and the magnesium backbone Dreams 2G (Biotronik SE & Co. KG, Berlin, Germany) have both lower strut thickness and greater distensibility than Absorb BVS™. These devices are only at the initial development phase, and large clinical studies with long-term follow-up are awaited to assess clinical performance of these coronary scaffolds.75,76
Conclusion
Available clinical data suggest that the Absorb BVS™ appears to be a safe technology. The main data come from observational registries in which the BVS performance has been satisfactory, even in challenging anatomical and clinical subsets. Recently, the Absorb BVS™ has proved to be noninferior to second-generation DES also in randomized controlled trials.
Major concerns have arisen about the ST rate. In the registries and randomized trials, it is numerically higher than expected. Moreover, in meta-analysis, it has been significantly more frequent with BVS than with DES.
Refined selection of the lesions to be treated and use of optimal implantation technique may be helpful to prevent the occurrence of thrombotic events. The new Absorb GT1 BVS™ has some technical improvements in the catheter delivery system and could facilitate scaffold implantation.
More randomized clinical trials with longer follow-up are necessary to definitively assess the real BVS performance and pitfalls.
Disclosure
The authors report no conflicts of interest in this work.
References
Hamid H, Coltart J. ‘Miracle stents’–A future without restenosis. Mcgill J Med. 2007;10(2):105–111. | ||
Sabate M, Cequier A, Iñiguez A, et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012;380:1482–1490. | ||
Serruys PW, Farooq V, Kalesan B, et al. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. JACC Cardiovasc Interv. 2013;6:777–789. | ||
Sabaté M, Räber L, Heg D, et al. Comparison of newer-generation drug-eluting with bare-metal stents in patients with acute ST-segment elevation myocardial infarction: a pooled analysis of the EXAMINATION (clinical Evaluation of the Xience-V stent in Acute Myocardial INfArcTION) and COMFORTABLE-AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction) trials. JACC Cardiovasc Interv. 2014;7:55–63. | ||
Sabaté M, Brugaletta S, Cequier A, et al. Clinical outcomes in patients with ST-segment elevation myocardial infarction treated with everolimus-eluting stents versus bare-metal stents (EXAMINATION): 5-year results of a randomised trial. Lancet. 2016;387:357–366. | ||
Wiebe J, Nef HM, Hamm CW. Current status of bioresorbable scaffolds in the treatment of coronary artery disease. J Am Coll Cardiol. 2014;64:2541–2551. | ||
Abbott Vascular web site. Data available from: http://www.abbottvascular.com/int/products/coronary-intervention/absorb-bioresorbable-scaffold.html?reie0e2vs4i. Accessed June 8, 2016. | ||
Giacchi G, Ortega-Paz L, Brugaletta S, Ishida K, Sabaté M. Bioresorbable vascular scaffold implantation in acute coronary syndromes: clinical evidence, tips and tricks. Postepy Kardiol Interwencyjnej. 2015;11:161–169. | ||
Onuma Y, Serruys PW, Gomez J, et al. Comparison of in vivo acute stent recoil between the bioresorbable everolimus-eluting coronary scaffolds (revision 1.0 and 1.1) and the metallic everolimus-eluting stent. Catheter Cardiovasc Interv. 2011;78:3–12. | ||
Brugaletta S, Garcia-Garcia HM, Diletti R, et al. Comparison between the first and second generation bioresorbable vascular scaffolds: a six month virtual histology study. EuroIntervention. 2011;6:1110–1116. | ||
Oberhauser JP, Hossainy S, Rapoza RJ. Design principles and performance of bioresorbable polymeric vascular scaffolds. EuroIntervention. 2009;5 (Suppl F):F15–F22. | ||
Onuma Y, Dudek D, Thuesen L, et al. Five-year clinical and functional multislice computed tomography angiographic results after coronary implantation of the fully resorbable polymeric everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB cohort A trial. JACC Cardiovasc Interv. 2013;6:999–1009. | ||
Serruys PW, Onuma Y, Dudek D, et al. Evaluation of the second generation of a bioresorbable everolimus-eluting vascular scaffold for the treatment of de novo coronary artery stenosis: 12-month clinical and imaging outcomes. J Am Coll Cardiol. 2011;58:1578–1588. | ||
Zhang Y, Bourantas CV, Farooq V, et al. Bioresorbable scaffolds in the treatment of coronary artery disease. Med Devices (Auckl). 2013;6:37–48. | ||
Lu C, Filion KB, Eisenberg MJ. The safety and efficacy of absorb bioresorbable vascular scaffold: a systematic review. Clin Cardiol. 2016;39:48–55. | ||
Lipinski MJ, Escarcega RO, Baker NC, et al. Scaffold thrombosis after percutaneous coronary intervention with ABSORB bioresorbable vascular scaffold: a systematic review and meta-analysis. JACC Cardiovasc Interv. 2016;9:12–24. | ||
Diletti R, Karanasos A, Muramatsu T, et al. Everolimus-eluting bioresorbable vascular scaffolds for treatment of patients presenting with ST-segment elevation myocardial infarction: BVS STEMI first study. Eur Heart J. 2014;35:777–786. | ||
Kajiya T, Liang M, Sharma RK, et al. Everolimus-eluting bioresorbable vascular scaffold (BVS) implantation in patients with ST-segment elevation myocardial infarction (STEMI). EuroIntervention. 2013;9:501–504. | ||
Rzeszutko Ł, Siudak Z, Włodarczak A, et al. Use of bioresorbable vascular scaffolds in patients with stable angina and acute coronary syndromes. Polish National Registry. Kardiol Pol. 2014;72:1394–1399. | ||
Clinical trials web site. Data available from: https://clinicaltrials.gov/ct2/show/record/NCT01759290. Accessed June 8, 2016. | ||
Eeckhout E, Seth A, Mao VW, et al. TCT-613 ABSORB FIRST: An interim report on baseline characteristics and acute performance on the first 1,200 patients from a prospective, multi-center, global registry. J Am Coll Cardiol. 2014;64(11_S). | ||
Cortese B, Ielasi A, Romagnoli E, et al. Clinical comparison with short-term follow-up of bioresorbable vascular scaffold versus everolimus-eluting stent in primary percutaneous coronary interventions. Am J Cardiol. 2015;116:705–710. | ||
Kočka V, Malý M, Toušek P, et al. Bioresorbable vascular scaffolds in acute ST-segment elevation myocardial infarction: a prospective multicentre study ‘Prague 19’. Eur Heart J. 2014;35:787–794. | ||
Gori T, Schulz E, Hink U, et al. Early outcome after implantation of Absorb bioresorbable drug-eluting scaffolds in patients with acute coronary syndromes. EuroIntervention. 2014;9:1036–1041. | ||
Wiebe J, Möllmann H, Most A, et al. Short-term outcome of patients with ST-segment elevation myocardial infarction (STEMI) treated with an everolimus-eluting bioresorbable vascular scaffold. Clin Res Cardiol. 2014;103:141–148. | ||
Capranzano P, Gargiulo G, Capodanno D, et al. Treatment of coronary bifurcation lesions with bioresorbable vascular scaffolds. Minerva Cardioangiol. 2014;62:229–234. | ||
Grundeken MJ, Hassell ME, Kraak RP, et al. Treatment of coronary bifurcation lesions with the Absorb bioresorbable vascular scaffold in combination with the Tryton dedicated coronary bifurcation stent: evaluation using two- and three-dimensional optical coherence tomography. EuroIntervention. 2015;11:877–884. | ||
Wiebe J, Liebetrau C, Dörr O, et al. Feasibility of everolimus-eluting bioresorbable vascular scaffolds in patients with chronic total occlusion. Int J Cardiol. 2015;179:90–94. | ||
Vaquerizo B, Barros A, Pujadas S, et al. One-year results of bioresorbable vascular scaffolds for coronary chronic total occlusions. Am J Cardiol. 2016;117(6):906–917. | ||
Ojeda S, Pan M, Romero M, et al. Outcomes and computed tomography scan follow-up of bioresorbable vascular scaffold for the percutaneous treatment of chronic total coronary artery occlusion. Am J Cardiol. 2015;115:1487–1493. | ||
La Manna A, Chisari A, Giacchi G, et al. Everolimus-eluting bioresorbable vascular scaffolds versus second generation drug-eluting stents for percutaneous treatment of chronic total coronary occlusions: Technical and procedural outcomes from the GHOST-CTO registry. Catheter Cardiovasc Interv. Epub 2016 Jan 12. | ||
Ielasi A, Latib A, Naganuma T, et al. Early results following everolimus-eluting bioresorbable vascular scaffold implantation for the treatment of in-stent restenosis. Int J Cardiol. 2014;173:513–514. | ||
Moscarella E, Varricchio A, Stabile E, et al. Bioresorbable vascular scaffold implantation for the treatment of coronary in-stent restenosis: results from a multicenter Italian experience. Int J Cardiol. 2015;199:366–372. | ||
Jaguszewski M, Ghadri JR, Zipponi M, et al. Feasibility of second-generation bioresorbable vascular scaffold implantation in complex anatomical and clinical scenarios. Clin Res Cardiol. 2015;104:124–135. | ||
Mattesini A, Secco GG, Dall’Ara G, et al. ABSORB biodegradable stents versus second-generation metal stents: a comparison study of 100 complex lesions treated under OCT guidance. JACC Cardiovasc Interv.2014;7:741–750. | ||
Kraak RP, Hassell ME, Grundeken MJ, et al. Initial experience and clinical evaluation of the absorb bioresorbable vascular scaffold (BVS) in real-world practice: the AMC Single Centre Real World PCI Registry. EuroIntervention. 2015;10:1160–1168. | ||
Capodanno D, Gori T, Nef H, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention. 2015;10:1144–1153. | ||
EuroPCR Congress 2015 web site. Data available from: http://www.pcronline.com/Lectures/2015/Ghost-EU-update. Accessed June 8, 2016. | ||
Tamburino C, Capranzano P, Gori T, et al. 1-Year outcomes of everolimus-eluting bioresorbable scaffolds versus everolimus-eluting stents: a propensity-matched comparison of the GHOST-EU and XIENCE V USA registries. JACC Cardiovasc Interv. 2016;9:440–449. | ||
Wöhrle J, Naber C, Schmitz T, et al. Beyond the early stages: insights from the ASSURE registry on bioresorbable vascular scaffolds. EuroIntervention. 2015;11:149–156. | ||
Costopoulos C, Crowson MC, Brown AJ, et al. Mid-term clinical outcomes of ABSORB bioresorbable vascular scaffold implantation in a real-world population: A single-center experience. Cardiovasc Revasc Med. 2015;16:461–464. | ||
Robaei D, Back L, Ooi SY, Pitney M, Jepson N. Twelve-month outcomes with a bioresorbable everolimus-eluting scaffold: results of the ESHC-BVS registry at two Australian centers. J Invasive Cardiol. 2015. | ||
Gil RJ, Bil J, Pawłowski T, et al. The use of bioresorbable vascular scaffold Absorb BVS® in patients with stable coronary artery disease: one-year results with special focus on the hybrid BVS and DES treatment. Kardiol Pol. Epub 2016 Jan 18. | ||
Kawamoto H, Panoulas VF, Sato K, et al. Short-term outcomes following “full-plastic jacket” everolimus-eluting bioresorbable scaffold implantation. Int J Cardiol. 2014;177:607–609. | ||
Kochman J, Tomaniak M, Kołtowski Ł, et al. A 12-month angiographic and optical coherence tomography follow-up after bioresorbable vascular scaffold implantation in patients with ST-segment elevation myocardial infarction. Catheter Cardiovasc Interv. 2015;86:E180–E189. | ||
Gori T, Schulz E, Hink U, et al. Clinical, angiographic, functional, and imaging outcomes 12 months after implantation of drug-eluting bioresorbable vascular scaffolds in acute coronary syndromes. JACC Cardiovasc Interv. 2015;8:770–777. | ||
Dudek D, Rzeszutko Ł, Zasada W, et al. Bioresorbable vascular scaffolds in patients with acute coronary syndromes: the POLAR ACS study. Pol Arch Med Wewn. 2014;124:669–677. | ||
Felix C, Fam JM, Onuma Y. TCT-527 long-term clinical outcomes of patients treated with the everolimus-eluting bioresorbable vascular scaffold. The BVS Expand Study. J Am Coll Cardiol. 2015;66(15_S). | ||
Clinical trials web site. Data available from: https://clinicaltrials.gov/ct2/show/record/NCT01023789?term=NCT01023789&rank=1. Accessed June 8, 2016. | ||
Abizaid A, Ribamar Costa J, Bartorelli AL, et al. The ABSORB EXTEND study: preliminary report of the twelve-month clinical outcomes in the first 512 patients enrolled. EuroIntervention. 2015;10:1396–1401. | ||
TCTmd web site. Data available from: http://www.tctmd.com/show.aspx?id=125719. Accessed June 8, 2016. | ||
Serruys PW, Ormiston J, van Geuns RJ, et al. A polylactide bioresorbable scaffold eluting everolimus for treatment of coronary stenosis: 5-year follow-up. J Am Coll Cardiol. 2016;67:766–776. | ||
Costopoulos C, Latib A, Naganuma T, et al. Comparison of early clinical outcomes between ABSORB bioresorbable vascular scaffold and everolimus-eluting stent implantation in a real-world population. Catheter Cardiovasc Interv. 2015;85:E10–E15. | ||
Sato K, Latib A, Panoulas VF, et al. Procedural feasibility and clinical outcomes in propensity-matched patients treated with bioresorbable scaffolds vs new-generation drug-eluting stents. Can J Cardiol. 2015;31:328–334. | ||
Muramatsu T, Onuma Y, van Geuns RJ, et al. 1-year clinical outcomes of diabetic patients treated with everolimus-eluting bioresorbable vascular scaffolds: a pooled analysis of the ABSORB and the SPIRIT trials.JACC Cardiovasc Interv. 2014;7:482–493. | ||
Dangas GD, Serruys PW, Kereiakes DJ, et al. Meta-analysis of everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease: final 3-year results of the SPIRIT clinical trials program (Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv. 2013;6:914–922. | ||
Brugaletta S, Gori T, Low AF, et al. Absorb bioresorbable vascular scaffold versus everolimus-eluting metallic stent in ST-segment elevation myocardial infarction: 1-year results of a propensity score matching comparison: the BVS-EXAMINATION Study (bioresorbable vascular scaffold-a clinical evaluation of everolimus eluting coronary stents in the treatment of patients with ST-segment elevation myocardial infarction). JACC Cardiovasc Interv. 2015;8:189–197. | ||
Puricel S, Arroyo D, Corpataux N, et al. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds. J Am Coll Cardiol. 2015;65:791–801. | ||
Sabaté M, Windecker S, Iñiguez A, et al. Everolimus-eluting bioresorbable stent vs. durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction: results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI II trial. Eur Heart J. 2016;37:229–240. | ||
Serruys PW, Chevalier B, Dudek D, et al. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet. 2015;385:43–54. | ||
Kimura T, Kozuma K, Tanabe K, et al. A randomized trial evaluating everolimus-eluting Absorb bioresorbable scaffolds vs. everolimus-eluting metallic stents in patients with coronary artery disease: ABSORB Japan. Eur Heart J. 2015;36:3332–3342. | ||
Ellis SG, Kereiakes DJ, Metzger DC, et al. Everolimus-eluting bioresorbable scaffolds for coronary artery disease. N Engl J Med. 2015;373:1905–1915. | ||
Gao R, Yang Y, Han Y, et al. Bioresorbable vascular scaffolds versus metallic stents in patients with coronary artery disease: ABSORB China Trial. J Am Coll Cardiol. 2015;66:2298–2309. | ||
Stone GW, Gao R, Kimura T, et al. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease: a patient-level, pooled meta-analysis. Lancet. 2016;387(10025):1277–1289. | ||
Cassese S, Byrne RA, Ndrepepa G, et al. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: a meta-analysis of randomised controlled trials. Lancet. 2016;387(10018):537–544. | ||
Tamburino C, Latib A, van Geuns RJ, et al. Contemporary practice and technical aspects in coronary intervention with bioresorbable scaffolds: a European perspective. EuroIntervention. 2015;11:45–52. | ||
Everaert B, Felix C, Koolen J, et al. Appropriate use of bioresorbable vascular scaffolds in percutaneous coronary interventions: a recommendation from experienced users : a position statement on the use of bioresorbable vascular scaffolds in the Netherlands. Neth Heart J. 2015;23:161–165. | ||
Giacchi G, Ortega-Paz L, Brugaletta S, Ishida K, Sabaté M. Bioresorbable vascular scaffold implantation in acute coronary syndromes: clinical evidence, tips and tricks. Postepy Kardiol Interwencyjnej. 2015;11:161–169. | ||
Räber L, Brugaletta S, Yamaji K, et al. Very late scaffold thrombosis: intracoronary imaging and histopathological and spectroscopic findings. J Am Coll Cardiol. 2015;66:1901–1914. | ||
Capodanno D, Joner M, Zimarino M. What about the risk of thrombosis with bioresorbable scaffolds? EuroIntervention. 2015;11 (Suppl V):V181–V184. | ||
Puricel S, Cuculi F, Weissner M, et al. Bioresorbable coronary scaffold thrombosis: multicenter comprehensive analysis of clinical presentation, mechanisms, and predictors. J Am Coll Cardiol. 2016;67:921–931. | ||
Abbott web site. Data available from: http://www.abbottvascular.com/int/products/coronary-intervention/absorb-bioresorbable-scaffold.html?ebm0vu6jemi. Accessed June 8, 2016. | ||
Clinical trials web site. Data available from: https://clinicaltrials.gov/ct2/show/NCT02632292?term=absorb+gt1&rank=1. Accessed June 8, 2016. | ||
Verheye S, Ormiston JA, Stewart J, et al. A next-generation bioresorbable coronary scaffold system: from bench to first clinical evaluation: 6- and 12-month clinical and multimodality imaging results. JACC Cardiovasc Interv. 2014;7:89–99. | ||
Giacchi G, Ortega-Paz L, Brugaletta S, et al. Bioresorbable vascular scaffolds in clinical practice: state-of-the-art. Panminerva Med. 2016;58:130–142. | ||
All data from TCTmd slide presentations, JIM 2016 meeting. Available from: http://www.tctmd.com/list.aspx?fid=1046680. Accessed June 8, 2016. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



