Back to Journals » International Journal of Nanomedicine » Volume 18
Bioprinting-Enabled Biomaterials: A Cutting-Edge Strategy for Future Osteoarthritis Therapy
Authors Yang X, Liu P, Zhang Y, Lu J, Zhao H
Received 26 July 2023
Accepted for publication 17 October 2023
Published 1 November 2023 Volume 2023:18 Pages 6213—6232
DOI https://doi.org/10.2147/IJN.S432468
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Xinquan Yang,* Peilong Liu,* Yan Zhang, Jun Lu, Hongmou Zhao
Department of Foot and Ankle Surgery, Honghui Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, 710054, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongmou Zhao, Department of Foot and Ankle Surgery, Honghui Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, 710054, People’s Republic of China, Email [email protected]
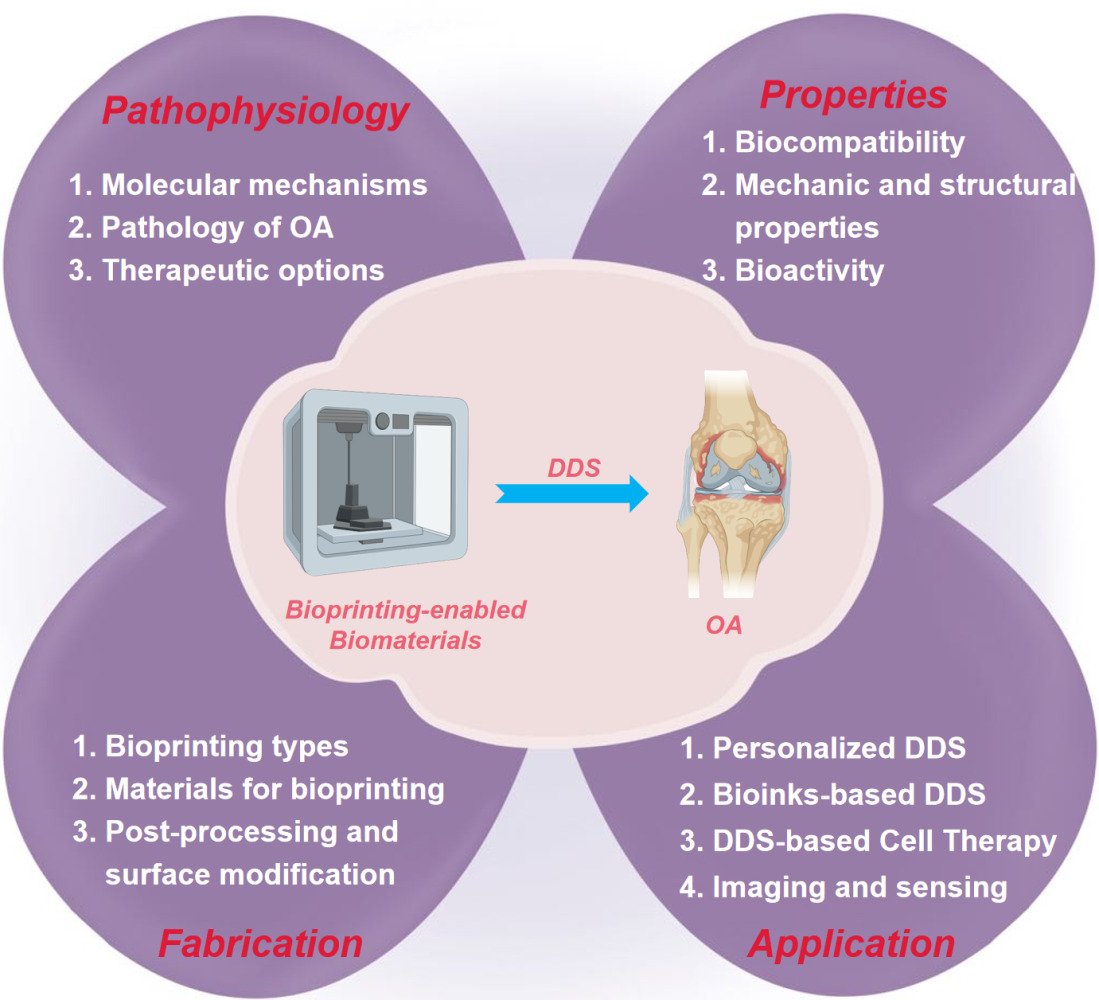
Abstract: Bioprinting is an advanced technology that allows for the precise placement of cells and biomaterials in a controlled manner, making significant contributions in regenerative medicine. Notably, bioprinting-enabled biomaterials have found extensive application as drug delivery systems (DDS) in the treatment of osteoarthritis (OA). Despite the widespread utilization of these biomaterials, there has been limited comprehensive research summarizing the recent advances in this area. Therefore, this review aims to explore the noteworthy developments and challenges associated with utilizing bioprinting-enabled biomaterials as effective DDS for the treatment of OA. To begin, we provide an overview of the complex pathophysiology of OA, highlighting the shortcomings of current treatment modalities. Following this, we conduct a detailed examination of various bioprinting technologies and discuss the wide range of biomaterials employed in DDS applications for OA therapy. Finally, by placing emphasis on their transformative potential, we discuss the incorporation of crucial cellular components such as chondrocytes and mesenchymal stem cells into bioprinted constructs, which play a pivotal role in promoting tissue regeneration and repair.
Keywords: drug delivery, bioprinting, osteoarthritis, biomaterials, translation
Graphical Abstract:
Introduction
Osteoarthritis (OA) is a chronic joint disease that primarily affects the elderly, often leading to disability and a substantial decline in their quality of life during their later years.1 The existing treatment modalities for OA have marked limitations, including their primarily symptomatic relief, systemic side effects, limited disease-modifying capabilities, invasive procedures, lack of personalization, temporary relief, and sometimes prohibitive costs. Moreover, OA treatment options face challenges in promoting effective tissue regeneration due to the limited regenerative capacity of damaged joint tissues. In this context, bioprinting-enabled drug delivery systems (DDS) offer a promising alternative, with the potential to provide precise, localized drug delivery, sustained release, tissue regeneration support, and personalized treatment options, addressing many of these limitations and improving the overall management of OA.2
Unlike conventional treatment options, which are often limited in their efficacy, bioprinting-enabled biomaterials offer a transformative avenue for combating OA.3 This innovative technology allows for the fabrication of intricate 3D structures that closely mimic the complexity of native tissues. With precision and finesse, bioprinting enables the controlled deposition of cells and biomaterials, resulting in the construction of structures that bear an eminent resemblance to the natural tissue microenvironment.4 By replicating the intricate architecture of the affected joint, bioprinting holds the potential to support the regeneration of damaged tissue and even impede the relentless progression of the disease.
One of the most critical aspects of bioprinting is the ability to design treatment to the specific needs of individual patients.5 The ability of customizing bioprinting-enabled biomaterials according to the individual’s personal characteristics, provides a paradigm shift in treatment outcomes. By considering factors such as the patient’s physiology, severity of the condition, and other individual characteristics, bioprinting paves the way for personalized interventions that yield improved outcomes.6 Furthermore, bioprinting technology offers a multitude of advantages that transcend the limitations of traditional therapies. By reducing the necessity for invasive procedures, which are often accompanied by risks and complications, bioprinting offers a safer and less intrusive route to recovery.7
OA primarily affects specific joints, and its chronic nature necessitates long-term therapy. Bioprinting offers precise drug placement directly into the affected joint space, reducing systemic exposure and minimizing systemic side effects.8 Furthermore, bioprinted constructs can control drug release kinetics, providing sustained therapeutic agents over an extended period, aligning with the chronicity of OA. Moreover, OA is characterized by the progressive loss of articular cartilage, inflammation, and tissue degeneration. Bioprinting enables the incorporation of chondrocytes or mesenchymal stem cells (MSCs) within scaffolds, promoting cartilage regeneration and tissue repair. By releasing anti-inflammatory agents or biological agents locally, bioprinted constructs effectively manage the chronic joint inflammation associated with OA.4 Additionally, the ability to customize bioprinted constructs to match the unique needs of each patient ensures personalized treatment, a crucial aspect in OA management.9
In this review, we conducted a comprehensive literature search using relevant databases such as PubMed, Scopus, and Web of Science. The search was focused on peer-reviewed articles and research papers published within the last ten years (from 2013 to 2023). We utilized specific keywords related to “bioprinting-enabled biomaterials”, “3D biomaterials”, “drug delivery system”, and “osteoarthritis therapy” to retrieve relevant studies. We aim to reveal the emerging developments and the inevitable challenges that arise when harnessing the excellent function of bioprinting-enabled biomaterials as an effective DDS for the treatment of OA. By closely linking the excellent advantages of bioprinting with the regenerative potential of these biomaterials-based DDS, we provide a promising strategy towards novel therapies for improving the therapeutic outcome of OA patients.
Pathophysiology of OA
The intricacies of OA unveil a multifaceted landscape, characterized by a variety of factors that contribute to its onset, advance, and severity. The earliest signs of OA pathology manifest on the surface of the articular cartilage, where focal regions experiencing maximum load display the telltale signs of fibrillation.10 In response to the loss of the cartilage’s supportive matrix, chondrocytes—the sole cell type present in cartilage—undergo a dramatic proliferation, striving to compensate for the damage incurred.10 However, some chondrocytes undergo a phenotypic shift, transforming into hypertrophic chondrocytes akin to those found in the hypertrophic zones of the growth plate.
With the relentless progression of OA, the matrix degradation and loss intensify, fueled by the continuous production of proteases instigated by proinflammatory cytokines. These cytokines, acting in an autocrine and paracrine manner, stimulate chondrocytes to produce even more cytokines and proteases, perpetuating a vicious cycle of destruction. The bone changes that occur in OA are equally significant.11 Subchondral sclerosis, characterized by an increase in collagen production, becomes evident, heralding the progression of the disease. At more advanced stages, osteophytes make their presence known, emerging as bone and cartilage outgrowths in the joint area. The growth pattern of osteophytes is sensitive to factors such as size and local cartilage narrowing, although exceptions exist, such as the lateral tibia and medial patella.11 Biomechanical forces play a pivotal role in facilitating osteophyte development, further entwining the intricate web of OA pathology.
Although synovial inflammation and hypertrophy are commonly observed in most patients with symptomatic OA, it is crucial to note that synovitis inflammation does not serve as the initiating factor for primary OA.12 Instead, it contributes to the progression of pain and disease, exacerbating the burdensome symptoms experienced by those afflicted with OA.
With the great stride in understanding the OA pathophysiology, it becomes increasingly apparent that a multitude of factors intertwines in the development and progression of the disease. Inflammatory mediators, proteinases, cell proliferation, and an array of biochemical parameters have all emerged as crucial players within this complex narrative (Figure 1). By elucidating the multifaceted nature of OA, we pave the way for comprehensive approaches to treatment and management, bringing in a new era of personalized and targeted interventions that hold the potential to alleviate the burden faced by those affected by this debilitating condition.
 |
Figure 1 The pathophysiology of OA. OA is a complex disease with multi-factors and causes, which affects not only the cartilage tissue but also the surrounding tissues. (A) Pathological knee. (B) OA not only impacts cartilage but also exerts its effects on the surrounding tissues. This results in the modulation of the subchondral plate, synovial tissue inflammation, and the development of erosion and osteophytes in the supporting bone. (C) The primary etiological event responsible for OA remains largely elusive. Several risk factors have been proposed to augment susceptibility to OA, including aging, chronic mechanical overloading, traumatic injury, genetic predisposition, sex, and hormonal variations. (D) Numerous regulatory pathways contribute to the initiation and progression of OA, although not all are necessarily involved in every disease phenotype. (E) Cells within the affected tissues actively participate in the initiation and propagation of OA. (F) During the course of OA, these cells undergo phenotypic changes, leading to altered transcriptome profiles that perpetuate an unending cycle of inflammation and tissue deterioration. Reproduced with permission from Foo JB, Looi QH, How CW, et al. Mesenchymal Stem Cell-Derived Exosomes and MicroRNAs in Cartilage Regeneration: biogenesis, Efficacy, miRNA Enrichment and Delivery. Pharmaceuticals. 2021;14(11):56.13 Copyright 2021, MDPI. Creative Commons. |
Functional Properties of Bioprinting-Enabled Biomaterials
The inherent properties of bioprinting-enabled biomaterials form the foundation of their effectiveness as a promising treatment option for OA. These properties not only define the structural integrity of the constructs but also enhance their interaction with the surrounding biological environment, ultimately influencing their therapeutic efficacy.14 First, bioprinting-enabled biomaterials exhibit exceptional biocompatibility, closely integrating with the host tissues. By mimicking the native tissue microenvironment, these biomaterials provide a beneficial milieu for cellular growth and proliferation, supporting the restoration of the damaged joint structures. Furthermore, the mechanical properties of bioprinting-enabled biomaterials are functionally engineered to withstand the dynamic forces and stresses encountered within the joint environment.15 By withstanding the rigors of daily joint activities, these biomaterials promote long-term therapeutic success, offering relief to individuals suffering from OA. Moreover, these biomaterials can act as carriers, capable of encapsulating a diverse range of pharmaceutical compounds, including anti-inflammatory agents, growth factors, and even cells.16 This controlled release mechanism enables targeted and sustained delivery of therapeutic payloads to the affected joint, optimizing treatment efficacy while reducing systemic side effects.
Specific Drugs and Materials Used in OA Therapy
OA treatment encompasses a range of drugs and pharmaceutical agents aimed at managing its symptoms and, more recently, modifying disease progression.17 Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), such as ibuprofen and naproxen, are frequently employed to alleviate OA-related pain and inflammation by inhibiting enzymes involved in the inflammatory process.18 However, long-term NSAID use can lead to gastrointestinal issues and cardiovascular concerns, necessitating careful monitoring. Disease-Modifying Osteoarthritis Drugs (DMOADs) represent a promising avenue, as they target the underlying processes contributing to cartilage degradation and joint inflammation.19 Compounds like sprifermin and strontium ranelate are under investigation for their potential to slow OA progression, offering hope for disease modification rather than mere symptom relief.20,21
Corticosteroid injections are another commonly utilized option, providing short-term relief by directly addressing joint inflammation.22 However, their prolonged use may lead to joint deterioration, emphasizing the importance of judicious administration. Hyaluronic acid injections, which mimic a natural joint component, aim to lubricate and cushion the affected joint, reducing pain and enhancing joint function.23 Nonetheless, the effectiveness of this treatment can vary among individuals. Emerging biologic therapies hold promise in OA management. These therapies harness biological agents like monoclonal antibodies and growth factors to specifically target pathways contributing to OA.24
Moreover, in bioprinting-enabled DDS for OA, the choice of biomaterials plays a pivotal role in determining the success of these therapeutic approaches.25,26 Natural materials such as collagen, chitosan, and hyaluronic acid have gained prominence due to their inherent biocompatibility and resemblance to the extracellular matrix of joint tissues. Collagen, being a ubiquitous protein in connective tissues, serves as an excellent scaffold and drug carrier, closely mimicking the native tissue environment.27 Chitosan, derived from chitin in crustacean shells, offers biodegradability and can be tailored to control drug release rates, providing sustained therapeutic benefits.28 Hyaluronic acid, a vital component of synovial fluid, is instrumental in lubricating and cushioning joints. In bioprinting applications, it fosters the creation of drug-loaded hydrogels that mimic the joint’s natural milieu, ensuring improved drug retention and diffusion within the target site.29 Synthetic polymers like poly(lactic-co-glycolic acid) (PLGA) and polyethylene glycol (PEG) bring versatility to DDS design, allowing for fine-tuned control over drug release kinetics and mechanical properties.4,30 PLGA, for example, can encapsulate drugs, enabling gradual, controlled release that reduces the necessity for frequent injections.30 Composite materials that blend natural and synthetic components offer synergistic advantages, such as enhanced structural integrity, controlled drug release, and biocompatibility.31 By harnessing the potential of these biomaterials, bioprinting-enabled DDS for OA not only provides a precise platform for localized drug delivery, minimizing systemic side effects, but also fosters an environment conducive to tissue regeneration and pain relief, ultimately advancing the therapeutic landscape for OA patients.
Biocompatibility and Degradability
Bioprinting techniques, while crucial for the precise deposition and spatial control of these materials, do not inherently alter the fundamental biocompatibility and biodegradability characteristics of the biomaterials. Good biocompatibility ensures that the biomaterial is well-tolerated by the body, facilitating a harmonious interaction between the construct and the surrounding biological milieu.32,33 By promoting a favorable cellular response, biocompatible biomaterials provide an environment beneficial to tissue regeneration and healing within the affected joint, effectively addressing the challenges posed by OA.34
Concomitantly, biodegradability plays a vital role in the context of OA treatment, which is crucial for the natural healing and remodeling processes within the joint. As the bioprinting-enabled biomaterial gradually degrades, it paves the way for the regeneration and integration of new-bone tissue, successfully replacing the damaged structures.35 The controlled degradation of the biomaterial complies with the body’s own healing mechanisms, facilitating a beneficial transition and minimizing the risk of complications.
The utilization of various biocompatible and biodegradable materials in bioprinting-enabled biomaterials for OA treatment has attracted considerable attention.34,35 Natural polymers, such as collagen, chitosan, hyaluronic acid, and alginate, have been extensively explored for their excellent biocompatibility and ability to support cellular growth.36 These materials closely mimic the native extracellular matrix, providing a favorable microenvironment for chondrocyte proliferation and differentiation. Additionally, their biodegradable nature ensures a gradual degradation over time, complying with the healing process of the joint and promoting tissue regeneration.
Synthetic polymers, including polycaprolactone (PCL), PLGA, and PEG, have also demonstrated considerable potential in OA treatment.37 These materials offer tunable properties, enabling precise control over their mechanical strength, degradation rate, and release kinetics. Their biocompatibility, coupled with their controlled degradation capability, enables customized interventions that align with the specific requirements of individual patients.38 By leveraging these synthetic polymers, researchers can design constructs that provide mechanical support to the damaged joint while facilitating the regeneration of functional tissue.
The exploration of biocompatible and biodegradable materials in OA treatment provides considerable possibilities for enhancing therapeutic interventions. By combining these properties with the precision of bioprinting technology, researchers can develop constructs that not only mimic the native tissue microenvironment but also actively promote tissue regeneration and healing. With ongoing research and innovation in bioprinting technique, the landscape of OA therapy is poised to witness transformative developments, where biocompatible and biodegradable bioprinting-enabled biomaterials play a pivotal role in restoring joint function, alleviating pain, and enhancing the quality of life for individuals living with OA.
Superior Mechanical and Structural Advantages
Mechanical and structural properties are critical for bioprinting-enabled biomaterials, which can mediate the performance and stability of these biomaterials within the complex environment of the human body.39 These properties serve as critical foundations for achieving optimal functionality and support, mimicking the eminent characteristics of natural cartilage and addressing the unique challenges presented by OA.
The mechanical properties of bioprinting-enabled biomaterials play a pivotal role in their success as a viable OA treatment option. These properties should closely mimic the intricate attributes of natural cartilage, including its stiffness, elasticity, and load-bearing capacity.39 By utilizing the mechanical properties to match those of the native tissue, the biomaterials can provide the joint with the necessary support and functionality to endure the dynamic forces and stresses encountered during daily activities.40 This simulation of natural cartilage mechanics not only enhances the performance of the biomaterial but also fosters an integration with the surrounding tissues, promoting long-term therapeutic success.
Similarly, the structural properties of bioprinting-enabled biomaterials are also crucial for their function, which demand diligent design to mimic the complex architecture of native cartilage tissue.41 Natural cartilage possesses an intricate arrangement of collagen fibers and proteoglycans, conferring strength, resilience, and lubrication to the joint. By mimicking this sophisticated structure, bioprinting-enabled biomaterials can closely integrate with the surrounding tissues and facilitate the formation of functional cartilage-like tissue. The high reproduction of the structural properties ensures optimal integration and enables the regenerated tissue to function effectively within the joint.41
To obtain the desired mechanical and structural properties in bioprinting-enabled biomaterials, certain printing parameters should be well-scheduled, including printing speed, nozzle diameter, and layer thickness. For instance, techniques like photopolymerization harness light to solidify the biomaterial, enabling the fabrication of constructs with high stiffness and strength that closely resemble natural cartilage properties.42 On the other hand, extrusion-based approaches afford flexibility in adjusting the porosity and pore size of the constructs, allowing for the incorporation of desired structural features that mimic the complexity of native cartilage tissue.43
The utilization of various bioprinting techniques, such as photopolymerization, extrusion, and inkjet printing, further broadens the repertoire of mechanical and structural possibilities.43 Each technique offers distinct advantages in modulating the properties of the biomaterials. Photopolymerization, for instance, empowers the fabrication of biomaterials with exceptional stiffness and strength, providing precise control over their mechanical properties.42 Extrusion-based methods, on the other hand, offer the versatility to tailor the porosity and pore size of the constructs, enabling the incorporation of desired structural characteristics.43
Through manipulation of mechanical and structural properties, we can fabricate bioprinting-enabled biomaterials that highly mimic the properties of natural cartilage. These biomaterials not only provide the required support, functionality, and structural resemblance but also foster successful tissue regeneration and integration within the joint. As the field of bioprinting continues to advance at a rapid pace, the ability to precisely tailor these properties provides huge potential for the development of personalized and effective OA therapies.
Excellent Bioactivity and Functionality
Owing to the eminent ability to foster cell growth and efficiently deliver therapeutic agents, bioactivity and functionality are crucial for bioprinting-enabled biomaterials.40 These properties play a pivotal role in OA treatment, as they determine the biomaterials’ potential to induce cartilage regeneration and alleviate pain.
To enhance the bioactivity and functionality of bioprinting-enabled biomaterials for OA treatment, diverse strategies have been employed. One such strategy involves the incorporation of growth factors and/or stem cells, like transforming growth factor-beta (TGF-β) and MSCs into the biomaterials.44 These growth factors act as catalysts, triggering cellular growth and differentiation, thereby facilitating the regeneration of damaged cartilage (Figure 2).25 Moreover, the encapsulation of therapeutic agents within the biomaterials offers an additional dimension of relief for OA patients. By incorporating NSAIDs and corticosteroids, these biomaterials effectively suppress inflammation in the joints, providing significant improvement from pain.45 The combination of therapeutic agents within the bioprinting-enabled biomaterials offers a comprehensive approach to OA treatment, addressing both the regenerative and pain management aspects. Currently, the ongoing developments in bioprinting technique have paved the way for innovative strategies in OA treatment.
 |
Figure 2 Schematic illustration of bioprinting scaffold fabrication and scaffold treatment for osteochondral defects in OA. (A) The fabrication of 3D-bioprinted MSC-laden biomimetic multiphasic scaffolds. (B) The designed scaffold was implanted into a severe joint injury rat model with osteochondral defects for evaluation. Reprinted from Biomaterials, 279, Liu Y, Peng L, Li L, et al 3D-bioprinted BMSC-laden biomimetic multiphasic scaffolds for efficient repair of osteochondral defects in an osteoarthritic rat model. 121216. Copyright 2021, with permission from Elsevier.25 |
Fabrication Methods of Bioprinting-Enabled Biomaterials
During the past decades, a variety of bioprinting techniques have emerged as promising tools for fabricating biomaterials. These cutting-edge developments offer a diverse range of fabrication methods, each with its own unique approach to constructing bioprinting-enabled biomaterials.46–48 3D printed constructs hold great potential in OA therapy due to their unique capabilities. These constructs can be precisely tailored to match the specific anatomical and mechanical requirements of the affected joint, offering a personalized approach to treatment. They serve as a delivery platform for various drugs and therapeutic agents, enabling controlled and localized release directly into the joint space, which can reduce systemic side effects and improve drug efficacy. Additionally, 3D printed constructs can provide structural support to damaged joint tissues, promoting tissue regeneration and repair. Their ability to incorporate key cellular components like chondrocytes and mesenchymal stem cells further enhances their therapeutic potential, as these cells contribute to cartilage restoration.
In this section, we summarize and discuss the intricacies of these fabrication methods, exploring the various bioprinting techniques, and the subsequent post-processing and surface modification techniques. The integration of different bioprinting techniques, appropriately selected materials, and feasible post-processing and surface modification techniques shows the ingenuity and innovation driving the OA treatment.
Types of Bioprinting Techniques
Various bioprinting technologies offer distinct advantages and limitations in the context of DDS applications for OA treatment. Inkjet bioprinting, known for its precision and high-speed capabilities, allows for the rapid deposition of cells and biomaterials, making it suitable for high-throughput production. However, its layer-by-layer approach can limit the complexity of 3D tissue constructs.49–51 Extrusion bioprinting, on the other hand, excels in versatility by accommodating a wide range of biomaterials, including cell-laden bioinks, and produces constructs with favorable mechanical properties.52 Nevertheless, achieving high-resolution printing may be challenging due to nozzle diameter restrictions, and the shear forces involved can potentially affect cell viability.53 Laser-based bioprinting stands out for its high precision and minimal cellular damage, making it ideal for intricate, highly detailed constructs.54 However, it can be relatively slow and require specialized equipment, which may not be suitable for large, thick constructs needed in OA therapy. Stereolithography (SLA) bioprinting, with its capacity to create detailed 3D structures, offers an advantage in fine detail-oriented applications but may entail longer processing times, particularly when dealing with complex constructs.55 Electrospinning bioprinting, although primarily used for scaffold fabrication, provides nanofiber-based scaffolds resembling the extracellular matrix, making it valuable in combination with other bioprinting techniques to create hybrid structures.54 While these technologies address OA therapy challenges by enabling precise drug delivery, sustained release, and personalized constructs, careful consideration of their limitations, including printing resolution, speed, and material compatibility, is essential to selecting the most appropriate bioprinting approach for OA treatment.
Inkjet bioprinting, an effective technique, involves the deposition of minuscule droplets of bioink onto a substrate, layering them to construct a 3D structure.50 The bioink utilized in this process typically comprises a combination of cells and biomaterials that can be delicately crosslinked or cured, solidifying into a stable and coherent architecture.51 Renowned for its speed and efficiency, inkjet bioprinting excels in producing small-scale structures with excellent resolution, empowering researchers to explore the intricacies of tissue engineering with precision (Figure 3).49
 |
Figure 3 Schematic illustration of the main content of inkjet bioprinting. (A) Concise depiction of the cellular environment reveals the presence of cell-fate associated biomolecules that are secreted by cells, existing in both liquid and solid phases within the living organism. (B) High resolution and overprinting strategy developed inkjet bioprinting. (C) Concentration gradient, pattern, and molecular pattern alone or their combination can be printed by the inkjet printer. (D) Through the application of printing technology, biomolecule patterns have been utilized in the study of cellular behavior and tissue regeneration. Reproduced with permission from Li X, Liu B, Pei B, et al. Inkjet Bioprinting of Biomaterials. Chem Rev. 2020;120(19):10793–10833.49 Copyright 2020, American Chemical Society. |
Another noteworthy technique, extrusion bioprinting, harnesses the controlled extrusion of bioink onto a substrate using a syringe or nozzle.52 This method relies on a viscous polymer or hydrogel-based bioink that can be strategically crosslinked or cured, ultimately yielding a robust and structurally sound construct. Notably, extrusion bioprinting offers the advantage of creating large-scale structures with varying levels of complexity. This technique has captured the attention of researchers seeking to fabricate biomaterials for diverse applications in tissue regeneration and organ engineering.53
Laser-assisted bioprinting represents another feasible technique, employing the precision of a laser to selectively transfer bioink onto a substrate. Typically, a cell-laden hydrogel-based bioink is employed, which can be intricately crosslinked or cured to solidify into a stable and well-defined structure.54 Laser-assisted bioprinting distinguishes itself by its excellent precision and adaptability, enabling the fabrication of complex and intricate structures while maintaining high cell viability.50
Materials Used for Bioprinting
The selection of materials for bioprinting-enabled biomaterials is crucial for tissue engineering applications. Among the available options, natural polymers, synthetic polymers, and ceramics stand out as commonly utilized materials for bioprinting, each possessing distinct properties that can be tailored to meet the specific requirements of the target tissue.
Natural polymers, derived from biological sources, offer an inherent biocompatibility in the biological environment. Materials such as collagen, fibrin, and hyaluronic acid exhibit excellent biodegradability and bioactivity, promoting cellular adhesion, proliferation, and tissue regeneration.56 These natural polymers mimic the extracellular matrix (ECM), providing a supportive scaffold for cell growth and facilitating the restoration of damaged tissues.41 However, their mechanical strength and stability may be comparatively lower, requiring reinforcement or combination with other materials for optimal performance in OA treatment.
On the other hand, synthetic polymers, such as PLGA, PEG, and PCL, offer excellent mechanical properties and can be utilized to meet specific requirements.57 Synthetic polymers provide stability, structural integrity, and tunable degradation rates, allowing researchers to design biomaterials with precise mechanical characteristics. Additionally, the versatility of synthetic polymers enables the incorporation of bioactive molecules and controlled release of therapeutic agents within the constructs, facilitating targeted tissue regeneration and pain relief.58 However, synthetic polymers may lack the natural bioactivity and recognition by cells, necessitating additional modifications or the combination of different materials to enhance cell-material interactions.
Ceramics, including hydroxyapatite and tricalcium phosphate, exhibit excellent biocompatibility and mimic the mineral component of natural bone. These materials possess favorable osteoconductivity, promoting bone growth and integration with the surrounding tissues.59 Ceramics provide structural support, stability, and longevity to the bioprinted constructs, making them particularly suitable for OA treatment where the regeneration of damaged cartilage and bone is crucial.51 However, the brittleness and limited versatility of ceramics may require their combination with other materials to achieve the desired mechanical properties and functionality.
Post-Processing and Surface Modification
Post-processing and surface modification techniques offer valuable ways for enhancing the properties of bioprinting-enabled biomaterials tailored for OA treatment. These techniques allow for modifying and optimizing the biomaterials to improve their mechanical strength, porosity, degradation rate, cell adhesion, and tissue regeneration capabilities.60
A variety of methods have been employed to bolster the functional performance of bioprinted constructs. Crosslinking, for instance, indicates instrumental in enhancing the mechanical strength and stability of the printed structure by forming robust chemical bonds between the polymer chains. This process reinforces the biomaterial, empowering it to withstand physiological forces and promoting long-term durability.61 Similarly, annealing serves as a valuable technique for ceramic-based biomaterials, enhancing their crystallinity and mechanical properties through controlled heating and cooling. By optimizing the material’s structure, annealing contributes to improved functionality and performance.62
Furthermore, solvent removal presents an effective means of constructing porous structures within the biomaterials.63 This controlled removal of solvents allows for the formation of interconnected pores, facilitating cell infiltration and nutrient exchange. The resulting porous architecture promotes cellular adhesion, proliferation, and tissue ingrowth, facilitating the regeneration process within the damaged joint. This technique is promising in augmenting the biomaterials’ capacity to promote tissue integration and functional restoration.
Surface modification techniques play a pivotal role in tailoring the biomaterials’ surface properties to optimize their interaction with cells and the surrounding environment. Incorporating bioactive molecules onto the surface of the printed structure offers a promising approach to stimulate specific cellular responses.64 Growth factors and ECM proteins, for example, can be strategically introduced, promoting cell adhesion, differentiation, and tissue regeneration.65 These bioactive molecules act as signals that guide cellular behavior, facilitating the formation of functional tissues and accelerating the healing process.
Additional surface modification techniques include plasma treatment, which enhances the wettability and surface energy of the printed structure. Plasma treatment alters the surface chemistry, promoting improved cell attachment and spreading.66 Moreover, nanotopography is well-documented beneficial in facilitating cell adhesion and migration. The incorporation of precise surface patterns or textures enhances the biomaterials’ capacity to interact with cells, providing cues that promote favorable cellular responses and tissue integration.67
By utilizing post-processing and surface modification techniques, bioprinting-enabled biomaterials can be optimized for OA therapy. These techniques enable researchers to modulate the material’s properties, bolster mechanical strength, fabricate porous structures for enhanced cellular infiltration, and customize the surface to elicit desired cellular responses. Through the convergence of these techniques, the development of biomaterials that promote tissue regeneration, alleviate pain, and restore function in OA joints becomes increasingly achievable.
The Applications of Bioprinting DDS in OA Therapy
The application of bioprinting in DDS has attracted considerable attention in OA therapy.68 By utilizing the capabilities of bioprinting technology, researchers are exploring innovative approaches to develop targeted and efficient DDS for OA treatment. Bioprinting enables the precise fabrication of complex 3D structures, allowing for the incorporation of different compartments, channels, and reservoirs within the constructs. This versatility provides possibilities for designing DDS that can be tailored to the specific needs of individual patients. By utilizing patient-specific data, such as joint anatomy and disease severity, bioprinting can enable the fabrication of customized DDS that optimize the localization and dosage of therapeutic agents.69
Bioprinting also allows for the encapsulation of drugs within biomaterial-based carriers, known as bioinks, which can be precisely deposited and patterned.69 These bioinks can be formulated with biodegradable polymers, hydrogels, or nanoparticles, providing controlled release mechanisms for the drugs. This controlled release allows for sustained and targeted delivery of therapeutic agents to the affected joint, minimizing systemic side effects and maximizing therapeutic efficacy.
Furthermore, bioprinting enables the incorporation of cells into the drug delivery systems.70 The incorporation of chondrocytes and MSCs into bioprinted constructs represents a promising strategy for promoting tissue regeneration and repair in OA treatment. Chondrocytes, being the native cells of articular cartilage, play a pivotal role in maintaining the cartilage matrix. In OA, the loss of chondrocyte function is a hallmark, leading to cartilage degeneration. By incorporating chondrocytes into bioprinted constructs, a milieu resembling the natural cartilage environment is recreated, enabling these cells to resume their critical function of producing extracellular matrix components.71 This, in turn, promotes the regeneration of damaged cartilage, restoring both its structural and functional integrity. On the other hand, MSCs bring their unique regenerative properties to the equation. Their ability to differentiate into chondrocytes within the construct contributes directly to cartilage regeneration. Moreover, MSCs possess anti-inflammatory and immunomodulatory properties, providing a favorable environment by mitigating local inflammation, supporting tissue healing, and stimulating the activity of endogenous chondrocytes.39 The synergistic use of chondrocytes and MSCs in bioprinted constructs addresses OA’s multifaceted challenges, encompassing cartilage degradation and chronic inflammation, ultimately offering a comprehensive and promising approach to rebuild damaged joint tissues and improve the long-term outcomes of OA therapy.39
Bioprinting Personalized DDS in OA Therapy
Bioprinting, a cutting-edge technology that combines three dimensional (3D) printing with biology, has revolutionized various fields of medicine, including tissue engineering, regenerative medicine, and drug delivery. One promising application of bioprinting is the development of personalized DDS for OA treatment. Personalized DDS aim to address the limitations of conventional strategies by providing targeted and controlled drug release directly to the affected joint.72 Bioprinting technique offers a unique approach to create such systems by using bioinks composed of living cells, biomaterials, and therapeutic agents.72
During the printing process, the therapeutic agents are integrated into the bioink in a spatially controlled manner, allowing for the construction of complex drug release patterns.73 These patterns can be tailored to match the specific needs of the patient, targeting areas of cartilage damage or inflammation. Additionally, the bioink can be designed to mimic the mechanical properties of native cartilage, providing mechanical support and promoting tissue regeneration.73
Once the personalized DDS is printed, it can be implanted directly into the affected joint using minimally invasive techniques. The system gradually releases the therapeutic agents over time, providing sustained and localized drug delivery (Figure 4).74 This targeted approach minimizes systemic side effects and enhances the therapeutic efficacy compared to oral medications or traditional injections.
 |
Figure 4 Schematic representation of the GelHACS-MA bioink design and the 3D bioprinting process. (A) Gelatin was functionalized by attaching amino groups with methacrylates, resulting in the formation of Gel-MA. The red text “RGD” signifies the incorporation of arginine-glycine-aspartic acid (RGD) into the GelHACS-MA bioink preparation. (B) Hyaluronic acid underwent functionalization by attaching methacrylates to its hydroxyl groups, yielding HA-MA. (C) Chondroitin sulfate was also modified by methacrylate incorporation into its hydroxymethyl groups, resulting in CS-MA. (D) GelHACS-MA hydrogels were subsequently formed, wherein Gel-MA, HA-MA, and CS-MA prepolymers were covalently cross-linked in the presence of the LAP photoinitiator, facilitated by blue light exposure, to establish a stable hydrogel network. (E) Isolation and extraction of synovial membrane-derived mesenchymal stem cells (SMSCs). The red text “Synovium” highlights the primary pathological changes in the synovium of the OA joint. (F) The outline of the 3D bioprinting strategy employing GelHACS-MA bioink. Reproduced with permission from Sang S, Mao X, Cao Y, et al 3D Bioprinting Using Synovium-Derived MSC-Laden Photo-Cross-Linked ECM Bioink for Cartilage Regeneration. ACS Appl Mater Interfaces. 2023.74 Copyright 2023, American Chemical Society. |
Furthermore, bioprinting enables the fabrication of multifunctional DDS.75 For example, the bioinks can be engineered to respond to specific triggers, such as changes in pH or temperature, allowing for on-demand drug release in response to the joint’s microenvironment.76 This responsiveness can further optimize the therapeutic outcomes and minimize the need for repeated interventions.
Although bioprinting personalized DDS for OA therapy is still in the early stages of development, it has great potential for the future of OA treatment. The ability to modulate drug release profiles, incorporate patient-specific factors, and promote tissue regeneration has the potential to transform the management of OA, providing personalized and targeted therapies that improve patient outcomes and quality of life.
Bioprinting personalized DDS in OA therapy represents a promising approach to address the limitations of current treatment options. By combining advanced 3D printing technologies with biological materials and therapeutic agents, bioprinting offers the potential for customized, targeted, and regenerative treatments that could significantly improve the lives of individuals suffering from OA.
Bioinks-Based DDS for Promotion of Cartilage Regeneration in OA
Bioinks-based DDS have emerged as a promising approach for promoting cartilage regeneration in OA. These innovative systems combine the principles of bioprinting and drug delivery to provide localized and controlled release of therapeutic agents directly to the damaged cartilage, aiming to enhance tissue regeneration and alleviate OA symptoms.
The development of a bioink for cartilage regeneration involves the formulation of a biomaterial that serves as the carrier for both the cells and the therapeutic agents.77 The bioink is functionally engineered to possess appropriate mechanical properties, biocompatibility, and the ability to support cell growth and tissue formation. Typically, hydrogels or other biocompatible polymers are used as the base material for the bioink.
Within the bioink, chondrocytes or MSCs are embedded to provide a cellular component for cartilage regeneration. These cells have the potential to differentiate into chondrocyte-like cells, which are responsible for producing and maintaining the cartilage ECM (Figure 5).78 The presence of these cells within the bioink helps to promote tissue regeneration and enhance the efficacy of the therapy.
 |
Figure 5 Schematic representation of the one-step DDS designed to facilitate osteochondral defect regeneration. The DDS involves stereolithography-based bioprinting of ECM/gelatin methacryloyl/exosome construct, followed by implantation into the osteochondral defect. (A) Utilizing stereolithography, an ECM combined with Gelatin Methacryloyl and exosome-based bioprinting is employed, followed by the implantation of the construct into the osteochondral defect. (B) Chondrocytes are observed migrating to the regions of the defect. (C) The controlled administration of exosomes is achieved through the use of 3D printed scaffolds. (D) The scaffolds lead to enhanced mitochondrial biogenesis in chondrocytes. Reproduced under the terms of a Creative Commons Attribution 4.0 International License from Chen P, Zheng L, Wang Y, et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9(9):2439–2459.78 Copyright 2019, The Authors. |
In addition to the cells, the bioink is loaded with therapeutic agents that can facilitate cartilage regeneration and alleviate OA symptoms.79 These therapeutic agents may include growth factors, anti-inflammatory drugs, cytokines, or other bioactive molecules. The choice of therapeutic agents depends on the specific goals of the treatment, such as promoting cell proliferation, stimulating matrix synthesis, reducing inflammation, or modulating the immune response.
The bioprinting process enables precise spatial control over the deposition of the bioink within the damaged cartilage region. Using a computer-aided design (CAD) model, the bioprinter deposits layers of the bioink onto the affected area, formulating a structure that mimics the native cartilage tissue.79 The incorporation of the therapeutic agents within the bioink allows for controlled release kinetics, ensuring a sustained and localized delivery of the drugs to the targeted site. Once implanted into the affected joint, the bioinks-based DDS promotes cartilage regeneration through various mechanisms. The therapeutic agents stimulate the resident cells or the seeded cells within the bioink to proliferate, differentiate, and produce cartilage-specific ECM components. This process helps to rebuild the damaged cartilage tissue, restore its structural integrity, and improve joint function.
Furthermore, the localized and controlled release of therapeutic agents from the bioink minimizes systemic side effects associated with conventional drug delivery methods. By delivering the drugs directly to the damaged site, the bioinks-based DDS maximizes the therapeutic efficacy while minimizing the dosage required and reducing the risk of adverse reactions. Although the field of bioinks-based DDS for cartilage regeneration in OA is still in its early stages, it holds tremendous potential for improving the treatment outcomes of OA patients.80 Various bioink formulations, cell sources, and therapeutic agents have been developed to optimize the effectiveness of these advanced DDS.
Bioprinting DDS-Based Cell Therapy in OA Joint
Bioprinting DDS for cell therapy in OA joints have emerged as a cutting-edge approach to enhance the efficacy of cell-based treatments and promote cartilage regeneration.81 This innovative technique combines the principles of bioprinting, drug delivery, and cell therapy to provide advanced targeted therapies for OA.
Cell therapy, which involves the transplantation of healthy cells into the damaged joint, provides promising direction for regenerating cartilage and alleviating the progression of OA.82 However, the success of cell therapy depends on various factors, including the survival and engraftment of the transplanted cells, their proper integration into the host tissue, and the controlled release of bioactive molecules to support tissue regeneration.
Bioprinting DDS provide a solution to these challenges by enabling precise spatial distribution of cells and therapeutic agents within the damaged joint. During the bioprinting process, the bioink is deposited layer by layer onto the damaged joint area according to the 3D model. This precise deposition allows for the spatial distribution of cells and therapeutic agents in a controlled manner, targeting specific regions of cartilage damage or inflammation (Figure 6).83,84 The bioprinted construct serves as a scaffold that provides mechanical support, promotes cell attachment and proliferation, and facilitates the integration of transplanted cells with the host tissue.85
 |
Figure 6 An example for bioprinting cell therapy for Cartilage Repair. (A) 3D-bioprinted cartilage scaffold for implantation. (a) Schematic representation of the fabricated scaffolds for cartilage repair in rabbit knee. (b) 3D computer-aided design (CAD) renderings of individual layers of the cartilage scaffold. (c-d) Dispensing path (outlined in yellow box in b) for the aligned PCL (Polycaprolactone) and hydrogel (outlined in green box in c). The GDF5-conjugated BMSC-laden hydrogel was dispensed into the space between PCL fibers. (e) Macroscopic view of GDF5-conjugated BMSC-laden hydrogel printed using OPUS. (f) Microscopic observation of the hydrogel under light microscopy. (g) Macroscopic view of the cartilage scaffold incorporating GDF5-conjugated BMSC-laden hydrogel and PCL as the supporting structure. (h) Scanning electron microscopy (SEM) images of GDF5-conjugated PLGA μS (Poly(lactic-co-glycolic acid) microspheres). (i) Implantation process of the cartilage scaffold into the defect site in a rabbit knee. (j) High-resolution image of the implanted scaffold shown in (i). (k) Microscopic visualization of the alignment of PCL and hydrogel within the scaffold. (l) High-resolution image of the area outlined in the blue box in (k). (m-p) Evaluation of minimal toxicity and distribution of PLGA μS within the BMSC-laden hydrogel in the scaffolds. (B) Mechanical properties of different components and the cross-linked hydrogel measured at 17°C. (C) Dynamic thermal rheological observations of the cross-linkage of GFHG. (D) Degradation rate of BMSC-laden hydrogel. E. Degradation rate of PCL in vitro and in vivo. Reproduced under the terms of a Creative Commons Attribution 4.0 International License from Sun Y, You Y, Jiang W, Zhai Z, Dai K. 3D-bioprinting a genetically inspired cartilage scaffold with GDF5-conjugated BMSC-laden hydrogel and polymer for cartilage repair. Theranostics. 2019;9(23):6949–6961.84 Copyright 2019, The Authors. |
Bioprinting DDS-based cell therapy represents a promising strategy to address the challenges of cartilage regeneration in OA. By combining bioprinting technology, cell therapy, and controlled drug delivery, this approach offers the potential for personalized, targeted, and regenerative treatments that can significantly improve the outcomes for individuals with OA.
Bioprinting-Enabled Imaging and Sensing Strategies in OA Therapy
Bioprinting-enabled imaging and sensing strategies in OA therapy combine the power of bioprinting technology with advanced imaging and sensing techniques to enhance the diagnosis, treatment, and monitoring of OA. These strategies offer innovative approaches to improve the accuracy, efficiency, and effectiveness of OA therapy.
Bioprinting-enabled imaging techniques, such as magnetic resonance imaging (MRI) and computed tomography (CT), are used to obtain high-resolution images of the affected joints.86 These images provide detailed information about the cartilage structure, damage extent, and overall joint condition. By integrating bioprinting technology, these imaging modalities can assist in creating patient-specific models or “blueprints” for the fabrication of customized tissue constructs for OA treatment.71 The combination of bioprinting and imaging allows for the generation of personalized tissue constructs tailored to the individual’s joint anatomy. By using imaging data to guide the bioprinting process, tissue constructs can be designed to precisely match the patient’s joint geometry and cartilage defects. This personalized approach improves the integration and functionality of the bioprinted constructs, enhancing the chances of successful tissue regeneration.
Bioprinting-enabled sensing strategies play a vital role in monitoring the progress of tissue regeneration following implantation. By incorporating sensors into the bioprinted constructs, various parameters can be monitored in real-time.87 For instance, sensors can measure mechanical properties like strain, stress, and stiffness of the regenerating tissue. These measurements provide valuable insights into the structural integrity and functionality of the engineered tissue, enabling clinicians to evaluate the success of the therapy and make necessary adjustments if needed.88 Furthermore, the incorporation of sensing capabilities into bioprinted constructs can also facilitate the monitoring of drug delivery for OA therapy. By integrating sensors capable of measuring factors such as pH, oxygen levels, or the release of therapeutic agents, clinicians can assess the effectiveness of drug treatments and adjust dosages as required.88 This real-time monitoring ensures optimal drug delivery and enhances treatment outcomes.
Moreover, bioprinting-enabled imaging and sensing strategies enable longitudinal tracking of OA progression and treatment outcomes.89 By periodically imaging and monitoring the implanted bioprinted constructs, clinicians can assess the evolution of the regenerating tissue over time. This longitudinal data helps in understanding the effectiveness of the therapy, identifying potential issues, and optimizing treatment strategies based on individual responses.
Utilization of Green Nanomaterials for Enhancing DDS in OA Therapy
Green nanomaterials represent a promising avenue for enhancing DDS within the context of OA treatment, offering both substantial advantages and notable environmental benefits.90 These materials, often derived from natural sources such as cellulose, chitosan, or plant extracts, exhibit exceptional biocompatibility, a critical factor when designing DDS systems for implantation or injection into the human body. Beyond their compatibility with biological systems, green nanomaterials contribute to sustainability by being sourced from renewable resources, effectively replacing petroleum-based counterparts and thereby mitigating the environmental impact associated with nanomaterial production.91 Furthermore, the biodegradability of many green nanomaterials ensures their eventual natural decomposition, reducing the potential for long-term environmental pollution—a key consideration when contemplating the disposal of medical devices and implants.92
In the specific context of OA treatment, the application of green nanomaterials within bioprinting-enabled DDS systems provides exciting possibilities.90 These materials can act as carriers for drugs or growth factors that promote cartilage regeneration, helping to address the fundamental challenge of cartilage degeneration in OA patients. Their biocompatibility ensures that they do not provoke inflammation or tissue rejection, making them an excellent choice for use within the human body. Moreover, green nanomaterials can facilitate controlled and sustained drug release, optimizing therapeutic effects while reducing potential side effects—an especially crucial consideration in chronic conditions like OA where long-term medication may be necessary.91 Additionally, the advent of bioprinting technology allows for the construction of customized implants that match a patient’s specific joint anatomy precisely.16 Integrating green nanomaterials into the implant matrix not only enhances its structural integrity but also ensures it is biocompatible, further enhancing its suitability for osteoarthritis treatment.
Challenges and Future Directions
Bioprinting is a rapidly developing field that has shown huge potential for the development of biomaterials for the treatment of OA. Although this technology offers many benefits, there are also significant challenges that should be addressed to fully realize its potential for clinical use.
One of the primary challenges in bioprinting is achieving precise control over the deposition of cells and biomaterials in 3D environment. The printing process should be optimized to ensure that the printed structures are uniform, reproducible, and biologically functional. This requires developments in printing technologies, such as nozzle design, printing speed, and resolution, to achieve high-precision printing of complex structures.
Furthermore, the mechanical properties of the printed structures should be carefully controlled. They need to be compatible with the surrounding tissue and able to support the physiological loads placed on them. Achieving the right balance of stiffness and elasticity is crucial for proper integration and functionality of the printed constructs within the joint.
Another technical challenge in bioprinting is the development of biomaterials suitable for printing. The biomaterials used should be biocompatible, biodegradable, and able to support the growth and differentiation of cells. They should possess appropriate mechanical properties, such as elasticity and strength, to withstand the mechanical demands of the joint environment. Increasing studies are focusing on exploring various biomaterial formulations, including natural polymers, synthetic polymers, and hybrid materials, to optimize their suitability for bioprinting in OA therapy.
Safety concerns are also significant in bioprinting. One primary concern is the potential for immune rejection. The cells used in the printing process may be recognized as foreigners by the recipient’s immune system, leading to an immune response that can cause tissue damage and rejection of the printed structures. To address this dilemma, researchers are trying to utilize induced pluripotent stem cells (iPSCs) derived from the patient’s own cells, reducing the risk of immune rejection.
In addition, regulatory approval from health authorities like the FDA or EMA is paramount, demanding comprehensive safety, efficacy, and quality control assessments. Standardization of bioprinting processes, including cell sourcing, bioink formulation, and quality assurance, is essential for consistency. Ensuring biocompatibility and safety, particularly concerning biomaterials and cells, is critical to avoid adverse reactions. Scalability is crucial for transitioning from research to clinical-scale production. Robust quality control measures are vital, as are rigorous clinical trials to validate safety and efficacy in human patients. Ethical considerations, cost-effectiveness assessments, and infrastructure and training requirements should also be addressed.
Despite the considerable challenges and limitations, the potential benefits of bioprinting for OA treatment are attracting increasing interest. Developments in printing techniques are being explored to produce complex structures with higher resolution and precision. Integration of advanced imaging technologies, such as MRI and CT, can help generate detailed 3D models of the affected joint, improving the accuracy of the printed constructs. The therapeutic potential of bioprinting-enabled DDS in the treatment of OA has shown significant promise, as evidenced by several key findings from studies. Bioprinting facilitates enhanced drug delivery precision, allowing for localized and controlled drug release within the affected joint, reducing systemic side effects. Importantly, it enables sustained drug release, ensuring therapeutic levels persist within the joint over time, offering prolonged pain relief and potential disease-modifying effects. Moreover, bioprinted constructs, often incorporating chondrocytes or MSCs, contribute to tissue regeneration and repair, with studies demonstrating the formation of cartilage-like tissue within these constructs.25 Personalized approaches, tailored to individual patients’ OA severity and joint geometry, have emerged as a hallmark of bioprinting applications. Inflammatory responses, common in OA, have been addressed by incorporating anti-inflammatory agents into bioprinted constructs, reducing pain and inflammation.29 Preclinical studies employing animal models and in vitro experiments have consistently demonstrated the feasibility and therapeutic potential of bioprinting for OA treatment.4,30 However, the transition to widespread clinical adoption will require further validation through well-designed clinical trials, and ongoing advances in bioprinting continue to shape the evolving landscape of OA therapy.
Conclusion
In conclusion, the development of bioprinting-enabled biomaterials for OA treatment represents a promising direction for providing advanced DDS for customized and patient-specific therapies. This technology offers huge potential to revolutionize the current OA therapeutic options with limited efficacy. However, there are still several challenges and technical limitations that need to be overcome to fully realize the potential of bioprinting in clinical applications. To overcome these challenges, future research should focus on developing more sophisticated bioprinting technologies. Multi-material and multi-cell printing approaches can enable the fabrication of complex tissue structures with multiple cell types and biomaterials, closely resembling the native joint environment. Finally, with persistent effort in improving bioprinting technique, bioprinting DDS has considerable potential to revolutionize the treatment of OA and improve the lives of individuals affected by this debilitating condition.
Data Sharing Statement
No data was used for the research described in this review paper.
Acknowledgments
This review was supported by Scientific research project of Xi’an Jiaotong University (xzy012022130) and Xi’an Talent Plan Project (XAYC210060). The authors would like to thank Figdraw software (http://www.figdraw.com) for its picture source assistance for the graphical abstract during the preparation of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–2126. doi:10.1016/S0140-6736(11)60243-2
2. Abramoff B, Caldera FE. Osteoarthritis: pathology, Diagnosis, and Treatment Options. Med Clin North Am. 2020;104(2):293–311. doi:10.1016/j.mcna.2019.10.007
3. Armiento AR, Stoddart MJ, Alini M, Eglin D. Biomaterials for articular cartilage tissue engineering: learning from biology. Acta Biomater. 2018;65:1–20. doi:10.1016/j.actbio.2017.11.021
4. Liang Q, Ma Y, Yao X, Wei W. Advanced 3D-Printing Bioinks for Articular Cartilage Repair. Int J Bioprint. 2022;8(3):511. doi:10.18063/ijb.v8i3.511
5. Zelinka A, Roelofs AJ, Kandel RA, De Bari C. Cellular therapy and tissue engineering for cartilage repair. Osteoarthritis Cartilage. 2022;30(12):1547–1560. doi:10.1016/j.joca.2022.07.012
6. Lafuente-Merchan M, Ruiz-Alonso S, Garcia-Villen F, et al. Progress in 3D Bioprinting Technology for Osteochondral Regeneration. Pharmaceutics. 2022;14(8):1578. doi:10.3390/pharmaceutics14081578
7. Martinez-Moreno D, Venegas-Bustos D, Rus G, Galvez-Martin P, Jimenez G, Marchal JA. Chondro-Inductive b-TPUe-Based Functionalized Scaffolds for Application in Cartilage Tissue Engineering. Adv Healthc Mater. 2022;11(19):e2200251. doi:10.1002/adhm.202200251
8. Tang M, Rich JN, Chen S. Biomaterials and 3D Bioprinting Strategies to Model Glioblastoma and the Blood-Brain Barrier. Adv Mater. 2021;33(5):e2004776. doi:10.1002/adma.202004776
9. Wang Z, Wang Y, Yan J, et al. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv Drug Deliv Rev. 2021;174:504–534. doi:10.1016/j.addr.2021.05.007
10. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. doi:10.1038/nrrheum.2010.196
11. Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2(1):16072. doi:10.1038/nrdp.2016.72
12. Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–257. doi:10.1016/j.bone.2012.02.012
13. Foo JB, Looi QH, How CW, et al. Mesenchymal Stem Cell-Derived Exosomes and MicroRNAs in Cartilage Regeneration: biogenesis, Efficacy, miRNA Enrichment and Delivery. Pharmaceuticals. 2021;14(11):56. doi:10.3390/ph14111093
14. Ostrovidov S, Salehi S, Costantini M, et al. 3D Bioprinting in Skeletal Muscle Tissue Engineering. Small. 2019;15(24):e1805530. doi:10.1002/smll.201805530
15. Wan Z, Zhang P, Liu Y, Lv L, Zhou Y. Four-dimensional bioprinting: current developments and applications in bone tissue engineering. Acta Biomater. 2020;101:26–42. doi:10.1016/j.actbio.2019.10.038
16. Shavandi A, Hosseini S, Okoro OV, Nie L, Eghbali Babadi F, Melchels F. 3D Bioprinting of Lignocellulosic Biomaterials. Adv Healthc Mater. 2020;9(24):e2001472. doi:10.1002/adhm.202001472
17. Zhu J, Yang S, Qi Y, et al. Stem cell-homing hydrogel-based miR-29b-5p delivery promotes cartilage regeneration by suppressing senescence in an osteoarthritis rat model. Sci Adv. 2022;8(13):eabk0011. doi:10.1126/sciadv.abk0011
18. Cooper C, Jordan KM. Topical NSAIDs in osteoarthritis. BMJ. 2004;329(7461):304–305. doi:10.1136/bmj.329.7461.304
19. Oo WM, Little C, Duong V, Hunter DJ. The Development of Disease-Modifying Therapies for Osteoarthritis (DMOADs): the Evidence to Date. Drug Des Devel Ther. 2021;15:2921–2945. doi:10.2147/DDDT.S295224
20. Gregori D, Giacovelli G, Minto C, et al. Association of Pharmacological Treatments With Long-term Pain Control in Patients With Knee Osteoarthritis: a Systematic Review and Meta-analysis. JAMA. 2018;320(24):2564–2579. doi:10.1001/jama.2018.19319
21. Han W, Fan S, Bai X, Ding C. Strontium ranelate, a promising disease modifying osteoarthritis drug. Expert Opin Investig Drugs. 2017;26(3):375–380. doi:10.1080/13543784.2017.1283403
22. Stitik TP, Kumar A, Foye PM. Corticosteroid injections for osteoarthritis. Am J Phys Med Rehabil. 2006;85(11 Suppl):S51-65; quiz S6–8. doi:10.1097/01.phm.0000245508.35730.f3
23. Belk JW, Kraeutler MJ, Houck DA, Goodrich JA, Dragoo JL, Mccarty EC. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: a Systematic Review and Meta-analysis of Randomized Controlled Trials. Am J Sports Med. 2021;49(1):249–260. doi:10.1177/0363546520909397
24. Zheng S, Hunter DJ, Xu J, Ding C. Monoclonal antibodies for the treatment of osteoarthritis. Expert Opin Biol Ther. 2016;16(12):1529–1540. doi:10.1080/14712598.2016.1229774
25. Liu Y, Peng L, Li L, et al. 3D-bioprinted BMSC-laden biomimetic multiphasic scaffolds for efficient repair of osteochondral defects in an osteoarthritic rat model. Biomaterials. 2021;279:121216. doi:10.1016/j.biomaterials.2021.121216
26. Bagherifard A, Joneidi Yekta H, Akbari Aghdam H, et al. Improvement in osseointegration of tricalcium phosphate-zircon for orthopedic applications: an in vitro and in vivo evaluation. Med Biol Eng Comput. 2020;58(8):1681–1693. doi:10.1007/s11517-020-02157-1
27. Gatenholm B, Lindahl C, Brittberg M, Simonsson S. Collagen 2A Type B Induction after 3D Bioprinting Chondrocytes In Situ into Osteoarthritic Chondral Tibial Lesion. Cartilage. 2021;13(2_suppl):1755S–69S. doi:10.1177/1947603520903788
28. Li S, Liu J, Liu S, Jiao W, Wang X. Chitosan oligosaccharides packaged into rat adipose mesenchymal stem cells-derived extracellular vesicles facilitating cartilage injury repair and alleviating osteoarthritis. J Nanobiotechnology. 2021;19(1):343. doi:10.1186/s12951-021-01086-x
29. Shi W, Fang F, Kong Y, et al. Dynamic hyaluronic acid hydrogel with covalent linked gelatin as an anti-oxidative bioink for cartilage tissue engineering. Biofabrication. 2021;14(1). doi:10.1088/1758-5090/ac42de.
30. Zhang J, Wehrle E, Vetsch JR, Paul GR, Rubert M, Muller R. Alginate dependent changes of physical properties in 3D bioprinted cell-laden porous scaffolds affect cell viability and cell morphology. Biomed Mater. 2019;14(6):065009. doi:10.1088/1748-605X/ab3c74
31. Yang J, Zhang YS, Yue K, Khademhosseini A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1–25. doi:10.1016/j.actbio.2017.01.036
32. Wang Z, Wang L, Li T, et al. 3D bioprinting in cardiac tissue engineering. Theranostics. 2021;11(16):7948–7969. doi:10.7150/thno.61621
33. Sahmani S, Khandan A, Saber-Samandari S, Mohammadi Aghdam M. Effect of magnetite nanoparticles on the biological and mechanical properties of hydroxyapatite porous scaffolds coated with ibuprofen drug. Mater Sci Eng C Mater Biol Appl. 2020;111:110835. doi:10.1016/j.msec.2020.110835
34. Tolabi H, Davari N, Khajehmohammadi M, et al. Progress of Microfluidic Hydrogel-Based Scaffolds and Organ-on-Chips for the Cartilage Tissue Engineering. Adv Mater. 2023;35(26). doi:10.1002/adma.202208852:e2208852
35. Qasim M, Chae DS, Lee NY. Advancements and frontiers in nano-based 3D and 4D scaffolds for bone and cartilage tissue engineering. Int J Nanomedicine. 2019;14:4333–4351. doi:10.2147/IJN.S209431
36. Eftekhari A, Kryschi C, Pamies D, et al. Natural and synthetic nanovectors for cancer therapy. Nanotheranostics. 2023;7(3):236–257. doi:10.7150/ntno.77564
37. Bedell ML, Navara AM, Du Y, Zhang S, Mikos AG. Polymeric Systems for Bioprinting. Chem Rev. 2020;120(19):10744–10792. doi:10.1021/acs.chemrev.9b00834
38. Han X, Chang S, Zhang M, Bian X, Li C, Li D. Advances of Hydrogel-Based Bioprinting for Cartilage Tissue Engineering. Front Bioeng Biotechnol. 2021;9:746564. doi:10.3389/fbioe.2021.746564
39. Matai I, Kaur G, Seyedsalehi A, Mcclinton A, Laurencin CT. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. doi:10.1016/j.biomaterials.2019.119536
40. Mcgivern S, Boutouil H, Al-Kharusi G, Little S, Dunne NJ, Levingstone TJ. Translational Application of 3D Bioprinting for Cartilage Tissue Engineering. Bioengineering. 2021;8(10). doi:10.3390/bioengineering8100144
41. Rastogi P, Kandasubramanian B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication. 2019;11(4):042001. doi:10.1088/1758-5090/ab331e
42. Kim SH, Hong H, Ajiteru O, et al. 3D bioprinted silk fibroin hydrogels for tissue engineering. Nat Protoc. 2021;16(12):5484–5532. doi:10.1038/s41596-021-00622-1
43. Wang Y, Yuan X, Yao B, Zhu S, Zhu P, Huang S. Tailoring bioinks of extrusion-based bioprinting for cutaneous wound healing. Bioact Mater. 2022;17:178–194. doi:10.1016/j.bioactmat.2022.01.024
44. Jia L, Hua Y, Zeng J, et al. Bioprinting and regeneration of auricular cartilage using a bioactive bioink based on microporous photocrosslinkable acellular cartilage matrix. Bioact Mater. 2022;16:66–81. doi:10.1016/j.bioactmat.2022.02.032
45. Huang Y, Meng X, Zhou Z, et al. A naringin-derived bioink enhances the shape fidelity of 3D bioprinting and efficiency of cartilage defect repair. J Mater Chem B. 2022;10(36):7030–7044. doi:10.1039/D2TB01247B
46. Esmaeili S, Akbari Aghdam H, Motififard M, et al. A porous polymeric-hydroxyapatite scaffold used for femur fractures treatment: fabrication, analysis, and simulation. Eur J Orthop Surg Traumatol. 2020;30(1):123–131. doi:10.1007/s00590-019-02530-3
47. Angili SN, Morovvati MR, Kardan-Halvaei M, et al. Fabrication and finite element simulation of antibacterial 3D printed Poly L-lactic acid scaffolds coated with alginate/magnesium oxide for bone tissue regeneration. Int J Biol Macromol. 2023;224:1152–1165. doi:10.1016/j.ijbiomac.2022.10.200
48. Sahmani S, Saber-Samandari S, Khandan A, Aghdam MM. Influence of MgO nanoparticles on the mechanical properties of coated hydroxyapatite nanocomposite scaffolds produced via space holder technique: fabrication, characterization and simulation. J Mech Behav Biomed Mater. 2019;95:76–88. doi:10.1016/j.jmbbm.2019.03.014
49. Li X, Liu B, Pei B, et al. Inkjet Bioprinting of Biomaterials. Chem Rev. 2020;120(19):10793–10833. doi:10.1021/acs.chemrev.0c00008
50. Marques CF, Diogo GS, Pina S, Oliveira JM, Silva TH, Reis RL. Collagen-based bioinks for hard tissue engineering applications: a comprehensive review. J Mater Sci Mater Med. 2019;30(3):32. doi:10.1007/s10856-019-6234-x
51. Zhang J, Wehrle E, Rubert M, Muller R. 3D Bioprinting of Human Tissues: biofabrication, Bioinks, and Bioreactors. Int J Mol Sci. 2021;22(8):46.
52. Askari M, Afzali Naniz M, Kouhi M, Saberi A, Zolfagharian A, Bodaghi M. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: a comprehensive review with focus on advanced fabrication techniques. Biomater Sci. 2021;9(3):535–573. doi:10.1039/d0bm00973c
53. Messaoudi O, Henrionnet C, Bourge K, Loeuille D, Gillet P, Pinzano A. Stem Cells and Extrusion 3D Printing for Hyaline Cartilage Engineering. Cells. 2020;10(1):2. doi:10.3390/cells10010002
54. Li J, Chen M, Fan X, Zhou H. Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med. 2016;14(1):271. doi:10.1186/s12967-016-1028-0
55. Szychlinska MA, Bucchieri F, Fucarino A, Ronca A, D’amora U. Three-Dimensional Bioprinting for Cartilage Tissue Engineering: insights into Naturally-Derived Bioinks from Land and Marine Sources. J Funct Biomater. 2022;13(3):118. doi:10.3390/jfb13030118
56. Moghaddam AS, Khonakdar HA, Arjmand M, et al. Review of Bioprinting in Regenerative Medicine: naturally Derived Bioinks and Stem Cells. ACS Appl Bio Mater. 2021;4(5):4049–4070. doi:10.1021/acsabm.1c00219
57. Abbadessa A, Mouser VHM, Blokzijl MM, et al. A Synthetic Thermosensitive Hydrogel for Cartilage Bioprinting and Its Biofunctionalization with Polysaccharides. Biomacromolecules. 2016;17(6):2137–2147. doi:10.1021/acs.biomac.6b00366
58. Aljohani W, Ullah MW, Zhang X, Yang G. Bioprinting and its applications in tissue engineering and regenerative medicine. Int J Biol Macromol. 2018;107(Pt A):261–275. doi:10.1016/j.ijbiomac.2017.08.171
59. Hikita A, Chung UI, Hoshi K, Takato T. Bone Regenerative Medicine in Oral and Maxillofacial Region Using a Three-Dimensional Printer. Tissue Eng Part A. 2017;23(11–12):515–521. doi:10.1089/ten.tea.2016.0543
60. Abdulghani S, Morouco PG. Biofabrication for osteochondral tissue regeneration: bioink printability requirements. J Mater Sci Mater Med. 2019;30(2):20. doi:10.1007/s10856-019-6218-x
61. Yue K, Trujillo-de santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi:10.1016/j.biomaterials.2015.08.045
62. Flegeau K, Puiggali-Jou A, Zenobi-Wong M. Cartilage tissue engineering by extrusion bioprinting utilizing porous hyaluronic acid microgel bioinks. Biofabrication. 2022;14(3):034105. doi:10.1088/1758-5090/ac6b58
63. Sawyer SW, Takeda K, Alayoubi A, et al. 3D bioprinting optimization of human mesenchymal stromal cell laden gelatin-alginate-collagen bioink. Biomed Mater. 2022;18(1). doi:10.1088/1748-605X/aca3e7.
64. Chen C, Bang S, Cho Y, et al. Research trends in biomimetic medical materials for tissue engineering: 3D bioprinting, surface modification, nano/micro-technology and clinical aspects in tissue engineering of cartilage and bone. Biomater Res. 2016;20:10. doi:10.1186/s40824-016-0057-3
65. Yilmaz B, Tahmasebifar A, Baran ET. Bioprinting Technologies in Tissue Engineering. Adv Biochem Eng Biotechnol. 2020;171:279–319. doi:10.1007/10_2019_108
66. Luo C, Xie R, Zhang J, et al. Low-Temperature Three-Dimensional Printing of Tissue Cartilage Engineered with Gelatin Methacrylamide. Tissue Eng Part C Methods. 2020;26(6):306–316. doi:10.1089/ten.tec.2020.0053
67. Kavand H, Van Lintel H, Bakhshi Sichani S, et al. Cell-Imprint Surface Modification by Contact Photolithography-Based Approaches: direct-Cell Photolithography and Optical Soft Lithography Using PDMS Cell Imprints. ACS Appl Mater Interfaces. 2019;11(11):10559–10566. doi:10.1021/acsami.9b00523
68. Ding SL, Liu X, Zhao XY, et al. Microcarriers in application for cartilage tissue engineering: recent progress and challenges. Bioact Mater. 2022;17:81–108. doi:10.1016/j.bioactmat.2022.01.033
69. Groen WM, Diloksumpan P, Van Weeren PR, Levato R, Malda J. From intricate to integrated: biofabrication of articulating joints. J Orthop Res. 2017;35(10):2089–2097. doi:10.1002/jor.23602
70. Mandrycky C, Wang Z, Kim K, Kim DH. 3D bioprinting for engineering complex tissues. Biotechnol Adv. 2016;34(4):422–434. doi:10.1016/j.biotechadv.2015.12.011
71. Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34(3):312–319. doi:10.1038/nbt.3413
72. Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng. 2015;9(1):4. doi:10.1186/s13036-015-0001-4
73. Echave MC, Hernaez-Moya R, Iturriaga L, et al. Recent advances in gelatin-based therapeutics. Expert Opin Biol Ther. 2019;19(8):773–779. doi:10.1080/14712598.2019.1610383
74. Sang S, Mao X, Cao Y, et al. 3D Bioprinting Using Synovium-Derived MSC-Laden Photo-Cross-Linked ECM Bioink for Cartilage Regeneration. ACS Appl Mater Interfaces. 2023. doi:10.1021/acsami.2c19058
75. Zhu W, Cui H, Boualam B, et al. 3D bioprinting mesenchymal stem cell-laden construct with core-shell nanospheres for cartilage tissue engineering. Nanotechnology. 2018;29(18):185101. doi:10.1088/1361-6528/aaafa1
76. Arya N, Forget A, Sarem M, Shastri VP. RGDSP functionalized carboxylated agarose as extrudable carriers for chondrocyte delivery. Mater Sci Eng C Mater Biol Appl. 2019;99:103–111. doi:10.1016/j.msec.2019.01.080
77. Badhe RV, Chatterjee A, Bijukumar D, Mathew MT. Current advancements in bio-ink technology for cartilage and bone tissue engineering. Bone. 2023;171:116746. doi:10.1016/j.bone.2023.116746
78. Chen P, Zheng L, Wang Y, et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9(9):2439–2459. doi:10.7150/thno.31017
79. Tasnim N, De La Vega L, Anil Kumar S, et al. 3D Bioprinting Stem Cell Derived Tissues. Cell Mol Bioeng. 2018;11(4):219–240. doi:10.1007/s12195-018-0530-2
80. Farmani AR, Salmeh MA, Golkar Z, et al. Li-Doped Bioactive Ceramics: promising Biomaterials for Tissue Engineering and Regenerative Medicine. J Funct Biomater. 2022;13(4):162. doi:10.3390/jfb13040162
81. Chiesa-Estomba CM, Aiastui A, Gonzalez-Fernandez I, et al. Three-Dimensional Bioprinting Scaffolding for Nasal Cartilage Defects: a Systematic Review. Tissue Eng Regen Med. 2021;18(3):343–353. doi:10.1007/s13770-021-00331-6
82. Lopa S, Mondadori C, Mainardi VL, et al. Translational Application of Microfluidics and Bioprinting for Stem Cell-Based Cartilage Repair. Stem Cells Int. 2018;2018:6594841. doi:10.1155/2018/6594841
83. Qin Y, Ge G, Yang P, et al. An Update on Adipose-Derived Stem Cells for Regenerative Medicine: where Challenge Meets Opportunity. Adv Sci. 2023;10(20). doi:10.1002/advs.202207334:e2207334
84. Sun Y, You Y, Jiang W, Zhai Z, Dai K. 3D-bioprinting a genetically inspired cartilage scaffold with GDF5-conjugated BMSC-laden hydrogel and polymer for cartilage repair. Theranostics. 2019;9(23):6949–6961. doi:10.7150/thno.38061
85. Onofrillo C, Duchi S, O’connell CD, et al. Biofabrication of human articular cartilage: a path towards the development of a clinical treatment. Biofabrication. 2018;10(4):045006. doi:10.1088/1758-5090/aad8d9
86. Gao G, Cui X. Three-dimensional bioprinting in tissue engineering and regenerative medicine. Biotechnol Lett. 2016;38(2):203–211. doi:10.1007/s10529-015-1975-1
87. Vrana NE, Gupta S, Mitra K, et al. From 3D printing to 3D bioprinting: the material properties of polymeric material and its derived bioink for achieving tissue specific architectures. Cell Tissue Bank. 2022;23(3):417–440. doi:10.1007/s10561-021-09975-z
88. Rotbaum Y, Puiu C, Rittel D, Domingos M. Quasi-static and dynamic in vitro mechanical response of 3D printed scaffolds with tailored pore size and architectures. Mater Sci Eng C Mater Biol Appl. 2019;96:176–182. doi:10.1016/j.msec.2018.11.019
89. Loai S, Szulc DA, Cheng HM. Three-Dimensional Bioprinted MR-Trackable Regenerative Scaffold for Postimplantation Monitoring on T1-Weighted MRI. J Magn Reson Imaging. 2022;56(2):570–578. doi:10.1002/jmri.28057
90. Sacchetti C, Liu-Bryan R, Magrini A, Rosato N, Bottini N, Bottini M. Polyethylene-glycol-modified single-walled carbon nanotubes for intra-articular delivery to chondrocytes. ACS Nano. 2014;8(12):12280–12291. doi:10.1021/nn504537b
91. Famta P, Famta M, Kaur J, et al. Protecting the Normal Physiological Functions of Articular and Periarticular Structures by Aurum Nanoparticle-Based Formulations: an Up-to-Date Insight. AAPS PharmSciTech. 2020;21(3):95. doi:10.1208/s12249-020-1636-0
92. Yin XF, Wang LL, Chu XC. A novel chondroitin sulfate decorated nano platinum for the treatment of osteoarthritis. Mater Sci Eng C Mater Biol Appl. 2017;78:452–456. doi:10.1016/j.msec.2017.04.028
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
