Back to Journals » International Journal of Nanomedicine » Volume 18
Biomembrane-Based Nanostructure- and Microstructure-Loaded Hydrogels for Promoting Chronic Wound Healing
Authors Liu WS, Liu Y, Gao J, Zheng H, Lu ZM, Li M
Received 31 August 2022
Accepted for publication 20 December 2022
Published 19 January 2023 Volume 2023:18 Pages 385—411
DOI https://doi.org/10.2147/IJN.S387382
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Wen-Shang Liu,1,* Yu Liu,2,* Jie Gao,3,* Hao Zheng,4 Zheng-Mao Lu,4 Meng Li1
1Department of Dermatology, Shanghai Ninth People’s Hospital, Shanghai Jiaotong University, Shanghai, People’s Republic of China; 2Department of Gastroenterology, Jinling Hospital, Medical School of Nanjing University, Nanjing, People’s Republic of China; 3Changhai Clinical Research Unit, Shanghai Changhai Hospital, Naval Medical University, Shanghai, People’s Republic of China; 4Department of General Surgery, Shanghai Changhai Hospital, Naval Medical University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Meng Li, Department of Dermatology, Shanghai Ninth People’s Hospital, Shanghai Jiaotong University, Shanghai, People’s Republic of China, Tel +086-15000879978, Fax +086-021-23271699, Email [email protected] Zheng-Mao Lu, Department of General Surgery, Shanghai Changhai Hospital, Naval Medical University, Shanghai, People’s Republic of China, Tel +086-13651688596, Fax +086-021-31161589, Email [email protected]
Abstract: Wound healing is a complex and dynamic process, and metabolic disturbances in the microenvironment of chronic wounds and the severe symptoms they cause remain major challenges to be addressed. The inherent properties of hydrogels make them promising wound dressings. In addition, biomembrane-based nanostructures and microstructures (such as liposomes, exosomes, membrane-coated nanostructures, bacteria and algae) have significant advantages in the promotion of wound healing, including special biological activities, flexible drug loading and targeting. Therefore, biomembrane-based nanostructure- and microstructure-loaded hydrogels can compensate for their respective disadvantages and combine the advantages of both to significantly promote chronic wound healing. In this review, we outline the loading strategies, mechanisms of action and applications of different types of biomembrane-based nanostructure- and microstructure-loaded hydrogels in chronic wound healing.
Keywords: biomembrane, nanostructures, microstructures, hydrogels, chronic wound healing
Introduction
Wound healing is an incredibly complex process that includes sequential and overlapping phases of hemostasis, inflammation, proliferation and remodeling, with each phase involving multiple cells and cytokines.1,2 Hemostasis occurs within seconds to minutes of the initial injury. The damaged blood vessels immediately constrict to form a blood clot to prevent blood loss from vascular damage. Platelets coagulate and secrete and recruit various growth factors to form a blood clot to occupy the wound site.3 Subsequently, the inflammatory phase begins when chemokines produced by platelets attract an influx of neutrophils, macrophages and lymphocytes to the wound site.2 Macrophages are the main phagocytes in the inflammatory phase, which remove damaged cells and necrotic tissue and release various cytokines, chemokines and growth factors.4 They exert a wide range of effects on the proliferation of fibroblasts and endothelial cells, which in turn influence the formation of new blood vessels and regulate the transition from inflammation to proliferation.5 The proliferative phase is characterized by extensive activation of keratinocytes, fibroblasts, macrophages and endothelial cells, which regulate angiogenesis and granulation tissue formation for the repair of epithelial tissue. The proliferative phase includes fiber proliferation, re-epithelialization, angiogenesis and nerve regeneration.6 During the tissue modeling phase, all repair processes that begin during the inflammatory and proliferative phases are terminated. The vascular component of myofibroblasts in fibroblasts and granulation tissue is reduced, type III collagen is replaced by type I collagen and formed into parallel fibers, and the wound is completely closed.6 Depending on the severity of the injury, skin injuries can be classified into acute or chronic wounds.7 Chronic wounds are generally classified based on their causality, including chronic vascular disease, diabetes, and pressure injuries.8 The ease of access to diabetic wound models has made chronic wound research more focused on diabetic wounds. We focus on diabetic wound healing in this review.
The special in vivo environment in diabetes can interfere with chronic wound healing; therefore, the healing time is prolonged, and the difficulty in healing is increased.9,10 During the inflammatory phase, keratinocytes produce more interleukin 8 (IL-8) because of the increased generation of reactive oxygen species (ROS) in the hyperglycemic environment, which leads to increased infiltration of neutrophils recruited by IL-8 and enhances inflammation, thus making wound healing more challenging.11,12 The excessive production of ROS results in the release of proinflammatory cytokines, destruction of the extracellular matrix (ECM) and oxidative damage.13–15 These changes lead to the persistence of M1 macrophages (proinflammatory) and inhibit their transition to M2 macrophages (anti-inflammatory).16 This phenomenon eventually prevents the progression of the inflammatory phase; therefore, the wound healing process remains stagnant in the inflammatory phase.17 Moreover, these changes also affect the healing process in the future. Persistent secretion of inflammatory cytokines by macrophages may cause damage to keratinocytes and fibroblasts, thereby preventing angiogenesis and re-epithelialization.18 Studies have shown that fibroblasts isolated from chronic diabetic wounds have a diminished proliferative response to growth factors,19,20 which is an obstacle to wound healing. Besides what has already been mentioned above, like burns and infected wounds, diabetic wounds are prone to bacterial infections, which need to be managed properly.21–23 Clinically, wounds are usually managed by controlling blood sugar, removing necrotic tissue, and reducing infection with antibiotics. While conventional therapies are effective at managing diabetic wounds to a certain extent, a large number of diabetic wounds still persist, deteriorate, and require amputation.24 Firstly, the formation of crusts and blood clotting also hinders the penetration of medication and causes poor circulation in diabetic wounds. Secondly, while cleaning wounds or changing dressings, gauze often adheres to the wounded skin tissue and destroys the new epithelium and granulation tissue, causing bleeding.25,26 Thirdly, the long-term application of antibiotics can kill not only the causative bacteria but also the beneficial bacteria and may lead to the development of drug resistance.27,28 Moreover, higher pH levels caused by chronic wounds affect wound healing, because the alkaline microenvironment affects protease activity and drug stability, and ultimately leads to impaired ECM synthesis and angiogenesis.
With the development of biomedical engineering, various biomaterials have been used to promote the healing of skin wounds, such as microneedles,29 nanoparticles (NPs),30 sponges,31,32 niosomes33–35 and hydrogels.36–38 Hydrogels are a class of biomimetic biomaterials consisting of a hydrophilic cross-linked network with a high moisture content39,40 and are usually characterized by good biocompatibility, adhesion ability, permeability and maintenance of a moist environment to facilitate cell migration. These advantages create a desirable microenvironment for the proliferation and differentiation of cells, thus promoting wound healing and angiogenesis, which in turn contribute to efficient tissue repair and skin regeneration.41 However, owing to the water content of hydrogels, loading lipophilic drugs is difficult,42 and synthetic hydrogels are often biologically inactive.43 Moreover, several hydrogels have been found to interfere with rapid drug release owing to their water content, which makes them ineffective for treating chronic wounds.44 Biomembrane-based nanostructures and microstructures, mainly including liposomes, exosomes, cell membrane-coated nanostructures, living cells, bacteria and algae, have been extensively used in the treatment and management of chronic wounds because of their unique properties.20,45–47 Chronic wound healing is a long-term process, and it is challenging to deliver drugs close enough to the wound, maintain the drugs in an active state and consistently release substances that promote wound healing over time. Combining hydrogels with biomembrane-based nanostructures and microstructures to yield complementary advantages is an effective therapeutic measure. For example, liposomes can be loaded with lipophilic drugs that are difficult to encapsulate in hydrogels,48 the combination of algae and hydrogels helps to improve traumatic microvascular hypoxia,49,50 and hydrogels provide a good environment for exosomes, cells, bacteria and algae to ensure their stable activity over time.51–53 Therefore, biomembrane-based nanostructure- and microstructure-loaded hydrogels represent a promising strategy for promoting the healing of diabetic wounds. Figure 1 summarizes the characteristics and healing mechanisms of biomembrane-based nanostructure- and microstructure-loaded hydrogels for chronic wounds.
During the past decade, liposome- or exosome-loaded hydrogels have attracted considerable attention owing to their unique advantages, and several preclinical studies have reported encouraging results. To the best of our knowledge, no study has provided a comprehensive summary of biomembrane-based nanostructure- and microstructure-loaded hydrogels. This review focuses on the advantages and disadvantages of using hydrogels and biomembrane-based nanostructures and microstructures for chronic wound healing and summarizes the direction of development of biomembrane-based nanostructure- and microstructure-loaded hydrogels for chronic wounds. Table 1 shows some examples of the use of biomembrane-based nanostructure- and microstructure-loaded hydrogels in diabetic wounds.
 |
Table 1 Representative Examples of Biomembrane-Based Nano- and Microstructure-Loaded Hydrogels in Diabetic Wounds |
The Design Strategy of Biomembrane-Based Nanostructure- and Microstructure-Loaded Hydrogels
Biomembrane-based nanostructures and microstructures mainly comprise living cell systems (living cells, bacteria and algae) or components derived from living cell systems (exosomes and cell membranes) and biomimetics (liposomes), which share the characteristics of good biocompatibility and complex biological functions, which contribute to their widespread interest in the treatment of diseases. However, the common drawbacks are low stability owing to changes in the external environment and the inconvenience of drug delivery.51,54,55 Hydrogels have been one of the most investigated dressings for wound healing in recent years because of their good biocompatibility and injectability.56 However, their lack of bioactivity limits their application in wound healing.57,58 Therefore, loading biomembrane-based nanostructures and microstructures into hydrogels can result in a drug delivery platform with combined advantages, neutralizing their respective disadvantages.
Designing this combined loading strategy is challenging because the loaded cargo should be maintained in an active state and its stable release should be ensured without affecting the performance of the hydrogel. In terms of hydrogel materials, the ideal materials should be biocompatible and degradable. Owing to bacterial infection, cell modulation and cytokine regulation involved in wound healing, some active hydrogel materials may enhance the healing process. For example, chitosan has favorable hemostatic and antibacterial effects,59 hyaluronic acid can regulate the inflammatory response and promote cell adhesion,60 and calcium ions produced via the degradation of hydroxyapatite can regulate the dynamic balance of the skin and facilitate the proliferation and differentiation of cells.61 In terms of loading methods, biomembrane-based nanostructures and microstructures are loaded onto biomaterials based on their interaction, and two methods are usually used for loading these structures into hydrogels. The first strategy involves the simultaneous mixing of hydrogel materials, nanostructures or microstructures and cross-linkers. These microstructures or nanostructures are loaded into the three-dimensional network formed by the hydrogel, thereby forming a hydrogel with an interconnected network.62 The second strategy involves the injection of preformed hydrogels into a solution containing nanostructures and microstructures. This solution is subsequently freeze-dried to form the final structure.48,63 The latter method is usually more advantageous than the former because the conditions for hydrogel formation do not affect the activity of the nanostructures and microstructures. However, the first preparation method is easier to handle and results in a higher yield, making it ideal for forming gels in situ.64 Each method has unique characteristics and is suitable for fabricating different types of hydrogels.
The release of the cargo loaded in the hydrogel is influenced by the hydrogel network. The type of polymer affects the morphology, mechanical properties and degradability of the hydrogel network, and cross-linking has a significant effect on swelling of the hydrogel.65–68 Mixing alginate with biomembrane-based nanostructure and microstructure solutions can result in the formation of a stable structure with a three-dimensional network by binding to some divalent ions, such as calcium ions.69,70 When this method is used, the hydrogel formed has larger pores, which is more suitable for loading cargoes with larger particle sizes but less suitable for loading cargoes with smaller particle sizes. This problem can be overcome by adding other materials that form a new cross-linked network.71 In addition, the cargo can be coated to improve its interaction with the biological material to achieve a cumulative amount of drug permeation. Compared to lipid-soluble drugs, liquid-soluble drugs are more easily loaded into hydrogels.72 The results of a study showed that lipophilic fluconazole NPs coated with polyethylene glycol significantly improved the water solubility and enhanced the accumulation of drug penetration, which in turn improved the efficacy.73 Studies on nanostructure- and microstructure-loaded hydrogels are limited, and efforts are required to design ideal hydrogel delivery systems to facilitate the delivery of different carriers at different release rates. Hydrogel systems with stimulus-responsive properties are also of great interest at present.74 Nanostructures and microstructures can be released from hydrogels when exposed to certain stimuli, such as temperature, pH, enzymes and magnetic fields.75,76
It is essential to maintain the activity of micro/nanocarriers in hydrogels by selecting a biocompatible hydrogel and loading method.77,78 Hydrogel crosslink density plays an important role in ensuring the sustained release of microstructures and nanostructures from the hydrogel. In addition, it is necessary to examine the hydrogel material and the crosslinking method to determine the optimal combination of release rates.79
The Applications of Biomembrane-Based Nanostructure- and Microstructure-Loaded Hydrogels in Chronic Wounds
Liposome-Loaded Hydrogels
Owing to their high water content and rich physicochemical properties, hydrogels provide a conducive microenvironment for the proliferation and differentiation of cells and promote wound healing and angiogenesis, thus enabling efficient tissue repair and regeneration.80 However, the hydrophilic environment limits the application of lipophilic drugs. Moreover, some hydrogels with large pores do not have adequate sustained release properties, resulting in a very rapid release of the drug. Liposomes are closed vesicles with bilayer structures composed of amphiphilic molecules such as phospholipids dispersed in water.81 When amphiphilic molecules are dispersed in an aqueous phase, the hydrophobic side moves away from the aqueous phase, whereas the hydrophilic side is exposed to the aqueous phase.81 Owing to this bilayer structure, hydrophilic and hydrophobic drugs can be encapsulated in the inner aqueous and bilayer phases, respectively.81 In addition, this special structure of liposomes promotes sustained release82 and improves drug stability.83 Liposomes are typically liquid and cannot maintain a certain concentration locally, whereas hydrogels can enhance liposome delivery, allowing them to be locally administered. Therefore, the liposome-based hydrogel drug delivery system integrates the merits of liposomes and hydrogels, which can improve the drug release properties of hydrogels and maintain drug stability.
Liposomes can overcome certain disadvantages of hydrogels and compensate for their weakness in drug loading, drug release and mechanical strength; therefore, they have great application prospects.48,84 First, the phospholipid bilayer of liposomes allows for the integration of lipophilic substances, improves drug solubility and facilitates the retention of drugs in hydrogels. The combination of liposomes and hydrogels facilitates the loading of lipophilic drugs into the hydrogel and is a promising delivery method for topical skin treatment.85,86 A curcumin-loaded liposome–hydrogel system has been used for wound healing.48 Liposomes improve the solubility of the fat-soluble drug curcumin, and the integrated delivery system ensures continuous dermal administration of curcumin and increases its retention in the skin site. Second, the rapid release of drugs from hydrogels with large pores can be improved using liposomes. Billard et al87 prepared a chitosan hydrogel consisting of enwrapped phosphatidylcholine liposomes and encapsulated the water-soluble model drug carboxyfluorescein (FAM) in the liposomes to detect the release of FAM. The study validated that the liposome–hydrogel system had a stronger sustained-release ability than hydrogels. Third, the application of bioactive cytokine-loaded hydrogels for wound healing has received increasing attention.88–90 However, the bioactivity of these cytokines is often reduced owing to enzymatic degradation when exposed to trauma fluids.91 Liposomes can contain hydrophilic protein-based drugs in the inner aqueous phase, which increases stability and prolongs the release of their contents. Xu et al92 successfully prepared a novel liposome-loaded silk fibroin hydrogel (SF-LIP) encapsulating basic fibroblast growth factor (bFGF), which effectively maintained the activity of bFGF and accelerated wound healing. Furthermore, the addition of liposomes increases the viscosity and mechanical strength of hydrogels. Ruel-Gariépy et al84 modified chitosan-beta-glycerophosphate with liposomes to obtain a new class of hydrogels. They observed that liposomes prominently enhanced the viscosity and mechanical strength of the hydrogel. In addition to the abovementioned studies, additional studies have demonstrated that liposomes can facilitate the targeted delivery of drugs to specific skin layers by ensuring the sustained release of drugs/active substances.87,93
The liposome–hydrogel system may be more effective in promoting chronic wound healing. The migration of macrophages to tissues is important during wound healing.94 Specifically, macrophages can be divided into two types, namely, M1- and M2-type macrophages, according to their functions and the level of inflammatory factor secretion. M1-type macrophages promote inflammation, whereas M2-type macrophages reduce inflammation to aid in wound healing.95,96 The two types of macrophages can be interconverted when exposed to different cytokines.97 Chronic skin wounds are usually characterized by open wounds in a constant state of chronic inflammation.98 The recruitment of macrophages to the wound site and enhanced polarization of M1 macrophages to the M2 phenotype are beneficial measures for promoting chronic wound healing. Yu et al95 prepared a prohealing stromal cell-derived factor-1 alpha (SDF-1α)-loaded liposomal hydrogel and used it to treat diabetic wounds. They demonstrated that the vascular skin tissue regenerated successfully in mice with diabetes with sustained dorsal wounds. The hydrogel offered a favorable environment for wound healing and protected against external microorganisms, whereas SDF-1α recruited macrophages to the wound site and promoted their polarization to the M2 phenotype. In addition, liposomes protected the released SDF-1α from enzymatic degradation in the traumatic environment, thereby improving the therapeutic effect.
Liposomes facilitate the sustained release of drugs from the hydrogel, and this combined drug delivery system is highly conducive to long-term drug release in chronic wounds and protects the drug from enzymatic degradation in the wound microenvironment. However, the slow initial release rate is not conducive to maintaining initial hemostasis. Thrombin (Th) plays a regulatory role in multiple stages of wound healing. It promotes coagulation during the early hemostatic phase99 and induces the differentiation of fibroblasts into myofibroblasts during the subsequent proliferative and remodeling phases.100 Based on this phenomenon, Wang et al101 prepared a photocrosslinkable hydrogel and encapsulated free Th and Th-loaded liposomes into it (Figure 2). The results showed that free Th was the first to be released from the hydrogel and exerted a hemostatic effect during the early stages of trauma. In addition, the sustained release of Th from liposomes induced fibroblast differentiation during the proliferative phase and promoted the formation of granulation tissue and deposition of collagen. This different pattern of release can promote the progression of the initial hemostatic and the subsequent proliferative and remodeling phases.
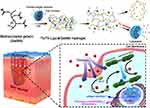 |
Figure 2 Anatomical and molecular mechanisms of Th and Th-Lipo-loaded GelMA hydrogel repairing and regenerating diabetic skin. Reprinted from Chemical Engineering Journal, 397, Wang C, Wu T, Liu G, et al. Promoting coagulation and activating SMAD3 phosphorylation in wound healing via a dual-release thrombin-hydrogel, 125414, Copyright © 2020, with permission from Elsevier B.V.101 Abbreviations: Th, thrombin; Th-Lipo, Th-loaded liposomes; GelMA, methacrylated gelatin. |
Liposomes are ideal drug carriers owing to properties such as sustained drug release and improved stability.102,103 However, their clinical application is limited owing to some disadvantages. For example, liquid formulations are difficult to deliver because of insufficient mechanical properties and cannot maintain a certain level of concentration locally.18 Hydrogels can facilitate the release of drugs directly at the wound site; however, it is difficult to load lipophilic drugs in hydrogels, and the active factors released by them are easily degraded in the wound microenvironment. Therefore, the combination of hydrogels and liposomes has great application potential in wound healing because they can compensate for their respective disadvantages, help to maintain a stable concentration of drugs in situ for a long time and are more advantageous than a single delivery system.104
Cell Membrane-Coated Nanostructure-Loaded Hydrogels
The metal ions or oxides of some metals possess some antibacterial properties, such as silver ions,105 copper ions,106 zinc oxide,107 etc. A major mechanism of inorganic NP antibacterial activity involves the alteration of bacterial membranes and disruption of organelles, which makes them widely used as antimicrobial dressings for wounds.108 Researchers have also found that some metal ions have a positive effect on angiogenesis, for example, copper ions induce revascularization mainly by upregulating the expression of angiogenic growth factors.109,110 However, a concentration of copper ions above physiological levels is toxic because it disrupts the dynamic balance of other metal ions and affects the metabolism of cells in the body.111 The researchers developed a hydrogel that slowly releases copper ions and boosts antimicrobial activity to address these problems and assist in wound healing.112 Furthermore, NPs can also be combined with photothermal therapy (PTT)113 and photodynamic therapy (PDT)114 to kill bacteria and promote vascularization and collagen deposition.
Although NPs can contribute to wound healing, the biocompatibility of NPs and the exclusion of immune cells in the wound area limit the effective action of NPs. In terms of biocompatibility, NPs can be modified by coating them with cell membranes to reduce immune recognition.115,116 An effective strategy is to combine synthetic NPs with natural or biomimetic materials. Cell membrane-encapsulated NPs have prominent physicochemical properties of NPs and complex properties of the cell membrane.117,118 For example, encapsulation of NPs with macrophage membranes can reduce the photolytic breakdown of NPs and prolong the cycling life.119 NPs encapsulated in leukocyte membranes can infiltrate the endothelium,120 and encapsulation of NPs with erythrocytes can increase immune evasion.121,122 Cell membrane-encapsulated NPs are increasingly used to harness the functions of natural cells to enhance the biocompatibility of NPs and improve therapeutic outcomes. In addition, the combination of specialized membranes or membrane types can enhance cellular functions. We previously reported that encapsulation of NPs with neutrophil–erythrocyte hybrid membranes prolonged their circulation and improved their targeted delivery.123
We speculate that the use of cell membranes to encapsulate NPs can improve the biocompatibility of NPs and facilitate immune escape, thus promoting effective chronic wound healing. Hydrogels provide an ideal microenvironment for the proliferation and differentiation of cells, leading to efficient tissue repair and regeneration.41 In addition, they can improve the stability of NPs124 and exert a sustained-release effect.125 Fan et al126 reported the preparation method and biological properties of a novel cell membrane-derived hydrogel scaffold (Figure 3). This scaffold is biocompatible and has large pores, which allow for the encapsulation and release of lipophilic model drugs. In addition, this scaffold can recruit a large number of anti-inflammatory macrophages, indicating the feasibility of drug delivery in the form of hydrogel scaffolds by basal cell membrane-encapsulated nanomaterials for chronic wound healing. Further development of nanotechnology and cell membrane encapsulation technology will promote the fabrication and use of cell membrane-coated nanostructure-loaded hydrogels for the treatment of chronic wounds in the future.
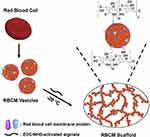 |
Figure 3 Diagram illustrating how the RBCM scaffold was fabricated. Reprinted from Biomaterials, 197, Fan Z, Deng J, Li PY, et al. A new class of biological materials: Cell membrane-derived hydrogel scaffolds, 244-254, Copyright © 2019, with permission from Elsevier.126 Abbreviations: RBCM, red blood cell membrane; EDC, 1-ethyl-3-(−3-dimethylamino-propyl) carbodiimide; NHS, N-hydroxysuccinimide. |
Exosome-Loaded Hydrogels
Despite significant advances in the treatment and management of wounds, the lack of bioactivity in hydrogels, especially synthetic hydrogels, limits their use in the clinical treatment of chronic wounds. Exosomes have been extensively investigated in recent years, and studies have reported that cells involved in wound healing can be modulated through exosomes derived from different sources.20 Specifically, exosomes accelerate wound healing by inhibiting inflammation,127 promoting angiogenesis128 and stimulating collagen deposition.129 However, the application of exosomes for wound treatment is challenging because they are mostly administered via injection and have a relatively short half-life and clearance time in vivo.130,131 A study reported that exosome-loaded hydrogels can preserve the stability of proteins and microRNAs in exosomes, which enhances their efficacy and prolongs their duration of action.132
Delivering exosomes to a wound site and maintaining their activity over time are major focus areas of research on diabetic wounds. The combination of exosomes and biomaterials can increase the retention of exosomes at the wound site without damaging their biological activity. Wang et al133 designed an antimicrobial, injectable, self-healing hydrogel encapsulated with active adipose mesenchymal stem cell-derived exosomes (Figure 4A), and the exosomes were released by the hydrogel at a lower pH (Figure 4B). The use of these hydrogels significantly increased the wound closure rate (Figure 4C) and accelerated angiogenesis, re-epithelialization and intrawound collagen deposition within diabetic wounds. Wound healing was better with the use of exosome-loaded hydrogels than with the use of either exosomes or hydrogels, indicating that the combination of exosomes and hydrogels effectively promotes the healing of diabetic wounds. Furthermore, exosomes are powerful bioactive carriers for drug delivery. Wang et al134 successfully loaded the small-molecule drug VH298 into epidermal stem cell-derived exosomes and administered it through a highly biocompatible GelMA hydrogel with sustained release. Exosomes promoted vascular regeneration and improved therapeutic effects when loaded with VH298, indicating that they can stimulate vascular regeneration. Overall, exosomes have great potential as active molecules and drug carriers in promoting wound healing.
 |
Figure 4 (A) Schematic diagram of the self-healing process of hydrogels. (B) pH-dependent release of exosomes from hydrogels. (C) The healing process of wounds treated with hydrogel, exosomes, exosome-loaded hydrogel and control wounds. Adapted with permission from Wang C, Wang M, Xu T, et al. Engineering Bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9:65–76, Open Access.133 (D) Hydrogel consisting of Cothe HA@MnO2/FGF-2/Exos is formed by the Schiff base reaction of hydrazides and aldehydes. (E) HA@MnO2/FGF-2/Exos hydrogel can be quickly formed by simple mixing injections, in which grafted ortho-quaternary ammonium groups have long-term antibacterial efficacy. The MnO2/ε-PL nanosheet is a nanoenzyme that catalyzes the oxidation of H2O2 into O2. The release of ExosM2@miR−223 mimicked persistent angiogenesis, and the release of FGF-2 enhanced epithelial formation. Reprinted with permission from Xiong Y, Chen L, Liu P, et al. All-in-One: multifunctional Hydrogel Accelerates Oxidative Diabetic Wound Healing through Timed-Release of Exosome and Fibroblast Growth Factor. Small. 2022;18:e2104229.© 2021 The Authors. Small published by Wiley-VCH GmbH.142 Abbreviations: F127, Pluronic F127; OHA, oxidative hyaluronic acid; EPL, Poly-ε-L-lysine; FHE, F127/OHA-EPL hydrogel; exo, exosomes; HAh, hydrazide-grafted HA; FGF-2, fibroblast growth factor 2. |
Because exosome-based chronic wound healing depends on the source of exosomes, it may alter some molecular pathways. Autologous dermal fibroblast-derived exosomes promote diabetic skin wound healing via the Akt/β-catenin pathway,135 human amniotic epithelial cell-derived exosomes increase the capillary density in mice with diabetes by activating the PI3K–Akt–mTOR pathway,136 and endothelial progenitor cell-derived exosomes activate the Erk1/2 pathway to cause angiogenesis.137 In addition, some studies have reported that mesenchymal stem cell-derived exosomes (MSC-Exos) have a long circulation time in vivo because they are protected from macrophage degradation and inhibit T-cell activation to maintain immune homeostasis.138,139 Moreover, the healing effect of exosomes derived from different sources also varies. Hoang et al140 compared MSC-Exos of the adipose tissue, bone marrow and umbilical cord and found that the effects of exosomes on cell proliferation and migration were related to the source and target cells of the exosomes.
Because numerous factors influence wound healing, a single treatment modality cannot promote rapid healing. Injured wounds are highly susceptible to infection caused by external bacteria. Although hydrogels are effective in covering wounds and protecting them from infection, their effectiveness is limited, and they cannot be used on infected wounds. In addition to the abovementioned treatment modalities, the introduction of antimicrobial strategies can further improve wound healing effectively. Geng et al141 reported a carboxyethyl chitosan (CEC)–bis-aldehyde carboxymethyl cellulose (DCMC) hydrogel encapsulated with bone marrow MSC-Exos. CECs can cross-link with DCMCs via the Schiff base reaction to form an antimicrobial self-healing hydrogel, whereas MSC-Exos can facilitate the polarization of M1 macrophages to M2 macrophages, modulate the traumatic inflammatory microenvironment and promote angiogenesis in rats with diabetes. Therefore, the study of multifunctional hydrogels has received significant attention. Xiong et al142 prepared a multifunctional hydrogel encapsulated with MnO2/ε-PL nanosheets, fibroblast growth factor-2 (FGF2) and M2-derived exosomes (M2-Exos) (Figure 4D). This hydrogel facilitated rapid hemostasis and long-term antibacterial action via local injection. In addition, M2-Exos and FGF2 stimulated vascular regeneration and epithelialization, respectively, and MnO2/ε-PL nanosheets catalyzed the conversion of hydrogen peroxide to oxygen at the diabetic wound sites. Therefore, M2-Exos, FGF2 and MnO2/ε-PL nanosheets synergistically acted to facilitate chronic wound healing (Figure 4D and E). This multifunctional hydrogel, which combines antibacterial effects, antioxidant effects, vascular regeneration and epithelialization, may improve ROS damage; induce angiogenesis; promote proliferation, granulation tissue formation and collagen deposition; and work in multiple ways to facilitate chronic wound healing.
Hydrogels are good wound dressings; however, their inherently low bioactivity limits their application. Exosomes can contribute to the adjustment of chronic wounds at various stages of wound healing;143 however, their short half-life and administration mode (injection) limit their use in chronic wound healing. However, the combination of exosomes and hydrogels can neutralize their respective disadvantages and combine their respective advantages for the treatment of wounds. Specifically, exosomes can enhance the biological activity of hydrogels and improve the therapeutic effects on chronic wounds, whereas hydrogels can facilitate the sustained release of exosomes. In addition, exosomes can act as drug carriers and play a role in promoting healing, and drugs can be directly loaded onto exosomes, thus improving drug targeting through the homing mechanism and eventually enhancing therapeutic effects.134
Living Cell-Loaded Hydrogels
Cell therapy is a promising method to replace or repair damaged tissue with cells transplanted from different sources. Different types of cells are transplanted into wounds for wound healing and skin repair, including mesenchymal stem cells (MSCs),144 adipose-derived stromal cells145 and endothelial cells.146 Although small molecules, biologics or cells may serve as therapeutic agents, only cells can respond to stimuli in the wound healing environment.147,148 However, research into cell therapy for skin wounds is limited owing to the low survival rate of transplanted cells. In cell therapy, stem cells suspended in a solution are typically delivered through injection, which may lead to rapid cell death, resulting in low implantation rates and activity.149–151 Therefore, establishing a protective microenvironment is necessary for increasing the survival of transplanted cells. Hydrogels maintain a moist and supportive environment within the wound, thereby preventing cell and tissue death and reducing pain.152,153 The elastic and structural properties of hydrogels are similar to those of ECM, which may enhance biocompatibility after implantation.154 Therefore, hydrogel-based cell therapy for wound healing holds great promise.
MSCs regulate wound healing through a series of paracrine growth factors155 and differentiate into effector cells involved in wound healing,156 thereby promoting wound closure157 and accelerating angiogenesis,158 granulation tissue formation159 and re-epithelialization.158 Rustad et al160 examined the delivery of MSCs to wounds in a biomimetic pullulan–collagen hydrogel to improve wound healing. The results indicated that compared with stem cells delivered via injection, those delivered via hydrogels were more likely to survive, and their implantation was more efficient. Zhang et al161 synthesized an injectable hydrogel composed of sodium alginate (SA) and type I collagen (Col) as a tissue scaffold and loaded it with MSCs. The SA/Col hydrogel accelerated wound closure and tissue remodeling by enhancing the survival of MSCs, promoting the secretion of growth factors and inhibiting the inflammatory response of the wound. Therefore, compared with injectables, hydrogels result in better engraftment and survival rates for delivering live cells and have great potential as wound healing agents.
Hydrogels provide an adequately hydrated environment for stem cells, and the stronger biological activity of stem cells promotes rapid wound healing. However, proteases are produced in greater quantities around the inflammatory microenvironment when inflammatory cytokines are present at high concentrations, thus leading to the degradation of growth factors secreted by cells.91 Therefore, this therapy is not as effective in certain chronic ulcerated wounds as in normal wounds, especially in diabetic ulcers. In addition, the activity of MSCs can be impaired during this chronic inflammatory condition. To design more effective strategies for MSC delivery, the ideal hydrogel material should be biocompatible and inhibit the inflammatory response or protease activity for effective treatment and management of diabetic ulcers. Chen et al162 reported a biocompatible, temperature-sensitive hydrogel for the delivery of MSCs. This hydrogel could inhibit the expression of M1 macrophages, thereby improving the chronic inflammatory microenvironment and maintaining cell viability. After their release from the hydrogel, MSCs released growth factors around the wound surface, which regulated wound healing by releasing active factors (Figure 5). Furthermore, this system promoted the adhesion and differentiation of MSCs owing to the crosslinker containing an RGD-like motif.163 Furthermore, hydrogels fabricated using natural materials can promote the proliferation, migration and differentiation of cells. For example, hyaluronic acid-based hydrogels can enhance the adhesion of MSCs to facilitate their proliferation and differentiation,164 and functionalized silk-based hydrogels can facilitate cell adhesion and survival.165,166
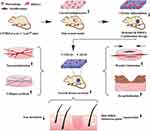 |
Figure 5 Diagram of MSC-loaded hydrogels for chronic wounds. Adapted with permission from Chen S, Shi J, Zhang M, et al. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci Rep. 2015;5:18104, Open Access.162 Abbreviation: BMSCs, bone marrow mesenchymal stem cells. |
Pluripotent stem cells can accelerate wound healing because of their proliferative potential, differentiation capacity and ability to secrete a wide variety of nutrient factors for wound healing.156 These properties eventually help to stimulate wound closure, angiogenesis, re-epithelialization and collagen deposition by reducing immunogenicity and increasing therapeutic effects.167 Hydrogels delivering live cells have received significant attention because of their effectiveness in improving the transplantation and survival of cells for promoting wound healing. Some hydrogel materials should be intensively investigated for their ability to promote the proliferation and differentiation of cells.168
Bacteria-Loaded Hydrogels
Bacteriotherapy, a strategy that functions through bacterial disruption and immunomodulation for treating various inflammatory and immunopathological diseases, has garnered a great deal of interest in recent years.169 In particular, certain beneficial bacteria can produce large quantities of metabolites and antimicrobials to create a unique microenvironment. For instance, Bacillus subtilis is used to treat fungal infections.170 However, the application of bacteria is largely limited by their rapid proliferation, which may lead to uncontrollable invasion. Hydrogels not only provide a physical barrier against bacterial invasion of damaged skin but also restrict the living region of bacteria to a certain area. Therefore, optimizing bacterial therapy using hydrogels is an effective measure.
Certain bacteria can secrete antimicrobial agents and create an environment with a lower pH to combat bacterial infection in the wound. Bacteria can be encapsulated within hydrogels, which can be administered topically to accelerate wound healing. Ming et al171 proposed a treatment modality that can accelerate the healing of infected wounds through beneficial bacteria that secrete antimicrobial substances. Lactobacillus reuteri, as a live strain, was encapsulated in hydrogel microspheres that formed a hydrogel in the wound and inhibited pathogenic bacteria by decreasing the local pH (Figure 6A and B) and producing antimicrobial agents during metabolism (Figure 6C). The experimental results indicated that this hydrogel promoted the closure of infected wounds and the regeneration of new tissue. Furthermore, the photothermal properties of certain bacteria can be exploited to combat external bacterial infections and accelerate wound healing through PTT.172,173 Zhao et al174 were the first to use nontoxic photosynthetic bacteria (PSB) as a photothermal agent (PTA) and encapsulated PSB into an ECM gel to inactivate bacteria by increasing the temperature after near-infrared light irradiation (Figure 7A and D). PSB have good photothermal properties (Figure 7B and C) and are a superior alternative to typical PTAs because they overcome many problems associated with cytotoxicity, high cost and cumbersome production processes.175 PSB can be considered not only PTAs but also anti-inflammatory agents that can enhance wound healing because of their antioxidant metabolites. Hydrogels contain various growth factors and nutrients, and the combination of hydrogels and PSB can accelerate cell migration and promote wound healing (Figure 7E). PSB are not only nontoxic but also easy to manufacture and available on a large scale, with a strong potential for clinical translation. Some bacteria can be genetically modified to secrete certain substances that can promote wound healing.176 Lu et al177 used a temperature-sensitive hydrogel to encapsulate gene-edited Lactococcus lactis and used the fabricated hydrogel for chronic wound healing. The engineered bacteria secreted vascular endothelial growth factor (VEGF), which facilitated endothelial cell proliferation, migration and angiogenesis, thereby accelerating wound healing. Furthermore, tumor-derived lactic acid can induce “phenotypic transformation” of macrophages, thus promoting tumor growth.178 Therefore, in a study, the effects of lactic acid production by Lactobacillus lactis on macrophages were investigated, and it was found that lactic acid has a better ability to act as a metabolite to induce M2 macrophage polarization in a dose-dependent manner, thereby modulating the wound microenvironment and complementing the angiogenic function of engineered bacteria to facilitate tissue regeneration.
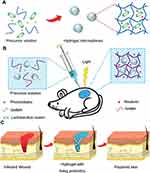 |
Figure 6 Schematic illustration of the preparation and acceleration of wound healing while using living probiotic hydrogels. (A) The process of encapsulating bacteria in microspheres. (B) Covalent cross-linking within light-irradiated microspheres containing active probiotics. (C) Procedure for treating wounds with live bacterial hydrogel. Reprinted from Ming Z, Han L, Bao M, et al. Living bacterial hydrogels for accelerated infected wound healing. Adv Sci. 2021;8:e2102545, © 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.171 Abbreviation: HAMA, hyaluronic acid methacryloyl. |
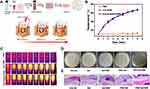 |
Figure 7 (A) Schematic for fabricating PSB gel dressings to eliminate MRSA and accelerate wound healing. (B) Curves of photothermal temperature for PBS, PSB, and inactivated PSB irradiated with an 808 nm (1 W/cm2) laser. (C) Images of PSB, inactivated PSB, and phosphate-buffered saline exposed to an 808 nm laser (1 W/cm2) infrared thermography. (D) Antibacterial properties of PBS in vitro. (E) H&E staining of wound tissue. The data were presented as the mean ± standard deviation (SD) of measurements (*p < 0.05, **p < 0.01, and ***p< 0.001). Adapted from Acta Biomater, 140, Zhao E, Liu H, Jia Y, et al. Engineering a photosynthetic bacteria-incorporated hydrogel for infected wound healing. 302–313, Copyright 2022, with permission from Elsevier.174 Abbreviation: PSB, photosynthetic bacteria. |
In addition to the abovementioned hydrogels, algae–bacterial hydrogels have also been used for wound healing.53 This symbiotic hydrogel system modulates ROS to reduce chronic inflammation and increase acute inflammation, thus effectively advancing the wound healing process to the proliferative and remodeling phases.53 However, research into the use of bacteriotherapy to accelerate chronic wound healing is limited, and many challenges remain unaddressed. There have been no studies done to determine whether the biomaterials can cause skin irritation or damage. Further exploration and research on the role of bacteria in wound healing is needed.
Algae-Loaded Hydrogels
When the skin is damaged, the damaged blood vessels prevent the transport of oxygen to the wound site, contributing to a hypoxic environment.179,180 Although acute hypoxia is beneficial to cell proliferation and tissue repair, angiogenesis is impaired in wounds with chronic hypoxia.181 A primary mechanism underlies diabetes-related angiogenesis disorders caused by impaired function of hypoxia-inducible factor 1α (HIF-1α)/VEGF.182,183 Briefly, the high-glucose environment affects the stability of HIF-1α, leading to a failure to promptly upregulate VEGF in the ischemic tissue of diabetic wounds, thus resulting in impaired angiogenesis. Therefore, the treatment of chronic wounds can be improved by increasing the oxygenation of injured tissues. Hyperbaric oxygen therapy (HBOT) has been used for wound healing;184 however, it is not suitable for healing chronic wounds because oxygen levels in the wound decrease rapidly after treatment.185 In recent years, it has been found that long-term oxygenation of the trauma surface can be achieved by hydrogels loaded with single-cell algae, which is beneficial in promoting healing of chronic wounds.62 Algae can continuously produce oxygen through photosynthesis, and hydrogels provide an environment for the survival of algae and allow them to release the oxygen produced directly into the wound. The combination of algae and hydrogels offers a treatment strategy that holds great promise as an alternative to HBOT.
Microalgae usually refer either to eukaryotic (microalgae) or prokaryotic (cyanobacteria) organisms that are capable of oxygenic photosynthesis.49 Microalgae are widely and conveniently available in aquatic and terrestrial habitats, and various techniques are available for their artificial cultivation.186,187 They can continuously release oxygen through photosynthesis, which can be of great advantage in improving the chronic hypoxic environment of wounds. Hydrogels can provide a convenient way for microalgae to attach to wounds and deliver the oxygen produced by microalgae to the wounded tissue, thus providing an opportunity for microalgae to help promote wound healing. Chen et al62 reported an SA gel patch for promoting chronic wound healing using a single-cell cyanobacterium, PCC7942, as an oxygen source (Figure 8A and B). Through the photosynthesis of PCC7942, the moist environment of hydrogels absorbed and permeated gas from the wounded tissue, which further facilitated oxygen diffusion and stimulated aerobic metabolism and angiogenesis in the original hypoxic tissue. Histological evaluation of the wounds revealed that this combination treatment modality facilitated wound closure, granulation tissue formation, collagen deposition and angiogenesis (Figure 8C-G). In addition, PCC7942 can promote cell proliferation and migration in the absence of light.62 In a study, hydrogels made from silk maintained slow proliferation of microalgae cells over a long period and had higher mechanical strength and stability than SA hydrogels.188 Li et al189 reported the use of chitosan–hyaluronic acid hydrogels prepared from the polysaccharide paramylon extracted from the microalga Euglena gracilis. The results showed that both live Euglena gracilis cells and their extracts promoted wound healing without generating excessive inflammatory responses. Certain compounds in microalgae may have a wound healing effect even in the absence of photosynthesis. Recent studies have reported that certain compounds in microalgae enhance wound healing and have excellent antioxidant and anti-inflammatory effects, thereby controlling ROS secretion190,191 and the release of antibacterial products.192 Overall, the development of microalgae-based wound dressings is an effective measure to avoid the development of chronic inflammation and/or complications caused by skin infections to enhance the healing of chronic wounds.
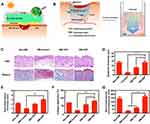 |
Figure 8 (A) Diagram illustrating the preparation of AGP and the treatment of diabetic wounds by dissolved oxygen release in response to light. (B) Design scheme of the wound-healing process using algae gel. (C) Different groups of wounds showed regenerated skin by H&E and Masson staining at day 12. (D-G) Quantification of the epithelial gap (D), granulation tissue (E), collagen deposition (F) and average microvessel densities in different groups (G). Significantly different (one-way ANOVA): *P<0.05, **P<0.01, and ***P<0.001. Adapted from Chen H, Cheng Y, Tian J, et al. Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes. Sci Adv. 2020;6:eaba4311, Open Access.62 Abbreviations: AGP, microalga-hydrogel patch; PU; polyurethane; PTFE, polytetrafluoroethylene. |
Furthermore, algae-loaded hydrogels can be combined with PDT to kill bacteria in wounds in the presence of oxygen. Li et al193 prepared a hydrogel encapsulated with Spirulina platensis (SP) using carboxymethyl chitosan. SP accelerates vascular regeneration by releasing oxygen through photosynthesis. In addition, in a bacterial infection model, SP released the natural photosensitiser chlorophyll to produce ROS after laser irradiation, thereby killing bacteria in the infected area of the wound. Overall, the SP–hydrogel system with laser irradiation treatment has good antimicrobial and vascular regenerative effects and significantly promotes wound healing, which has significant implications for the development of suitable wound dressings for chronic wounds. In addition to improving the hypoxic state of the wound microenvironment, the oxygen produced by photosynthesis facilitates the antimicrobial effects of PDT by producing ROS. This treatment strategy accelerates wound healing and greatly promotes its efficiency.
The use of algae-loaded hydrogels for accelerating wound healing holds great promise. In addition to generating oxygen and improving hypoxia in chronic wounds, some compounds in algae have strong antibacterial and anti-inflammatory effects. In addition, the combination of algae and PDT exerts antibacterial and vascular regenerative effects. Overall, microalgae play a major role in antimicrobial activity and enhancing chronic wound healing. However, microalgae, like bacteria-loaded hydrogels, need further testing to ensure their safety on the skin. The use of microalgae on skin may be explored using different loading strategies in future studies to ensure that microalgae are effective in accelerating wound healing and maintaining skin safety.
Mechanisms of Biomembrane-Based Nano- and Microstructure Loaded Hydrogels in Wounds
Diabetic wounds often affect the healing process owing to their complex environment. Biomembrane-based nanostructure- and microstructure-loaded hydrogels often modulate the healing process of chronic wounds by inhibiting bacterial infection, modulating inflammation, and promoting angiogenesis.
Inhibition of Bacterial Infection
Wound injuries are often caused by external bacterial microorganisms and result in wound deterioration and delayed healing,194,195 and the ensuing bacterial resistance restricts antibiotic use.196 Antibiotic-loaded hydrogels for topical administration are an effective measure for reducing bacterial resistance.197 However, the unsuitability of hydrogels for loading lipophilic drugs limits the use of lipophilic antibiotics. Liposomes are extremely efficient drug carriers, and both hydrophobic and hydrophilic drugs can be encapsulated in liposomes. In addition, liposomes can facilitate the sustained release of drugs, which can reduce the dosage of antibiotics in clinical settings.63,198 Antimicrobial therapy based on NPs encapsulated in cell membranes is also a promising approach because cell membranes enhance the biocompatibility and reduce the immunogenicity of NPs, thus improving their efficacy in the traumatic microenvironment.199,200 In addition, NPs encapsulated in platelet membranes can target bacteria and improve the antibacterial effect.201 Because the presence of a large number of immune cells in the traumatic microenvironment affects the efficiency of NPs, we speculate that hydrogels based on NP-encapsulated cell membranes offer equally promising strategies for fighting bacterial infections on traumatic surfaces. Certain substances isolated from bacteria and algae and PTT- or PDT-based antimicrobials have also been used to suppress wound infections. In addition, inherent antimicrobial hydrogel materials have been developed, such as chitosan and modified chitosan. The antibacterial properties of chitosan-based hydrogels are mainly due to the presence of protonated amino groups on the chitosan backbone, which can interact electrostatically with the phosphoryl groups of the phospholipid components of the cell membrane, influencing the permeability of the bacterial cell wall and thus leading to an imbalance in bacterial internal permeation.202,203 Their inherent antimicrobial properties reduce bacterial infection at the wound site and promote wound healing.204–206
Regulation of Inflammation
Macrophages play a dominant role in the inflammatory phase of wound healing.207 In the early phase of inflammation, M1 macrophages produce a proinflammatory environment and remove dead cells. During the subsequent inflammatory phase, the number of M2 macrophages gradually increases to promote angiogenesis and facilitate the transition to the proliferative phase by secreting cytokines.96 However, in diabetic wounds, M1-type macrophages consistently release inflammatory cytokines and ROS to promote inflammation,96,208 which results in the failure of macrophage polarization and prevents the progression of the persistent chronic inflammatory state. Enhanced polarization of M1 macrophages to the anti-inflammatory M2 phenotype contributes to the anti-inflammatory response and promotes recovery of diabetic wounds.141 Exosomes can increase the rate of M2/M1 polarization to facilitate wound healing.209 For example, macrophage-derived exosomes promote angiogenesis in high-glucose-cultured human umbilical vein endothelial cells in vitro and reduce the infiltration of inflammatory cells in diabetic wounds in rats in vivo by inhibiting the secretion of proinflammatory cytokines, thereby preventing insufficient and delayed diabetic wound healing to some extent.127 Furthermore, exosomes also overexpress NF-E2-related factor 2, which reduces inflammation and oxidative stress.210 Exosomes can be encapsulated in hydrogels to maintain their activity and modify the mode of drug delivery. Exosome-based hydrogels can transform the chronic phase of inflammation into the acute phase, thus facilitating a smooth transition to the proliferative phase. An algal–bacterial symbiotic hydrogel has also been used to promote the expression of antioxidant enzymes and inhibit inflammatory factors through hydrogen production.53
Promotion of Angiogenesis
When the skin is damaged, the damaged blood vessels impede the delivery of oxygen to the wound site, creating a hypoxic environment. The formation of new blood vessels plays an important role in wound healing by providing oxygen and nutrients to cells associated with the healing and maintenance of new tissues.211 Although direct cell transplantation is a promising method for repair and rejuvenation of the skin, it is not preferred because of some risks.212 Hydrogels provide a physiological microenvironment for transplanted cells as well as mechanical support and physical protection to natural immune cells. They are a good alternative to cell transplantation.52 In addition, some hydrogel materials can promote vascular regeneration.213 Although the mechanism underlying cell therapy for wound healing remains unclear, several experimental studies have shown that hydrogel-based cell therapy has great promise in promoting wound healing.52,161,214–217 In addition, exosomes are immune tolerant, perform functions similar to those of the original cells and may promote wound healing.218 For example, exosomes derived from human amniotic epithelial cells can increase the capillary density in mice with diabetes by activating the PI3K–Akt–mTOR pathway,136 and exosomes derived from endothelial progenitor cells can activate the Erk1/2 pathway to accelerate angiogenesis.137 MSC-Exos can benefit almost all phases of wound healing, such as by controlling the immune response, suppressing inflammation and promoting cell proliferation and angiogenesis.219 Exosomes can be used as an alternative to MSCs and are widely involved in intercellular signaling. They are more readily endocytosed and more efficiently loaded at the nanoscale than cells.131,220 Therefore, owing to the good biocompatibility and self-healing properties of hydrogels, exosome-loaded hydrogels can accelerate wound healing by synergistically promoting angiogenesis and restoring the complete structure and function of the traumatized skin.221
Fibroblasts play a key role in regulating endothelium-mediated angiogenesis.222,223 HBOT promotes the proliferation of fibroblasts and induces the production of the angiogenic regulators SDF-1 and VEGF, which in turn stimulate the expression of their receptors CXCR4 and VEGFR, respectively, in endothelial cells, thereby stimulating endothelial cell migration and angiogenesis.184 However, the oxygen content of the wound decreases after treatment, and it is difficult to continuously provide sufficient oxygen via HBOT. Some adverse clinical effects have also been observed owing to this hypoxic environment.185 Algae can produce oxygen through photosynthesis and provide long-term oxygen to wounds. Algae-loaded hydrogels can slowly release oxygen via photosynthesis, which improves the hypoxic microenvironment of the wounds and provides an opportunity to promote angiogenesis.
Different types of nanoparticles also can promote angiogenesis in several ways.224 Some metal ions have attracted much attention for their proangiogenic effects.225,226 Copper ions can stimulate angiogenesis by secreting VEGF,227 and zinc ions can reduce ROS in wounds and increase the expression of HIF-1α and VEGF,228 promoting the migration of human umbilical vein endothelial cells. In addition, some responsive NPs have been used to promote angiogenesis. Tang et al229 synthesized a ROS-responsive nanoparticle loaded with SDF-1α, which was effectively released from the nanoparticle in the presence of ROS and was effectively released and targeted to the wound. Overall, angiogenesis is a critical event in wound healing and is influenced by a variety of factors. Various strategies can be devised to accelerate angiogenesis at the wound site and thus effectively accelerate the rate of wound healing.
Conclusion and Outlook
Wound healing is a complex process that involves multiple cells and cytokines. Efforts have been made to accelerate the healing of chronic wounds by exploring various drug delivery strategies. Recent advances in biomembrane-based nanostructure- and microstructure-loaded hydrogels for treating chronic wounds have been greatly encouraged. Considerable progress has been made in developing biomembrane-based nanostructure- and microstructure-loaded hydrogels for chronic wound healing. In this review, we compared the advantages of biomembrane-based nanostructure- and microstructure-loaded hydrogels with those of either hydrogels or biomembrane-based nanostructures and microstructures and summarized their applications in chronic trauma.
Biomembrane-based nanostructure- and microstructure-loaded hydrogels can overcome the respective shortcomings of hydrogels and nanostructures or microstructures and enhance therapeutic efficacy. Liposome-loaded hydrogels can effectively encapsulate hydrophilic and lipophilic drugs and increase their stability. In addition, liposomes enhance the viscosity and mechanical strength of hydrogels;84 however, these effects remain controversial.48 It is necessary to study the effects of liposomes on the viscosity of hydrogels. Encapsulation of NPs in cell membranes can improve the biocompatibility of NPs and prevent their degradation by the complex microenvironment of wounds, which may enhance the role of NPs in promoting wound healing.230 Although therapeutic strategies targeting drug-loaded cell membranes in combination with hydrogels have not yet been used for wound healing, it is reasonable to assume that they can be used for the treatment of chronic wounds, given their progress in the treatment of other diseases. Exosome-loaded hydrogels can prolong drug release and increase bioactivity, thereby improving therapeutic efficacy. Living cell-loaded hydrogels offer an effective alternative strategy to cell transplantation for wound healing. They can also be designed to improve the inflammatory microenvironment and reduce the killing of transplanted cells in the inflammatory state, thereby improving transplantation efficiency and reducing mortality. In recent years, bacteria- and algae-loaded hydrogels have also been developed to provide a suitable living environment for both to promote wound healing by secreting certain substances or using other properties, thus improving the state of chronic wounds and accelerating wound healing. There is evidence that microstructure-loaded hydrogels exhibit good rheological properties, despite potentially poor mechanical stability.174 Detailed reasons for this have not yet been established by research, and this issue will need to be addressed in the development of microstructure-loaded hydrogels. In conclusion, a hydrogel can act as a carrier for biomembrane-based nanostructures and microstructures, allowing them to effectively contact the wound surface to act on it, whereas biomembrane-based nanostructures and microstructures have exceptional bioactivity.
In terms of delivery strategies, various types of hydrogels have been used as delivery systems while protecting bioactive molecules from protease degradation.231 Both natural and synthetic materials have different characteristics, and the selection of a suitable material for the delivery of biomembrane-based nanostructures and microstructures is difficult. The main challenge is to ensure that the biologically active molecule remains stable over time and can be released slowly or responsively under specific conditions. Researchers have attempted to overcome the deficiency of mechanical properties of natural hydrogels by modifying natural materials232 and by combining natural and synthetic materials to fabricate integrated hydrogels,233,234 thus neutralizing the disadvantages of both to some extent. Furthermore, hydrogel materials that promote the adhesion, migration and proliferation of cells, which can further enhance the therapeutic effects of live cell-loaded hydrogels, have also attracted considerable attention.
However, despite significant advancements and breakthroughs, achieving optimal results is difficult. Some challenges include the potential toxicity of cross-linkers that do not completely react during the preparation of hydrogels, needle clogging owing to the poor performance of injection-responsive hydrogels and changes in in vivo drug release profiles. In-depth research is required to overcome these problems. Other challenges include the high cost of exosomes, which cannot be prepared on a large scale, and the need for further testing of the safety of algae and bacteria on the skin, despite better results in cellular and animal experiments. These reasons have limited further research, resulting in them remaining in preclinical studies. The pathogenesis of diabetic trauma remains unclear. In the future, the characteristics of the pathogenesis of diabetic trauma should be explored further to design treatment strategies in a more comprehensive and clear manner. In recent years, the use of biomembrane-based nanostructure- and microstructure-loaded hydrogels has received significant attention as a drug delivery strategy to improve chronic wound healing. The healing of chronic wounds relies on the complex coordination of multiple cells and cytokines, and future studies should focus on the following aspects of biomembrane-based nanostructure- and microstructure-loaded hydrogels for drug delivery: 1) designing controlled-release liposome-loaded hydrogels for the release of drugs in response to changes in the trauma microenvironment; 2) developing novel drug delivery systems that integrate antibacterial effects and modulation of the trauma microenvironment; 3) in-depth exploration of the interaction between hydrogels and biomembrane-based nanostructures and microstructures and discovery or synthesis of hydrogel materials that can maintain higher activity or retention time; and 4) combining hydrogels with other advanced manufacturing technologies, such as 3D printing,235 for modulation of the chronic trauma microenvironment and/or skin regeneration.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (grant number: 82003331), the National Natural Science Foundation of China (grant number: 81903233), the National Natural Science Foundation of China (grant number: 81874250) and the National Natural Science Foundation of China (grant number: 82072051).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bai Q, Han K, Dong K., et al. Potential applications of nanomaterials and technology for diabetic wound healing. Int J Nanomedicine. 2020;15:9717–9743. doi:10.2147/IJN.S276001
2. Xu Z, Han S, Gu Z, et al. Advances and impact of antioxidant hydrogel in chronic wound healing. Adv Healthc Mater. 2020;9:e1901502.
3. Giusti I, D’Ascenzo S, Macchiarelli G, et al. In vitro evidence supporting applications of platelet derivatives in regenerative medicine. Blood Transfus. 2020;18:117–129.
4. Kim SY, Nair MG. Macrophages in wound healing: activation and plasticity. Immunol Cell Biol. 2019;97:258–267.
5. Chen H, Li G, Liu Y, et al. Pleiotropic Roles of CXCR4 in Wound Repair and Regeneration. Front Immunol. 2021;12:668758.
6. Cañedo-Dorantes L, Cañedo-Ayala M. Skin acute wound healing: a comprehensive review. Int J Inflam. 2019;2019:3706315.
7. Masri S, Fauzi MB. Current insight of printability quality improvement strategies in natural-based bioinks for skin regeneration and wound healing. Polymers. 2021;13:1011.
8. Alesa Gyles D, Pereira Júnior AD, Diniz Castro L, et al. Polyacrylamide-metilcellulose hydrogels containing aloe barbadensis extract as dressing for treatment of chronic cutaneous skin lesions. Polymers. 2020;12:690.
9. van de Vyver M, Idensohn PJ, Niesler CU. A regenerative approach to the pharmacological management of hard-to-heal wounds. Biochimie. 2022;196:131–142.
10. Ahmed R, Augustine R, Chaudhry M, et al. Nitric oxide-releasing biomaterials for promoting wound healing in impaired diabetic wounds: state of the art and recent trends. Biomed Pharmacother. 2022;149:112707. doi:10.1016/j.biopha.2022.112707
11. Lan CC, Wu CS, Huang SM, et al. High-glucose environment enhanced oxidative stress and increased interleukin-8 secretion from keratinocytes: new insights into impaired diabetic wound healing. Diabetes. 2013;62:2530–2538.
12. Ezhilarasu H, Vishalli D, Dheen ST, et al. Nanoparticle-based therapeutic approach for diabetic wound healing. Nanomaterials. 2020;10:1234.
13. Amaral LSB, Souza CS, Volpini RA, et al. Previous exercise training reduces markers of renal oxidative stress and inflammation in streptozotocin-induced diabetic female rats. J Diabetes Res. 2018;2018:6170352.
14. Ye F, Wu Y, Chen Y, et al. Impact of moderate- and high-intensity exercise on the endothelial ultrastructure and function in mesenteric arteries from hypertensive rats. Life Sci. 2019;222:36–45.
15. Fan Y, Wu W, Lei Y, et al. Edaravone-loaded alginate-based nanocomposite hydrogel accelerated chronic wound healing in diabetic mice. Mar Drugs. 2019;17:548.
16. Matoori S, Veves A, Mooney DJ. Advanced bandages for diabetic wound healing. Sci Transl Med. 2021;13:998.
17. Li S, Mohamedi AH, Senkowsky J, et al. Imaging in Chronic Wound Diagnostics. Adv Wound Care. 2020;9:245–263.
18. Brazil JC, Quiros M, Nusrat A, et al. Innate immune cell-epithelial crosstalk during wound repair. J Clin Invest. 2019;129:2983–2993.
19. Ning J, Zhao H, Chen B, et al. Argon mitigates impaired wound healing process and enhances wound healing in vitro and in vivo. Theranostics. 2019;9:477–490.
20. Li D, Wu N. Mechanism and application of exosomes in the wound healing process in diabetes mellitus. Diabetes Res Clin Pract. 2022;187:109882.
21. Maslova E, Shi Y, Sjöberg F, et al. An invertebrate burn wound model that recapitulates the hallmarks of burn trauma and infection seen in mammalian models. Front Microbiol. 2020;11:998.
22. Chang MH, Hsiao YP, Hsu CY, et al. Photo-crosslinked polymeric matrix with antimicrobial functions for excisional wound healing in mice. Nanomaterials. 2018;8:791.
23. Golmohammadi R, Najar-Peerayeh S, Tohidi Moghadam T, et al. Synergistic antibacterial activity and wound healing properties of selenium-chitosan-mupirocin nanohybrid system: an in vivo study on rat diabetic staphylococcus aureus wound infection model. Sci Rep. 2020;10:2854.
24. Chen S, Yang J, Wei Y, et al. Epigenetic regulation of macrophages: from homeostasis maintenance to host defense. Cell Mol Immunol. 2020;17:36–49.
25. Chen A, An Y, Huang W, et al. Highly water-preserving zwitterionic betaine-incorporated collagen sponges with anti-oxidation and anti-inflammation for wound regeneration. Front Cell Dev Biol. 2020;8:491.
26. Cheng Y, Li Y, Huang S, et al. Hybrid freeze-dried dressings composed of epidermal growth factor and recombinant human-like collagen enhance cutaneous wound healing in rats. Front Bioeng Biotechnol. 2020;8:742.
27. Cieplak T, Soffer N, Sulakvelidze A, et al. A bacteriophage cocktail targeting Escherichia coli reduces E. coli in simulated gut conditions, while preserving a non-targeted representative commensal normal microbiota. Gut Microbes. 2018;9:391–399.
28. Farha MA, French S, Brown ED. Systems-level chemical biology to accelerate antibiotic drug discovery. Acc Chem Res. 2021;54:1909–1920.
29. Ma W, Zhang X, Liu Y, et al. Polydopamine Decorated Microneedles with Fe-MSC-Derived Nanovesicles Encapsulation for Wound Healing. Adv Sci. 2022;9:e2103317.
30. de Souza ML, Dos Santos WM, de Sousa A, et al. Lipid nanoparticles as a skin wound healing drug delivery system: discoveries and advances. Curr Pharm Des. 2020;26:4536–4550.
31. Crane MJ, Henry WL, Tran HL, et al. Assessment of Acute wound healing using the dorsal subcutaneous polyvinyl alcohol sponge implantation and excisional tail skin wound models. J Vis Exp. 2020:25:e60653.
32. Aravinthan A, Park JK, Hossain MA, et al. Collagen-based sponge hastens wound healing via decrease of inflammatory cytokines. Biotech. 2018;8:487.
33. Mansouri MB, Barzi SM, Zafari M, et al. Electrosprayed cefazolin-loaded niosomes onto electrospun chitosan nanofibrous membrane for wound healing applications. J Biomed Mater Res B Appl Biomater. 2022;110:1814–1826.
34. Jamaludin R, Mohd Daud N, Raja Sulong RS, et al. Andrographis paniculata-loaded niosome for wound healing application: characterisation and in vivo analyses. J Drug Deliv Sci Technol. 2021;63:102427.
35. Dadashzadeh A, Imani R, Moghassemi S, et al. Study of hybrid alginate/gelatin hydrogel-incorporated niosomal Aloe vera capable of sustained release of Aloe vera as potential skin wound dressing. Polymer Bulletin. 2020;77:387–403.
36. Maleki A, He J, Bochani S, et al. Multifunctional photoactive hydrogels for wound healing acceleration. ACS Nano. 2021;15:18895–18930.
37. Albarqi HA, Alqahtani AA, Ullah I, et al. Microwave-assisted physically cross-linked chitosan-sodium alginate hydrogel membrane doped with curcumin as a novel wound healing platform. AAPS PharmSciTech. 2022;23:72.
38. Kim H, Joo Y, Kook YM, et al. On-demand local immunomodulation via epigenetic control of macrophages using an inflammation-responsive hydrogel for accelerated wound healing. ACS Appl Mater Interfaces. 2022;14:4931–4945.
39. Dhaliwal K, Lopez N. Hydrogel dressings and their application in burn wound care. Br J Community Nurs. 2018;23:S24–s27.
40. Zhang A, Liu Y, Qin D, et al. Research status of self-healing hydrogel for wound management: a review. Int J Biol Macromol. 2020;164:2108–2123.
41. Madl CM, LeSavage BL, Dewi RE, et al. Matrix remodeling enhances the differentiation capacity of neural progenitor cells in 3d hydrogels. Adv Sci. 2019;6:1801716.
42. Han Z, Wang P, Mao G, et al. Dual pH-responsive hydrogel actuator for lipophilic drug delivery. ACS Appl Mater Interfaces. 2020;12:12010–12017.
43. Vasile C, Pamfil D, Stoleru E, et al. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules. 2020;25:548.
44. Fan R, Tong A, Li X, et al. Enhanced antitumor effects by docetaxel/LL37-loaded thermosensitive hydrogel nanoparticles in peritoneal carcinomatosis of colorectal cancer. Int J Nanomedicine. 2015;10:7291–7305.
45. Gauthier A, Fisch A, Seuwen K, et al. Glucocorticoid-loaded liposomes induce a pro-resolution phenotype in human primary macrophages to support chronic wound healing. Biomaterials. 2018;178:481–495.
46. Tellechea A, Leal EC, Kafanas A, et al. Mast cells regulate wound healing in diabetes. Diabetes. 2016;65:2006–2019.
47. Wang Y, Xue Y, Zhang T, et al. Photosynthetic biomaterials: applications of photosynthesis in algae as oxygenerator in biomedical therapies. Bio-Design and Manufacturing. 2021;4:596–611.
48. Ternullo S, Schulte Werning LV, Holsæter AM, et al. Curcumin-in-deformable liposomes-in-chitosan-hydrogel as a novel wound dressing. Pharmaceutics. 2019;12:963.
49. Miguel SP, Ribeiro MP, Otero A, et al. Application of microalgae and microalgal bioactive compounds in skin regeneration. Algal Res. 2021;58:102395.
50. Hu H, Zhong D, Li W, et al. Microalgae-based bioactive hydrogel loaded with quorum sensing inhibitor promotes infected wound healing. Nano Today. 2022;42:101368.
51. Yang J, Chen Z, Pan D, et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int J Nanomedicine. 2020;15:5911–5926.
52. Sivaraj D, Chen K, Chattopadhyay A, et al. Hydrogel Scaffolds to Deliver Cell Therapies for Wound Healing. Front Bioeng Biotechnol. 2021;9:660145.
53. Chen H, Guo Y, Zhang Z, et al. Symbiotic algae-bacteria dressing for producing hydrogen to accelerate diabetic wound healing. Nano Lett. 2022;22:229–237.
54. Li A, Yang F, Xin J, et al. An efficient and long-acting local anesthetic: ropivacaine-loaded lipid-polymer hybrid nanoparticles for the control of pain. Int J Nanomedicine. 2019;14:913–920.
55. Guo X, Zhu X, Liu D, et al. Continuous delivery of propranolol from liposomes-in-microspheres significantly inhibits infantile hemangioma growth. Int J Nanomedicine. 2017;12:6923–6936.
56. Guan T, Li J, Chen C, et al. Self-assembling peptide-based hydrogels for wound tissue repair. Adv Sci. 2022;9:e2104165.
57. Chen YH, Rao ZF, Liu YJ, et al. Multifunctional Injectable hydrogel loaded with cerium-containing bioactive glass nanoparticles for diabetic wound healing. Biomolecules. 2021;11:354.
58. Negro A, Cherbuin T, Lutolf MP. 3D inkjet printing of complex, cell-laden hydrogel structures. Sci Rep. 2018;8:17099.
59. Deng P, Yao L, Chen J, et al. Chitosan-based hydrogels with injectable, self-healing and antibacterial properties for wound healing. Carbohydr Polym. 2022;276:118718.
60. Tiwari S, Bahadur P. Modified hyaluronic acid based materials for biomedical applications. Int J Biol Macromol. 2019;121:556–571.
61. Zhang Q, Gerlach JC, Nettleship I, et al. Calcium-infiltrated biphasic hydroxyapatite scaffolds for human hematopoietic stem cell culture. Tissue Eng Part A. 2018;24:1563–1573.
62. Chen H, Cheng Y, Tian J, et al. Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes. Sci Adv. 2020;6:eaba4311.
63. Lyu D, Chen S, Guo W. Liposome Crosslinked Polyacrylamide/DNA Hydrogel: a Smart Controlled-Release System for Small Molecular Payloads. Small. 2018;14:e1704039.
64. Ishikawa S, Iijima K, Matsukuma D, et al. Interpenetrating polymer network hydrogels via a one-pot and in situ gelation system based on peptide self-assembly and orthogonal cross-linking for tissue regeneration. Chem Mater. 2020;32:2353–2364.
65. Liang X, Kozlovskaya V, Chen Y, et al. Thermosensitive multilayer hydrogels of poly(N-vinylcaprolactam) as nanothin films and shaped capsules. Chem Mater. 2012;24:3707–3719.
66. Wang LL, Chung JJ, Li EC, et al. Injectable and protease-degradable hydrogel for siRNA sequestration and triggered delivery to the heart. J Control Release. 2018;285:152–161.
67. Asadpour S, Kargozar S, Moradi L, et al. Natural biomacromolecule based composite scaffolds from silk fibroin, gelatin and chitosan toward tissue engineering applications. Int J Biol Macromol. 2020;154:1285–1294.
68. Su J, Li J, Liang J, et al. Hydrogel preparation methods and biomaterials for wound dressing. Life. 2021;11:53.
69. Tan C, Wang J, Sun B. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: recent advances. Biotechnol Adv. 2021;48:107727.
70. Ma W, Ma H, Qiu P, et al. Sprayable β-FeSi(2) composite hydrogel for portable skin tumor treatment and wound healing. Biomaterials. 2021;279:121225.
71. Ota A, Istenič K, Skrt M, et al. Encapsulation of pantothenic acid into liposomes and into alginate or alginate–pectin microparticles loaded with liposomes. J Food Eng. 2018;229:21–31.
72. Larrañeta E, Stewart S, Ervine M, et al. Hydrogels for hydrophobic drug delivery. classification, synthesis and applications. J Funct Biomater. 2018;9:215.
73. Abdellatif AA, El-Telbany DFA, Zayed G, et al. Hydrogel containing PEG-coated fluconazole nanoparticles with enhanced solubility and antifungal activity. J Pharm Innov. 2019;14:112–122.
74. Zhou J, Yao D, Qian Z, et al. Bacteria-responsive intelligent wound dressing: simultaneous In situ detection and inhibition of bacterial infection for accelerated wound healing. Biomaterials. 2018;161:11–23.
75. Dosmar E, Vuotto G, Su X, et al. Compartmental and COMSOL Multiphysics 3D Modeling of Drug Diffusion to the Vitreous Following the Administration of a Sustained-Release Drug Delivery System. Pharmaceutics. 2021;13:1862.
76. Wen H, Wang B, Zhu H, et al. Security-enhanced 3D data encryption using a degradable ph-responsive hydrogel. Nanomaterials. 2021;11:1744.
77. Rehman SRU, Augustine R, Zahid AA, et al. Reduced graphene oxide incorporated gelma hydrogel promotes angiogenesis for wound healing applications. Int J Nanomedicine. 2019;14:9603–9617.
78. Shepard JA, Virani FR, Goodman AG, et al. Hydrogel macroporosity and the prolongation of transgene expression and the enhancement of angiogenesis. Biomaterials. 2012;33:7412–7421.
79. Anumolu SS, DeSantis AS, Menjoge AR, et al. Doxycycline loaded poly(ethylene glycol) hydrogels for healing vesicant-induced ocular wounds. Biomaterials. 2010;31:964–974.
80. Madl CM, LeSavage BL, Dewi RE, et al. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat Mater. 2017;16:1233–1242.
81. Antimisiaris SG, Marazioti A, Kannavou M, et al. Overcoming barriers by local drug delivery with liposomes. Adv Drug Deliv Rev. 2021;174:53–86.
82. Zhang Z, Yang J, Yang Q, et al. Fabrication of non-phospholipid liposomal nanocarrier for sustained-release of the fungicide cymoxanil. Front Mol Biosci. 2021;8:627817.
83. Mao W, Wu F, Lee RJ, et al. Development of a stable single-vial liposomal formulation for vincristine. Int J Nanomedicine. 2019;14:4461–4474.
84. Ruel-Gariépy E, Leclair G, Hildgen P, et al. Thermosensitive chitosan-based hydrogel containing liposomes for the delivery of hydrophilic molecules. J Control Release. 2002;82:373–383.
85. Wang S, Wang Z, Xu C, et al. PEG-α-CD/AM/liposome @amoxicillin double network hydrogel wound dressing-Multiple barriers for long-term drug release. J Biomater Appl. 2021;35:1085–1095.
86. Xu HL, Chen PP, Wang LF, et al. Skin-permeable liposome improved stability and permeability of bFGF against skin of mice with deep second degree scald to promote hair follicle neogenesis through inhibition of scar formation. Colloids Surf B Biointerfaces. 2018;172:573–585.
87. Billard A, Pourchet L, Malaise S, et al. Liposome-loaded chitosan physical hydrogel: toward a promising delayed-release biosystem. Carbohydr Polym. 2015;115:651–657.
88. Li MN, Yu HP, Ke QF, et al. Gelatin methacryloyl hydrogels functionalized with endothelin-1 for angiogenesis and full-thickness wound healing. J Mater Chem B. 2021;9:4700–4709.
89. He Y, Li Y, Sun Y, et al. A double-network polysaccharide-based composite hydrogel for skin wound healing. Carbohydr Polym. 2021;261:117870.
90. Lin S, Pei L, Zhang W, et al. Chitosan-poloxamer-based thermosensitive hydrogels containing zinc gluconate/recombinant human epidermal growth factor benefit for antibacterial and wound healing. Mater Sci Eng C Mater Biol Appl. 2021;130:112450.
91. Nishiguchi A, Taguchi T. Designing an anti-inflammatory and tissue-adhesive colloidal dressing for wound treatment. Colloids Surf B Biointerfaces. 2020;188:110737.
92. Xu HL, Chen PP, ZhuGe DL, et al. Liposomes with silk fibroin hydrogel core to stabilize bfgf and promote the wound healing of mice with deep second-degree scald. Adv Healthc Mater. 2017;6:1744.
93. Banerjee R. Overcoming the stratum corneum barrier: a nano approach. Drug Deliv Transl Res. 2013;3:205–208.
94. Martin KE, García AJ. Macrophage phenotypes in tissue repair and the foreign body response: implications for biomaterial-based regenerative medicine strategies. Acta Biomater. 2021;133:4–16.
95. Yu JR, Varrey P, Liang BJ, et al. Liposomal SDF-1 alpha delivery in nanocomposite hydrogels promotes macrophage phenotype changes and skin tissue regeneration. ACS Biomater Sci Eng. 2021;7:5230–5241.
96. Hesketh M, Sahin KB, West ZE, et al. Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci. 2017;18:548
97. Mortezaee K, Majidpoor J. Roles for macrophage-polarizing interleukins in cancer immunity and immunotherapy. Cell Oncol. 2022;45:333–353.
98. Chang M, Nguyen TT. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc Chem Res. 2021;54:1080–1093.
99. Shi Z, Lan G, Hu E, et al. Puff pastry-like chitosan/konjac glucomannan matrix with thrombin-occupied microporous starch particles as a composite for hemostasis. Carbohydr Polym. 2020;232:115814.
100. Chen H-Y, Lin C-H, Chen B-C. ADAM17/EGFR-dependent ERK activation mediates thrombin-induced CTGF expression in human lung fibroblasts. Exp Cell Res. 2018;370(1):39–45. doi:10.1016/j.yexcr.2018.06.008
101. Wang C, Wu T, Liu G, et al. Promoting coagulation and activating SMAD3 phosphorylation in wound healing via a dual-release thrombin-hydrogel. Chem Eng J. 2020;397:125414. doi:10.1016/j.cej.2020.125414
102. Yellepeddi VK, Joseph A, Nance E. Pharmacokinetics of nanotechnology-based formulations in pediatric populations. Adv Drug Deliv Rev. 2019;151-152:44–55. doi:10.1016/j.addr.2019.08.008
103. Gonzalez Gomez A, Hosseinidoust Z. Liposomes for Antibiotic Encapsulation and Delivery. ACS Infect Dis. 2020;6(5):896–908. doi:10.1021/acsinfecdis.9b00357
104. Veloso SRS, Andrade RGD, Castanheira EMS. Review on the advancements of magnetic gels: towards multifunctional magnetic liposome-hydrogel composites for biomedical applications. Adv Colloid Interface Sci. 2021;288:102351.
105. Nie X, Gao F, Wang F, et al. Charge-reversal silver clusters for targeted bacterial killing. J Mater Chem B. 2021;9:4006–4014.
106. Liu W, Tao Z, Wang D, et al. Immobilization of Cu (II) via a graphene oxide-supported strategy for antibacterial reutilization with long-term efficacy. J Hazard Mater. 2021;410:124601.
107. Gharpure S, Ankamwar B. Synthesis and Antimicrobial Properties of Zinc Oxide Nanoparticles. J Nanosci Nanotechnol. 2020;20:5977–5996.
108. Leudjo Taka A, Fosso-Kankeu E, Naidoo EB, et al. Recent development in antimicrobial activity of biopolymer-inorganic nanoparticle composites with water disinfection potential: a comprehensive review. Environ Sci Pollut Res Int. 2021;28:26252–26268.
109. Yin M, Lin X, Ren T, et al. Cytocompatible quaternized carboxymethyl chitosan/poly(vinyl alcohol) blend film loaded copper for antibacterial application. Int J Biol Macromol. 2018;120:992–998.
110. Kong N, Lin K, Li H, et al. Synergy effects of copper and silicon ions on stimulation of vascularization by copper-doped calcium silicate. J Mater Chem B. 2014;2:1100–1110.
111. Lu Y, Li L, Zhu Y, et al. Multifunctional copper-containing carboxymethyl chitosan/alginate scaffolds for eradicating clinical bacterial infection and promoting bone formation. ACS Appl Mater Interfaces. 2018;10:127–138.
112. Wang M, Huang H, Ma X, et al. Copper metal-organic framework embedded carboxymethyl chitosan-g-glutathione/polyacrylamide hydrogels for killing bacteria and promoting wound healing. Int J Biol Macromol. 2021;187:699–709.
113. Chen S, Tang F, Tang L, et al. Synthesis of Cu-Nanoparticle Hydrogel with Self-Healing and Photothermal Properties. ACS Appl Mater Interfaces. 2017;9:20895–20903.
114. Mao C, Xiang Y, Liu X, et al. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano. 2017;11:9010–9021.
115. Zhen X, Cheng P, Pu K. Recent Advances in Cell Membrane-Camouflaged Nanoparticles for Cancer Phototherapy. Small. 2019;15:e1804105.
116. Chen X, Liu B, Tong R, et al. Orchestration of biomimetic membrane coating and nanotherapeutics in personalized anticancer therapy. Biomater Sci. 2021;9:590–625.
117. Li SY, Cheng H, Xie BR, et al. Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy. ACS Nano. 2017;11:7006–7018.
118. Zhu JY, Zheng DW, Zhang MK, et al. Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett. 2016;16:5895–5901.
119. Xuan M, Shao J, Dai L, et al. Macrophage cell membrane camouflaged au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl Mater Interfaces. 2016;8:9610–9618.
120. Parodi A, Quattrocchi N, van de Ven AL, et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8:61–68.
121. Liu B, Wang W, Fan J, et al. RBC membrane camouflaged prussian blue nanoparticles for gamabutolin loading and combined chemo/photothermal therapy of breast cancer. Biomaterials. 2019;217:119301.
122. Bao Y, Chen J, Qiu H, et al. Erythrocyte Membrane-Camouflaged PCN-224 Nanocarriers Integrated with Platinum Nanoparticles and Glucose Oxidase for Enhanced Tumor Sonodynamic Therapy and Synergistic Starvation Therapy. ACS Appl Mater Interfaces. 2021;13:24532–24542.
123. Xue X, Liu H, Wang S, et al. Neutrophil-erythrocyte hybrid membrane-coated hollow copper sulfide nanoparticles for targeted and photothermal/anti-inflammatory therapy of osteoarthritis. Composites Part B. 2022;237:109855.
124. Mahmoud NN, Hikmat S, Abu Ghith D, et al. Gold nanoparticles loaded into polymeric hydrogel for wound healing in rats: effect of nanoparticles’ shape and surface modification. Int J Pharm. 2019;565:174–186.
125. Masood N, Ahmed R, Tariq M, et al. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int J Pharm. 2019;559:23–36.
126. Fan Z, Deng J, Li PY, et al. A new class of biological materials: cell membrane-derived hydrogel scaffolds. Biomaterials. 2019;197:244–254.
127. Li M, Wang T, Tian H, et al. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif Cells Nanomed Biotechnol. 2019;47:3793–3803.
128. Mi B, Chen L, Xiong Y, et al. Saliva exosomes-derived UBE2O mRNA promotes angiogenesis in cutaneous wounds by targeting SMAD6. J Nanobiotechnology. 2020;18:68.
129. Zhao B, Zhang X, Zhang Y, et al. Human exosomes accelerate cutaneous wound healing by promoting collagen synthesis in a diabetic mouse model. Stem Cells Dev. 2021;30:922–933.
130. Li L, Zhang Y, Mu J, et al. Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. 2020;20:4298–4305.
131. Liu X, Yang Y, Li Y, et al. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9:4430–4438.
132. Zhang K, Zhao X, Chen X, et al. Enhanced therapeutic effects of mesenchymal stem cell-derived exosomes with an injectable hydrogel for hindlimb ischemia treatment. ACS Appl Mater Interfaces. 2018;10:30081–30091.
133. Wang C, Wang M, Xu T, et al. Engineering Bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9:65–76.
134. Wang Y, Cao Z, Wei Q, et al. VH298-loaded extracellular vesicles released from gelatin methacryloyl hydrogel facilitate diabetic wound healing by HIF-1α-mediated enhancement of angiogenesis. Acta Biomater. 2022;147:342–355.
135. Han X, Wu P, Li L, et al. Exosomes derived from autologous dermal fibroblasts promote diabetic cutaneous wound healing through the Akt/β-catenin pathway. Cell Cycle. 2021;20:616–629.
136. Wei P, Zhong C, Yang X, et al. Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via PI3K-AKT-mTOR-mediated promotion in angiogenesis and fibroblast function. Burns Trauma. 2020;8:tkaa020.
137. Chen L, Qin L, Chen C, et al. Serum exosomes accelerate diabetic wound healing by promoting angiogenesis and ECM formation. Cell Biol Int. 2021;45:1976–1985.
138. Baquir B, Hancock RE. Exosomes, your body’s answer to immune health. Ann Transl Med. 2017;5:81.
139. Casado JG, Blázquez R, Vela FJ, et al. Mesenchymal stem cell-derived exosomes: immunomodulatory evaluation in an antigen-induced synovitis porcine model. Front Vet Sci. 2017;4:39.
140. Hoang DH, Nguyen TD, Nguyen HP, et al. Differential wound healing capacity of mesenchymal stem cell-derived exosomes originated from bone marrow, adipose tissue and umbilical cord under serum- and xeno-free condition. Front Mol Biosci. 2020;7:119.
141. Geng X, Qi Y, Liu X, et al. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Mater Sci Eng C Mater Biol Appl. 2021;112613.
142. Xiong Y, Chen L, Liu P, et al. All-in-One: multifunctional Hydrogel Accelerates Oxidative Diabetic Wound Healing through Timed-Release of Exosome and Fibroblast Growth Factor. Small. 2022;18:e2104229.
143. He X, Dong Z, Cao Y, et al. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019;2019:7132708.
144. Magne B, Dedier M, Nivet M, et al. IL-1β-Primed Mesenchymal Stromal Cells Improve Epidermal Substitute Engraftment and Wound Healing via Matrix Metalloproteinases and Transforming Growth Factor-β1. J Invest Dermatol. 2020;140:688–698.e621.
145. Shang Q, Chu Y, Li Y, et al. Adipose-derived mesenchymal stromal cells promote corneal wound healing by accelerating the clearance of neutrophils in cornea. Cell Death Dis. 2020;11:707.
146. Kaushik K, Das A. TWIST1-reprogrammed endothelial cell transplantation potentiates neovascularization-mediated diabetic wound tissue regeneration. Diabetes. 2020;69:1232–1247.
147. Kosaric N, Kiwanuka H, Gurtner GC. Stem cell therapies for wound healing. Expert Opin Biol Ther. 2019;19:575–585.
148. Cho H, Blatchley MR, Duh EJ, et al. Acellular and cellular approaches to improve diabetic wound healing. Adv Drug Deliv Rev. 2019;146:267–288.
149. Rustad KC, Gurtner GC. Mesenchymal Stem Cells Home to Sites of Injury and Inflammation. Adv Wound Care. 2012;1:147–152.
150. Tartarini D, Mele E. Adult stem cell therapies for wound healing: biomaterials and computational models. Front Bioeng Biotechnol. 2015;3:206.
151. Ma K, Kwon SH, Padmanabhan J, et al. Controlled delivery of a focal adhesion kinase inhibitor results in accelerated wound closure with decreased scar formation. J Invest Dermatol. 2018;138:2452–2460.
152. Kamoun EA, Kenawy ES, Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res. 2017;8:217–233.
153. Youngblood RL, Truong NF, Segura T, et al. It’s All in the Delivery: designing Hydrogels for Cell and Non-viral Gene Therapies. Mol Ther. 2018;26:2087–2106.
154. Xu X, Xia X, Zhang K, et al. Bioadhesive hydrogels demonstrating pH-independent and ultrafast gelation promote gastric ulcer healing in pigs. Sci Transl Med. 2020;12:98.
155. Lasocka I, Jastrzębska E, Szulc-Dąbrowska L, et al. The effects of graphene and mesenchymal stem cells in cutaneous wound healing and their putative action mechanism. Int J Nanomedicine. 2019;14:2281–2299.
156. Zhao Y, Wang M, Liang F, et al. Recent strategies for enhancing the therapeutic efficacy of stem cells in wound healing. Stem Cell Res Ther. 2021;12:588.
157. Baez-Jurado E, Hidalgo-Lanussa O, Guio-Vega G, et al. Conditioned medium of human adipose mesenchymal stem cells increases wound closure and protects human astrocytes following scratch assay in vitro. Mol Neurobiol. 2018;55:5377–5392.
158. Guillamat-Prats R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells. 2021;10:9653.
159. Jiang D, Scharffetter-Kochanek K. Mesenchymal stem cells adaptively respond to environmental cues thereby improving granulation tissue formation and wound healing. Front Cell Dev Biol. 2020;8:697.
160. Rustad KC, Wong VW, Sorkin M, et al. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials. 2012;33:80–90.
161. Zhang Z, Li Z, Li Y, et al. Sodium alginate/collagen hydrogel loaded with human umbilical cord mesenchymal stem cells promotes wound healing and skin remodeling. Cell Tissue Res. 2021;383:809–821.
162. Chen S, Shi J, Zhang M, et al. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci Rep. 2015;5:18104.
163. Liu Q, Zheng S, Ye K, et al. Cell migration regulated by RGD nanospacing and enhanced under moderate cell adhesion on biomaterials. Biomaterials. 2020;263:120327.
164. Wang AT, Zhang QF, Wang NX, et al. Cocktail of hyaluronic acid and human amniotic mesenchymal cells effectively repairs cartilage injuries in sodium iodoacetate-induced osteoarthritis rats. Front Bioeng Biotechnol. 2020;8:87.
165. Wang F, Wang Y, Tian C, et al. Fabrication of the FGF1-functionalized sericin hydrogels with cell proliferation activity for biomedical application using genetically engineered Bombyx mori (B. mori) silk. Acta Biomater. 2018;79:239–252.
166. Qi C, Liu J, Jin Y, et al. Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials. 2018;163:89–104.
167. da Silva LP, Reis RL, Correlo VM, et al. Hydrogel-based strategies to advance therapies for chronic skin wounds. Annu Rev Biomed Eng. 2019;21:145–169.
168. Hanjaya-Putra D, Bose V, Shen YI, et al. Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood. 2011;118:804–815.
169. O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057.
170. Lufton M, Bustan O, Eylon B, et al. Living bacteria in thermoresponsive gel for treating fungal infections. Adv Funct Mater. 2018;28:1801581.
171. Ming Z, Han L, Bao M, et al. Living bacterial hydrogels for accelerated infected wound healing. Adv Sci. 2021;8:e2102545.
172. Chen Y, Gao Y, Chen Y, et al. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J Control Release. 2020;328:251–262.
173. Wang H, Guo Y, Gan S, et al. Photosynthetic microorganisms‐based biophotothermal therapy with enhanced immune response. Small. 2021;17:2007734.
174. Zhao E, Liu H, Jia Y, et al. Engineering a photosynthetic bacteria-incorporated hydrogel for infected wound healing. Acta Biomater. 2022;140:302–313.
175. Sarkar S, Levi-Polyachenko N. Conjugated polymer nano-systems for hyperthermia, imaging and drug delivery. Adv Drug Deliv Rev. 2020;163-164:40–64.
176. Mao N, Cubillos-Ruiz A, Cameron DE, et al. Probiotic strains detect and suppress cholera in mice. Sci Transl Med. 2018;10:48.
177. Lu Y, Li H, Wang J, et al. Engineering bacteria-activated multifunctionalized hydrogel for promoting diabetic wound healing. Adv Funct Mater. 2021;31:2105749.
178. Chen QW, Wang JW, Wang XN, et al. Inhibition of tumor progression through the coupling of bacterial respiration with tumor metabolism. Angew Chem Int Ed Engl. 2020;59:21562–21570.
179. Kim J, Kim B, Kim SM, et al. Hypoxia-Induced Epithelial-To-Mesenchymal Transition Mediates Fibroblast Abnormalities via ERK Activation in Cutaneous Wound Healing. Int J Mol Sci. 2019;20:2546.
180. Comino-Sanz IM, López-Franco MD, Castro B, et al. Antioxidant dressing therapy versus standard wound care in chronic wounds (the REOX study): study protocol for a randomized controlled trial. Trials. 2020;21:505.
181. Davis FM, Kimball A, Boniakowski A, et al. Dysfunctional wound healing in diabetic foot ulcers: new crossroads. Curr Diab Rep. 2018;18:2.
182. Cerychova R, Pavlinkova G. HIF-1, metabolism, and diabetes in the embryonic and adult heart. Front Endocrinol (Lausanne). 2018;9:460.
183. Zhu Y, Wang Y, Jia Y, et al. Roxadustat promotes angiogenesis through HIF-1α/VEGF/VEGFR2 signaling and accelerates cutaneous wound healing in diabetic rats. Wound Repair Regen. 2019;27:324–334.
184. Huang X, Liang P, Jiang B, et al. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 2020;259:118246.
185. Heyboer M 3rd, Sharma D, Santiago W, et al. Hyperbaric Oxygen Therapy: side Effects Defined and Quantified. Adv Wound Care. 2017;6:210–224.
186. Johnson TJ, Katuwal S, Anderson GA, et al. Photobioreactor cultivation strategies for microalgae and cyanobacteria. Biotechnol Prog. 2018;34:811–827.
187. Schipper K, Al-Jabri H, Wijffels RH, et al. Techno-economics of algae production in the Arabian Peninsula. Bioresour Technol. 2021;331:125043.
188. Fu Y, Xie X, Wang Y, et al. Sustained photosynthesis and oxygen generation of microalgae-embedded silk fibroin hydrogels. ACS Biomater Sci Eng. 2021;7:2734–2744.
189. Li J, Zheng Z, Du M, et al. Euglena gracilis and its aqueous extract constructed with chitosan-hyaluronic acid hydrogel facilitate cutaneous wound healing in mice without inducing excessive inflammatory response. Front Bioeng Biotechnol. 2021;9:713840.
190. Xu H, Zheng Y-W, Liu Q, et al. Reactive oxygen species in skin repair, regeneration, aging, and inflammation. Reactive Oxygen Species. 2018;8;69–87.
191. Hussein RA, Salama AA, El Naggar ME, et al. Medicinal impact of microalgae collected from high rate algal ponds; phytochemical and pharmacological studies of microalgae and its application in medicated bandages. Biocatalysis Agr Biotechnol. 2019;20:101237.
192. Sun J, Zhao J, Fu D, et al. Extraction, optimization and antimicrobial activity of IWSP from oleaginous microalgae Chlamydomonas sp. YB-204. Food Sci Technol Res. 2017;23:819–826.
193. Li W, Wang S, Zhong D, et al. A Bioactive Living Hydrogel: photosynthetic Bacteria Mediated Hypoxia Elimination and Bacteria-Killing to Promote Infected Wound Healing. Adv Therapeutics. 2021;4:2000107.
194. Han HM, Ko S, Cheong MJ, et al. Myxinidin2 and myxinidin3 suppress inflammatory responses through STAT3 and MAPKs to promote wound healing. Oncotarget. 2017;8:87582–87597.
195. Kalantari K, Mostafavi E, Afifi AM, et al. Wound dressings functionalized with silver nanoparticles: promises and pitfalls. Nanoscale. 2020;12:2268–2291.
196. Pompilio A, Galardi G, Verginelli F, et al. Myroides odoratimimus forms structurally complex and inherently antibiotic-resistant biofilm in a wound-like in vitro model. Front Microbiol. 2017;8:2591.
197. Zhang F, Han X, Guo C, et al. Fibrous aramid hydrogel supported antibacterial agents for accelerating bacterial-infected wound healing. Mater Sci Eng C Mater Biol Appl. 2021;121:111833.
198. Dawoud MHS, Yassin GE, Ghorab DM, et al. Insulin mucoadhesive liposomal gel for wound healing: a formulation with sustained release and extended stability using quality by design approach. AAPS PharmSciTech. 2019;20:158.
199. Wang C, Wang Y, Zhang L, et al. Pretreated macrophage-membrane-coated gold nanocages for precise drug delivery for treatment of bacterial infections. Adv Mater. 2018;30:e1804023.
200. Lin A, Liu Y, Zhu X, et al. Bacteria-Responsive Biomimetic Selenium Nanosystem for Multidrug-Resistant Bacterial Infection Detection and Inhibition. ACS Nano. 2019;13:13965–13984.
201. Hu CM, Fang RH, Wang KC, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118–121.
202. Simões D, Miguel SP, Ribeiro MP, et al. Recent advances on antimicrobial wound dressing: a review. Eur J Pharm Biopharm. 2018;127:130–141.
203. Hamedi H, Moradi S, Hudson SM, et al. Chitosan based hydrogels and their applications for drug delivery in wound dressings: a review. Carbohydr Polym. 2018;199:445–460.
204. Liang S, Dang Q, Liu C, et al. Characterization and antibacterial mechanism of poly(aminoethyl) modified chitin synthesized via a facile one-step pathway. Carbohydr Polym. 2018;195:275–287.
205. Du X, Liu Y, Wang X, et al. Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Mater Sci Eng C Mater Biol Appl. 2019;104:109930.
206. Jin X, Fu Q, Gu Z, et al. Chitosan/PDLLA-PEG-PDLLA solution preparation by simple stirring and formation into a hydrogel at body temperature for whole wound healing. Int J Biol Macromol. 2021;184:787–796.
207. Peiseler M, Kubes P. Macrophages play an essential role in trauma-induced sterile inflammation and tissue repair. Eur J Trauma Emerg Surg. 2018;44:335–349.
208. Ogle ME, Segar CE, Sridhar S, et al. Monocytes and macrophages in tissue repair: implications for immunoregenerative biomaterial design. Exp Biol Med (Maywood). 2016;241:1084–1097.
209. Dalirfardouei R, Jamialahmadi K, Jafarian AH, et al. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13:555–568.
210. Li X, Xie X, Lian W, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50:1–14.
211. Kang M, Jin S, Lee D, et al. MRI Visualization of Whole Brain Macro- and Microvascular Remodeling in a Rat Model of Ischemic Stroke: a Pilot Study. Sci Rep. 2020;10:4989.
212. Chu GY, Chen YF, Chen HY, et al. Stem cell therapy on skin: mechanisms, recent advances and drug reviewing issues. J Food Drug Anal. 2018;26:14–20.
213. Silva LP, Pirraco RP, Santos TC, et al. Neovascularization induced by the hyaluronic acid-based spongy-like hydrogels degradation products. ACS Appl Mater Interfaces. 2016;8:33464–33474.
214. Tyeb S, Shiekh PA, Verma V, et al. Adipose-Derived Stem Cells (ADSCs) Loaded Gelatin-Sericin-Laminin Cryogels for Tissue Regeneration in Diabetic Wounds. Biomacromolecules. 2020;21:294–304.
215. Viezzer C, Mazzuca R, Machado DC, et al. A new waterborne chitosan-based polyurethane hydrogel as a vehicle to transplant bone marrow mesenchymal cells improved wound healing of ulcers in a diabetic rat model. Carbohydr Polym. 2020;231:115734.
216. Moon KC, Suh HS, Kim KB, et al. Potential of allogeneic adipose-derived stem cell-hydrogel complex for treating diabetic foot ulcers. Diabetes. 2019;68:837–846.
217. Xue J, Sun N, Liu Y. Self-assembled nano-peptide hydrogels with human umbilical cord mesenchymal stem cell spheroids accelerate diabetic skin wound healing by inhibiting inflammation and promoting angiogenesis. Int J Nanomedicine. 2022;17:2459–2474.
218. Ferguson SW, Nguyen J. Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. J Control Release. 2016;228:179–190.
219. Vu NB, Nguyen HT, Palumbo R, et al. Stem cell-derived exosomes for wound healing: current status and promising directions. Minerva Med. 2021;112:384–400.
220. Xu N, Wang L, Guan J, et al. Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int J Biol Macromol. 2018;117:102–107.
221. Wang C, Liang C, Wang R, et al. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater Sci. 2019;8:313–324.
222. Cao J, Ehling M, März S, et al. Polarized actin and VE-cadherin dynamics regulate junctional remodelling and cell migration during sprouting angiogenesis. Nat Commun. 2017;8:2210.
223. Di Francia R, Berretta M, Benincasa G, et al. Pharmacogenetic-based interactions between nutraceuticals and angiogenesis inhibitors. Cells. 2019;8:876.
224. Nosrati H, Aramideh Khouy R, Nosrati A, et al. Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. J Nanobiotechnology. 2021;19:1.
225. Dong C, Feng W, Xu W, et al. The Coppery Age: copper (Cu)-Involved Nanotheranostics. Adv Sci. 2020;7:2001549.
226. Zhu D, Su Y, Zheng Y, et al. Zinc regulates vascular endothelial cell activity through zinc-sensing receptor ZnR/GPR39. Am J Physiol Cell Physiol. 2018;314:C404–c414.
227. Zhou W, Zi L, Cen Y, et al. Copper Sulfide nanoparticles-incorporated hyaluronic acid injectable hydrogel with enhanced angiogenesis to promote wound healing. Front Bioeng Biotechnol. 2020;8:417.
228. Chen Z, Duan J, Diao Y, et al. ROS-responsive capsules engineered from EGCG-Zinc networks improve therapeutic angiogenesis in mouse limb ischemia. Bioact Mater. 2021;6:1–11.
229. Tang T, Jiang H, Yu Y, et al. A new method of wound treatment: targeted therapy of skin wounds with reactive oxygen species-responsive nanoparticles containing SDF-1α. Int J Nanomedicine. 2015;10:6571–6585.
230. Qi X, Huang Y, You S, et al. Engineering robust ag-decorated polydopamine nano-photothermal platforms to combat bacterial infection and prompt wound healing. Adv Sci. 2022;9:e2106015.
231. Kharaziha M, Baidya A, Annabi N. Rational design of immunomodulatory hydrogels for chronic wound healing. Adv Mater. 2021;33:e2100176.
232. Li R, Liu Q, Wu H, et al. Preparation and characterization of in-situ formable liposome/chitosan composite hydrogels. Mater Lett. 2018;220:289–292.
233. Ahmed R, Afreen A, Tariq M, et al. Bone marrow mesenchymal stem cells preconditioned with nitric-oxide-releasing chitosan/PVA hydrogel accelerate diabetic wound healing in rabbits. Biomed Mater. 2021;16:6596.
234. Wang K, Dong R, Tang J, et al. Exosomes laden self-healing injectable hydrogel enhances diabetic wound healing via regulating macrophage polarization to accelerate angiogenesis. Chem Eng J. 2022;430:132664.
235. Li J, Wu C, Chu PK, et al. 3D printing of hydrogels: rational design strategies and emerging biomedical applications. Mater Sci Eng. 2020;140:100543.
236. Yu JR, Varrey P, Liang BJ, et al. Liposomal SDF-1 Alpha Delivery in Nanocomposite Hydrogels Promotes Macrophage Phenotype Changes and Skin Tissue Regeneration. ACS Biomater Sci Eng. 2021;7:5230.
237. Hu Y, Wu B, Xiong Y, et al. Cryogenic 3D printed hydrogel scaffolds loading exosomes accelerate diabetic wound healing. Chem Eng J. 2021;426:130634.
238. Geng X, Qi Y, Liu X, et al. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Mater Sci Eng. 2021;112613.
239. Chen S, Shi J, Zhang M, et al. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci Rep. 2015;5:1–12.
240. Kaisang L, Siyu W, Lijun F, et al. Adipose-derived stem cells seeded in Pluronic F-127 hydrogel promotes diabetic wound healing. J Surg Res. 2017;217:63–74.
241. Chen H, Cheng Y, Tian J, et al. Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes. Sci Adv. 2020;6:eaba4311.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.