Back to Journals » International Journal of Nanomedicine » Volume 18
Biomedical Applications of Biosynthesized Nickel Oxide Nanoparticles
Authors Berhe MG, Gebreslassie YT
Received 31 March 2023
Accepted for publication 10 July 2023
Published 27 July 2023 Volume 2023:18 Pages 4229—4251
DOI https://doi.org/10.2147/IJN.S410668
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Mearg Gidey Berhe,1 Yemane Tadesse Gebreslassie2
1Department of Physics, College of Natural and Computational Science, Adigrat University, Adigrat, Ethiopia; 2Department of Chemistry, College of Natural and Computational Science, Adigrat University, Adigrat, Ethiopia
Correspondence: Yemane Tadesse Gebreslassie, Tel +251 910027187, Email [email protected]; [email protected]
Abstract: Nickel oxide nanoparticles have gained tremendous attention recently in a variety of scientific domains thanks to their characteristic chemical, physical, optical, and biological properties. Due to the diversity of applications in various fields, different physicochemical methods have been used to synthesize nickel oxide nanoparticles. However, most conventional methods use hazardous chemicals during synthesis and become liable for potential health risks, while others are expensive and require a lot of energy to synthesize nanoparticles. As a result, the nanoparticles become less biocompatible and biologically inefficient. Biogenic synthesis of nanoparticles is currently proposed as a valuable alternative to the physical and chemical methods, as it is a simple, non-toxic, cheap, green and facile approach. This synthetic method uses biological substrates such as plant extracts, microorganisms, and other biological products to synthesize nickel oxide nanoparticles. The various phytochemicals from plant extracts, enzymes or proteins from microorganisms, and other biological derivatives play as reducing, stabilizing, and capping agents to provide bioactive and biocompatible nickel oxide nanoscale material. This review discusses current findings and trends in the biogenic synthesis of nickel oxide nanoparticles and their biological activities such as antibacterial, antifungal, antileishmanial, and anticancer, with an emphasis on antimicrobial and anticancer activity along with their mechanistic elucidation. Overall, this thorough study provides insight into the possibilities for the future development of green nickel oxide nanoparticles as therapeutic agents for a variety of ailments.
Keywords: nickel oxide nanoparticles, biogenic synthesis, antimicrobial activity, antileishmanial activity, anticancer activity
Graphical Abstract:
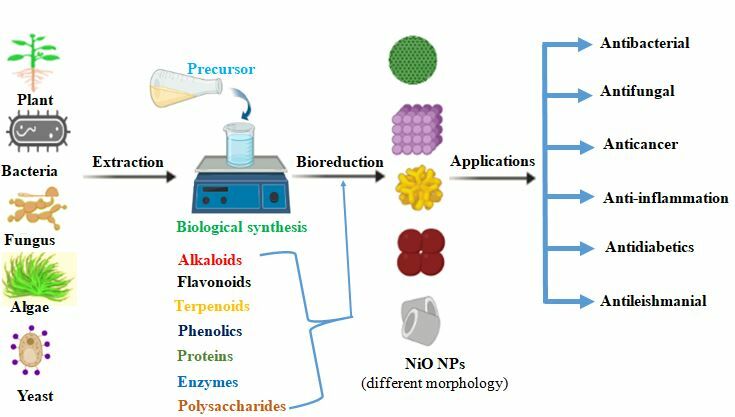
Introduction
The design, production, surface analysis, and application of nanoscale materials, typically with diameters ranging from 1 to 100 nm, are the focus of the rapidly expanding field of nanotechnology. These nanoscale materials, also known as nanoparticles, differ significantly from their bulk counterparts in terms of their physicochemical and biological properties. This is because of their incredibly small size and high surface-to-volume ratio.1–3 Nanoparticles’ amazing and intriguing qualities have made it possible to conduct multidisciplinary research and find solutions to a variety of issues. They have improved many aspects of human existence, including food production, pharmaceuticals, cosmetics, sensor, electronics, catalysis, energy production, medical care, and the environment.4–7 In the medical field, they have been utilized for drug delivery, formulations of innovative medications, disease diagnostics, and treatment.8–10
Over the past decades, many inorganic nanoparticles have been studied extensively on account of their practical applications in different areas such as magnetic devices, sensors, photocatalysis, and biomedicine.11–13 Among the numerous available nanomaterials, nickel oxide (NiO) nanoparticles have gained a great deal of attention mostly because of their superb stability and outstanding magnetic, electronic, optical, and catalytic properties14,15 as well as their multifarious applications, including gas sensors,16 electrochromic devices,17 dye-sensitized solar cells,18 batteries,19 fuel cell,20 solar energy absorber,21 and magnetic recording devices.22 Furthermore, NiO nanoparticles, unlike other metal oxide nanoparticles, are extremely inexpensive, non-toxic, and highly stable conductive materials with a broad bandgap of 3.6–4.0 eV.23,24 These nanoparticles were utilized for medical applications, including imaging, drug delivery, biomedical detection, and antibiotics.25,26 Apart from the previously mentioned usage, NiO NPs can also be successfully utilized for the removal of organic and inorganic pollutants, as a result, they play a crucial role in environmental protection. Photocatalytic degradation of Evans blue, methylene blue, 4-chlorophenol, cyanide, etc., through NiO nanoparticles, has been reported.15,27–29 NiO nanoparticles can also promote seed germination. These are known to increase the growth rate of seedlings.30 In doing so, they confirm their ability to modernize agriculture.
Recently, the distinctive nature of nanoparticles has prompted many researchers to develop inexpensive, environmentally friendly, time-efficient, and sustainable protocols for producing technologically important nanomaterials. Among the various synthesis routes utilized to fabricate NiO nanoparticles, biogenic synthesis (also called green synthesis), has received more attention, mainly due to its availability, simplicity, cost-effectiveness, and environment-friendly approach.31–33 Biological synthesis of nanoparticles is economically advantageous and offers natural reducing, capping and stabilizing agents, thereby preventing the agglomeration of nanoparticles.34 Green synthesized NiO nanoparticles have demonstrated considerable antibacterial, antioxidant, anticancer, and anti-inflammatory properties, making them promising tools for biomedical applications.35–37 NiO nanoparticles have been found to have fungicidal activity against many fungal strains.35 Several studies have described the anticancer activities of green synthesized NiO nanoparticles, including cytotoxicity studies towards HT-29, MCF-7, HepG2, A549, and Hela cancer cell lines.38–40 Furthermore, these nanoparticles have shown excellent antiparasitic properties towards Leishmania tropica.41 Other studies have also revealed the anti-diabetic, anti-inflammatory, and antioxidant nature of NiO nanoparticles.42,43 The biocompatibility of green synthesized NiO nanoparticles was examined against freshly isolated macrophages and RBCs and found to be safe at a lower concentration.28,35 NiO nanoparticles have shown toxicity towards different microbes and cancer cell lines by producing excess reactive oxygen species (ROS) and releasing nickel (II) ions that result in apoptosis.44 Few reviews dealing with the synthesis of NiO nanoparticles have been published in the last decade.45–49 However, most of these reviewers focus on several physicochemical and green synthetic methods, characterization techniques, and their applications. This review, unlike the previous reviews, provides a detailed overview of the biological synthesis, synthesis conditions, formation mechanisms, and biomedical applications, and predicts the antimicrobial and anticancer mechanism of NiO nanoparticles. In addition, the knowledge gaps, limitations, challenges and perspectives are pointed out to direct further research.
Synthesis of Nickel Oxide Nanoparticles
In general, the methods involved in the preparation of nickel oxide nanoparticles can be divided into “top-down” and “bottom-up” approaches (Figure 1). In the top-down pathway, nanoparticles are produced by decomposing bulk materials into nanosized materials through various chemical and physical methods.50 Examples of this method are pyrolysis, mechanical milling, laser ablation, sputtering, and nanolithography. A major advantage of the top-down approach is the ability to synthesize nanomaterials in large quantities in a short period. But the disadvantage associated with this approach is surface imperfections of nanomaterials, which can affect their surface chemistry and physical properties.3,51 Consequently, scientists prefer a bottom-up pathway where nanomaterials are prepared by self-assembly of small particles such as atoms, molecules, or clusters. Chemical methods such as sol-gel, precipitation, hydrothermal, solvothermal, and so on as well as biological synthesis are examples of bottom-up methods. The greater possibility of obtaining nanoparticles with minimum defect, homogeneous chemical composition and surface structure is the major benefit of the bottom-up approach.3
 |
Figure 1 General methods of nanoparticles synthesis. |
Synthesis of nickel oxide nanoparticles using several physicochemical and biological methods has been reported. Physical methods include mechanical milling,52,53 sputtering,16 spray pyrolysis,54 chemical vapor deposition,55 laser ablation,56–58 and so forth. Among the physical methods, pulsed laser ablation has received much attention owing to its capability to monitor the shape, size and physicochemical properties of the nanoparticles. Moreover, unlike other methods of synthesis, pulsed laser ablation does not need high-vacuum pumping systems, costly chambers and post-synthesis purification of nanoparticles.57,58 In this method, a pulsed high-power laser is essential for sample surface ablation. Variations of the laser ablation parameters played a major role in the fabrication of nanoparticles of desired morphology and size.56,57 Pulsed laser ablation was used for the synthesis of NiO NPs using Ni as a precursor.57 The NiO nanoparticles along with amoxicillin had a synergetic effect against different bacterial pathogens. Among other methods, NiO nanoparticles of average particle size 25 to 30 nm were prepared using spray pyrolysis of nickel chloride hexahydrate.59 Chemical vapour deposition technique was utilized for the preparation of NiO nanoparticles.55 NiO nanoparticles with a spherical shape and an average size of 25 nm have been successfully synthesized using the anodic arc plasma method.60 Nevertheless, the majority of the above methods demand a large amount of energy, robust equipment and skilled manpower, which pose major obstacles to the synthesis of NiO nanoparticles.
Chemical methods, such as hydrothermal,61 sonochemical,62 solvothermal,63–65 chemical reduction,66,67 sol-gel,68,69 microemulsion,70 and so on have also been utilized for the fabrication of NiO nanoparticles. Amongst the aforesaid methods, the sol-gel technique is the most popular for the synthesis of NiO nanoparticles because it is simple, inexpensive, and has relatively mild conditions of synthesis. In this method, the formation of the nickel-containing gels is monitored by using chemical reagents and the gel is then exposed to heat treatment up to 1000 °C to produce NiO nanoparticles. Cubic NiO nanoparticles were formed by utilizing chemical stabilizers like ethylene glycol and isopropanol and nickel nitrate hexahydrate as a precursor.71 In this study, surfactant TritonX-100 detergent was added to avoid aggregation. In other studies, Yang et al72 and Li et al73 described the synthesis of NiO nanoparticles using malic acid and citric acid, respectively without adding any surfactants and reducing agents. NiO nanoparticles of size 25 nm were fabricated using Ni(octa)2-oleylamine complex by thermal decomposition at 200 °C.74 It was reported that oleylamine (C18H37N) and triphenylphosphine (C18H15P) were used as surfactants and the C18H37N was used as both the medium and the stabilizing agent. Alcohols and hydrazine were also used as complexing agents during the production of NiO nanoparticles.75 NiO nanoparticles were synthesized by simple solvothermal synthesis protocol using nickel nitrate as a precursor and citric acid as a chelating agent.65 Abdullah et al76 synthesized NiO nanoparticles by chemical precipitation without using stabilizing, capping agent or surfactant. In another study, Srikesh et al synthesized NiO nanoparticles through combustion by utilizing organic fuels.77 However, many of the mentioned methods are time taking, labour-intensive, and require special conditions (eg, high temperature and pressure).78–80 Another drawback of chemical synthesis methods is the use of harmful chemicals, combustible organic reagents, and non-biodegradable materials, which can be hazardous to humans and the environment. Moreover, the conventional physicochemical methods are not environmentally friendly, mainly due to the high production costs, low reaction yield, harmful side products, and high energy demands.81–83 Additionally, the nanoparticles produced in this way cannot be used medically for health reasons, as these aggressive chemicals are adsorbed on their surface. Thus, recently there is a growing need to produce nanoparticles that are free of harmful side products. This can be achieved by green synthesis which is considered a sustainable, safe and inexpensive method.
Biological Synthesis of Nickel Oxide Nanoparticles
Biological synthesis is an example of a bottom-up approach that synthesizes nanoparticles using natural reducing and stabilizing agents. This process uses natural substrates such as microorganisms, plant extracts and biomolecules instead of chemical reducing agents and stabilizers. Most often, the reduction and stabilization of metal ions occur via various biomolecules present in these extracts, including enzymes/proteins, polysaccharides, amino acids, and vitamins.84 The biological method is eco-friendly, sustainable, and low-cost, and the nanoparticles so generated do not contain toxic chemicals and are suitable for biomedical applications (Figure 2).84,85 The development and significant interest of this method is not only due to the absence of toxic chemicals or because of its low energy consumption compared to some physicochemical synthesis methods but also because it can be used to produce nanoparticles with well-defined size and morphology.81,86
 |
Figure 2 Benefits of biological synthesis of nanoparticles. |
Plant-Mediated Synthesis of NiO Nanoparticles
The utilization of plant and plant extracts to generate nanoparticles has received a lot of attention in recent years because it is simple, rapid, inexpensive, and eco-friendly. Numerous plant species have been described for the biological fabrication of NiO nanoparticles. In this process, the reduction of nickel ions occurs by the phytochemicals present in the extracts.28,36 For instance; the polyphenols and hydroxyl group of flavonoids as well as the carbonyl and hydroxyl groups of amino acids serve as reducing agents and stabilize the synthesized nanoparticles. During the preparation of NiO nanoparticles, solutions of nickel salt and plant extracts are mixed and heated with constant stirring. Centrifuge the mixture after the reaction is complete. The clear supernatant solution is then discarded and the remaining pellets are washed, oven-dried, and calcined to obtain NiO nanoparticles. Finally, the formed nanoparticles were analyzed using various spectroscopic and microscopic techniques, such as dynamic light scattering (DLS), differential thermal analysis (DTA), energy-dispersive X-ray spectroscopy (EDX), UV-Vis spectrophotometer, Fourier-transform infrared spectroscopy (FT-IR), high-resolution scanning electron microscopy (HR-SEM), energy dispersion analysis of X-ray (EDX), high-resolution transmission electron microscopy (HR-TEM), X-ray diffraction (XRD), X-ray photoelectron microscopy (XPS), photoluminescence analysis (PL), particle size analyzer (PSA), selective area electron diffraction (SAED), and thermal gravimetric analysis (TGA) (Figure 3).
 |
Figure 3 Schematic illustration of the biological synthesis of NiO NPs. |
NiO nanocrystals of size about 50 nm were successfully prepared using Nephelium lappaceum L. extract.85 The phenolic compounds present in the extract were responsible for the formation of NiO nanocrystals. Furthermore, the nanocrystals treated cotton fabric showed strong antibacterial properties. Moringa oleifera leaf extract was utilized for the biosynthesis of NiO NPs.39 The TEM and EDX analysis indicated the production of spherical nickel oxide nanoparticles. Moreover, the resultant NiO showed significant antibacterial activity and cytotoxic activity. Oblong-shaped NiO NPs with 12 nm size were prepared using Azadirachta indica extract and showed outstanding antibacterial activity against E. coli and S. aureus.87
NiO NPs have been produced using a green and cost-effective method utilizing an aqueous leaf extract of Aegle marmelos.28 The plant extracts have been suggested to play as capping and reducing agents. Spectroscopic and microscopic examinations revealed the formation of spherical NiO nanoparticles ranging in size from 8 to 10 nm. Moreover, the nanoparticles showed considerable anticancer activity against A549 cell lines. Until now, several plant extracts with distinct compositions have been exploited for the biosynthesis of NiO NPs of different shapes and sizes (Table 1). Although different plants and plant extracts have been utilized for this purpose, the variation in the concentration of nickel ion and plant extract, temperature, pH and contact time may result in the formation of NiO NPs of different morphologies and sizes. For instance, the crystal size of NiO NPs was increased from 32.14 nm to 33.24 nm when the annealing temperature was increased from 350 °C to 450 °C.87
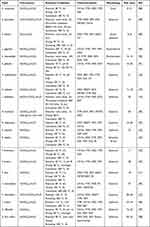 |
Table 1 Plant Extracts Used for the Biosynthesis of NiO Nanoparticles with Their Size, Morphology, and Brief Experimental Conditions |
Microbes-Mediated Biosynthesis of NiO Nanoparticles
Microbial-mediated fabrication of nanoparticles occurs via either intracellular or extracellular approaches. Intracellular synthesis generally involves the transport of metal ions into the microbial cell and the formation of nanoparticles by proteins, coenzymes, and heterocyclic derivatives present within the cell. In extracellular synthesis, metal ions are trapped on the surface of the microbial cell and the enzymes and/or proteins present on the surface reduce the metal ions and are involved in the stabilization of the nanoparticles.12,99 Green syntheses of NiO NPs by microorganisms is a simple, inexpensive and environmentally friendly approach because no toxic and dangerous chemicals are used in the synthesis.15 Various microbes such as algae, bacteria, fungi, and yeasts were used for the green synthesis of nickel oxide nanoparticles. For example, Mycobacterium sp isolated from an electroplating waste solution was used for the extracellular fabrication of NiO NPs.100 The produced NiO NPs were flower-like and ranged in size from 100 to 500 nm. Mycobacterium sp cells were found to have a nickel removal efficiency of 95% using a nickel-containing industrial effluent. This demonstrates the significant impact of Mycobacterium sp cells in the bioremediation of nickel-containing wastewater and their ability to produce NiO NPs.
Fungi are a potential candidate for nanoparticle production because it has limited toxicity, do not require nutrients or growth media, and can be stored for long periods. In addition, it can withstand metal toxicity by attaching metal ions on the cell wall composed of hydroxides, phospholipids, amino-based phosphates, chitin, chitosan, sulfates, and so forth.101,102
NiO NPs in film form were synthesized using Aspergillus aculeatus.101 Among the three types of fungal biomass used in the experiments, the dead biomass was found to exhibit the highest adsorption capacity and thus the greatest resistance to nickel toxicity. The NiO NPs synthesized were predominantly spherical with a size of 5.8 nm. XPS and EDS analysis demonstrated the presence of proteins that are supposed to be involved in the capping and/or stabilizing and organization of the nanoparticles. In another study,102 the fungus Hypocre lixii has been used to synthesize NiO NPs. The TEM results indicated the presence of spherical nanoparticles inside and outside the cells. The additional peaks of C, N, and O in the EDS spectra of the resulting nanoparticles showed the existence of macromolecules in the fungal cell wall, which acts as a capping material. Rhizopus nigricans, as a reducing agent and stabilizer, was also used for the fabrication of NiO NPs using nickel nitrate as a metal precursor.103
Another green and inexpensive method for synthesizing NiO NPs was reported by Sabouri et al104 They synthesized membranous Ni/NiO NPs via Rhodotorula mucilaginosa yeast. The Ni/NiO NPs were found to be spherical with a size of 5.5 nm. The authors suggested that yeast proteins play a crucial role in organizing the nanoparticles into films.
In addition to bacterial-, fungi-, and yeast-based synthesis of NiO NPs, algae-mediated synthesis has also been described. Spherical NiO NPs with a size of 32.64 nm were formed by red marine algal extract and NiCl2.6H2O solution as a precursor.105 Additionally, the nanoparticles were found to be an efficient catalyst for the preparation of pyridopyrimidine derivatives. In another study, spirogyra sp was utilized for synthesizing quasi-spherical NiO NPs.106 The biosynthesized NiO NPs exhibited strong bactericidal activity.
Other Green Source-Mediated Syntheses of NiO Nanoparticles
In addition to the synthesis of NiO NPs via plants and microorganisms, researchers have explored other low-cost, benign, and environmentally friendly methods using tannic acid, gums, chitosan, polysaccharides, amino acids, etc. (Table 2). An environmentally benign and biodegradable natural substance has been utilized for the biogenic synthesis of spherical NiO NPs and Ag-NiO nanocomposite.107 It was reported that tannic acid act as stabilizing agent and NiO NPs exhibited excellent photocatalytic activity towards methyl violet. A similar method of synthesis has been reported for NiO nanostructures.108 TEM spectra revealed the production of spherical NiO NPs with particle sizes of about 10–12 nm. Guar gum, a polysaccharide substance, was used as a stabilizing and capping agent during the synthesis of NiO NPs.109 FTIR analysis indicated that the hydroxyl, carbonyl and carboxyl groups of the gum were involved in the reduction of the metal ions as well as stabilization of the nanoparticles. Moreover, the nanoparticles demonstrated tremendous photocatalytic effects towards nitroarenes. Arabic gum was used to fabricate NiO NPs of spherical shape and an average size of about 59 nm.110 The produced NiO NPs exhibited strong anticancer activity against U87MG cells, revealing the significant therapeutic potential of the nanoparticles. An eco-friendly, biocompatible, non-toxic approach was followed for the green synthesis of NiO NPs using chitosan as a green template.111 Chitosan has been reported to play a key role in polymerization and as an endpoint agent in the particle growth stage. It also minimizes toxicity, increases stability and prevents accumulation. NiO NPs having cubic morphology with uniform particle distribution were prepared using Egg white.112 Furthermore, the nanoparticles exhibited considerable cytotoxicity against U87MG cells. Table 2 represents some of the biomolecules and other biological products that have been employed for the biosynthesis of nickel oxide nanoparticles.
 |
Table 2 Biomolecules or Other Green Sources Used for Biosynthesis of NiO Nanoparticles with Shape, Size, and Brief Experimental Conditions |
Mechanism of Bio-Mediated Nanoparticles Production
In recent years, several plant extracts and microbes were utilized for the biosynthesis of metal and metal oxide nanoparticles. In plant-mediated synthesis, the phytochemicals/secondary metabolites of the plant extracts are responsible for the reduction and stabilization of metal ions into their respective metal/metal oxide nanoparticles.81,115 However, the difference in concentration and composition of these biologically active compounds as well as pH, reaction time, temperature, and concentration of analyte has an extensive influence on the size and morphology of the synthesized nanoparticles.116 The plant-mediated biosynthesis of nickel nanoparticles follows three phases, namely, the activation phase, growth phase and termination phase. The activation phase involves the reduction of nickel ions by the action of plant metabolites followed by the nucleation of the reduced atoms. This is followed by the growth phase that involves the spontaneous coalescence of the seceded metal atoms into larger-sized nanoparticles and further bioreduction of metal ions (Ostwald ripening), which in turn, increases the thermodynamic stability of nanoparticles. On the other hand, the increase in the duration of the growth phase leads to the aggregation of nanoparticles. The last step in the plant-mediated biosynthesis of nanoparticles is the termination phase. In this step, the nanoparticles acquire the most energetically favorable morphology, with this process being strongly influenced by the ability of a plant extract to stabilize metal nanoparticles.2,117 In the case of metal oxides, the final product is oxidized by subjecting to air drying/calcination to get metal oxide nanoparticles.48 Regarding the biosynthesis of NiO NPs, published research suggests that the active compounds present in the plant extract react with a nickel salt to reduce or form complexes with the metal ion.85,93,98 For instance, the hydroxyl groups present in the phenolic compounds (such as corilagin, geranin, ellagic acid, and ellagitannins) of the plant extract are bound with the nickel ions to form a nickel phenolate complex (nickel-ellagate complex) through chelating effect. The formed complex undergoes decomposition during calcination and forms NiO nanoparticles.93 The polyphenols of Nephelium lappaceum peel extract were found to chelate with nickel(II) ions and form a metal coordinated complex that is further thermally treated to form NiO NPs.85 The antioxidants present in Aspalathus linearis extract also chelate with nickel(II) ions and form nickel oxide nanoparticles after thermal treatment.118 The possible mechanism for plant-mediated biosynthesis of nickel oxide nanoparticles is shown in Figure 4.
 |
Figure 4 Mechanism of plant-mediated synthesis of Ni/NiO NPs. Reproduced from Imran Din M, Rani A. Recent advances in the synthesis and stabilization of nickel and nickel oxide nanoparticles: a green adeptness. Int J Anal Chem. 2016;2016:1–14.48 |
The microbial-mediated synthesis mechanism of metal and metal oxide nanoparticles is also described in three steps: (1) Electrostatic interactions between negatively charged cell walls and positively charged metal cations trap metal ions in the cell walls of bacteria and fungi. (2) The cell wall then releases the enzyme reductase, which reduces the metal cation to a metal atom. (3) These atoms assemble to form metal nanoparticles. The nanoparticles formed can be capped by bacterial or fungal biomolecules, preventing further aggregation of the metal nanoparticles and allowing the eventually formed nanoparticles to diffuse out of the cell wall.119 For metal oxide nanoparticles, the final product is air dried or air calcined to obtain the final metal oxide nanoparticles. Figure 5 shows a proposed mechanism of the microbes-mediated synthesis of nanoparticles.
 |
Figure 5 Mechanism of microbe-mediated synthesis of Ni/NiO NPs. Reproduced from Imran Din M, Rani A. Recent advances in the synthesis and stabilization of nickel and nickel oxide nanoparticles: a green adeptness. Int J Anal Chem. 2016;2016:1–14.48 |
Biomedical Applications
Antimicrobial Activity
In impoverished countries with slow socio-economic growth, infectious diseases brought on by microorganisms pose a severe threat to public health. Many drugs in the form of antibiotics have been developed to treat such infections, but the development of resistance to another class of antibiotics has limited the ability of conventional drugs to fight against microbial infections. Therefore alternative antimicrobial treatments are urgently needed.120 Recently, nanotechnology-based therapies have been extensively utilized to diagnose and treat diseases and formulate novel drugs.2,121 Among the various kinds of nanoparticles, NiO NPs have been screened against different human pathogenic microorganisms and showed substantial outcomes.28,91 NiO NPs biosynthesized using the leaves extract of Moringa Oleifera exhibited strong bactericidal potential towards multidrug-resistant gram-positive and gram-negative bacteria.39 NiO NPs synthesized using Aegle marmelos leaf extract also showed similar bactericidal activity towards the same bacterial strains.28 This variation may be due to the polarity difference between their membranes. Gram-positive bacteria have excess positive charge than Gram-negative bacteria, resulting in easy penetration of negatively charged-free radicals, causing more cell damage and cell death in Gram-positive than Gram-negative bacteria.122,123 Another possible reason could be the structural complexity of the cell wall in Gram-negative bacteria. Gram-positive bacteria have a membrane surrounding the cell and a cell wall consisting of multiple layers of thick peptidoglycan, whereas Gram-negative bacteria have a single layer of thin peptidoglycan and an outer membrane consisting mainly of lipopolysaccharide. Thus, in Gram-negative bacteria, the outer membrane acts as a permeability barrier that reduces the entry of ROS into the cell.124
The antibiofilm and bactericidal activities of green synthesized nickel oxide nanoparticles towards S. aureus, P. aeruginosa, and E. coli were well established.91 NiO NPs treated bacterial cells showed shrinking, fragmentation, and disorganization of outer surfaces including the formation of pits and gaps, revealing the substantial inhibitory effect of the nanoparticles. Furthermore, P. aeruginosa showed higher morphological change compared to S. aureus which may be due to the disparity in their peptidoglycan layer. In another study, cotton fabrics inoculated with S. aureus and E. coli were used to examine the antibacterial activity of NiO nanocrystals.85 The findings showed a higher zone of inhibition against S. aureus (35 mm) than E. coli (25 mm). Furthermore, a significant antibacterial potential was observed even after repeated washing of the NiO nanocrystal-treated cotton fabrics. Calotropis gigantea was utilized for the biosynthesis of NiO NPs and displayed almost similar bactericidal activity with standard drug chloramphenicol towards P. aeruginosa, implying the potential of these nanoparticles as reliable antibacterial agents.125 The Sageretia thea leaf extract-mediated synthesized NiO NPs were evaluated against six pathogenic bacterial strains with and without UV illumination.41 Among the strains tested, B. subtilis was the most sensitive, whereas K. pneumonia and P. aeruginosa were the least susceptible bacterial strains. Additionally, an increasing antibacterial impact was observed after exposure to UV light. Similarly, the antibacterial effect of Rhamnus virgata-mediated NiO NPs significantly increased on UV illumination.82 The MIC value of P. aeruginosa before and after UV illumination was found to be 125 μg/mL and 31.5 μg/mL, respectively. The highest bactericidal effect of NiO NPs under UV illumination may be because of the mass generation of reactive oxygen species. NiO NPs prepared using R. triquetra and G. wallichianum also showed similar results.36,42 Table 3 summarizes the antibacterial effect of NiO NPs synthesized using various biological entities.
 |
Table 3 Antibacterial Effect of Biosynthesized NiO Nanoparticles |
The antifungal properties of bio-inspired nickel oxide nanoparticles have rarely been investigated compared to their antibacterial activity. The biosynthesis of NiO NPs using Sageretia thea leaf extract was reported and the formed nanoparticles were examined against five pathogenic fungal strains.41 The findings showed that the fungal strains were suppressed by the green-produced NiO NPs in a dose-dependent manner. Furthermore, A. flavus was the least sensitive strain, whereas R. solani and M. racemosus were determined to be the most sensitive, as demonstrated by inhibition rates of 63.2% and 64%, respectively. As a result of the nanoparticles being internalized, ROS were produced, which damaged mitochondria and DNA, contributing to the antifungal action. NiO NPs showed remarkable antifungal efficacy against a variety of fungal strains, including A. flavus, A. niger, C. Albicans, F. solani, M. racemosus.35 Among the fungal strains, M. racemosus was found to be the most susceptible, and A. flavus was the least susceptible. In another study,42 NiO NPs synthesized using Rhamnus triquetra exhibited a concentration-dependent fungicidal activity against the same fungal strains. The study revealed that A. niger was the most susceptible strain, while A. flavus was the least susceptible. According to a review article, NiO NPs have been shown to have antimicrobial activity against various bacteria.127 However, there are some challenges associated with using NiO NPs as antimicrobial agents. One of the challenges is that the NiO nanoparticles can cause oxidative stress and inflammation in human cells.128 Another challenge is that the nanoparticles can cause oxidative stress in bacterial cells. This can lead to the development of resistance in bacteria. Additionally, nanoparticles can be unstable and can aggregate in solution.128 Another study found that NiO nanoparticles can cause lung inflammation and fibrosis in rats.129 Therefore, it is important to use caution when handling NiO NPs and to follow proper safety protocols. Table 4 summarizes the antifungal activity of NiO NPs produced using plants, microorganisms and other natural sources.
 |
Table 4 Antifungal Activity of Green Synthesized NiO Nanoparticles |
The actual mechanism for the antimicrobial activity of green synthesized NiO NPs is not clearly understood and is still under investigation. Nevertheless, many ways of action such as 1) generation of reactive oxygen species due to light illumination, 2) direct contact of NiO NPs with microbial cells, resulting in plasma membrane destruction, and 3) release of antimicrobial ions mainly nickel (II) ions have been suggested (Figure 6).35,41,85 Researchers have unveiled that the stress elicited by the creation of ROS is the main cause for the antimicrobial potential of NiO NPs.35,36,39,41 Generation of reactive oxygen species such as superoxide (O2−), hydrogen peroxide (H2O2) and hydroxyl radical (OH) is attributed to the activation of NiO NPs by ultraviolet and visible light.35,85 Superoxide and hydroxyl radicals consist of excess negative charge and therefore do not penetrate the cell membrane; however, hydrogen peroxide readily enters cells85 and induces cell death by disrupting cell membrane integrity and damaging DNA, mitochondria, and proteins within the cell.42,85 The antimicrobial impact of NiO NPs depends on their surface area, morphology, and surface defects. For example, the larger the surface areas of NiO NPs, the more ROS are produced on the surface and thus the greater antimicrobial property.28,37 Besides the ROS generation, the direct contact of NiO NPs with the bacterial membranes and damage to the microbial surface have been described as factors responsible for the antimicrobial property of these nanoparticles.39 The cytotoxic behaviour of NiO NPs led to the formation of pores, shrinking and cell membrane fragmentation.91 Likewise, surface defects in the nanoparticles’ symmetry and adsorption of nanoparticles on the surface of cells can also cause injury to cells and account for the antibacterial potential.36,43
 |
Figure 6 Antimicrobial mechanism of green synthesized NiO NPs. |
In addition to the aforementioned mechanism, the significant antimicrobial impact of NiO NPs was also thought to be a result of the intermolecular interactions between the nickel (II) ions and the cell surface.41 Ezhilarasi et al studied the antimicrobial effect of NiO NPs and pointed out that the binding of nickel (II) ions on the cell surface is more likely to affect membrane permeability, enable nanoparticle uptake, and ultimately inhibit cell growth.39 The nickel (II) ion that enters the microbial cell interrupts transmembrane electron transport, thereby inhibiting the growth of the microbial species.39,40,126 The internalization of NiO NPs into the microbial cells intensifies the inhibition of microbial growth by interfering with electron transport, damaging DNA by rupturing proteins by changing the tertiary structure and harming the mitochondria by generating ROS. This ultimately results in cell death.43 Moreover, the uncoupling of ATP production, the loss of protein motive force, and the interference with the phosphate efflux mechanism brought on by the interaction of NiO NPs with thiol groups of cellular proteins results in the separation of the cell membrane from the cytoplasm.91 The condensation of genetic materials, loss of replication, and finally the release of intracellular contents may result from these processes occurring alone or together. Generally speaking, NiO NPs’ bactericidal potential is ascribed to their tiny size, unique morphology, low band energy, and strong electrostatic properties. Despite the increasing knowledge on the antimicrobial activity of NiO nanoparticles, much remains unknown about their exact mechanism of encountering bacteria, toxicity, in vivo studies, and environmental concerns, which need to be addressed before utilizing them for clinical settings.
Anticancer Activity
Biosynthesized NiO NPs showed substantial anticancer properties, especially against colon, breast, liver, and lung cancers (Table 5).36,38,40 The anticancer properties of NiO NPs prepared with Geranium wallichianum were investigated against liver cancer cells using 3 - (4, 5 – dimethylthiazol - 2 - yl) - 2, 5 - diphenyltetrazolium bromide (MTT) assay.36 In their study, the cancer cells were maintained at 37 °C with a continuous supply of 5% CO2 in their respective media and seeded in a 96-plate before treatment. Exposure of human hepatocarcinoma (HepG2) cells to various concentrations of NiO NPs (500–3.9 µg/mL) for 24 hr showed concentration-dependent inhibition of the cell lines. NiO NPs prepared using Andrographis paniculata leaf extract also showed a dose-dependent inhibition against human breast cancer (MCF-7) cell lines.38 NiO NPs fabricated with Moringa Oleifera exhibited higher cytotoxicity against human colorectal adenocarcinoma cancer (HT-29) cell lines, and a gradual decrease in cell viability was observed with increasing nanoparticle dose.39 The significant anticancer activity of these nanoparticles towards HT-29 cell lines was attributed to their large surface area to volume ratio. Moreover, the level of nickel (II) ions released was found to be high, thereby inducing the massive generation of ROS through mitochondria dysfunction, and subsequently, cell death. The Euphorbia heterophylla leaf extract-mediated synthesized NiO NPs showed a concentration-dependent anticancer activity towards HepG2 and human lung cancer (A549) cell lines.40 Rhamnus virgata extract orchestrated NiO NPs were examined for their anticancer potential towards HepG2 cell lines.35 Findings showed the anticancer activity of NiO NPs increased with increasing doses. Furthermore, the biosynthesized NiO NPs exhibited remarkable cytotoxicity towards brine shrimps with an IC50 value of 43.73 µg/mL. The biocompatible nature of the NiO nanoparticles to freshly isolated macrophages and human RBCs was also investigated and revealed that NiO NPs at a concentration of 2 µg/mL were biocompatible and could be used in different treatments. Green synthesized NiO NPs demonstrated a dose-dependent cytotoxic effect and fluorescence microscopic analysis confirmed the nanoparticles induced apoptosis, which might be due to the production of ROS.126 The Salvia macrosiphon extract-mediated synthesized NiO NPs exhibited dose-dependent toxicity toward Neuro2A cell lines.95 The significant toxicity of NiO NPs could be due to their tendency to release nickel (II) ions inside the cell ultimately leading to cell death. NiO NPs synthesized using egg white also showed significant cytotoxicity towards U87MG cell lines, with an IC50 value of 15.62 µg/mL.112
 |
Table 5 Anticancer Effects of Biosynthesized NiO Nanoparticles |
The anticancer mechanism of nanoparticles is quite complex and still under investigation. Many studies proposed that the plausible anticancer mechanism of NiO nanoparticles could be due to the reactive oxygen species-dependent and caspase-mediated apoptosis in cancer cell lines (Figure 7).28 When the nanoparticles come into contact with the cancer cell membrane, the cell surface triggers nanoparticle invagination via endocytosis to generate intracellular membrane-bound vesicles.133 This allows the endocytosed nanoparticles to enter the intracellular space without being eliminated. They are then released to generate ROS which causes mitochondria dysfunction, nuclear destruction, protein oxidation, DNA damage, decreasing major non-protein free radical scavengers and finally apoptosis.28,134 Particularly, the ROS are likely to cause cell cycle arrest during growth and preparation for the mitosis and meiosis phases.135 This process is completed by stimulating caspases-3, −8, and −9 (proteins associated with apoptosis). Many studies have also shown that ROS increases levels of the tumor protein P53, which is known to inhibit cancer cells.135,136 Another possible reason could be the internalization of nickel ion into the cells which activates the calcium-dependent cascades that disrupt the DNA repair pathways and ultimately causes apoptosis.28,137 The amount of nickel (II) ions released will be higher for nanosized compared to the micron-sized particles. Thus, the smaller particle size of NiO NPs may be the reason for the higher cytotoxic activity towards A549 cell lines.28 Based on the aforementioned mechanism, NiO nanoparticles may significantly contribute to cancer cell killing by reactive oxygen agents.
 |
Figure 7 Anticancer mechanism of green synthesized NiO NPs. |
Antileishmanial Activity
The parasite Leishmania tropica is the source of the neglected disease caused by Leishmania tropica. The commonly used antileishmanial medications have some drawbacks, including toxicity, side effects, and decreased efficacy due to drug resistance.138 Nowadays, many researchers are engaged in developing alternative routes for its treatment. Recently, the antileishmanial activity of bio-inspired NiO NPs was tested against amastigote and promastigote cultures of Leishmania tropica using MTT cytotoxic assay.41 The IC50 values against promastigotes and amastigotes cultures were 24.13 µg/mL and 26 µg/mL, respectively, indicating that both cultures were efficiently suppressed in a dose-dependent manner. Similarly, NiO NPs synthesized using Rhamnus virgata showed considerable antileishmanial potential against amastigotes and promastigotes with IC50 values of 10.62 µg/mL and 27.58 µg/mL respectively.35,36 NiO NPs prepared using R. triquetra also showed a dose-dependent antileishmanial activity.40 The concentration-dependent and lower IC50 values of the nanoparticles indicated that they could be used in future medicine for potent drug delivery against Leishmania.
Anti-Diabetic Activity
NiO nanoparticles synthesized using Averrhoa bilimbi exhibit potent anti-diabetic activity on α-amylase inhibitory effectiveness with IC50 of 311.26 µg/ mL.139 Similarly, the as-synthesized NiO NPs also showed significant anti-diabetic activity compared to Metformin.130 These findings suggest the potential of biogenic NiO Nanoparticles for the treatment of various diseases in the future.
Challenges and Future Perspectives
In recent years, nanoparticles have been used in biomedicine as antimicrobials, anticancer, and drug delivery agents by encapsulating drugs or binding therapeutic agents for more effective delivery to target tissues or cells.140,141 They are produced with a very small size so that they can move freely within the human body and target cancer cells.142 The Food and Drug Administration (FDA) has approved several nanoparticles, such as Au, Ag, zinc oxide, nickel oxide, and silica nanoparticles, for biomedical applications for the treatment of chronic diseases and cancers.142,143 These nanoparticles are used to treat liver, breast, cervical, and lung cancers.143
Research results on the green synthesis of nanoparticles have grown gradually within the last few decades. However, the focus is primarily on antimicrobials, with less emphasis on anticancer agents.144 Extensive in vitro studies have shown the therapeutic potential of biologically synthesized NiO nanoparticles, but these nanoparticles are still far from clinical trials due to limited in vivo data. Further detailed studies on the toxicity of these nanoparticles should be undertaken before they can be utilized in clinical settings. Since nanomaterials exhibit much more variable behavior than bulk materials, during biosynthesis the variables affecting the physicochemical and biological properties of NiO nanoparticles need to be monitored to better understand the underlying influencing mechanisms. Additionally, the underlying mechanism of NiO nanoparticles in various disease models should be investigated. The biggest obstacle limiting the application of nanoparticles is the lack of standardized regulations required for their use. Their uncontrolled use without uniform regulation will cause many unavoidable consequences. These challenges need to be addressed before large-scale commercialization of NiO nanoparticles can be made. Despite these challenges, the current promising data demonstrate the potential of NiO nanoparticles for the treatment of cancer and other disease in the future.
Conclusions
Nanotechnology is a highly developing field due to its extensive application in various fields of science and technology. Various physicochemical methods have been used to synthesize NiO nanoparticles. However, conventional chemical and physical methods have certain limitations in the form of chemical impurities during the synthesis process or in subsequent applications. Various chemicals (reducing agents) are used to chemically reduce NiO nanoparticles, most of which are toxic and cannot be easily discarded for environmental reasons. In many other cases, synthesis takes place at high temperatures, requires high energy and is very expensive. In recent years, the biological method of NiO nanoparticle synthesis is increasingly being developed and is gradually replacing the physicochemical methods with economic, environmental and safety advantages. In this paper, we have reviewed the recent trends and understandings of biogenic NiO NPs for possible use in biomedical applications, with a particular emphasis on antimicrobial and anticancer activity. Different types of natural extracts (such as plants, bacteria, algae, and fungus) and other biological products have been discussed with their synthesis condition and mechanism of formation. From this review, it can be seen that microbe-mediated synthesis is not industrially viable due to the requirement of strong sterile conditions and their maintenance. Therefore, the use of plant extracts for this purpose is more beneficial than microbes due to their ease of modification, fewer biological hazards, and laborious process of maintaining cell cultures. Furthermore, the use of plant extracts also reduces the cost of isolating micro-organisms and their culture media, making them more cost competitive compared to microorganism-mediated nanoparticle synthesis. Soon, nanoparticles fabricated using plant extracts may be integrated into large-scale production as they are reliable, eco-friendly, simple, and cost-effective. These strengths may open up new commercial opportunities for biological nanoparticles, hence lowering the production cost. The superior properties, small size, and biocompatibility nature of NiO NPs have led to their wide use in biomedical fields. They have shown promising results against multidrug-resistant microbes and may be used as a potential antimicrobial agent against such intractable pathogens in the future. In addition, these nanoparticles have also shown remarkable antioxidant, anti-diabetic, antileishmanial, and anticancer effects, however, further studies need to be conducted in vivo models to clarify the full mechanism and side effects. Furthermore, a detailed study on the future application of biogenic NiO NPs in magnetic resonance imaging, cell separation, drug delivery and biomedical detection is highly needed. Hence, understanding the current progress in the biosynthesis of NiO NPs and their biomedical applications will help drive future research on NiO NPs and their large-scale production.
Abbreviations
NiO NPs, Nickel oxide nanoparticles; UV-Vis, Ultraviolet-visible spectroscopy; SEM, Scanning electron microscopy; FE-SEM, Field emission scanning electron microscopy; TEM, Transmission electron microscopy; HR-TEM, High-resolution transmission electron microscopy; FTIR, Fourier-transform infrared spectroscopy; EDS, Energy dispersive X-ray analysis; DLS, Dynamic light scattering; PSA, Particle size analyzer; XPS, X-ray photoemission spectroscopy; SAED, Selective area electron diffraction; TG/DTA, Thermal gravimetric/differential thermal analysis; ROS, Reactive oxygen species; ZOI, Zone of inhibition; MIC, Minimum inhibitory concentration.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this manuscript.
References
1. Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7(1):17–28. doi:10.1016/j.jare.2015.02.007
2. Akbar S, Tauseef I, Subhan F, et al. An overview of the plant-mediated synthesis of zinc oxide nanoparticles and their antimicrobial potential. Inorg Nano Met. 2020;50(4):257–271. doi:10.1080/24701556.2019.1711121
3. Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine. 2010;6(2):257–262. doi:10.1016/j.nano.2009.07.002
4. Kubik T, Bogunia-Kubik K, Sugisaka M. Nanotechnology on duty in medical applications. Curr Pharm Biotechnol. 2005;6(1):17–33. doi:10.2174/1389201053167248
5. Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 2008;10:507–517. doi:10.1007/s11051-007-9275-x
6. Dehghani F, Shahmoradi S, Naghizadeh M, et al. Magnetic graphite-ODA@ CoFe2O4: attempting to produce and characterize the development of an innovative nanocomposite to investigate its antimicrobial properties. Appl Phys A. 2022;128(3):250. doi:10.1007/s00339-022-05387-2
7. Xu Y, Li C, Ma X, et al. Long wavelength–emissive Ru (II) metallacycle–based photosensitizer assisting in vivo bacterial diagnosis and antibacterial treatment. Proc Natl Acad Sci. 2022;119(32):e2209904119. doi:10.1073/pnas.2209904119
8. Smith DM, Simon JK, Baker Jr JR Jr. Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13(8):592–605. doi:10.1038/nri3488
9. Abbasi M, Gholizadeh R, Kasaee SR, et al. An intriguing approach toward antibacterial activity of green synthesized Rutin-templated mesoporous silica nanoparticles decorated with nanosilver. Sci Rep. 2023;13(1):5987. doi:10.1038/s41598-023-33095-1
10. Mosleh-Shirazi S, Kasaee SR, Dehghani F, et al. Investigation through the anticancer properties of green synthesized spinel ferrite nanoparticles in present and absent of laser photothermal effect. Ceram Int. 2023;49(7):11293–11301. doi:10.1016/j.ceramint.2022.11.329
11. Pakzad K, Alinezhad H, Nasrollahzadeh M. Green synthesis of Ni@ Fe3O4 and CuO nanoparticles using Euphorbia maculata extract as photocatalysts for the degradation of organic pollutants under UV-irradiation. Ceram Int. 2019;45(14):17173–17182. doi:10.1016/j.ceramint.2019.05.272
12. Gebreslassie YT, Gebretnsae HG. Green and cost-effective synthesis of tin oxide nanoparticles: a review on the synthesis methodologies, mechanism of formation, and their potential applications. Nanoscale Res Lett. 2021;16(1):97. doi:10.1186/s11671-021-03555-6
13. Gupta AK, Gupta M. Cytotoxicity suppression and cellular uptake enhancement of surface modified magnetic nanoparticles. Biomaterials. 2005;26(13):1565–1573. doi:10.1016/j.biomaterials.2004.05.022
14. Chen F, Zhou W, Yao H, et al. Self-assembly of NiO nanoparticles in lignin-derived mesoporous carbons for supercapacitor applications. Green Chem. 2013;15(11):3057–3063. doi:10.1039/c3gc4108
15. Kannan K, Radhika D, Nikolova MP, Sadasivuni KK, Mahdizadeh H, Verma U. Structural studies of bio-mediated NiO nanoparticles for photocatalytic and antibacterial activities. Inorg Chem Commun. 2020;113:107755. doi:10.1016/j.inoche.2019.107755
16. Hotovy I, Huran J, Spiess L, Romanus H, Buc D, Kosiba R. NiO-based nanostructured thin films with Pt surface modification for gas detection. Thin Solid Films. 2006;515(2):658–661. doi:10.1016/j.tsf.2005.12.232
17. He J, Lindström H, Hagfeldt A, Lindquist S-E. Dye-sensitized nanostructured p-type nickel oxide film as a photocathode for a solar cell. J Phys Chem B. 1999;103(42):8940–8943. doi:10.1021/jp991681r
18. Bandara J, Weerasinghe H. Solid-state dye-sensitized solar cell with p-type NiO as a hole collector. Sol Energy Mater Sol. 2005;85(3):385–390. doi:10.1016/j.solmat.2004.05.010
19. Rai AK, Anh LT, Park C-J, Kim J. Electrochemical study of NiO nanoparticles electrode for application in rechargeable lithium-ion batteries. Ceram Int. 2013;39(6):6611–6618. doi:10.1016/j.ceramint.2013.01.097
20. Cheng J, Liping D, Zhang B, Ping S, Guangyao M. Properties and microstructure of NiO/SDC materials for SOFC anode applications. Rare Met. 2007;26(2):110–117. doi:10.1016/S1001-0521(07)60169-7
21. Kaneko R, Chowdhury TH, Wu G, et al. Cobalt-doped nickel oxide nanoparticles as efficient hole transport materials for low-temperature processed perovskite solar cells. Sol Energy. 2019;181:243–250. doi:10.1016/j.solener.2019.01.097
22. Ichiyanagi Y, Wakabayashi N, Yamazaki J, et al. Magnetic properties of NiO nanoparticles. Physica B Condens Matter. 2003;329:862–863. doi:10.1016/S0921-4526(02)02578-4
23. Hosny NM. Synthesis, characterization and optical band gap of NiO nanoparticles derived from anthranilic acid precursors via a thermal decomposition route. Polyhedron. 2011;30(3):470–476. doi:10.1016/j.poly.2010.11.020
24. Sasi B, Gopchandran K, Manoj P, Koshy P, Rao PP, Vaidyan V. Preparation of transparent and semiconducting NiO films. Vacuum. 2002;68(2):149–154. doi:10.1016/S0042-207X(02)00299-3
25. Jaji N-D, Lee HL, Hussin MH, Akil HM, Zakaria MR, Othman MBH. Advanced nickel nanoparticles technology: from synthesis to applications. Nanotechnol Rev. 2020;9(1):1456–1480. doi:10.1515/ntrev-2020-0109
26. Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2012;64:24–36. doi:10.1016/j.addr.2012.09.006
27. Sabouri Z, Akbari A, Hosseini HA, Hashemzadeh A, Darroudi M. Eco-friendly biosynthesis of nickel oxide nanoparticles mediated by okra plant extract and investigation of their photocatalytic, magnetic, cytotoxicity, and antibacterial properties. J Cluster Sci. 2019;30:1425–1434. doi:10.1007/s10876-019-01584-x
28. Ezhilarasi AA, Vijaya JJ, Kaviyarasu K, Kennedy LJ, Ramalingam RJ, Al-Lohedan HA. Green synthesis of NiO nanoparticles using Aegle marmelos leaf extract for the evaluation of in-vitro cytotoxicity, antibacterial and photocatalytic properties. Biology. 2018;180:39–50. doi:10.1016/j.jphotobiol.2018.01.023
29. Bashir A, Razanamahandry LC, Nwanya AC, et al. Biosynthesis of NiO nanoparticles for photodegradation of free cyanide solutions under ultraviolet light. J Phys Chem Solids. 2019;134:133–140. doi:10.1016/j.jpcs.2019.05.048
30. Uddin S, Safdar LB, Anwar S, et al. Green synthesis of nickel oxide nanoparticles from Berberis balochistanica stem for investigating bioactivities. Molecules. 2021;26(6):1548. doi:10.3390/molecules26061548
31. Rafique M, Sadaf I, Rafique MS, Tahir MB. A review on green synthesis of silver nanoparticles and their applications. Artif Cells Nanomed Biotechnol. 2017;45(7):1272–1291. doi:10.1080/21691401.2016.1241792
32. Herlekar M, Barve S, Kumar R. Plant-mediated green synthesis of iron nanoparticles. J Nanopart. 2014;2014:1–9. doi:10.1155/2014/140614
33. Mittal J, Batra A, Singh A, Sharma MM. Phytofabrication of nanoparticles through plant as nanofactories. Adv Nat Sci. 2014;5(4):043002.
34. Ahmed S, Ikram S, Ikram S, Yudha S S. Biosynthesis of gold nanoparticles: a green approach. Biology. 2016;161:141–153. doi:10.1016/j.jphotobiol.2016.04.034
35. Iqbal J, Abbasi BA, Mahmood T, Hameed S, Munir A, Kanwal S. Green synthesis and characterizations of Nickel oxide nanoparticles using leaf extract of Rhamnus virgata and their potential biological applications. Appl Organomet Chem. 2019;33(8):e4950. doi:10.1002/aoc.4950
36. Abbasi BA, Iqbal J, Mahmood T, Ahmad R, Kanwal S, Afridi S. Plant-mediated synthesis of nickel oxide nanoparticles (NiO) via Geranium wallichianum: characterization and different biological applications. Mater Res Express. 2019;6(8):0850a7. doi:10.1088/2053-1591/ab23e1
37. Ramalingam R, Fazil MH, Verma NK, Arunachalam KD. Green synthesis, characterization and antibacterial evaluation of electrospun nickel oxide nanofibers. Mater Lett. 2019;256:126616. doi:10.1016/j.matlet.2019.126616
38. Karthik K, Shashank M, Revathi V, Tatarchuk T. Facile microwave-assisted green synthesis of NiO nanoparticles from Andrographis paniculata leaf extract and evaluation of their photocatalytic and anticancer activities. Mol Cryst Liq Cryst. 2019;47:1926–1954. doi:10.1080/02678292.2019.1622158
39. Ezhilarasi AA, Vijaya JJ, Kaviyarasu K, Maaza M, Ayeshamariam A, Kennedy LJ. Green synthesis of NiO nanoparticles using Moringa oleifera extract and their biomedical applications: cytotoxicity effect of nanoparticles against HT-29 cancer cells. Biology. 2016;164:352–360. doi:10.1016/j.jphotobiol.2016.10.003
40. Lingaraju K, Naika HR, Nagabhushana H, Jayanna K, Devaraja S, Nagaraju G. Biosynthesis of nickel oxide nanoparticles from Euphorbia heterophylla (L.) and their biological application. Arab J Chem. 2020;13(3):4712–4719. doi:10.1016/j.arabjc.2019.11.003
41. Khalil AT, Ovais M, Ullah I, et al. Sageretia thea (Osbeck.) modulated biosynthesis of NiO nanoparticles and their in vitro pharmacognostic, antioxidant and cytotoxic potential. Artif Cells Nanomed Biotechnol. 2018;46(4):838–852. doi:10.1080/21691401.2017.1345928
42. Iqbal J, Abbasi BA, Ahmad R, et al. Phytogenic synthesis of nickel oxide nanoparticles (NiO) using fresh leaves extract of Rhamnus triquetra (wall.) and investigation of its multiple in vitro biological potentials. Biomedicines. 2020;8(5):117. doi:10.3390/biomedicines8050117
43. Srihasam S, Thyagarajan K, Korivi M, Lebaka VR, Mallem SPR. Phytogenic generation of NiO nanoparticles using Stevia leaf extract and evaluation of their in-vitro antioxidant and antimicrobial properties. Biomolecules. 2020;10(1):89. doi:10.3390/biom10010089
44. Gong N, Shao K, Feng W, Lin Z, Liang C, Sun Y. Biotoxicity of nickel oxide nanoparticles and bio-remediation by microalgae Chlorella vulgaris. Chemosphere. 2011;83(4):510–516. doi:10.1016/j.chemosphere.2010.12.059
45. Sivagami M, Asharani I. Phyto-mediated Ni/NiO NPs and their catalytic applications-a short review. Inorg Chem Commun. 2022;145:110054. doi:10.1016/j.inoche.2022.110054
46. Narender SS, Varma VVS, Srikar CS, Ruchitha J, Varma PA, Praveen BVS. Nickel oxide nanoparticles: a brief review of their synthesis, characterization, and applications. Chem Eng Technol. 2022;45(3):397–409. doi:10.1002/ceat.202100442
47. Ahmad W, Bhatt SC, Verma M, Kumar V, Kim H. A review on current trends in the green synthesis of nickel oxide nanoparticles, characterizations, and their applications. Environ Nanotechnol Monit Manag. 2022;18:100674. doi:10.1016/j.enmm.2022.100674
48. Imran Din M, Rani A. Recent advances in the synthesis and stabilization of nickel and nickel oxide nanoparticles: a green adeptness. Int J Anal Chem. 2016;2016:1–14. doi:10.1155/2016/3512145
49. Kumari A, Pandey A. A review on green synthesis of nickel oxide nanoparticles and their photocatalytic activities. Mater Today. 2023;2023:1.
50. Singh S, Mishra S, Srivastava R, Gopal R. Optical properties of selenium quantum dots produced with laser irradiation of water suspended Se nanoparticles. J Phys Chem C. 2010;114(41):17374–17384. doi:10.1021/jp105037w
51. Pareek V, Bhargava A, Gupta R, Jain N, Panwar J. Synthesis and applications of noble metal nanoparticles: a review. Adv Sci Eng Med. 2017;9(7):527–544. doi:10.1166/asem.2017.2027
52. Ahmadisoltansaraei K, Moghaddam J. Preparation of NiO nanoparticles from Ni (OH) 2· NiCO 3· 4H 2 O precursor by mechanical activation. Int J Miner Metall Mater. 2014;21:726–735. doi:10.1007/s12613-014-0964-z
53. Balamurugan S, Philip A, Kiruba V, Veluraja K. Simple and efficient way of synthesizing NiO nanoparticles by combustion followed by ball milling method. Nanosci Nanotechnol Lett. 2015;7(2):89–93. doi:10.1166/nnl.2015.1889
54. Ukoba K, Eloka-Eboka A, Inambao F. Review of nanostructured NiO thin film deposition using the spray pyrolysis technique. Renew Sust Energ Rev. 2018;82:2900–2915. doi:10.1016/j.rser.2017.10.041
55. Moravec P, Keskinen H, Jyrki M, Bakardjieva S, Levdansky VV, Levdansky VV. NiOx nanoparticle synthesis by chemical vapor deposition from nickel acetylacetonate. Mater Sci Appl. 2011;2(04):258. doi:10.4236/msa.2011.24033
56. Gondal M, Saleh TA, Drmosh Q. Synthesis of nickel oxide nanoparticles using pulsed laser ablation in liquids and their optical characterization. Appl Surf Sci. 2012;258(18):6982–6986. doi:10.1016/j.apsusc.2012.03.147
57. Khashan KS, Sulaiman GM, Hamad AH, Abdulameer FA, Hadi A. Generation of NiO nanoparticles via pulsed laser ablation in deionised water and their antibacterial activity. Appl Phys A. 2017;123:1–10. doi:10.1007/s00339-017-0826-4
58. Baig U, Khan A, Gondal MA, Dastageer MA, Falath WS. Laser induced anchoring of nickel oxide nanoparticles on polymeric graphitic carbon nitride sheets using pulsed laser ablation for efficient water splitting under visible light. Nanomaterials. 2020;10(6):1098. doi:10.3390/nano10061098
59. Reguig B, Khelil A, Cattin L, Morsli M, Bernede J. Properties of NiO thin films deposited by intermittent spray pyrolysis process. Appl Surf Sci. 2007;253(9):4330–4334. doi:10.1016/j.apsusc.2006.09.046
60. Wei Z, Qiao H, Yang H, Zhang C, Yan X. Characterization of NiO nanoparticles by anodic arc plasma method. J Alloys Compd. 2009;479(1–2):855–858. doi:10.1016/j.jallcom.2009.01.064
61. Safa S, Hejazi R, Rabbani M, Azimirad R. Hydrothermal synthesis of NiO nanostructures for photodegradation of 4-nitrophenol. Desalin Water Treat. 2016;57(46):21982–21989. doi:10.1080/19443994.2015.1125799
62. Parsaee Z. Synthesis of novel amperometric urea-sensor using hybrid synthesized NiO-NPs/GO modified GCE in aqueous solution of cetrimonium bromide. Ultrason Sonochem. 2018;44:120–128. doi:10.1016/j.ultsonch.2018.02.021
63. Qing Z, Haixia L, Huali L, Yu L, Huayong Z, Tianduo L. Solvothermal synthesis and photocatalytic properties of NiO ultrathin nanosheets with porous structure. Appl Surf Sci. 2015;328:525–530. doi:10.1016/j.apsusc.2014.12.077
64. Xu J, Wang M, Liu Y, Li J, Cui H. One-pot solvothermal synthesis of size-controlled NiO nanoparticles. Adv Powder Technol. 2019;30(4):861–868. doi:10.1016/j.apt.2019.01.016
65. Nathan T, Aziz A, Noor A, Prabaharan S. Nanostructured NiO for electrochemical capacitors: synthesis and electrochemical properties. J Solid State Electrochem. 2008;12:1003–1009. doi:10.1007/s10008-007-0465-3
66. Deng X, Chen Z. Preparation of nano-NiO by ammonia precipitation and reaction in solution and competitive balance. Mater Lett. 2004;58(3–4):276–280. doi:10.1016/S0167-577X(03)00469-5
67. Khairnar SD, Shrivastava VS. Facile synthesis of nickel oxide nanoparticles for the degradation of Methylene blue and Rhodamine B dye: a comparative study. J Taibah Univ Sci. 2019;13(1):1108–1118. doi:10.1080/16583655.2019.1686248
68. Tadic M, Nikolic D, Panjan M, Blake GR. Magnetic properties of NiO (nickel oxide) nanoparticles: blocking temperature and Neel temperature. J Alloys Compd. 2015;647:1061–1068. doi:10.1016/j.jallcom.2015.06.027
69. Zayim EO, Turhan I, Tepehan F, Ozer N. Sol–gel deposited nickel oxide films for electrochromic applications. Sol Energy Mater Sol. 2008;92(2):164–169. doi:10.1016/j.solmat.2007.03.034
70. Han D, Yang H, Shen C, Zhou X, Wang F. Synthesis and size control of NiO nanoparticles by water-in-oil microemulsion. Powder Technol. 2004;147(1–3):113–116. doi:10.1016/j.powtec.2004.09.024
71. Zorkipli NNM, Kaus NHM, Mohamad AA. Synthesis of NiO nanoparticles through sol-gel method. Procedia Chem. 2016;19:626–631. doi:10.1016/j.proche.2016.03.062
72. Yang Q, Sha J, Ma X, Yang D. Synthesis of NiO nanowires by a sol-gel process. Mater Lett. 2005;59(14–15):1967–1970. doi:10.1016/j.matlet.2005.02.037
73. J-f LI, Bo X, L-j DU, Rong Y, Liang TD. Preparation of nano-NiO particles and evaluation of their catalytic activity in pyrolyzing cellulose. J Fuel Chem Technol. 2008;36(1):42–47. doi:10.1016/S1872-5813(08)60010-9
74. Fereshteh Z, Salavati-Niasari M, Saberyan K, Hosseinpour-Mashkani SM, Tavakoli F. Synthesis of nickel oxide nanoparticles from thermal decomposition of a new precursor. J Cluster Sci. 2012;23:577–583. doi:10.1007/s10876-012-0477-8
75. El-Kemary M, Nagy N, El-Mehasseb I. Nickel oxide nanoparticles: synthesis and spectral studies of interactions with glucose. Mater Sci Semicond Process. 2013;16(6):1747–1752. doi:10.1016/j.mssp.2013.05.018
76. Abdallah A, Basma H, Awad R. Preparation, characterization, and application of nickel oxide nanoparticles in glucose and lactose biosensors. Mod Appl Sci. 2019;13(6):99. doi:10.5539/mas.v13n6p99
77. Srikesh G, Nesaraj AS. Synthesis and characterization of phase pure NiO nanoparticles via the combustion route using different organic fuels for electrochemical capacitor applications. J Electrochem Sci Technol. 2015;6(1):16–25. doi:10.33961/JECST.2015.6.1.16
78. Jain N, Bhargava A, Majumdar S, Tarafdar J, Panwar J. Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: a mechanism perspective. Nanoscale. 2011;3(2):635–641. doi:10.1039/C0NR00656D
79. Thema F, Manikandan E, Gurib-Fakim A, Maaza M. Single phase Bunsenite NiO nanoparticles green synthesis by Agathosma betulina natural extract. J Alloys Compd. 2016;657:655–661. doi:10.1016/j.jallcom.2015.09.227
80. Mittal AK, Bhaumik J, Kumar S, Banerjee UC. Biosynthesis of silver nanoparticles: elucidation of prospective mechanism and therapeutic potential. J Colloid Interface Sci. 2014;415:39–47. doi:10.1016/j.jcis.2013.10.018
81. Mittal AK, Chisti Y, Banerjee UC. Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31(2):346–356. doi:10.1016/j.biotechadv.2013.01.003
82. Dauthal P, Mukhopadhyay M. Noble metal nanoparticles: plant-mediated synthesis, mechanistic aspects of synthesis, and applications. Ind Eng Chem Res. 2016;55(36):9557–9577. doi:10.1021/acs.iecr.6b00861
83. Sood R, Chopra DS. Metal–plant frameworks in nanotechnology: an overview. Phytomedicine. 2018;50:148–156. doi:10.1016/j.phymed.2017.08.025
84. Khan SA, Shahid S, Ayaz A, Alkahtani J, Elshikh MS, Riaz T. Phytomolecules-coated NiO nanoparticles synthesis using abutilon indicum leaf extract: antioxidant, antibacterial, and anticancer activities. Int J Nanomedicine. 2021;16:1757. doi:10.2147/IJN.S294012
85. Yuvakkumar R, Suresh J, Nathanael AJ, Sundrarajan M, Hong S. Rambutan (Nephelium lappaceum L.) peel extract assisted biomimetic synthesis of nickel oxide nanocrystals. Mater Lett. 2014;128:170–174. doi:10.1016/j.matlet.2014.04.112
86. Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13(10):2638–2650. doi:10.1039/c1gc15386b
87. Helan V, Prince JJ, Al-Dhabi NA, et al. Neem leaves mediated preparation of NiO nanoparticles and its magnetization, coercivity and antibacterial analysis. Results Phys. 2016;6:712–718. doi:10.1016/j.rinp.2016.10.005
88. Wardani M, Yulizar Y, Abdullah I, Bagus Apriandanu DO. Synthesis of NiO nanoparticles via green route using Ageratum conyzoides L. leaf extract and their catalytic activity.
89. Mayedwa N, Mongwaketsi N, Khamlich S, Kaviyarasu K, Matinise N, Maaza M. Green synthesis of nickel oxide, palladium and palladium oxide synthesized via Aspalathus linearis natural extracts: physical properties & mechanism of formation. Appl Surf Sci. 2018;446:266–272. doi:10.1016/j.apsusc.2017.12.116
90. Kumar CR, Betageri VS, Nagaraju G, Pujar G, Suma B, Latha M. Photocatalytic, nitrite sensing and antibacterial studies of facile bio-synthesized nickel oxide nanoparticles. J Sci. 2020;5(1):48–55.
91. Saleem S, Ahmed B, Khan MS, Al-Shaeri M, Musarrat J. Inhibition of growth and biofilm formation of clinical bacterial isolates by NiO nanoparticles synthesized from Eucalyptus globulus plants. Microb Pathog. 2017;111:375–387. doi:10.1016/j.micpath.2017.09.019
92. Kganyago P, Mahlaule-Glory L, Mathipa M, et al. Synthesis of NiO nanoparticles via a green route using Monsonia burkeana: the physical and biological properties. Biology. 2018;182:18–26. doi:10.1016/j.jphotobiol.2018.03.016
93. Adinaveen T, Karnan T, Selvakumar SAS. Photocatalytic and optical properties of NiO added Nephelium lappaceum L. peel extract: an attempt to convert waste to a valuable product. Heliyon. 2019;5(5):e01751. doi:10.1016/j.heliyon.2019.e01751
94. Zahra T, Ahmad KS. Structural, optical and electrochemical studies of organo-templated wet synthesis of cubic shaped nickel oxide nanoparticles. Optik. 2020;205:164241. doi:10.1016/j.ijleo.2020.164241
95. Sabouri Z, Fereydouni N, Akbari A, et al. Plant-based synthesis of NiO nanoparticles using salvia macrosiphon Boiss extract and examination of their water treatment. Rare Met. 2020;39:1134–1144. doi:10.1007/s12598-019-01333-z
96. Nasseri M, Ahrari F, Zakerinasab B. A green biosynthesis of NiO nanoparticles using aqueous extract of Tamarix serotina and their characterization and application. Appl Organomet Chem. 2016;30(12):978–984. doi:10.1002/aoc.3530
97. Haider A, Ijaz M, Ali S, et al. Green synthesized phytochemically (Zingiber officinale and Allium sativum) reduced nickel oxide nanoparticles confirmed bactericidal and catalytic potential. Nanoscale Res Lett. 2020;15:1–11. doi:10.1186/s11671-020-3283-5
98. Gebretinsae H, Tsegay M, Nuru Z. Biosynthesis of nickel oxide (NiO) nanoparticles from cactus plant extract. Mater Today. 2020;2020:1.
99. Li X, Xu H, Chen Z-S, Chen G. Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomater. 2011;2011:1–16. doi:10.1155/2011/910539
100. Sathyavathi S, Manjula A, Rajendhran J, Gunasekaran P. Extracellular synthesis and characterization of nickel oxide nanoparticles from Microbacterium sp. MRS-1 towards bioremediation of nickel electroplating industrial effluent. Bioresour Technol. 2014;165:270–273. doi:10.1016/j.biortech.2014.03.031
101. Salvadori MR, Nascimento CAO, Corrêa B. Nickel oxide nanoparticles film produced by dead biomass of filamentous fungus. Sci Rep. 2014;4(1):1–6. doi:10.1038/srep06404
102. Salvadori MR, Ando RA, Oller Nascimento CA, Correa B. Extra and intracellular synthesis of nickel oxide nanoparticles mediated by dead fungal biomass. PLoS One. 2015;10(6):e0129799. doi:10.1371/journal.pone.0129799
103. Ullah M, Naz A, Mahmood T, Siddiq M, Bano A. Biochemical synthesis of nickel & cobalt oxide nano-particles by using biomass waste. Int J Enhanc Res Sci Technol Eng. 2014;3:415–422.
104. Salvadori MR, Ando RA, Muraca D, Knobel M, Nascimento CAO, Corrêa B. Magnetic nanoparticles of Ni/NiO nanostructured in film form synthesized by dead organic matrix of yeast. RSC Adv. 2016;6(65):60683–60692. doi:10.1039/C6RA07274G
105. Moavi J, Buazar F, Sayahi MH. Algal magnetic nickel oxide nanocatalyst in accelerated synthesis of pyridopyrimidine derivatives. Sci Rep. 2021;11(1):1–14. doi:10.1038/s41598-021-85832-z
106. Singh Y, Sodhi RS, Singh PP, Kaushal S. Biosynthesis of NiO nanoparticles using Spirogyra sp. cell-free extract and their potential biological applications. Mater Adv. 2022;3(12):4991–5000. doi:10.1039/D2MA00114D
107. Devi HS, Singh TD, Singh NR. Green synthesis and catalytic activity of composite NiO-Ag nanoparticles for photocatalytic degradation of dyes. J Indian Chem Soc. 2017;94(2):159–169.
108. Suvith V, Devu V, Philip D. Tannic acid mediated synthesis of nanostructured NiO and SnO 2 for catalytic degradation of methylene blue. Opt Quantum Electron. 2020;52:1–17. doi:10.1007/s11082-019-2131-2
109. Baranwal K, Dwivedi LM, Singh V, Singh V. Guar gum mediated synthesis of NiO nanoparticles: an efficient catalyst for reduction of nitroarenes with sodium borohydride. Int J Biol Macromol. 2018;120:2431–2441. doi:10.1016/j.ijbiomac.2018.09.013
110. Sabouri Z, Akbari A, Hosseini HA, Khatami M, Darroudi M. Green-based bio-synthesis of nickel oxide nanoparticles in Arabic gum and examination of their cytotoxicity, photocatalytic and antibacterial effects. Green Chem Lett Rev. 2021;14(2):404–414. doi:10.1080/17518253.2021.1923824
111. Choo CK, Goh TL, Shahcheraghi L, et al. Synthesis and characterization of NiO nano‐spheres by templating on chitosan as a green precursor. J Am Ceram Soc. 2016;99(12):3874–3882. doi:10.1111/jace.14411
112. Sabouri Z, Akbari A, Hosseini HA, Khatami M, Darroudi M. Egg white-mediated green synthesis of NiO nanoparticles and study of their cytotoxicity and photocatalytic activity. Polyhedron. 2020;178:114351. doi:10.1016/j.poly.2020.114351
113. Williams L, Prasad AR, Sowmya P, Joseph A. Characterization and temperature dependent DC conductivity study of bio templated nickel oxide nanoparticles (NiO) and their composites using polyaniline (PANI). Mater Chem Phys. 2020;242:122469. doi:10.1016/j.matchemphys.2019.122469
114. Sabouri Z, Akbari A, Hosseini HA, Hashemzadeh A, Darroudi M. Bio-based synthesized NiO nanoparticles and evaluation of their cellular toxicity and wastewater treatment effects. J Mol Struct. 2019;1191:101–109. doi:10.1016/j.molstruc.2019.04.075
115. Raveendran P, Fu J, Wallen SL. Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125(46):13940–13941. doi:10.1021/ja029267j
116. Malik P, Shankar R, Malik V, Sharma N, Mukherjee TK. Green chemistry based benign routes for nanoparticle synthesis. J Nanopart. 2014;2014. doi:10.1155/2014/302429
117. Makarov V, Love A, Sinitsyna O, et al. “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Nat. 2014;6(20):35–44. doi:10.32607/20758251-2014-6-1-35-44
118. Diallo A, Kaviyarasu K, Ndiaye S, et al. Structural, optical and photocatalytic applications of biosynthesized NiO nanocrystals. Green Chem Lett Rev. 2018;11(2):166–175. doi:10.1080/17518253.2018.1447604
119. Rai M, Duran N. Metal Nanoparticles in Microbiology. Springer Science & Business Media; 2011.
120. Engler AC, Wiradharma N, Ong ZY, Coady DJ, Hedrick JL, Yang -Y-Y. Emerging trends in macromolecular antimicrobials to fight multi-drug-resistant infections. Nano Today. 2012;7(3):201–222. doi:10.1016/j.nantod.2012.04.003
121. Nadeem M, Abbasi BH, Younas M, Ahmad W, Khan T. A review of the green syntheses and anti-microbial applications of gold nanoparticles. Green Chem Lett Rev. 2017;10(4):216–227. doi:10.1080/17518253.2017.1349192
122. Gordon T, Perlstein B, Houbara O, Felner I, Banin E, Margel S. Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Colloids Surf. 2011;374(1–3):1–8. doi:10.1016/j.colsurfa.2010.10.015
123. Sonohara R, Muramatsu N, Ohshima H, Kondo T. Difference in surface properties between Escherichia coli and Staphylococcus aureus as revealed by electrophoretic mobility measurements. Biophys Chem. 1995;55(3):273–277. doi:10.1016/0301-4622(95)00004-H
124. Espitia PJP, Soares Nd FF, Coimbra JS, de Andrade NJ, Cruz RS, Medeiros EAA. Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications. Food Bioproc Tech. 2012;5:1447–1464. doi:10.1007/s11947-012-0797-6
125. Din MI, Nabi AG, Rani A, Aihetasham A, Mukhtar M. Single step green synthesis of stable nickel and nickel oxide nanoparticles from Calotropis gigantea: catalytic and antimicrobial potentials. Environ Nanotechnol Monit Manag. 2018;9:29–36. doi:10.1016/j.enmm.2017.11.005
126. Vijaya Kumar P, Jafar Ahamed A, Karthikeyan M. Synthesis and characterization of NiO nanoparticles by chemical as well as green routes and their comparisons with respect to cytotoxic effect and toxicity studies in microbial and MCF-7 cancer cell models. SN Appl Sci. 2019;1:1–15. doi:10.1007/s42452-019-1113-0
127. Mba IE, Nweze EI. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: research progress, challenges, and prospects. World J Microbiol Biotechnol. 2021;37:1–30. doi:10.1007/s11274-021-03070-x
128. Makabenta JMV, Nabawy A, C-H L, Schmidt-Malan S, Patel R, Rotello VM. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat Rev Microbiol. 2021;19(1):23–36. doi:10.1038/s41579-020-0420-1
129. Jeong M-J, Jeon S, H-S Y, et al. Exposure to nickel oxide nanoparticles induces acute and chronic inflammatory responses in rat lungs and perturbs the lung microbiome. Int J Environ Res Public Health. 2022;19(1):522. doi:10.3390/ijerph19010522
130. Shwetha UR, Rajith Kumar CR, Virupaxappa S, et al. Biogenic synthesis of NiO nanoparticles using areca catechu leaf extract and their antidiabetic and cytotoxic effects. Molecules. 2021;26(9):2448. doi:10.3390/molecules26092448
131. Sabouri Z, Akbari A, Hosseini HA, Khatami M, Darroudi M. Tragacanth-mediate synthesis of NiO nanosheets for cytotoxicity and photocatalytic degradation of organic dyes. Bioprocess Biosyst Eng. 2020;43:1209–1218. doi:10.1007/s00449-020-02315-7
132. Zhang Y, Mahdavi B, Mohammadhosseini M, et al. Green synthesis of NiO nanoparticles using Calendula officinalis extract: chemical characterization, antioxidant, cytotoxicity, and anti-esophageal carcinoma properties. Arab J Chem. 2021;14(5):103105. doi:10.1016/j.arabjc.2021.103105
133. Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi:10.1146/annurev.biochem.78.081307.110540
134. Bethu MS, Netala VR, Domdi L, Tartte V, Janapala VR. Potential anticancer activity of biogenic silver nanoparticles using leaf extract of Rhynchosia suaveolens: an insight into the mechanism. Artif Cells Nanomed Biotechnol. 2018;46(sup1):104–114. doi:10.1080/21691401.2017.1414824
135. Patil MP, Kim G-D. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl Microbiol Biotechnol. 2017;101:79–92. doi:10.1007/s00253-016-8012-8
136. Kim YJ, Perumalsamy H, Castro-Aceituno V, et al. Photoluminescent and self-assembled hyaluronic acid-zinc oxide-ginsenoside Rh2 nanoparticles and their potential caspase-9 apoptotic mechanism towards cancer cell lines. Int J Nanomedicine. 2019;14:8195–8208. doi:10.2147/IJN.S221328
137. Horie M, Nishio K, Fujita K, et al. Protein adsorption of ultrafine metal oxide and its influence on cytotoxicity toward cultured cells. Chem Res Toxicol. 2009;22(3):543–553. doi:10.1021/tx800289z
138. Götte M, Berghuis A, Matlashewski G, Wainberg MA, Sheppard D. Handbook of Antimicrobial Resistance. Springer; 2017.
139. Haritha V, Gowri S, Janarthanan B, Faiyazuddin M, Karthikeyan C, Sharmila S. Biogenic synthesis of nickel oxide nanoparticles using Averrhoa bilimbi and investigation of its antibacterial, antidiabetic and cytotoxic properties. Inorg Chem Commun. 2022;144:109930. doi:10.1016/j.inoche.2022.109930
140. Jahangirian H, Lemraski EG, Webster TJ, Rafiee-Moghaddam R, Abdollahi Y. A review of drug delivery systems based on nanotechnology and green chemistry: green nanomedicine. Int J Nanomedicine. 2017;12:2957. doi:10.2147/IJN.S127683
141. Lam P-L, Wong W-Y, Bian Z, Chui C-H, Gambari R. Recent advances in green nanoparticulate systems for drug delivery: efficient delivery and safety concern. Nanomedicine. 2017;12(4):357–385. doi:10.2217/nnm-2016-0305
142. Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):1–33. doi:10.1186/s12951-017-0328-8
143. Navya P, Kaphle A, Srinivas S, Bhargava SK, Rotello VM, Daima HK. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019;6:1–30. doi:10.1186/s40580-018-0172-z
144. Fierascu I, Fierascu IC, Brazdis RI, Baroi AM, Fistos T, Fierascu RC. Phytosynthesized metallic nanoparticles—between nanomedicine and toxicology. A brief review of 2019′ s findings. Materials. 2020;13(3):574. doi:10.3390/ma13030574
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
