Back to Journals » Drug Design, Development and Therapy » Volume 17
Biomaterial-Based Gene Delivery: Advanced Tools for Enhanced Cartilage Regeneration
Authors Chen H, Li Z, Li X, Lu J, Chen B, Wang Q, Wu G
Received 25 July 2023
Accepted for publication 9 November 2023
Published 4 December 2023 Volume 2023:17 Pages 3605—3624
DOI https://doi.org/10.2147/DDDT.S432056
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Hongfeng Chen,1 Zhen Li,1 Xiaoqi Li,1 Jiongjiong Lu,1 Beibei Chen,1 Qiongchao Wang,1 Guangliang Wu2
1Department of Foot and Ankle Surgery, The Second Affiliated Hospital of Luohe Medical College, Luohe, Henan, 462300, People’s Republic of China; 2Department of Orthopaedics, The Second Affiliated Hospital of Luohe Medical College, Luohe, Henan, 462300, People’s Republic of China
Correspondence: Guangliang Wu, Department of Orthopaedics, The Second Affiliated Hospital of Luohe Medical College, Western Haihe Road, Luohe, Henan, 462300, People’s Republic of China, Email [email protected]
Abstract: Gene therapy has emerged as a promising and innovative approach in cartilage regeneration. Integrating biomaterials into gene therapy offers a unique opportunity to enhance gene delivery efficiency, optimize gene expression dynamics, modulate immune responses, and promote tissue regeneration. Despite the rapid progress in biomaterial-based gene delivery, there remains a deficiency of comprehensive discussions on recent advances and their specific application in cartilage regeneration. Therefore, this review aims to provide a thorough overview of various categories of biomaterials employed in gene delivery, including both viral and non-viral vectors, with discussing their distinct advantages and limitations. Furthermore, the diverse strategies employed in gene therapy are discussed and summarized, such as the utilization of growth factors, anti-inflammatory cytokines, and chondrogenic genes. Additionally, we highlights the significant challenges that hinder biomaterial-based gene delivery in cartilage regeneration, including immune response modulation, gene delivery efficiency, and the sustainability of long-term gene expression. By elucidating the functional properties of biomaterials-based gene therapy and their pivotal roles in cartilage regeneration, this review aims to enhance further advances in the design of sophisticated gene delivery systems for improved cartilage regeneration outcomes.
Keywords: cartilage, regeneration, delivery systems, biomaterials, osteoarthritis
Introduction
Osteoarthritis (OA) is a progressive degenerative joint disorder with a global prevalence, inflicting millions of individuals and causing debilitating symptoms such as pain, joint stiffness, and limited range of motion.1 This pathological condition arises from the gradual deterioration of the protective cartilage within joints, resulting in bone-on-bone friction and subsequent inflammation.2 While a definitive cure for OA remains elusive, numerous treatment modalities are available to alleviate its symptoms and manage its progression.3 Primary therapeutic options for OA includes pharmacological interventions, physical therapy, and surgical interventions. Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids are commonly employed to mitigate pain and reduce inflammatory responses.4 Furthermore, physical therapy interventions aim to enhance joint functionality, flexibility, and muscular strength. In cases of severe OA, surgical interventions, such as arthroscopy and joint replacement, are assumed necessary to restore mobility and alleviate discomfort.5
Gene therapy has emerged as a promising therapeutic strategy, holding potential to target the fundamental mechanisms underlying OA, including inflammation, cartilage degradation, and bone remodeling.6–9 In this context, the combination of biomaterials and gene therapy offers a potential approach to foster a conducive microenvironment for gene therapy vectors, augment gene delivery efficiency to target cells, and sustain prolonged gene expression.10 The selection of appropriate biomaterials for gene therapy delivery assumes considerable significance, as it profoundly affects the efficacy and safety of the treatment.11 Notably, a diverse range of biomaterials, including synthetic polymers, natural polymers, and hybrid materials, has been explored in the context of gene therapy for OA.10 These biomaterial platforms can be engineered to provide controlled release of therapeutic genes, protect genes from degradation, and selectively target specific cells and tissues. Encouragingly, biomaterial-based gene therapy has demonstrated promising outcomes in preclinical investigations, exhibiting the potential to modify disease progression and improve joint function in the context of OA.10 Despite this, more studies need to be performed to summarize and discuss the recent advances and the specific application of biomaterial-based gene therapy in cartilage regeneration and OA therapy.
Herein, we present a summary of advanced strategies for enhancing cartilage regeneration through the utilization of diverse biomaterials-based gene delivery systems. First, we perform a discussion of the various types of biomaterials employed in gene delivery systems, highlighting their distinctive properties and functionalities. Second, the diverse strategies employed in gene therapy are summarized, followed by discussing recently developed therapeutic modalities of biomaterials-based gene delivery systems in OA. Finally, advanced cartilage regeneration technologies and their potential for clinical translation are evaluated, and emerging biomaterials-based gene delivery systems directions for OA therapy are discussed.
Types of Therapeutic Agents Used for Cartilage Gene Therapy
Gene therapy represents a promising therapeutic approach in OA treatment, including the delivery of therapeutic agents to target cells with the aim of facilitating tissue repair and regeneration. In the context of OA, the therapeutic agents employed in gene therapy can be generally classified into four categories: growth factors, anti-inflammatory factors, therapeutic genes, and non-coding RNAs.12
Growth Factors
Growth factors represent a class of vital proteins that exert significant effect on cell proliferation, differentiation, and tissue repair processes. Within the context of gene therapy for OA, numerous growth factors have been used to elucidate their potential therapeutic potential. Notably, transforming growth factor-beta (TGF-β) and insulin-like growth factor-1 (IGF-1) have exhibited significant influence in preclinical studies.13,14
Overexpression of TGF-β was well-documented with the capacity to enhance articular cartilage regeneration in animal models of OA.15 In a prior study, Ye et al developed a functionalized self-assembling peptide Ac-(RADA)4-GG-LIANAK-CONH2 (RAD-CM) by connecting the TGF-β1-simulating peptide LIANAK (CM) with the self-assembling peptide Ac-(RADA)4-CONH2 (RAD).16 This composite scaffold RAD/RAD-CM/DCM (R/C/D) exhibited excellent bioactivity and structural stability. Importantly, it demonstrated satisfactory performance in promoting neocartilage restoration and reconstructing the osteochondral unit. This study presents a promising strategy for in situ cartilage regeneration through the stable presentation of a TGF-β1-simulating peptide (Figure 1).
Similarly, IGF-1, characterized by its potent mitogenic and chondrogenic properties, has displayed potential as a therapeutic candidate for OA gene therapy. Overexpression of IGF-1 has been observed to stimulate the proliferation and differentiation of chondrocytes, thereby promoting cartilage repair.14 Gene therapy has been proposed to enhance chondrocyte transplantation therapies by increasing the expression of growth factors such as IGF-I. In a previous study, Aguilar et al performed a comparison between endogenous and exogenous IGF-I in promoting matrix production in neonatal and mature chondrocytes.17 In vitro, the results showed a clear correlation between the amount of IGF-I produced by the cells and their biosynthetic response. Both neonatal and mature chondrocytes exhibited this relationship, although the sensitivities varied significantly.17 These findings suggest that IGF-I gene therapy may be more advantageous when using younger cell sources. Both types of chondrocytes showed reduced sensitivity to exogenous IGF-I compared to endogenous IGF-I.
Anti-Inflammatory Factors
Anti-inflammatory factors include a class of molecules that exert inhibitory effects on inflammation, a hallmark feature of OA pathology. Inflammation contributes significantly to cartilage destruction and the progressive nature of OA. Extensive investigations have been conducted on several anti-inflammatory factors with the aim of exploring their potential utilization in gene therapy for OA.18 Notably, interleukin-1 receptor antagonist (IL-1Ra) has garnered considerable attention in this context.19
FX201 is an example of a novel gene therapy under development for the treatment of OA, where IL-1Ra is delivered directly into the affected joint.20 Senter et al assessed the effectiveness, distribution, and safety of a rat surrogate of FX201, called helper-dependent adenovirus (HDAd)-ratIL-1Ra, in an animal model of OA.20 The researchers administered a single intra-articular injection of HDAd-ratIL-1Ra seven days after surgery in rats. The treatment significantly reduced OA-related changes in cartilage, bone, and the synovial membrane at 12 weeks after the surgery. FX201 and HDAd-ratIL-1Ra remained detectable in the injected joint and nearby tissues for at least 92 days, with minimal evidence of spreading to other areas. HDAd-ratIL-1Ra showed therapeutic and disease-modifying effects locally without causing significant adverse effects, supporting the further clinical development of FX201 as a potential treatment for OA.
Another promising anti-inflammatory factor is tissue inhibitor of metalloproteinase-1 (TIMP-1), which acts as an inhibitor of matrix metalloproteinases (MMPs).21 MMPs are enzymes responsible for the degradation of the extracellular matrix (ECM) in cartilage.21 In a prior study, Tamura et al. Investigated the role of rhein in regulation of the production of MMPs and TIMP-1 in rabbit articular chondrocytes.22 The results demonstrates that rhein, as an active metabolite of diacerein, downregulates the gene expression and production of proMMPs while upregulating the production of TIMP-1.22 The chondroprotective effect of rhein, observed in this study, may contribute to the therapeutic effects of diacerein in the treatment of OA.22
Therapeutic Genes
Genes associated with the maintenance of cartilage homeostasis are integral to the health and proper functioning of cartilage tissue. Among these genes, such as aggrecan, collagen type II, and SOX-9, significant research has been conducted to explore their potential applications in gene therapy for OA.23
SOX-9, for instance, has been thoroughly examined for its therapeutic potential in OA gene therapy.24 Jeong et al developed nanoparticles consisting of dexamethasone-conjugated polyethylenimine (DEX PEI) complexed with a minicircle plasmid (MC) carrying the SOX-9, −6 and small hairpin RNA targeting ANGPTL4 (shANG).25 Adipose-derived stem cells (ADSCs) transfected with MC SOX9/6/shANG (referred to as MC SOX9/6/shANG-tADSCs) demonstrated significantly higher expression of the COL2 gene and protein compared to ADSCs transfected with MC SOX9/6 (referred to as MC SOX9/6-tADSCs) during in vitro chondrogenesis.25 Both MC SOX9/6/shANG-tADSCs and MC SOX9/6-tADSCs enhanced chondrogenesis even without the addition of growth factors, in comparison to negative controls. Moreover, in vivo experiments using rats with surgically-induced OA, synovial fluid analysis revealed significantly lower levels of cyclooxygenase (COX-2) and MMP13 in the MC SOX9/6/shANG-tADSC-treated rats compared to the MC SOX9/6-tADSC-treated rats.25 These findings demonstrate that the use of dual-functional nanoparticles containing the SOX9/6 can enhance chondrogenesis in ADSCs and reduce inflammation in OA.
Non-Coding RNAs
Non-coding RNAs, including microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), hold significant therapeutic potential in gene therapy for OA,26 primarily through their involvement in gene expression regulation and modulation of various cellular processes such as differentiation, proliferation, and apoptosis.27 These non-coding RNAs have emerged as potential targets for OA treatment.
MiRNAs, a class of small non-coding RNAs, regulate gene expression post-transcriptionally by binding to the 3’-untranslated region (UTR) of target mRNAs, leading to mRNA degradation or translational repression.27 Several miRNAs have been identified as potential therapeutic targets for OA, including miR-140 and miR-483.28,29 For an example of MiR-140, a chondrocyte-specific miRNA, plays a critical role in cartilage homeostasis by modulating the expression of genes involved in ECM synthesis and degradation.30 Overexpression of miR-140 has demonstrated the capacity to promote chondrogenesis and cartilage repair in animal models of OA.30 In a prior study, Liang et al introduced chondrocyte-targeting exosomes as vehicles for the delivery of miR-140, offering a novel approach for OA treatment.31 To achieve chondrocyte-specific delivery, the researchers engineered exosomes by fusing a chondrocyte-affinity peptide (CAP) with the lysosome-associated membrane glycoprotein 2b protein on the exosome surface. These modified exosomes, termed CAP-exosomes, efficiently encapsulated miR-140 and demonstrated the ability to specifically enter chondrocytes and deliver the cargo in vitro. Furthermore, CAP-genes successfully delivered miR-140 to deep cartilage regions by penetrating the dense mesochondrium. This targeted delivery resulted in the inhibition of cartilage-degrading proteases and alleviation of OA progression in a rat model.
LncRNAs are RNA molecules longer than 200 nucleotides that do not encode proteins but play a role in regulating gene expression at the transcriptional or post-transcriptional level. Several lncRNAs have been identified as potential therapeutic targets for OA, including H19, MALAT1, and MEG3.32–34 Suppression of MALAT1 has shown promise in promoting chondrogenesis and facilitating cartilage repair in OA animal models. For example, as a bioactive molecule, docosahexaenoic acid (DHA) exhibited anti-inflammatory and chondroprotective effects in OA chondrocytes.35 Of note, emerging evidence suggests Malat1 is the key mediator of DHA’s anti-inflammatory, chondroprotective, and chondrogenic effects.35 In a surgical model of OA in mice, the researchers found DHA effectively mitigated cartilage loss and damage, and led to a reduction in elevated serum levels of Malat1 associated with OA.35 These findings demonstrate that DHA exerts anti-inflammatory, chondroprotective, and chondrogenic effects possibly through the regulation of Malat1 levels.
CircRNAs are circular RNAs generated by back-splicing of pre-mRNAs, and their circular structure renders them resistant to degradation by RNA exonucleases. Several circRNAs have been identified as potential therapeutic targets for OA, including circRNA-UBE2G1 and circRNA.33186.36,37 For instance, Zhou et al investigated the function of circRNA.33186 in OA.37 They observed a significant upregulation of circRNA.33186 in chondrocytes treated with IL-1β and in cartilage tissues from a mouse model of OA induced by destabilized medial meniscus (DMM).37 CircRNA.33186 knockdown promoted chondrocyte proliferation and inhibited apoptosis in IL-1β-treated cells.37 In vivo silencing of circRNA.33186 effectively alleviated OA in the DMM mouse model.37 These findings demonstrate the significant role of circRNA.33186 in the progression of OA and highlight its potential as a therapeutic target for OA treatment.
Delivery Strategies for Biomaterial-Based Gene Therapy
Biomaterials assume a critical role as carriers or delivery vehicles for transporting gene therapies to targeted tissues affected by OA.38,39 The choice of suitable biomaterials hinges on several crucial factors, notably biocompatibility, biodegradability, and mechanical properties. Thoughtful evaluation of these elements guarantees the effective and efficient delivery of therapeutic genes to the intended target site.38
Lipid-Based Nanoparticles (LNPs)
LNPs have emerged as a highly versatile and extensively employed class of delivery vehicles within the domain of gene therapy.40 Their broad adoption stems from their exceptional biocompatibility, low toxicity profiles, and impressive efficacy in protecting and efficiently delivering nucleic acids.41 LNPs are composed of a well-defined lipid bilayer structure encompassing a hydrophobic core, providing a conducive environment for the encapsulation and preservation of nucleic acids, thereby shielding them from degradation encountered within the bloodstream.40
The unique architectural design of LNPs enables them to effectively shield nucleic acids during systemic circulation, protecting their integrity and bioactivity until they reach the target tissues.42 The lipid bilayer confers stability to the LNPs, preventing premature release of the encapsulated nucleic acids and ensuring their sustained protection until reaching the desired site of action.43 Moreover, LNPs exhibit excellent adaptability through the ability to be modified with ligands, such as targeting moieties or specific receptors, to facilitate cell- or tissue-specific delivery.43 This capability enhances the precision and efficacy of gene delivery, enabling LNPs to navigate through biological barriers, selectively interact with target cells, and improve the uptake efficiency of the delivered nucleic acids.42 This targeted approach holds significant promise for gene therapy applications in OA, as it allows for the preferential delivery of therapeutic genes to the affected joint tissues, mitigating off-target effects and optimizing treatment outcomes.
Polymeric Nanoparticles (PNPs)
PNPs serve as another prevalent and highly versatile biomaterial employed in gene therapy applications.44 Consisting of polymers that can autonomously assemble into nanoparticles, they provide unique advantages, including the controlled release of gene therapies and customized surface modifications to improve tissue targeting.45
The self-assembly property of polymeric nanoparticles facilitates the formation of stable structures that effectively encapsulate and protect the therapeutic genetic material.46 This protective encapsulation shields the nucleic acids from enzymatic degradation and maintains their stability during circulation, ensuring their intact delivery to the target tissues.47
Furthermore, polymeric nanoparticles can be engineered with various surface modifications to optimize their targeting capabilities towards specific tissues or cell types.48 Through the attachment of ligands or targeting moieties, such as antibodies or peptides, the nanoparticles can selectively interact with receptors on the desired cells, thereby enhancing their uptake and intracellular delivery of the therapeutic genes.49
Polyethyleneimine (PEI) is a frequently utilized cationic polymer for gene delivery due to its capacity to create complexes with negatively charged nucleic acids through electrostatic interactions.50 This complexation enables efficient packaging and protection of the genetic material during delivery.51 However, it is important to note that PEI can exhibit toxicity at high doses, thereby necessitating careful optimization of the polymer concentration to strike a balance between delivery efficiency and potential cytotoxic effects.
Hydrogels
Hydrogels, intricate three-dimensional (3D) networks comprising crosslinked hydrophilic polymers, possess exceptional properties that make them an attractive biomaterial in gene therapy.52–54 One prominent characteristic of hydrogels is their ability to absorb and retain substantial amounts of water within their structure. This unique water-absorbing capacity allows hydrogels to serve as effective vehicles for the encapsulation and protection of nucleic acids, crucial for preserving the integrity and bioactivity of gene therapies.55
The biocompatibility of hydrogels further contributes to their suitability for gene therapy applications.56 Hydrogels can provide a favorable environment for cells and tissues, minimizing adverse immune responses and promoting cell viability and function. Their biocompatible nature ensures compatibility with the surrounding biological environment, reducing the risk of host rejection or inflammation.
One significant advantage of hydrogels is their tunable mechanical properties.57 Through the regulation of polymer composition, crosslinking density, and formulation parameters, it is possible to customize the mechanical properties of hydrogels to align with the particular demands of the target tissue or application. This adaptability empowers hydrogels to replicate the mechanical attributes of natural tissues, promoting their integration and providing the necessary scaffolding for cellular growth and tissue regeneration.
Additionally, hydrogels can be modified to allow for sustained release of gene therapy over time.58 By incorporating drug delivery mechanisms into the hydrogel structure, controlled and prolonged release of therapeutic genes can be achieved. This sustained release feature is particularly advantageous in gene therapy, as it ensures a continuous and controlled supply of therapeutic agents, enhancing their efficacy and potentially reducing the frequency of administration.57,59
The unique combination of encapsulation and protection of nucleic acids, biocompatibility, tunable mechanical properties, and sustained release capabilities makes hydrogels an appealing choice for gene therapy applications. Their potential in facilitating efficient and controlled delivery of therapeutic genes holds promise for various fields, including OA, where hydrogels can contribute to promoting cartilage regeneration and tissue repair.38
Viral Vectors
Viral vectors, which are genetically modified viruses, represent a powerful tool in gene therapy as they possess inherent capabilities for efficient gene delivery to target cells.60 However, it is important to recognize both the advantages and potential challenges associated with their utilization.
Viral vectors exhibit excellent efficiency in delivering therapeutic genes to target cells, owing to their natural ability to infect and enter host cells.61 Their viral structure and genetic modifications allow for the precise insertion and expression of therapeutic genes within the host genome. This efficient gene delivery mechanism enhances the potential for successful gene therapy outcomes.61
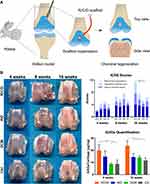
Among the viral vectors utilized in OA gene therapy, adeno-associated virus (AAV) and lentivirus have obtained considerable attention.62 AAV, a non-pathogenic virus, has demonstrated notable advantages due to its ability to mediate long-term gene expression in both dividing and non-dividing cells.63 This characteristic is particularly advantageous in the context of OA, as it allows for sustained therapeutic gene expression within the joint tissues. For instance, Ji et al presented compelling evidence establishing the indispensable role of hematopoietic pre-B cell leukemia transcription factor-interacting protein (HPIP) in the development of OA.64 Specifically, in vivo experiments involving intra-articular administration of AAV containing HPIP-specific short hairpin RNA demonstrated attenuation of histological manifestations associated with OA. Moreover, the in vitro analyses utilizing RNA-sequencing and chromatin immunoprecipitation sequencing profiles have identified HPIP as a crucial modulator of cartilage degeneration in OA, primarily through the transcriptional activation of Wnt target genes (Figure 2). These findings collectively suggest that HPIP represents a promising target within the regulatory network governing OA.
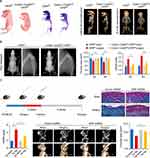 |
Figure 2 Representative example of AAV-based gene therapy in OA. (A) Col2a1-CreERT2; HPIPf/f mice exhibit skeletal and cartilage abnormalities. (B) Plain radiographs of the whole body (left) and knee (right) were taken for both HPIPf/f and Col2a1-CreERT2; HPIPf/f littermates at 8 weeks of age. And the immunohistochemistry assays were conducted using specific antibodies on mouse samples obtained 8 weeks after anterior cruciate ligament transection. (C) The experimental design involved the utilization of AAV containing HPIP-specific shRNA for the treatment of OA in mice. Safranin O/fast green staining was performed on the entire tibias of mice. The staining was carried out two weeks after surgery, where 8-week-old C57/BL6J mice underwent intra-articular injection of AAV carrying HPIP-specific shRNA. *p<0.05. Reproduced from Ji Q, Xu X, Kang L, Xu Y, Xiao J, Goodman SB, et al. Hematopoietic PBX-interacting protein mediates cartilage degeneration during the pathogenesis of osteoarthritis. Nat Commun. 2019;10(1):313.http://creativecommons.org/licenses/by/4.0/. Copyright © The Author(s) 2019.64 |
Lentivirus, another commonly employed viral vector, possesses high gene-carrying capacity and the ability to infect a broad range of cell types, including both dividing and non-dividing cells.65 These properties render lentivirus an attractive option for gene delivery in OA gene therapy, facilitating effective transduction of target cells.66
Nevertheless, it is important to address potential concerns associated with viral vectors, including immunogenicity and adverse reactions.67 Due to their viral origin, viral vectors can elicit an immune response in the host, leading to the generation of neutralizing antibodies. In some cases, this immune response can limit the effectiveness of subsequent gene therapy treatments.67 Additionally, the immunogenicity of viral vectors can result in unwanted immune reactions, potentially impacting patient safety and treatment outcomes.68 Careful consideration of the immune response and strategies to mitigate adverse reactions is essential in the development and implementation of viral vector-based gene therapies.
Non-Viral Vectors
Non-viral vectors, which encompass a diverse range of biomaterials, represent an alternative approach to gene delivery in comparison to viral vectors.69 They offer distinct advantages, including enhanced safety profiles, ease of production, and reduced immunogenicity, although their gene delivery efficiency may be comparatively lower than that of viral vectors.70
One commonly used non-viral vector is naked plasmid DNA, which involves the direct delivery of DNA without the use of a carrier system.71 Naked plasmid DNA possesses the advantage of simplicity in design and production, making it a convenient option for gene delivery.72 In a recent study, Cai et al developed biomimetic cupper sulfide@phosphatidylcholine (CuS@PC) NPs loaded with plasmid DNA encoding TGF-β1 to enhance cartilage regeneration.13 Notably, CTP-MSCs demonstrated the ability to inhibit extracellular matrix degradation in chondrocytes induced by IL-1β.Furthermore, through intra-articular administration, CTP-MSCs significantly enhanced the repair of damaged cartilage in a mouse model (Figure 3). These findings highlight the potential of our novel non-viral vectors as a strategy to overcome the limitations of current stem cell therapies for treating OA.
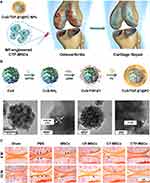 |
Figure 3 A representative example of non-viral vector-based gene delivery for OA therapy. (A) CTP-MSCs were intra-articularly injected into the knee and beneficially enhanced cartilage regeneration. (B) Schematic illustration of the preparation and characterizations of the NPs. The white arrows indicate the presence of a thin layer, approximately 2 nanometers thick, of phosphatidylcholine (PC) on the nanoparticles, signifying the successful surface modification of PC. (C) Safranin-O/Fast green staining staining of the knee joints after receiving different treatments. The black arrows point to the cartilage lesions. Reproduced from Cai Y, Wu C, Ou Q, et al. Enhanced osteoarthritis therapy by nanoengineered mesenchymal stem cells using biomimetic CuS nanoparticles loaded with plasmid DNA encoding TGF-beta1. Bioact Mater. 2023;19:444–457.Creative Commons Attribution 4.0 International License creativecommons.org/licenses/by-nc-nd/4.0/.13 © 2022 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co. Ltd. |
NPs, another category of non-viral vectors, comprise a diverse array of materials such as polymers, lipids, and metals.73–76 These nanoparticles can encapsulate and protect the therapeutic genes, facilitating their delivery to target cells. The surface properties of nanoparticles can be modified to enhance their stability, target specific cell types or tissues, and facilitate cellular uptake.74 While non-viral nanoparticles may exhibit lower gene delivery efficiency compared to viral vectors, they offer advantages such as ease of production, tunability, and lower immunogenicity.73,75 Researchers continue to refine nanoparticle design and formulation to optimize their gene delivery capabilities.
In cartilage regeneration, the choice between viral and non-viral vectors for gene delivery is a critical decision, influenced by a multitude of factors and inherent challenges. Viral vectors offer the distinct advantage of high transduction efficiency, which can be vital for introducing therapeutic genes into the relatively avascular and dense cartilage tissue. However, their use comes with significant limitations.77–79 One prominent concern is immunogenicity; many viral vectors, particularly adenoviral and lentiviral vectors, can provoke an immune response when introduced into the body. This immune response may lead to inflammation and hinder the healing process, a particularly sensitive issue when dealing with the delicate cartilage.80 Another concern is the risk of insertional mutagenesis, which is primarily associated with retroviral vectors. This feature, while valuable in certain applications, raises concerns in the context of cartilage regeneration, as it could result in uncontrolled cell growth or other undesirable genetic changes.80 Moreover, the duration of transgene expression in cartilage can be limited by the immune response, resulting in the clearance of transduced cells over time.80 Additionally, many viral vectors have limited packaging capacities, which can be a significant obstacle when attempting to deliver larger therapeutic genes or multiple genes simultaneously, often required for complex cartilage regeneration.80
On the other hand, non-viral vectors are generally considered safer in terms of immunogenicity and insertional mutagenesis, as they lack viral components. While this enhanced safety is advantageous in cartilage regeneration to avoid complications, non-viral vectors tend to exhibit lower transfection efficiency, making it more challenging to deliver therapeutic genes to target cells within the dense and avascular cartilage tissue.79 Achieving sufficient transfection levels may require optimization. Customizability is another key advantage of non-viral vectors; they can be tailored for specific applications, including cartilage regeneration, allowing for improved targeting and controlled release of therapeutic genes.79 Taken together, the choice of vector for cartilage regeneration should be a carefully considered decision, taking into account the specific requirements of the application, the desired level of control over transgene expression, and potential safety concerns, striking a balance between efficiency and safety by optimizing the vector system for the specific needs of cartilage repair.
Delivery Administration
The efficacy of gene therapy in OA critically depends on the precise and efficient delivery of therapeutic genes to the affected tissues. To achieve this objective, investigators have undertaken extensive investigations into various delivery methods and routes for biomaterial-based gene therapy in OA. These strategies are designed to enhance gene delivery efficiency, mitigate off-target effects, and ensure the targeted deposition of therapeutic genes at the intended sites of action.
Intra-Articular Injection
Intra-articular injection stands out as a notable and extensively explored delivery method for biomaterial-based gene therapy in OA.81 This approach entails the direct administration of the gene therapy into the joint space, offering several distinct advantages. By delivering the therapeutic genes locally, intra-articular injection enables targeted delivery, ensuring a concentrated and immediate effect within the affected joint tissues.
Studies conducted in animal models of OA have demonstrated the efficacy of intra-articular injection in promoting therapeutic outcomes.16,82 This approach has shown promise in ameliorating disease progression, reducing cartilage degradation, and alleviating OA symptoms. Furthermore, the localized delivery provided by intra-articular injection contributes to minimizing potential systemic side effects, enhancing the safety profile of the treatment.83 For instance, Di Francesco et al. Introduced a treatment of OA using intra-articular injections of shape-defined poly(d,l-lactide-co-glycolide) acid microPlates (μPLs) loaded with dexamethasone (DEX).84 The researchers evaluated the therapeutic effects of DEX-loaded μPLs compared to free DEX. They found that a single intra-articular injection of DEX-μPLs reduced the expression of inflammatory mediators. This suggests that the sustained release of DEX from μPLs had a more prolonged and effective anti-inflammatory effect. Furthermore, the DEX-μPLs treatment was found to reduce load-induced histological changes in the articular cartilage and synovial tissues when compared to saline or free DEX treatments (Figure 4). This indicates that the μPLs not only provided sustained drug release but also had a mechanical benefit in protecting the joint tissues from damage caused by overload.
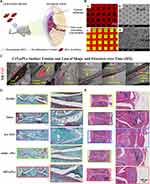 |
Figure 4 A representative example of gene therapy in OA through Intra-articular injection. (A) The schematic illustration of intra-articular injection of DEX-μPLs for treating OA. (B) The geometrical characterization of μPLs. (a) Utilizing confocal microscopy and (b) scanning electron microscopy (SEM), an examination was conducted on an unoccupied polyvinyl alcohol (PVA) template. Subsequently, (c) confocal microscopy was employed to scrutinize a PVA template, delineated in red, that had been imbued with a paste composed of poly(lactic-co-glycolic acid) (PLGA) and curcumin (CURC), resulting in the formation of CURC-loaded microscale particles (CURC-μPLs) displayed in green and yellow. Finally, (d) SEM was used to capture images of individual μPLs that had been liberated from the PVA template. The inset on the side displays an enlarged and angled perspective of the μPLs. (C) Confocal microscopy imaging of Cy5-μPLs within the mouse knee joint. TD = Transmission detector. The yellow arrows denote the names of the tissues. (D) Safranin-O staining of joint sections in a mouse model of OA. Safranin-O staining revealed several significant findings: cartilage erosion, indicated by arrows; cartilage fissures, marked with “#” (with “###” indicating a higher degree of fissures); and regions of reduced safranin-O staining, represented by “*”. (E) Hematoxylin and eosin (H&E) staining of joint sections in a mouse model of OA. Histological analysis unveiled several noteworthy features, including mineralization, designated by “#” (with “###” indicating a greater extent of mineralization); cellular infiltration, represented by “*”. Reproduced with permission from Di Francesco M, Bedingfield SK, Di Francesco V, Colazo JM, Yu F, Ceseracciu L, et al. Shape-Defined microPlates for the Sustained Intra-articular Release of Dexamethasone in the Management of Overload-Induced Osteoarthritis. ACS Appl Mater Interfaces. 2021;13(27):31379–92. Copyright © 2021 The Authors. Published by American Chemical Society. This publication is licensed under CC-BY 4.0.84 |
By employing intra-articular injection, researchers strive to capitalize on its localized delivery capabilities, promoting efficient gene uptake by the target cells within the joint environment.83,85 The ability to accurately position the gene therapy biomaterials within the joint space under imaging guidance further enhances the potential for successful outcomes in OA gene therapy. Continued advancements in this delivery method hold promise for optimizing treatment efficacy and advancing the translation of biomaterial-based gene therapies from preclinical studies to clinical applications for OA patients.
Systemic Delivery
Systemic delivery, encompassing intravenous administration or other systemic routes, represents a strategy for achieving widespread distribution of gene therapy throughout the body.86 This approach holds promise for delivering therapeutic genes to multiple affected joints in cases of OA or targeting systemic factors contributing to disease progression. However, the inherent challenges associated with systemic delivery necessitate careful consideration and ongoing research to ensure both the safety and efficacy of this approach.
By delivering the gene therapy systemically, therapeutic genes can reach not only the target joint but also other affected sites, allowing for a broader impact on disease pathology.87 This approach offers the potential to address systemic factors contributing to OA and offers a convenient non-invasive administration route.88 Moreover, systemic delivery can facilitate the treatment of bilateral OA or patients with multiple affected joints simultaneously.88
However, it is essential to notice that systemic delivery may be accompanied by potential drawbacks, including off-target effects and potential toxicity.87,89 The widespread distribution of the gene therapy throughout the body increases the likelihood of unintended gene expression in non-target tissues, which can lead to undesired effects. Additionally, the potential for systemic toxicity arises due to the exposure of various organs and tissues to the therapeutic genes or delivery vectors.
To ensure the safe and effective implementation of systemic delivery for OA gene therapy, further comprehensive studies are needed. Ongoing research aims to elucidate the optimal gene therapy formulations, dosing regimens, and delivery vectors to minimize off-target effects and maximize therapeutic efficacy. Rigorous evaluation of the safety profile, potential immune responses, and long-term effects of systemic delivery is essential for its successful translation into clinical practice.
Implantable Devices
Implantable devices have emerged as a promising approach for gene therapy in OA by enabling localized and sustained delivery of therapeutic genes within the affected joint.90 These devices, including hydrogels and nanoparticles, offer distinct advantages in terms of controlled release kinetics and targeted delivery, but their utility may be hindered by the invasiveness of the implantation procedure.12
Hydrogels, three-dimensional networks of crosslinked hydrophilic polymers, can be designed as implantable devices to encapsulate and protect the gene therapy within the joint.91 These hydrogel-based implants offer a localized depot for sustained release, allowing for a continuous and controlled delivery of therapeutic genes over an extended period. By modulating the properties of the hydrogel, such as its composition, degradation rate, and porosity, the release kinetics of the gene therapy can be tailored to match the desired therapeutic needs.91 Hydrogel implants hold great potential in promoting cartilage regeneration, reducing inflammation, and improving joint function.
Similarly, nanoparticles can be utilized as implantable devices for localized gene delivery within the joint. These small particles, typically composed of polymers or lipids, can be loaded with therapeutic genes and implanted directly into the affected joint.90 Once implanted, the nanoparticles release the therapeutic genes in a sustained manner, providing a localized and controlled delivery over an extended period. The surface properties of nanoparticles can be engineered to enhance their stability, cellular uptake, and target-specific interactions, enabling precise and efficient gene delivery to the desired cells within the joint tissues.
Despite their potential advantages, the use of implantable devices in gene therapy for OA may face limitations due to the invasiveness of the implantation procedure. Implanting these devices requires a surgical intervention, which adds complexity and potential risks associated with the procedure.92 Moreover, the implantation process may cause tissue damage, inflammation, or infection, thus necessitating careful consideration of the balance between therapeutic benefits and the invasiveness of the approach.
Various Strategies Utilized in Gene Delivery for OA Therapy
LNPs-Enhanced Gene Delivery
Numerous studies have demonstrated the effectiveness of LNPs in delivering therapeutic genes to chondrocytes, the primary cells responsible for maintaining cartilage integrity in joints.93 In a prior study, Jain et al prepared diacerein-loaded solid LNPs (SLNs) using stearic acid, a long-chain fatty acid, as the lipid component.94 Pluronic F68 and soya lecithin were used as surfactants, and citric acid was added to create an acidic environment for the drug. In vitro release studies showed a controlled and extended release profile of diacerein from the SLNs for up to 12 hours. The in vivo pharmacokinetic study revealed an enhanced oral bioavailability of approximately compared to free diacerein. Additionally, the study found that the use of diacerein-loaded SLNs reduced the diarrheal side effects of diacerein by up to 37% compared to free diacerein. These findings demonstrate that diacerein-loaded SLNs can efficiently provide controlled and prolonged drug release. The formulation enhances the oral bioavailability of diacerein, reduces its side effects, and potentially improves patient compliance for OA treatment.
To enhance targeting capabilities within OA, LNPs have been modified with ligands to selectively recognize and deliver genes to specific cells or tissues. For instance, Jain et al focused on the targeted delivery of the antiosteoarthritic drug diacerein to articular tissue using SLNs modified with soluble polysaccharide chondroitin sulfate (ChS), called ChS-DC-SLN.95 The in vitro and in vivo results suggest that ChS-DC-SLN has the potential to enhance the overall efficacy of OA treatment. The targeted delivery of diacerein to articular tissue using ChS-DC-SLN may provide a promising approach for improving the therapeutic outcomes in OA.
These studies underscore the potential of LNPs as a versatile and efficient tool for gene delivery in OA therapy. LNPs demonstrate the ability to safely and effectively deliver therapeutic genes to chondrocytes, promoting crucial cellular activities and providing promising outcomes for cartilage regeneration. Furthermore, the engineering of LNPs to achieve specific targeting enhances their utility in facilitating precise gene delivery to the desired cells or tissues in the context of OA treatment.
PNPs
PNPs are extensively employed for gene delivery, with polyethyleneimine (PEI) being a widely used polymer due to its cationic nature that facilitates condensation and protection of negatively charged nucleic acids.25 PEI, characterized by a high positive charge density, establishes strong electrostatic interactions with nucleic acids, resulting in the formation of stable complexes known as polyplexes.25 However, the toxicity associated with high molecular weight PEI restricts its clinical applications. Consequently, investigations have focused on exploring low molecular weight PEI and other biocompatible and biodegradable polymers, such as poly(lactic-co-glycolic acid) (PLGA), chitosan, and poly(beta-amino ester) (PBAE), for gene delivery purposes.96–98
To enhance targeting and cellular uptake, PNPs can be engineered with surface modifications. The functionalization of PNPs with ligands, including peptides or antibodies, improves their binding affinity to cell surface receptors, facilitating endocytosis and enhancing intracellular delivery of the gene therapy.24 In the context of OA treatment, PNPs have been functionalized with various ligands, such as hyaluronic acid, which exhibits binding affinity towards CD44 receptors expressed on chondrocytes and synovial cells.99 Additionally, integrin-targeting peptides have been employed, which can bind to αvβ3 integrin receptors expressed on the surface of inflamed cells within the joint.100
In addition to their targeting capabilities, PNPs can be engineered to enable controlled release of the gene therapy.98 This can be achieved through the degradation of the polymer matrix or by surface modification with stimuli-responsive moieties, such as pH or temperature-sensitive polymers. Such design strategies afford the ability to achieve sustained gene expression over an extended duration, reducing the frequency of repeated administrations.
Hydrogel-Based Delivery Systems
Hydrogels offer versatile options for gene delivery in OA, which can be administered through direct injection into the affected joint or via implantable devices, such as hydrogel scaffolds, that provide sustained release of the gene therapy.38,101 The selection of an appropriate hydrogel system depends on several crucial factors, including biocompatibility, biodegradability, mechanical properties, and the ability to encapsulate and release the therapeutic genes effectively.38
One noteworthy example of a hydrogel-based gene delivery system in OA involves the utilization of chitosan hydrogels. Chitosan, a biodegradable polysaccharide derived from chitin found in crustacean exoskeletons, has demonstrated biocompatible and biodegradable characteristics, making it an attractive candidate for gene delivery applications.102 Chitosan hydrogels have exhibited the ability to effectively protect and deliver nucleic acids.103 Moreover, their properties can be tailored through modifications with various chemical groups to enhance targeting capabilities towards specific tissues. Man et al constructed a hybrid scaffold consisting of chitosan hydrogel (CS) and demineralized bone matrix (DBM) for the repair of rabbit cartilage injuries.104 The in vitro and in vivo results demonstrated that the transplantation of allogenic chondrocytes using the CS/DBM scaffold successfully repaired rabbit cartilage injuries with a one-step procedure. This approach provides valuable insights into the field of cartilage tissue engineering and offers potential for clinical applications in cartilage repair and regeneration (Figure 5).
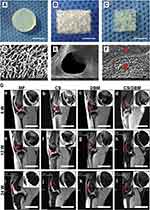 |
Figure 5 Chitosan hydrogel-based delivery systems for OA gene therapy. (A–F) Structural characterization of the different scaffolds. The red asterisk (*) designates the DBM component within the CS/DBM scaffold, whereas the red hash symbol (#) signifies the presence of the CS component within the same CS/DBM scaffold. (G). MRI examination of specimens after receiving the surgery of cartilage repair. (a-l) Te various lateral slices of the MRI examination. In the upper-right corner of each image, the inset displays an enlarged view of the corresponding zoomed region, enclosed by a red circle. Reproduced with permission from Man Z, Hu X, Liu Z, Huang H, Meng Q, Zhang X, et al. Transplantation of allogenic chondrocytes with chitosan hydrogel-demineralized bone matrix hybrid scaffold to repair rabbit cartilage injury. Biomaterials. 2016;108:157–67. © 2016 Elsevier Ltd. All rights reserved.104 |
Additionally, several other hydrogel systems have been explored for gene therapy in OA, including hyaluronic acid-based hydrogels, alginate-based hydrogels, and fibrin-based hydrogels.39,105,106 These hydrogel platforms have shown promising results in preclinical studies, exhibiting the ability to encapsulate and deliver therapeutic genes effectively. However, further research is necessary to comprehensively evaluate the safety, efficacy, and long-term effects of these hydrogel systems in clinical settings.
Viral Vectors for Cartilage Regeneration
Viral vectors represent an effective tool for delivering therapeutic genes to cells, exhibiting high efficiency in gene transfer and finding widespread application in OA gene therapy.80 AAV, a small non-enveloped virus, is capable of infecting both dividing and non-dividing cells, with low immunogenicity and a favorable safety profile.39 Importantly, AAV can integrate into the host genome at a precise location on chromosome 19, enabling stable and long-term expression of the therapeutic gene. Numerous studies have successfully utilized AAV-mediated gene therapy for OA, particularly in delivering genes encoding anti-inflammatory cytokines and growth factors.39,80,107
Lentiviruses, enveloped viruses with the ability to infect both dividing and non-dividing cells, similarly provide an advantageous platform for OA gene therapy.108 Like AAV, lentiviral vectors possess the ability to integrate into the host genome, ensuring sustained and long-term gene expression. Consequently, lentiviral vectors have been employed for gene therapy in OA to deliver genes encoding anti-inflammatory cytokines and chondrogenic factors, among others.80 It is worth noting that the use of lentiviral vectors can be constrained by potential immunogenicity and the risk of insertional mutagenesis. Insertional mutagenesis entails the activation of oncogenes or inactivation of tumor suppressor genes due to viral vector integration.
Despite the efficacy of viral vectors in gene delivery, concerns regarding immunogenicity and adverse reactions in some patients have prompted exploration of non-viral vectors as an alternative approach for gene therapy in OA. The development of non-viral vectors aims to overcome the limitations associated with viral vectors, offering potential advantages such as improved safety profiles and reduced immunogenic responses.
Non-Viral Vectors Delivery for OA
Non-viral vectors represent a class of biomaterials devoid of viral components and are generally considered safer alternatives to viral vectors in gene therapy applications.77 Naked plasmid DNA, nanoparticles, and liposomes are prominent examples of non-viral vectors. While these biomaterials are typically less efficient than viral vectors in terms of gene delivery, they offer advantages such as ease of production and reduced immunogenicity.78
Naked plasmid DNA stands out as a widely utilized non-viral vector in OA gene therapy.79 Delivery of naked plasmid DNA to the target tissue can be achieved through various techniques, including direct injection, electroporation, or gene gun-mediated delivery.109 It is important to note that naked plasmid DNA may trigger an immune response, which can hamper its efficacy in certain cases.
Nanoparticles, another type of non-viral vector, have garnered significant attention for their potential in gene delivery for OA.77 Notably, surface modifications can be employed to enhance their targeting abilities towards specific tissues. Liposomes, spherical lipid vesicles, have emerged as an appealing option for encapsulating and delivering nucleic acids to target cells.77,110 They possess desirable traits such as biodegradability and biocompatibility, and their surfaces can be modified with ligands to facilitate targeting of specific cells or tissues. Liposomes have been explored as potential gene delivery vehicles for OA treatment, although their utility has been somewhat limited by their relatively low transfection efficiency.
Biomaterial-based gene therapy for cartilage regeneration is a promising avenue in regenerative medicine. Various ongoing clinical trials and case studies have explored the practical applicability of this approach, with the goal of enhancing cartilage repair and regeneration. One notable study involved the integration of gene therapy with autologous chondrocyte implantation (ACI), where a biodegradable biomaterial scaffold loaded with plasmid DNA encoding for bone morphogenetic protein-2 (BMP-2) was employed to stimulate cartilage formation, showing promising results in a rabbit model.111 In addition, a study explored the safety and efficacy of a biomaterial-based gene therapy for OA, employing a gene-activated matrix (GAM) to deliver therapeutic genes to the affected joint.112 Moreover, researchers have been investigating the use of AAV vectors in combination with biomaterials to deliver therapeutic genes, modifying the local cellular environment to promote cartilage regeneration.61 As the field rapidly advances, ongoing clinical trials and emerging case studies continue to shape the practical landscape of biomaterial-based gene therapy for cartilage repair.
Challenges and Perspectives
One of the significant challenges in biomaterial-based gene therapy for OA lies in obtaining efficient and targeted delivery of therapeutic genes to the affected joint.23 Non-specific gene delivery can elicit undesired immune responses or toxic effects, impeding therapeutic efficacy. Moreover, the complex structure of the joint and the lack of blood vessels in cartilage present substantial obstacles to achieving effective gene delivery.81
Another significant challenge is the careful selection of suitable gene targets and the development of effective gene therapy strategies.83 A comprehensive understanding of OA’s pathogenesis and the identification of specific molecular targets are imperative for the successful development of gene therapy approaches.
Furthermore, gene therapy entails unique safety challenges, including the potential for insertional mutagenesis and immune reactions triggered by viral vectors employed for gene delivery.113 Thorough evaluation of the safety of gene therapy through preclinical studies is essential prior to initiating clinical trials.
To address technical challenges and limitations, researchers are exploring various approaches to enhance gene delivery and targeting. For instance, the use of viral vectors with improved specificity and reduced immunogenicity holds promise for enhancing the efficiency and safety of gene therapy.80 Additionally, the development of non-viral gene delivery systems, such as nanoparticles or liposomes, offers a potentially safer alternative for gene delivery.77 Another promising avenue entails the development of gene editing technologies, such as CRISPR-Cas9, which could rectify specific genetic mutations underlying OA.114 Gene editing holds the potential to provide a more targeted and precise therapy for OA compared to traditional gene therapy approaches.115
Addressing safety and regulatory considerations, continuous collaboration among researchers, regulatory bodies, and the pharmaceutical industry is crucial to guarantee the secure and efficient development and application of gene therapy products. Additionally, further research is imperative to gain a comprehensive understanding of the long-term safety and effectiveness of gene therapy for treating osteoarthritis.
Funding
This study was supported by Henan Province Science and Technology Tackling Key Issues Project (232102310060, 192102310397), Henan Provincial Department of Science and Technologyand Joint Co-construction Project of Medical Science and Technology Tackling Programme of Henan Province (LHGJ20210964)Health and Health Commission of Henan Province.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Abramoff B, Caldera FE. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104(2):293–311. doi:10.1016/j.mcna.2019.10.007
2. Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–2126. doi:10.1016/S0140-6736(11)60243-2
3. Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. doi:10.1038/nrdp.2016.72
4. Rannou F, Pelletier JP, Martel-Pelletier J. Efficacy and safety of topical NSAIDs in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4 Suppl):S18–21. doi:10.1016/j.semarthrit.2015.11.007
5. Cho Y, Jeong S, Kim H, et al. Disease-modifying therapeutic strategies in osteoarthritis: current status and future directions. Exp Mol Med. 2021;53(11):1689–1696. doi:10.1038/s12276-021-00710-y
6. Evans CH, Ghivizzani SC, Robbins PD. Osteoarthritis gene therapy in 2022. Curr Opin Rheumatol. 2023;35(1):37–43. doi:10.1097/BOR.0000000000000918
7. Madry H, Cucchiarini M. Gene therapy for human osteoarthritis: principles and clinical translation. Expert Opin Biol Ther. 2016;16(3):331–346. doi:10.1517/14712598.2016.1124084
8. Wang Y, Chu X, Wang B. Recombinant adeno-associated virus-based gene therapy combined with tissue engineering for musculoskeletal regenerative medicine. Biomater Transl. 2021;2(1):19–29. doi:10.3877/cma.j.issn.2096-112X.2021.01.004
9. Xia K, Wang F, Lai X, et al. AAV-mediated gene therapy produces fertile offspring in the Lhcgr-deficient mouse model of Leydig cell failure. Cell Rep Med. 2022;3(11):100792. doi:10.1016/j.xcrm.2022.100792
10. Aguilar IN, Trippel S, Shi S, Bonassar LJ. Customized biomaterials to augment chondrocyte gene therapy. Acta Biomater. 2017;53:260–267. doi:10.1016/j.actbio.2017.02.008
11. Yoon DS, Lee KM, Choi Y, et al. TLR4 downregulation by the RNA-binding protein PUM1 alleviates cellular aging and osteoarthritis. Cell Death Differ. 2022;29(7):1364–1378. doi:10.1038/s41418-021-00925-6
12. Madry H, Venkatesan JK, Carballo-Pedrares N, Rey-Rico A, Cucchiarini M. Scaffold-mediated gene delivery for osteochondral repair. Pharmaceutics. 2020;12(10). doi:10.3390/pharmaceutics12100930
13. Cai Y, Wu C, Ou Q, et al. Enhanced osteoarthritis therapy by nanoengineered mesenchymal stem cells using biomimetic CuS nanoparticles loaded with plasmid DNA encoding TGF-beta1. Bioact Mater. 2023;19:444–457. doi:10.1016/j.bioactmat.2022.04.021
14. Wu H, Peng Z, Xu Y, et al. Engineered adipose-derived stem cells with IGF-1-modified mRNA ameliorates osteoarthritis development. Stem Cell Res Ther. 2022;13(1):19. doi:10.1186/s13287-021-02695-x
15. Wang G, Chen S, Xie Z, et al. TGFbeta attenuates cartilage extracellular matrix degradation via enhancing FBXO6-mediated MMP14 ubiquitination. Ann Rheum Dis. 2020;79(8):1111–1120. doi:10.1136/annrheumdis-2019-216911
16. Ye W, Yang Z, Cao F, et al. Articular cartilage reconstruction with TGF-beta1-simulating self-assembling peptide hydrogel-based composite scaffold. Acta Biomater. 2022;146:94–106. doi:10.1016/j.actbio.2022.05.012
17. Aguilar IN, Trippel SB, Shi S, Bonassar LJ. Comparison of efficacy of endogenous and exogenous IGF-I in stimulating matrix production in neonatal and mature chondrocytes. Cartilage. 2015;6(4):264–272. doi:10.1177/1947603515578691
18. Hu Y, Gui Z, Zhou Y, Xia L, Lin K, Xu Y. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic Biol Med. 2019;145:146–160. doi:10.1016/j.freeradbiomed.2019.09.024
19. Stone A, Grol MW, Ruan MZC, et al. Combinatorial Prg4 and Il-1ra gene therapy protects against hyperalgesia and cartilage degeneration in post-traumatic osteoarthritis. Hum Gene Ther. 2019;30(2):225–235. doi:10.1089/hum.2018.106
20. Senter R, Boyce R, Repic M, et al. Efficacy and safety of FX201, a novel intra-articular IL-1Ra gene therapy for osteoarthritis treatment, in a rat model. Hum Gene Ther. 2022;33(9–10):541–549. doi:10.1089/hum.2021.131
21. Ko JH, Kang YM, Yang JH, et al. Regulation of MMP and TIMP expression in synovial fibroblasts from knee osteoarthritis with flexion contracture using adenovirus-mediated relaxin gene therapy. Knee. 2019;26(2):317–329. doi:10.1016/j.knee.2019.01.010
22. Tamura T, Kosaka N, Ishiwa J, Sato T, Nagase H, Ito A. Rhein, an active metabolite of diacerein, down-regulates the production of pro-matrix metalloproteinases-1, −3, −9 and −13 and up-regulates the production of tissue inhibitor of metalloproteinase-1 in cultured rabbit articular chondrocytes. Osteoarthritis Cartilage. 2001;9(3):257–263. doi:10.1053/joca.2000.0383
23. Evans CH, Gouze JN, Gouze E, Robbins PD, Ghivizzani SC. Osteoarthritis gene therapy. Gene Ther. 2004;11(4):379–389. doi:10.1038/sj.gt.3302196
24. Wei Y, Luo L, Gui T, et al. Targeting cartilage EGFR pathway for osteoarthritis treatment. Sci Transl Med. 2021;13(576). doi:10.1126/scitranslmed.abb3946
25. Jeong SY, Kang ML, Park JW, Im GI. Dual functional nanoparticles containing SOX duo and ANGPT4 shRNA for osteoarthritis treatment. J Biomed Mater Res B Appl Biomater. 2020;108(1):234–242. doi:10.1002/jbm.b.34383
26. Ali SA, Peffers MJ, Ormseth MJ, Jurisica I, Kapoor M. The non-coding RNA interactome in joint health and disease. Nat Rev Rheumatol. 2021;17(11):692–705. doi:10.1038/s41584-021-00687-y
27. Wu Y, Lu X, Shen B, Zeng Y. The therapeutic potential and role of miRNA, lncRNA, and circRNA in osteoarthritis. Curr Gene Ther. 2019;19(4):255–263. doi:10.2174/1566523219666190716092203
28. Cao F, Chen Y, Wang X, et al. Therapeutic effect and potential mechanisms of intra-articular injections of miR-140-5p on early-stage osteoarthritis in rats. Int Immunopharmacol. 2021;96:107786. doi:10.1016/j.intimp.2021.107786
29. Yan C, Wu J. Effect of Toddalia asiatica extract combined with miR-483 on proliferation, apoptosis and inflammatory factors expression of osteoarthritis chondrocyte. Pak J Pharm Sci. 2020;33(3(Special)):1333–1340.
30. Liang Y, Duan L, Xiong J, et al. E2 regulates MMP-13 via targeting miR-140 in IL-1beta-induced extracellular matrix degradation in human chondrocytes. Arthritis Res Ther. 2016;18(1):105. doi:10.1186/s13075-016-0997-y
31. Liang Y, Xu X, Li X, et al. Chondrocyte-targeted MicroRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12(33):36938–36947. doi:10.1021/acsami.0c10458
32. Mishra S, Verma SS, Rai V, et al. Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell Mol Life Sci. 2019;76(10):1947–1966. doi:10.1007/s00018-019-03053-0
33. Xing D, Liang JQ, Li Y, et al. Identification of long noncoding RNA associated with osteoarthritis in humans. Orthop Surg. 2014;6(4):288–293. doi:10.1111/os.12147
34. Xiong G, Wang S, Pan Z, et al. Long non-coding RNA MEG3 regulates the progress of osteoarthritis by regulating the miR-34a/Klotho axis. Ann Transl Med. 2022;10(8):454. doi:10.21037/atm-22-894
35. Feng L, Yang Z, Li Y, et al. Malat1 attenuated the rescuing effects of docosahexaenoic acid on osteoarthritis treatment via repressing its chondroprotective and chondrogenesis activities. Biomed Pharmacother. 2022;154:113608. doi:10.1016/j.biopha.2022.113608
36. Chen G, Liu T, Yu B, Wang B, Peng Q. CircRNA-UBE2G1 regulates LPS-induced osteoarthritis through miR-373/HIF-1a axis. Cell Cycle. 2020;19(13):1696–1705. doi:10.1080/15384101.2020.1772545
37. Zhou ZB, Huang GX, Fu Q, et al. circRNA.33186 contributes to the pathogenesis of osteoarthritis by sponging miR-127-5p. Mol Ther. 2019;27(3):531–541. doi:10.1016/j.ymthe.2019.01.006
38. Cucchiarini M, Madry H. Biomaterial-guided delivery of gene vectors for targeted articular cartilage repair. Nat Rev Rheumatol. 2019;15(1):18–29. doi:10.1038/s41584-018-0125-2
39. Maihofer J, Madry H, Rey-Rico A, et al. Hydrogel-guided, rAAV-mediated IGF-I overexpression enables long-term cartilage repair and protection against perifocal osteoarthritis in a large-animal full-thickness chondral defect model at one year in vivo. Adv Mater. 2021;33(16):e2008451. doi:10.1002/adma.202008451
40. Rehman M, Madni A, Ihsan A, et al. Solid and liquid lipid-based binary solid lipid nanoparticles of diacerein: in vitro evaluation of sustained release, simultaneous loading of gold nanoparticles, and potential thermoresponsive behavior. Int J Nanomedicine. 2015;10:2805–2814. doi:10.2147/IJN.S67147
41. Sagar S, Singh D, Gupta GD. Recent development in the management of osteoarthritis - overview of nanoformulation approaches. Pharm Nanotechnol. 2021;9(4):251–261. doi:10.2174/2211738509666210615165759
42. Chuang SY, Lin CH, Huang TH, Fang JY. Lipid-based nanoparticles as a potential delivery approach in the treatment of rheumatoid arthritis. Nanomaterials (Basel). 2018;8(1). doi:10.3390/nano8010042
43. Shang H, Younas A, Zhang N. Recent advances on transdermal delivery systems for the treatment of arthritic injuries: from classical treatment to nanomedicines. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022;14(3):e1778. doi:10.1002/wnan.1778
44. Pena SA, Iyengar R, Eshraghi RS, et al. Gene therapy for neurological disorders: challenges and recent advancements. J Drug Target. 2020;28(2):111–128. doi:10.1080/1061186X.2019.1630415
45. Kim J, Wilson DR, Zamboni CG, Green JJ. Targeted polymeric nanoparticles for cancer gene therapy. J Drug Target. 2015;23(7–8):627–641. doi:10.3109/1061186X.2015.1048519
46. Yang Q, Zhou Y, Chen J, Huang N, Wang Z, Cheng Y. Gene therapy for drug-resistant glioblastoma via lipid-polymer hybrid nanoparticles combined with focused ultrasound. Int J Nanomedicine. 2021;16:185–199. doi:10.2147/IJN.S286221
47. Kowalski PS, Rudra A, Miao L, Anderson DG. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol Ther. 2019;27(4):710–728. doi:10.1016/j.ymthe.2019.02.012
48. Lin G, Zhang H, Huang L. Smart polymeric nanoparticles for cancer gene delivery. Mol Pharm. 2015;12(2):314–321. doi:10.1021/mp500656v
49. Wang L, Wang Z, Pan Y, et al. Polycatechol-derived mesoporous polydopamine nanoparticles for combined ROS scavenging and gene interference therapy in inflammatory bowel disease. ACS Appl Mater Interfaces. 2022;14(17):19975–19987. doi:10.1021/acsami.1c25180
50. Zhou Y, Quan G, Wu Q, et al. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm Sin B. 2018;8(2):165–177. doi:10.1016/j.apsb.2018.01.007
51. Hong SJ, Ahn MH, Sangshetti J, Arote RB. Sugar alcohol-based polymeric gene carriers: synthesis, properties and gene therapy applications. Acta Biomater. 2019;97:105–115. doi:10.1016/j.actbio.2019.07.029
52. Cai C, Zhang X, Li Y, et al. Self-healing hydrogel embodied with macrophage-regulation and responsive-gene-silencing properties for synergistic prevention of peritendinous adhesion. Adv Mater. 2022;34(5):e2106564. doi:10.1002/adma.202106564
53. Zhang H, Wu S, Chen W, Hu Y, Geng Z, Su J. Bone/cartilage targeted hydrogel: strategies and applications. Bioact Mater. 2023;23:156–169. doi:10.1016/j.bioactmat.2022.10.028
54. Zhou Z, Cui J, Wu S, Geng Z, Su J. Silk fibroin-based biomaterials for cartilage/osteochondral repair. Theranostics. 2022;12(11):5103–5124. doi:10.7150/thno.74548
55. Wang S, Chen B, Ouyang L, et al. A novel stimuli-responsive injectable antibacterial hydrogel to achieve synergetic photothermal/gene-targeted therapy towards uveal melanoma. Adv Sci (Weinh). 2021;8(18):e2004721. doi:10.1002/advs.202004721
56. Cao H, Duan L, Zhang Y, Cao J, Zhang K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct Target Ther. 2021;6(1):426. doi:10.1038/s41392-021-00830-x
57. Gong Y, Chen W, Chen X, et al. An injectable epigenetic autophagic modulatory hydrogel for boosting umbilical cord blood NK cell therapy prevents postsurgical relapse of triple-negative breast cancer. Adv Sci (Weinh). 2022;9(23):e2201271. doi:10.1002/advs.202201271
58. Mo F, Jiang K, Zhao D, Wang Y, Song J, Tan W. DNA hydrogel-based gene editing and drug delivery systems. Adv Drug Deliv Rev. 2021;168:79–98. doi:10.1016/j.addr.2020.07.018
59. Fan L, Liu C, Chen X, et al. Exosomes-loaded electroconductive hydrogel synergistically promotes tissue repair after spinal cord injury via immunoregulation and enhancement of myelinated axon growth. Adv Sci (Weinh). 2022;9(13):e2105586. doi:10.1002/advs.202105586
60. Song H, Park KH. Regulation and function of SOX9 during cartilage development and regeneration. Semin Cancer Biol. 2020;67(Pt 1):12–23. doi:10.1016/j.semcancer.2020.04.008
61. Madry H, Gao L, Rey-Rico A, et al. Thermosensitive hydrogel based on PEO-PPO-PEO poloxamers for a controlled in situ release of recombinant adeno-associated viral vectors for effective gene therapy of cartilage defects. Adv Mater. 2020;32(2):e1906508. doi:10.1002/adma.201906508
62. Shen S, Yang Y, Shen P, et al. circPDE4B prevents articular cartilage degeneration and promotes repair by acting as a scaffold for RIC8A and MID1. Ann Rheum Dis. 2021;80(9):1209–1219. doi:10.1136/annrheumdis-2021-219969
63. Seol D, Choe HH, Zheng H, et al. Intra-articular adeno-associated virus-mediated proteoglycan 4 gene therapy for preventing posttraumatic osteoarthritis. Hum Gene Ther. 2022;33(9–10):529–540. doi:10.1089/hum.2021.177
64. Ji Q, Xu X, Kang L, et al. Hematopoietic PBX-interacting protein mediates cartilage degeneration during the pathogenesis of osteoarthritis. Nat Commun. 2019;10(1):313. doi:10.1038/s41467-018-08277-5
65. Guo Q, Chen X, Chen J, et al. STING promotes senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the NF-kappaB signaling pathway. Cell Death Dis. 2021;12(1):13. doi:10.1038/s41419-020-03341-9
66. Zhao C, Li X, Sun G, et al. CircFOXO3 protects against osteoarthritis by targeting its parental gene FOXO3 and activating PI3K/AKT-mediated autophagy. Cell Death Dis. 2022;13(11):932. doi:10.1038/s41419-022-05390-8
67. Kallert SM, Darbre S, Bonilla WV, et al. Replicating viral vector platform exploits alarmin signals for potent CD8(+) T cell-mediated tumour immunotherapy. Nat Commun. 2017;8:15327. doi:10.1038/ncomms15327
68. Ortved K, Wagner B, Calcedo R, Wilson J, Schaefer D, Nixon A. Humoral and cell-mediated immune response, and growth factor synthesis after direct intraarticular injection of rAAV2-IGF-I and rAAV5-IGF-I in the equine middle carpal joint. Hum Gene Ther. 2015;26(3):161–171. doi:10.1089/hum.2014.050
69. Qadir A, Gao Y, Suryaji P, et al. Non-viral delivery system and targeted bone disease therapy. Int J Mol Sci. 2019;20(3). doi:10.3390/ijms20030565
70. Pi Y, Zhang X, Shi J, et al. Targeted delivery of non-viral vectors to cartilage in vivo using a chondrocyte-homing peptide identified by phage display. Biomaterials. 2011;32(26):6324–6332. doi:10.1016/j.biomaterials.2011.05.017
71. Wang Y, Zhao X, Liu-Bryan R. Role of TLR2 and TLR4 in regulation of articular chondrocyte homeostasis. Osteoarthritis Cartilage. 2020;28(5):669–674. doi:10.1016/j.joca.2020.01.011
72. Moghadam NA, Bagheri F, Eslaminejad MB. Chondroitin sulfate modified chitosan nanoparticles as an efficient and targeted gene delivery vehicle to chondrocytes. Colloids Surf B Biointerfaces. 2022;219:112786. doi:10.1016/j.colsurfb.2022.112786
73. Cheng X, Lee RJ. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv Drug Deliv Rev. 2016;99(Pt A):129–137. doi:10.1016/j.addr.2016.01.022
74. Maier MA, Jayaraman M, Matsuda S, et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol Ther. 2013;21(8):1570–1578. doi:10.1038/mt.2013.124
75. Mukherjee A, Waters AK, Kalyan P, Achrol AS, Kesari S, Yenugonda VM. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: state of the art, emerging technologies, and perspectives. Int J Nanomedicine. 2019;14:1937–1952. doi:10.2147/IJN.S198353
76. Ruttkay-Nedecky B, Krystofova O, Nejdl L, Adam V. Nanoparticles based on essential metals and their phytotoxicity. J Nanobiotechnology. 2017;15(1):33. doi:10.1186/s12951-017-0268-3
77. Gantenbein B, Tang S, Guerrero J, et al. Non-viral gene delivery methods for bone and joints. Front Bioeng Biotechnol. 2020;8:598466. doi:10.3389/fbioe.2020.598466
78. Grol MW, Lee BH. Gene therapy for repair and regeneration of bone and cartilage. Curr Opin Pharmacol. 2018;40:59–66. doi:10.1016/j.coph.2018.03.005
79. Sun XD, Jeng L, Bolliet C, Olsen BR, Spector M. Non-viral endostatin plasmid transfection of mesenchymal stem cells via collagen scaffolds. Biomaterials. 2009;30(6):1222–1231. doi:10.1016/j.biomaterials.2008.10.020
80. Nagelli CV, Evans CH, De La Vega RE. Viral gene delivery in chondrocytes. Methods Mol Biol. 2023;2598:289–300.
81. Jones IA, Togashi R, Wilson ML, Heckmann N, Vangsness CT Jr. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2019;15(2):77–90. doi:10.1038/s41584-018-0123-4
82. Zhou X, Zheng Y, Sun W, et al. D-mannose alleviates osteoarthritis progression by inhibiting chondrocyte ferroptosis in a HIF-2alpha-dependent manner. Cell Prolif. 2021;54(11):e13134. doi:10.1111/cpr.13134
83. Mao L, Wu W, Wang M, et al. Targeted treatment for osteoarthritis: drugs and delivery system. Drug Deliv. 2021;28(1):1861–1876. doi:10.1080/10717544.2021.1971798
84. Di Francesco M, Bedingfield SK, Di Francesco V, et al. Shape-defined microplates for the sustained intra-articular release of dexamethasone in the management of overload-induced osteoarthritis. ACS Appl Mater Interfaces. 2021;13(27):31379–31392. doi:10.1021/acsami.1c02082
85. Chen X, Gong W, Shao X, et al. METTL3-mediated m(6)A modification of ATG7 regulates autophagy-GATA4 axis to promote cellular senescence and osteoarthritis progression. Ann Rheum Dis. 2022;81(1):87–99. doi:10.1136/annrheumdis-2021-221091
86. Bruno MC, Cristiano MC, Celia C, et al. Injectable drug delivery systems for osteoarthritis and rheumatoid arthritis. ACS Nano. 2022;16(12):19665–19690. doi:10.1021/acsnano.2c06393
87. Chen L, Wang Y, Sun L, Yan J, Mao HQ. Nanomedicine strategies for anti-inflammatory treatment of noninfectious arthritis. Adv Healthc Mater. 2021;10(11):e2001732. doi:10.1002/adhm.202001732
88. Zhang M, Hu W, Cai C, Wu Y, Li J, Dong S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater Today Bio. 2022;14:100223. doi:10.1016/j.mtbio.2022.100223
89. Rahimi M, Charmi G, Matyjaszewski K, Banquy X, Pietrasik J. Recent developments in natural and synthetic polymeric drug delivery systems used for the treatment of osteoarthritis. Acta Biomater. 2021;123:31–50. doi:10.1016/j.actbio.2021.01.003
90. Das P, Jana S, Kumar Nandi S. Biomaterial-based therapeutic approaches to osteoarthritis and cartilage repair through macrophage polarization. Chem Rec. 2022;22(9):e202200077. doi:10.1002/tcr.202200077
91. Wang Y, Chen Y, Wei Y. Osteoarthritis animal models for biomaterial-assisted osteochondral regeneration. Biomater Transl. 2022;3(4):264–279. doi:10.12336/biomatertransl.2022.04.006
92. Wang X, Han X, Li C, et al. 2D materials for bone therapy. Adv Drug Deliv Rev. 2021;178:113970. doi:10.1016/j.addr.2021.113970
93. Xu XL, Xue Y, Ding JY, et al. Nanodevices for deep cartilage penetration. Acta Biomater. 2022;154:23–48. doi:10.1016/j.actbio.2022.10.007
94. Jain A, Singh SK, Singh Y, Singh S. Development of lipid nanoparticles of diacerein, an antiosteoarthritic drug for enhancement in bioavailability and reduction in its side effects. J Biomed Nanotechnol. 2013;9(5):891–900. doi:10.1166/jbn.2013.1580
95. Jain A, Mishra SK, Vuddanda PR, Singh SK, Singh R, Singh S. Targeting of diacerein loaded lipid nanoparticles to intra-articular cartilage using chondroitin sulfate as homing carrier for treatment of osteoarthritis in rats. Nanomedicine. 2014;10(5):1031–1040. doi:10.1016/j.nano.2014.01.008
96. Lin T, Zhao Y, Chen J, et al. Carboxymethyl chitosan-assisted MnO(x) nanoparticles: synthesis, characterization, detection and cartilage repair in early osteoarthritis. Carbohydr Polym. 2022;294:119821. doi:10.1016/j.carbpol.2022.119821
97. Saeedi T, Prokopovich P. Poly beta amino ester coated emulsions of NSAIDs for cartilage treatment. J Mater Chem B. 2021;9(29):5837–5847. doi:10.1039/D1TB01024G
98. Zerrillo L, Que I, Vepris O, et al. pH-responsive poly(lactide-co-glycolide) nanoparticles containing near-infrared dye for visualization and hyaluronic acid for treatment of osteoarthritis. J Control Release. 2019;309:265–276. doi:10.1016/j.jconrel.2019.07.031
99. Cai Y, Lopez-Ruiz E, Wengel J, Creemers LB, Howard KA. A hyaluronic acid-based hydrogel enabling CD44-mediated chondrocyte binding and gapmer oligonucleotide release for modulation of gene expression in osteoarthritis. J Control Release. 2017;253:153–159. doi:10.1016/j.jconrel.2017.03.004
100. Connolly M, Veale DJ, Fearon U. Acute serum amyloid A regulates cytoskeletal rearrangement, cell matrix interactions and promotes cell migration in rheumatoid arthritis. Ann Rheum Dis. 2011;70(7):1296–1303. doi:10.1136/ard.2010.142240
101. Xiong Y, Mi BB, Lin Z, et al. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: from mechanism to therapeutic opportunity. Mil Med Res. 2022;9(1):65. doi:10.1186/s40779-022-00426-8
102. Oryan A, Sahvieh S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int J Biol Macromol. 2017;104(Pt A):1003–1011. doi:10.1016/j.ijbiomac.2017.06.124
103. Zheng D, Chen T, Han L, et al. Synergetic integrations of bone marrow stem cells and transforming growth factor-beta1 loaded chitosan nanoparticles blended silk fibroin injectable hydrogel to enhance repair and regeneration potential in articular cartilage tissue. Int Wound J. 2022;19(5):1023–1038. doi:10.1111/iwj.13699
104. Man Z, Hu X, Liu Z, et al. Transplantation of allogenic chondrocytes with chitosan hydrogel-demineralized bone matrix hybrid scaffold to repair rabbit cartilage injury. Biomaterials. 2016;108:157–167. doi:10.1016/j.biomaterials.2016.09.002
105. Almeida HV, Eswaramoorthy R, Cunniffe GM, Buckley CT, O’brien FJ, Kelly DJ. Fibrin hydrogels functionalized with cartilage extracellular matrix and incorporating freshly isolated stromal cells as an injectable for cartilage regeneration. Acta Biomater. 2016;36:55–62. doi:10.1016/j.actbio.2016.03.008
106. Jiang X, Liu J, Liu Q, et al. Therapy for cartilage defects: functional ectopic cartilage constructed by cartilage-simulating collagen, chondroitin sulfate and hyaluronic acid (CCH) hybrid hydrogel with allogeneic chondrocytes. Biomater Sci. 2018;6(6):1616–1626. doi:10.1039/C8BM00354H
107. Klinger P, Surmann-Schmitt C, Brem M, et al. Chondromodulin 1 stabilizes the chondrocyte phenotype and inhibits endochondral ossification of porcine cartilage repair tissue. Arthritis Rheum. 2011;63(9):2721–2731. doi:10.1002/art.30335
108. Wang P, Li Y, Meng T, et al. KDM6A promotes chondrogenic differentiation of periodontal ligament stem cells by demethylation of SOX9. Cell Prolif. 2018;51(3):e12413. doi:10.1111/cpr.12413
109. Gonzalez-Fernandez T, Tierney EG, Cunniffe GM, O’brien FJ, Kelly DJ. Gene delivery of TGF-beta3 and BMP2 in an MSC-laden alginate hydrogel for articular cartilage and endochondral bone tissue engineering. Tissue Eng Part A. 2016;22(9–10):776–787. doi:10.1089/ten.tea.2015.0576
110. Friedman B, Larranaga-Vera A, Castro CM, Corciulo C, Rabbani P, Cronstein BN. Adenosine A2A receptor activation reduces chondrocyte senescence. FASEB J. 2023;37(4):e22838. doi:10.1096/fj.202201212RR
111. Schillinger U, Wexel G, Hacker C, et al. A fibrin glue composition as carrier for nucleic acid vectors. Pharm Res. 2008;25(12):2946–2962. doi:10.1007/s11095-008-9719-8
112. Lee YH, Wu HC, Yeh CW, et al. Enzyme-crosslinked gene-activated matrix for the induction of mesenchymal stem cells in osteochondral tissue regeneration. Acta Biomater. 2017;63:210–226. doi:10.1016/j.actbio.2017.09.008
113. Frisbie DD, Mcilwraith CW. Gene therapy: future therapies in osteoarthritis. Vet Clin North Am Equine Pract. 2001;17(2):233–43, vi. doi:10.1016/S0749-0739(17)30059-7
114. Tanikella AS, Hardy MJ, Frahs SM, et al. Emerging gene-editing modalities for osteoarthritis. Int J Mol Sci. 2020;21(17). doi:10.3390/ijms21176046
115. Guo H, Huang X. Engineered exosomes for future gene-editing therapy. Biomater Transl. 2022;3(4):240–242. doi:10.12336/biomatertransl.2022.04.003
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.