Back to Journals » International Journal of General Medicine » Volume 17
Biomarkers in the Severity of Necrotizing Enterocolitis in Preterm Infants: A Pilot Study
Authors Meng W, Wang Q, Xu Q, Gao H, Zhou Y, Shao W
Received 24 October 2023
Accepted for publication 5 March 2024
Published 15 March 2024 Volume 2024:17 Pages 1017—1023
DOI https://doi.org/10.2147/IJGM.S446378
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandul Yasobant
Wei Meng,1 Qing Wang,1 Qingyu Xu,2 Hongli Gao,3 Yunjun Zhou,1 Wei Shao1
1Department of Pediatric Internal Medicine, Hongqi Hospital Affiliated to Mudanjiang Medical University, Mudanjiang, Heilongjiang, People’s Republic of China; 2Department of Pediatrics Surgery, Hongqi Hospital Affiliated to Mudanjiang Medical University, Mudanjiang, Heilongjiang, People’s Republic China; 3Department of Digestive Endoscopy Center, Hongqi Hospital Affiliated to Mudanjiang Medical University, Mudanjiang, Heilongjiang, People’s Republic of China
Correspondence: Wei Shao, Department of Pediatrics, Hongqi Hospital Affiliated to Mudanjiang Medical University, No. 5 Tongxiang Road, Aimin District, Mudanjiang, Heilongjiang, 157000, People’s Republic of China, Tel +86-4536602008, Email [email protected]
Background: The occurrence of necrotizing enterocolitis (NEC) is a common and severe disease of the digestive system in neonates. This study aims to assess the value of the intestinal tissue oxygen saturation (rintSO2) combined with the levels of procalcitonin (PCT) and mean platelet volume (MPV) in predicting the severity of NEC in preterm infants.
Methods: This experiment was a retrospective cohort study conducted in the Department of Pediatrics, Hongqi Hospital Affiliated to Mudanjiang Medical University between January 2017 and July 2022. Premature neonates with NEC were enrolled and divided into mild-moderate NEC group and severe NEC group according to Bell’s stage. The general information data, rintSO2 and blood parameters such as the white blood cell (WBC) count, platelet count (PLT), PCT, MPV, red blood cell distribution width (RDW), hemoglobin (Hb), C-reactive protein (CRP) were compared between the two groups.
Results: A total of 122 patients were enrolled, including 79 mild-moderate NEC and 43 severe NEC. The rintSO2 was lower in severe group than in mild-moderate group (P = 0.042), the PCT and MPV were both higher in severe group than in mild-moderate group (P = 0.048, P = 0.049). The results of logistic regression suggested that the rintSO2 (OR = 1.491, P = 0.003), PCT (OR = 3.071, P = 0.001) and MPV (OR = 4.027, P = 0.015) were independent predictive factors for severity of NEC. The area under the curve (AUC) of the rintSO2 combined with PCT and MPV showed good diagnostic ability in the severity of NEC.
Conclusion: The rintSO2 combined with PCT and MPV may be considered as the early biomarkers in the severity of NEC and could help us to diagnose the case early with early treatment with better prognosis.
Keywords: premature infants, necrotizing enterocolitis, intestinal tissue oxygen saturation, procalcitonin, mean platelet volume
Introduction
Necrotizing enterocolitis (NEC) is considered to be a common serious disease of the digestive system and an important cause of serious neonatal complication and death in neonates.1,2 Therefore, its early diagnosis and appropriate treatment are important. Generally, the severity of NEC is based on clinical and biochemical variables representing the infant’s intestinal and systemic condition, whereby the patient characteristics and the comorbidities of interest include postmenstrual age, weight, etc.3 But there is still a lack of indicators that can aid in the prediction of the severity of NEC.
The intestinal tissue oxygen saturation (rintSO2) is documented an important function in gastrointestinal damage and affection of the mucosa of the digestive tract in NEC.4 The near-infrared spectroscopy (NIRS) is a noninvasive tool used to identify preterm infants at risk of developing NEC or progression to complicated NEC.5–8 The technique measures rintSO2 to assess end-organ perfusion.9–11 Serum procalcitonin (PCT), a kind of the calcitonin precursor, is an important mediator produced by monocytes. It starts to increase within 2–3 hours following the affection by the bacterial endotoxin as most of the biomarkers need several hours or even days to rise in response to bacterial toxin, so 3 hours in PCT is a convenient and sensitive index for clinical application.12–14 The mean platelet volume (MPV) describes the average size of platelets in a blood sample and is a simple, economical and useful diagnostic marker for children.
In light of the previous studies, we aimed to predict rapid suspicion of the severity of NEC through determination of the rintSO2, serum levels of PCT and MPV.
Materials and Methods
Sample Selection
This retrospective study was conducted from January 2017 to July 2022 at the Department of Pediatrics of Hongqi Hospital Affiliated to Mudanjiang Medical University. A request of waiver of written consent was approved by the Ethical Committee of the Hongqi Hospital (2023049) after declaring that the data collection tool is anonymous and does not require collection of any identification data. All procedures were carried out according to the Declaration of Helsinki.
All data were accessed from the patients’ electronic medical records. In our study, the mild-moderate NEC was defined as Bell’s stage I and II, and the severe NEC was defined as Bell’s stage III.15 None of the included cases used probiotics. The exclusion criteria were as follows: (1) gestational age (GA) ≥ 38 weeks; (2) Congenital intestinal tract developmental malformations, inherited metabolic diseases, chromosomal diseases, severe asphyxia, severe congenital heart disease and other diseases; (3) Abdominal skin was damaged.16
Clinical Data and Laboratory Parameters
The patients’ records were reviewed to collect the following data on a standardized form: GA, gender, birth weight, mode of delivery, comorbidities during pregnancy, postnatal age, and weight at the time of NEC diagnosis, etc.
The rintSO2 values were obtained within 24 hours after the diagnosis of NEC and continued for 3 hours. For the purpose of routine clinical monitoring of the patients at our department, we used the MNIR-P100 NIRS (Mingxi Medical Equipment, Chongqing, China) in combination with neonatal SomaSensors (Suzhou AIQIN Bio-Medical Electronics, Suzhou, China). The instrument measured rSO2 every 5 seconds (0.2 Hz). The intestinal sensor was placed 0.5 to 1 cm below the umbilicus on the central abdomen and sheltered from light. Mepitel film (Mölnlycke, Sweden) was used below each sensor for skin protection and there was no adverse effects on NIRS integrity and validity in the previous research.11,17 Sensor placement was checked and documented several times a day by the nurse.
Blood samples were collected within the first time after the onset of clinical manifestations of NEC such as bloody stools, bloating. Laboratory parameters such as the white blood cell (WBC), platelet count (PLT), PCT, MPV, red blood cell distribution width (RDW), hemoglobin (Hb), C-reactive protein (CRP) and were measured.
Statistical Analysis
Statistical analysis was performed using SPSS19.0 software (SPSS, Chicago, USA). Categorical variables are expressed as proportions and were compared using the chi-square (χ2) test and Fisher’s exact test. Continuous variables with a non normal distribution were presented as the median and interquartile range (IQR) percentiles, and variables with a normal distribution were expressed as mean ± standard deviation (s.d.). The results of the parameters were compared using t-tests or Mann–Whitney U-tests. Logistic regression analysis was applied to assess the independent risk factors for the severity of NEC. The receiver operating characteristic (ROC) method was conducted to evaluate the utility of different variables in predicting the severity of NEC. The areas under the ROC curve (AUCs) and optimal cut-off points based on maximizing the sum of the sensitivity and specificity were calculated for each of the variables. P-value < 0.05 was considered significant.
Results
Patient Characteristics
Out of 862 premature infants hospitalized during the study period, 687 premature infants were identified with no health associated disease. Nineteen patients were excluded because of severe disease and abdominal skin damaged and 34 patients were excluded for missing data on rintSO2 or blood parameters. A total of 122 premature infants were enrolled in the study, including 79 mild-moderate NEC patients and 43 severe NEC patients. Eleven patients were treated with surgery in the severe group, and others and the mild-moderate patients were accepted conservative medical treatment (Figure 1).
 |
Figure 1 Flow chart of the study. |
We analyzed the baseline characteristics of patients with NEC, including their gender, gestational age (GA), < 28 weeks gestation, birth weight, age of onset, breastfeeding, delivery mode, premature rupture of membranes (PROM), maternal hypertension, maternal diabetes, Intra-uterine growth retardation (IUGR). No significant differences were observed regarding these data between the mild-moderate group and the severe group (P > 0.05, Table 1).
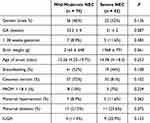 |
Table 1 The Baseline Clinical Characteristics of the Mild-Moderate and Severe NEC Groups |
Clinical Indicator Observations
The rintSO2 were lower in the severe NEC group than the mild-moderate NEC group, but the PCT and MPV were higher in the former (P = 0.042, P = 0.048 and P = 0.049). No significant differences in WBC, PLT, RDW, Hb and CRP were observed between the two groups (P > 0.05) (Table 2).
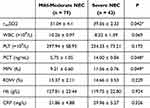 |
Table 2 Levels of rintSO2, WBC, PLT, PCT, MPV, RDW, Hb, CRP in Mild-Moderate NEC and Severe NEC Groups (*P < 0.05) |
Logistic Regression Analysis
The results of logistic regression suggested that the rintSO2 (OR = 1.491, P = 0.003), PCT (OR = 3.071, P = 0.001) and MPV (OR = 4.027, P = 0.015) were independent predictive factors for the severity of NEC (Table 3).
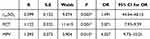 |
Table 3 Logistic Regression Analysis Results (*P < 0.05) |
The receiver operating characteristic (ROC) analysis showed that the area under the curve (AUC) was 0.749 for rintSO2 alone, 0.919 for PCT alone, 0.850 for MPV alone, and 0.970 for rintSO2 combined with PCT, 0.892 for rintSO2 combined with MPV, 0.935 for PCT+MPV, and 0.986 for rintSO2+PCT+MPV, suggesting that rintSO2 combined with PCT and MPV was better for predicting the severity of NEC in preterm infants (Table 4, Figure 2).
 |
Table 4 The AUC of rintSO2, PCT Combined with MPV (*P < 0.05, **P < 0.01) |
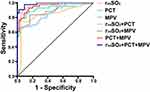 |
Figure 2 The ROC curve of rintSO2, PCT Combined with MPV for predicting the severity of NEC. |
Discussion
NEC is the most common and aggressive gastrointestinal emergency in neonates. Clinical features of NEC are complex and there is a lack of specific biomarkers corresponding to the development of the disease. It means that determining the severity of NEC in time and administering appropriate treatment is difficult and of utmost importance to physicians, parents and infants. Therefore, our research focuses on searching for effective biomarkers that can predict the severity of NEC among commonly used clinical indicators.
NIRS can analyze the ischemic-hypoxic injury which cannot be identified by traditional hemodynamic examination. It reflects the circulatory perfusion of specific organs, and guides the treatment with the goal of oxygen supply and restoration of organ blood flow. In previous studies, most of the current research on NIRS focused on the measurement of cerebral oxygen saturation (rcSO2). It dynamic monitored the value every week after birth is basically used to predict the development of late NEC, and the time of each monitoring is short.5,17–19 There are few studies on dynamic monitoring of rintSO2 in preterm infants with definite the severity of NEC. In our study, the rintSO2 was lower in the severe NEC group than mild-moderate NEC group at the early stage.
The PCT is a non-active procalcitonin peptide substance, which can predict the status of bacterial infection. The level of PCT is positively correlated with the degree of infection, so it is important for the judgment and prevention of inflammatory diseases.12 Cai et al16,20 showed that the serum PCT level of neonates with NEC was significantly higher than that of healthy neonates, and the level of PCT in stage III NEC was significantly higher than stage II NEC, and that in stage II NEC was significantly higher than stage I NEC. Our study found that PCT in the severe group was higher than that in the mild-moderate group. It was agreement with the results in the literature.
The MPV is an easy-to-measure surrogate for platelet activation that is provoked by inflammation.15 It describes platelet size and platelet production rate in a blood, and increased in the development of platelet-activated diseases and participates in the oxidative process related to inflammation. High MPV and the associated increase in platelet mass has been shown to promote ductus arteriosus thrombosis and anatomic closure in preterm infants.14 Cekmez et al21 noted that infants who went on to develop NEC had higher mean platelet volumes, possibly reflecting increased thrombopoiesis. In our study that MPV in the severe group was higher than that in the mild-moderate group. It may be possible that platelet activating factor was enhanced release and lead to platelet aggregation and microthrombus formation, leading to ischemic necrosis of the mesenteric arteries in the case of prematurity, infection, and hypoxic ischemia. At the same time, it promoted the inflammatory reaction.
However, to date, there are but limited data on their relationship with the severity of NEC. We found the result of the ROC curve analysis showed that the AUC was 0.986 for rintSO2+PCT+MPV, indicating that the biomarkers were very effective in judging the severity of NEC. To further confirm the validity, we calculated the Youden Index. The best interception value, sensitivity and specificity of the groups were obtained through the maximum Youden index. The best cut-off value obtained by the maximum Youden index (0.884) is 0.731 (sensitivity 97.7%; specificity 90.7%). It means rintSO2 combined with PCT and MPV was better at predicting the severity of NEC in preterm infants. They are the simple, economical and useful diagnostic markers for children in the clinical.
Limitation
Our study suffers from several limitations. Firstly, the study is retrospective in design and limited to the experience of a single center what were obtained by the clinical doctors. The second limitation is that, although our study included more participants than most other studies on this subject, the sample size remains small.
Conclusion
In summary, our results showed that rintSO2 combined with PCT and MPV could be considered an early biomarkers for diagnosed the severity of NEC in preterm infants. However, the value of rintSO2 may change rapidly in the early stage of NEC, our research was focused on within 24 hours. Follow-up multi-time, multi-factor and prospective research can be carried out to further determine its effectiveness.
Data Sharing Statement
The data and materials collected in this research are available from corresponding author when requested reasonably.
Author Contributions
All authors contributed significantly to the work that was published, whether this was in the conceptualization, study design, implementation, data collection, analysis, and interpretation or in all of these areas. They all also participated in writing, revising, or critically evaluating the article, gave their final approval for the version that would be published, agreed on the journal to which the article would be submitted, and agreed to be responsible for all aspects of the work.
Funding
There is no funding to report for this study.
Disclosure
The authors declare that there are no competing interests in this study.
References
1. Neu J. Necrotizing enterocolitis: the future. Neonatology. 2020;117(2):240–244. doi:10.1159/000506866
2. Rich BS, Dolgin SE. Necrotizing enterocolitis. Pediatr Rev. 2017;38(12):552–559. doi:10.1542/pir.2017-0002
3. Kessler U, Mungnirandr A, Nelle M, et al. A simple presurgical necrotizing enterocolitis-mortality scoring system. J Perinatol. 2006;26(12):764–768. doi:10.1038/sj.jp.7211613
4. Perrone S, Tataranno ML, Santacroce A, et al. The role of oxidative stress on necrotizing enterocolitis in very low birth weight infants. Curr Pediatr Rev. 2014;10(3):202–207. doi:10.2174/1573396309666140101235126
5. Schat TE, Schurink M, van der Laan ME, et al. Near-infrared spectroscopy to predict the course of necrotizing enterocolitis. PLoS One. 2016;11(5):e0154710. doi:10.1371/journal.pone.0154710
6. Schat TE, van Zoonen A, van der Laan ME, et al. Early cerebral and intestinal oxygenation in the risk assessment of necrotizing enterocolitis in preterm infants. Early Hum Dev. 2019;131:75–80. doi:10.1016/j.earlhumdev.2019.03.001
7. Patel AK, Lazar DA, Burrin DG, et al. Abdominal near-infrared spectroscopy measurements are lower in preterm infants at risk for necrotizing enterocolitis. Pediatr Crit Care Med. 2014;15(8):735–741. doi:10.1097/PCC.0000000000000211
8. Schat TE, Heida FH, Schurink M, et al. The relation between splanchnic ischaemia and intestinal damage in necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2016;101(6):F533–F539. doi:10.1136/archdischild-2015-309838
9. Marin T, Moore J. Understanding near-infrared spectroscopy. Adv Neonatal Care. 2011;11(6):382–388. doi:10.1097/ANC.0b013e3182337ebb
10. Cortez J, Gupta M, Amaram A, et al. Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. J Matern Fetal Neonatal Med. 2011;24(4):574–582. doi:10.3109/14767058.2010.511335
11. McNeill S, Gatenby JC, McElroy S, et al. Normal cerebral, renal and abdominal regional oxygen saturations using near-infrared spectroscopy in preterm infants. J Perinatol. 2011;31(1):51–57. doi:10.1038/jp.2010.71
12. Abdel GE, Alsharany W, Ali AA, et al. Anti-oxidant profiles and markers of oxidative stress in preterm neonates. Paediatr Int Child Health. 2016;36(2):134–140. doi:10.1179/2046905515Y.0000000017
13. Quadir AF, Britton PN. Procalcitonin and C-reactive protein as biomarkers for neonatal bacterial infection. J Paediatr Child Health. 2018;54(6):695–699. doi:10.1111/jpc.13931
14. Pravin CM, Kalaivani R, Venkatesh S, et al. Evaluation of procalcitonin as a diagnostic marker in neonatal sepsis. Indian J Pathol Microbiol. 2018;61(1):81–84. doi:10.4103/IJPM.IJPM_820_16
15. Zani A, Pierro A. Necrotizing enterocolitis: controversies and challenges. F1000Res. 2015;4:4. doi:10.12688/f1000research.6003.2
16. Cai N, Liao W, Chen Z, et al. The mean platelet volume combined with procalcitonin as an early accessible marker helps to predict the severity of necrotizing enterocolitis in preterm infants. Int J Gene Med. 2022;15(1):3789–3795. doi:10.2147/IJGM.S346665
17. Kuik SJ, Kalteren WS, Mebius MJ, et al. Predicting intestinal recovery after necrotizing enterocolitis in preterm infants. Pediatr Res. 2020;87(5):903–909. doi:10.1038/s41390-019-0634-y
18. Palleri E, Wackernagel D, Wester T, et al. Low splanchnic oxygenation and risk for necrotizing enterocolitis in extremely preterm newborns. J Pediatr Gastroenterol Nutr. 2020;71(3):401–406. doi:10.1097/MPG.0000000000002761
19. van der Heide M, Hulscher J, Bos AF, et al. Near-infrared spectroscopy as a diagnostic tool for necrotizing enterocolitis in preterm infants. Pediatr Res. 2021;90(1):148–155. doi:10.1038/s41390-020-01186-8
20. Kasirer Y, Shchors I, Hammerman C, et al. Platelet indices: universally available clinical adjunct for diagnosing necrotizing enterocolitis. Am J Perinatol. 2023;10(10):2053–7759.
21. Cekmez F, Tanju IA, Canpolat FE, et al. Mean platelet volume in very preterm infants: a predictor of morbidities? Eur Rev Med Pharmacol Sci. 2013;17(1):134–137.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
