Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Biological Role and Related Natural Products of SIRT1 in Nonalcoholic Fatty Liver
Authors Meng D, Zhang F , Yu W, Zhang X, Yin G, Liang P, Feng Y, Chen S, Liu H
Received 21 September 2023
Accepted for publication 26 November 2023
Published 8 December 2023 Volume 2023:16 Pages 4043—4064
DOI https://doi.org/10.2147/DMSO.S437865
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Decheng Meng,1 Fengxia Zhang,2 Wenfei Yu,1 Xin Zhang,1 Guoliang Yin,1 Pengpeng Liang,3 Yanan Feng,1 Suwen Chen,1 Hongshuai Liu1
1The First Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan, 250011, People’s Republic of China; 2Department of Neurology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, 250011, People’s Republic of China; 3Shenzhen Hospital, Shanghai University of Traditional Chinese Medicine, Shenzhen, 518001, People’s Republic of China
Correspondence: Fengxia Zhang, Department of Neurology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, 250011, People’s Republic of China, Tel +86-131-5317-5246, Email [email protected]
Abstract: Non-alcoholic fatty liver disease(NAFLD) is an umbrella term for a range of diseases ranging from hepatic fat accumulation and steatosis to non-alcoholic steatohepatitis (NASH) in the absence of excessive alcohol consumption and other definite liver damage factors. The incidence of NAFLD has increased significantly in recent years and will continue to grow in the coming decades. NAFLD has become a huge health problem and economic burden. SIRT1 is a member of Sirtuins, a group of highly conserved histone deacetylases regulated by NAD+, and plays a vital role in regulating cholesterol and lipid metabolism, improving oxidative stress, inflammation, and insulin resistance through deacetylating some downstream transcription factors and thus improving NAFLD. Although there are no currently approved drugs for treating NAFLD and some unresolved limitations in developing SIRT1 activators, SIRT1 holds promise as a proper therapeutic target for NAFLD and other metabolic diseases. In recent years, natural products have played an increasingly important role in drug development due to their safety and efficacy. It has been discovered that some natural products may be able to prevent and treat NAFLD by targeting SIRT1 and its related pathways. This paper reviews the mechanism of SIRT1 in the improvement of NALFD and the natural products that regulate NAFLD through SIRT1 and its associated pathways, and discusses the potential of SIRT1 as a therapeutic target for treating NAFLD and the effectiveness of these related natural products as clinical drugs or dietary supplements. These works may provide some new ideas and directions for finding new therapeutic targets for NAFLD and the development of anti-NAFLD drugs with good pharmacodynamic properties.
Keywords: NAFLD, SIRT1, natural products, FFA, TG
Graphical Abstract:
Introduction
NAFLD, a metabolic syndrome characterized by hepatic steatosis and fat accumulation, has developed into the most prevalent chronic liver disease worldwide. The incidence of NAFLD has increased significantly over the past decade, reaching 25% in the global population and 27% in Asia. In recent years, the increase in the incidence of metabolic complications such as obesity, type 2 diabetes, hyperlipidemia, and hypertension has also exacerbated the prevalence and burden of NALFD. NAFLD is most likely to eventually develop into end-stage liver disease such as hepatocellular carcinoma (HCC) and seriously endangers patients’ life safety and quality of life, causing severe economic and social burdens.1,2 Moreover, a population-based cohort study pointed out that NAFLD not only increases the risk of liver cancer such as HCC but may also increase the risk of cancers in the colorectum, kidney, bladder, and uterus.3 The development of NAFLD will cause changes in the physiological functions of the pancreas, brain and other organs, leading to insulin resistance. This may be one of the reasons why NAFLD leads to non-metastatic bladder cancer and other extrahepatic cancers common in the elderly.4 In recent years, the term “metabolic dysfunction-associated fatty liver disease (MAFLD)” has also been used internationally to define chronic liver disease, excluding other factors such as excessive drinking to reflect better the disease process as well as diagnose and treat it.5 Compared with “NAFLD”, “MAFLD” emphasizes the main role of metabolic disorders such as type 2 diabetes and obesity in the occurrence, development, diagnosis and treatment of the disease.This term has also been recognized by more and more institutions around the world.6 Although steady progress in research on the pathogenesis, therapeutic targets, and drug development of NAFLD, the development of some drugs has entered Phase 2 and Phase 3 clinical trials, and no drugs are currently approved to treat NAFLD.7 Therefore, due to the severe harm of NAFLD and the lack of existing therapeutic drugs, it is increasingly important to find new therapeutic targets to provide ideas and directions for the development of new anti-NAFLD drugs.
In the past, hepatic steatosis from insulin resistance and excess fatty acids was called “first-hit”. “Second-hit” mainly includes liver cell damage, inflammation, fibrosis, and other pathological changes caused by oxidative stress and lipid peroxidation. The “Second-hit” theory has gradually been replaced by the “multiple hits” theory as it is difficult to fully interpret the pathological changes and metabolic mechanisms in the development of NAFLD. The “multiple hits” theory based on the “second hits” theory also includes changes in gut microbiota, nutritional factors, endoplasmic reticulum stress, mitochondrial dysfunction, lipotoxicity, and genetic and epigenetic factors.8 Therefore, regulating lipid and cholesterol metabolism, reducing hepatic steatosis and oxidative Stress, anti-inflammation, and improving fatty acid oxidation(FAO) and insulin resistance(IR) is the focus of developing drugs for preventing and treating NAFLD to minimize potential morbidity and mortality in the patient population (Figure 1).
In recent studies, it has been found that sirtuins (SIRTs) play a crucial role in the course of treatment of diseases related to metabolic syndrome. Sirtuins have an essential role in the dynamic pathophysiology of NAFLD. SIRTs are NAD + (nicotinamide adenine dinucleotide) -dependent histone and protein deacetylases that are highly conserved and classified as class III histone deacetylases (HDACs). They also belong to silent information regulator 2 (Sir2). Previous studies have identified seven mammalian sirtuin homologs (SIRT1-7). There are different subcellular regions where they are located; among them, SIRT1 is distributed in the nucleus and cytoplasm. SIRT1 is a protein that regulates adipocyte accumulation and maturation, hepatic lipid metabolism, and systemic inflammation due to its therapeutic activity on insulin sensitivity, antihyperlipidemic activity on lipid homeostasis, anti-inflammatory activity, antiaging activity, and positive effects on autophagy, apoptosis and cancer. So far, SIRT1 is the most extensively studied among the sirtuins in the pathophysiology of many metabolic diseases, especially NAFLD. SIRT1 deacetylates cellular proteins, thereby linking the metabolic state of the cell to protein function.9 SIRT1 is expressed in the liver, pancreas, adipose tissue, muscle, and heart and plays a vital role in maintaining metabolic functions through its capacity for protein deacetylation. Recent studies have shown that SIRT1 is required for lipid/cholesterol homeostasis and insulin sensitivity through its protective actions on mitochondrial biogenesis and beta-oxidation.10 Moreover, SIRT1 and its activators indirectly reduce oxidative stress through its anti-inflammation effect and contribute to improving obesity, hypertension, cardiovascular protection, apoptosis, and autophagy. A recent clinical study has shown that levels of SIRT1 in patients with NAFLD were markedly reduced, and activating SIRT1 and its associated pathways with drugs can significantly reduce the severity of NAFLD.11
Although there are no FDA-approved SIRT1-related drugs, studies have shown that the activation of SIRT1 may play a role in the treatment of NAFLD by affecting the pathogenic molecular cascade and therapeutic mechanism of NAFLD. Besides, many natural products are reported to act on SIRT1 and its signaling pathway to prevent or alleviate NAFLD. In this review, we will summarize the mechanisms by which SIRT1 and its signaling pathways play a role in preventing and treating NAFLD and natural products in preventing and treating NAFLD via the SIRT1 and its pathway, and evaluate the possibility of SIRT1 as a target for the prevention and treatment of NAFLD and search for relevant natural products that have the potential to be developed as drugs for metabolic diseases.
The Role of SIRT1 and Its Downstream Targets in the Occurrence, Development and Treatment of NAFLD
De Novo Lipogenesis
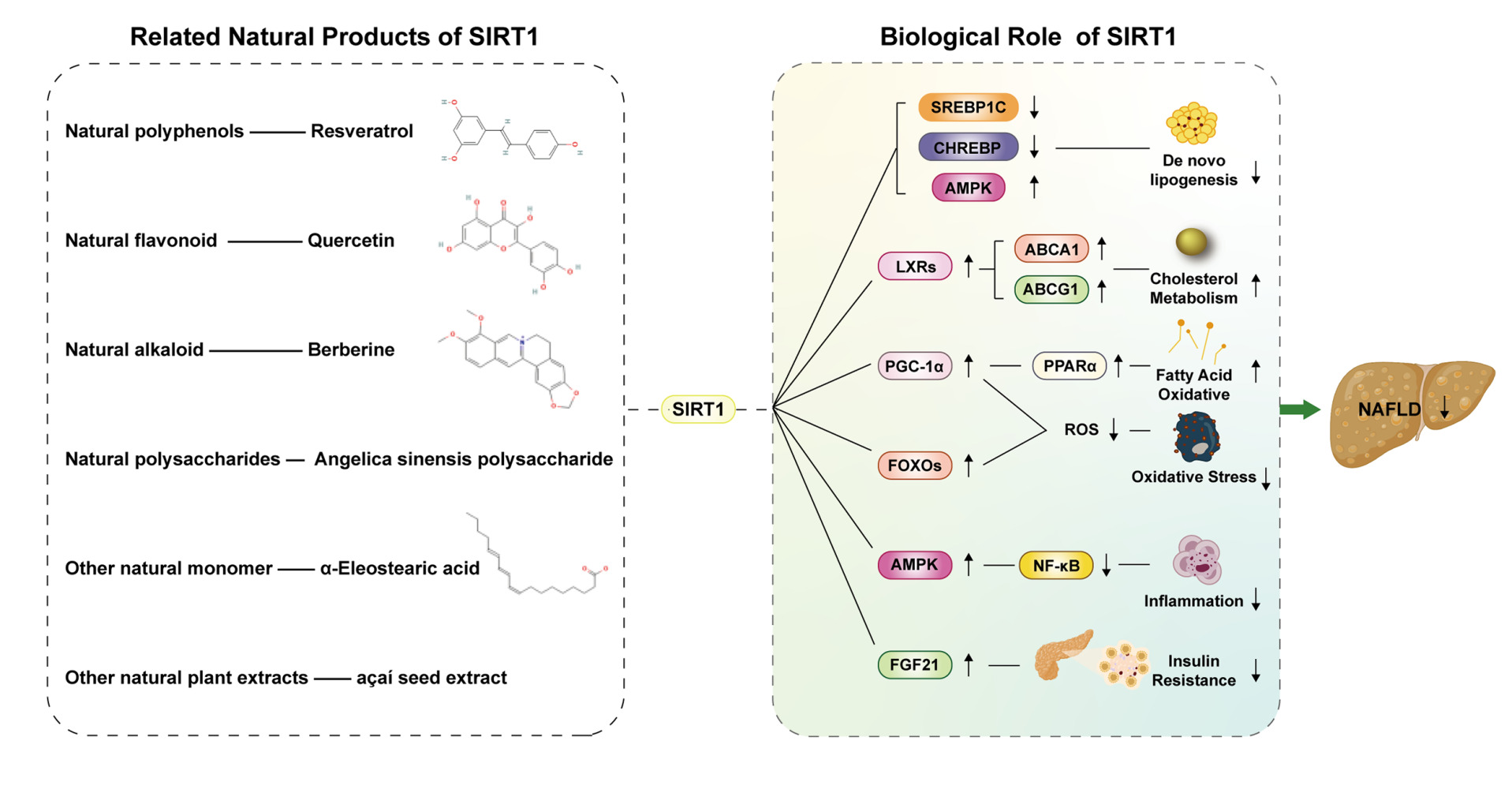
De novo lipogenesis(DNL), a metabolic pathway active primarily in the liver and adipose tissue, is the process of converting excess carbohydrates to fatty acids, followed by esterification into triglycerides(TG), and then provides energy to the body through beta-oxidation. DNL disorders are closely related to the occurrence and development of obesity, NAFLD and other metabolic syndromes.12 Hepatic DNL is one of the crucial factors for lipid accumulation in NAFLD patients, and its increased activity may lead to excessive release of fatty acids and hepatic steatosis. Therefore, DNL plays an essential role in the aetiology of fatty liver disease and is promising to be a therapeutic target for NAFLD.13 Sterol regulatory element-binding protein-1c (SREBP-1c), primarily expressed in the liver, can control glucose synthesis of lipids in the liver and promote hepatic TG synthesis.14 Carbohydrate response element-binding protein (ChREBP), a central regulator of de novo fatty acid synthesis in the liver, is another master lipogenic transcription factor and regulates the expression of critical genes involved in glucose and lipid metabolism.15 Under the induction of glucose flow and insulin signalling, ChREBP acts in synergy with SREBP-1c to fully induce the synthesis of TG and fatty acid, mainly through phosphorylation and acetylation.16 Because SREBP-1c and ChREBP bind to promoter gene targets in the nucleus, they are also positive regulators of lipogenic genes such as acetyl-CoA carboxylase 1 (ACC1), fatty acid synthase (FAS) and Stearoyl-CoA Desaturase 1 (SCD1).17,18 High-fat diet-induced hepatic steatosis promotes increased acetylation of SREBP-1C and CHREBP and leads to liver steatosis, insulin resistance and inflammation, which aggravates the development of NAFLD.19,20 Overexpression of SIRT1 enhances the expression of AMPK (Adenosine 5‘-monophosphate (AMP)-activated protein kinase) through the interaction of two proteins and inhibits lipogenesis by deacetylating SREBP-1C and CHREBP and blocking its downstream lipogenic genes.17,19 Thus, activation of SIRT1 inhibits DNL to reduce hepatic lipid accumulation and attenuate NAFLD.
In addition, SIRT1 also regulates hepatic fatty acid metabolism and lipid metabolism in hepatocytes by activating the LKB1 (Liver kinase B1) /AMPK signaling pathway and reducing FAS expression and lipid accumulation (Figure 2).
Cholesterol Metabolism
Excess cholesterol accumulation eventually leads to the development of NAFLD with steatosis, steatohepatitis, fibrosis and insulin resistance.21 The liver X receptors (LXRs) act as nuclear receptors, essential in regulating lipid homeostasis and hepatic fat metabolism, and play a preventive and therapeutic role in various metabolic and lipid-related diseases. LXRs promote reverse hepatic cholesterol transport by promoting the cholesterol efflux to apolipoprotein AI from peripheral tissues and cells and form high-density lipoprotein (HDL) via ATP-binding cassette transporter-A1 (ABCA1) and -G1 (ABCG1). Cholesterol is synthesized in the liver into bile acids, and then cholesterol and bile acids are excreted by LXR activation. Furthermore, LXRS can also promote the conversion of cholesterol into bile acids by upregulating the expression of cytochrome P450 7α-hydroxylase (CYP7A1).22 SIRT1 directly interacts with and deacetylates FXR at lysine K432 and increases the expression of LXRS and its downstream target genes.17 Although LXRs also promote hepatic lipogenesis mainly through increasing transcription of SREBP-1c,23 SIRT1-mediated deacetylation can downregulate the level of SREBP-1c, as mentioned above. At the same time, SIRT1 also regulates lipid/cholesterol homeostasis and improves lipid metabolic disorders by inhibiting the expression of SREBP target genes. Activation of SIRT1 leads to deacetylation of SREBP, thereby reducing hepatic lipid and cholesterol levels and attenuating hepatic steatosis.24 Therefore, SIRT1 show beneficial effects on hepatic cholesterol metabolism to improve hepatic steatosis by enhancing the expression of LXRS and inhibiting the expression of the SREBP gene (Figure 2).
Fatty Acid Oxidative
Fatty acid oxidative(FAO), the primary way for the liver to utilize fatty acid, is essential for energy homeostasis. Moore MP et al25 reported that increasing NAFLD severity in obese patients was closely related to mitochondrial dysfunction and FAO impairment. In hepatocytes, FAO deficiency leads to lipid accumulation, produces excess reactive oxygen species (ROS), and undergoes oxidative damage, thus leading to the generation or aggravation of oxidative stress and NALFD. At the same time, the increased severity of NAFLD will also exacerbate ROS production and impaired FAO and affect mitochondrial biogenesis, autophagy, mitophagy, fission and fusion. The PGC-1α/PPARα signaling pathway is vital in regulating this process.26 Peroxisome proliferator-activated receptor alpha (PPARα) is a ligand-activated transcription factor whose primary endogenous ligands include fatty acids. PPARα binding to fatty acids can activate fatty acids catabolic genes, such as CD36 and CTPa1, in the mitochondrial matrix and play a central role in metabolism.27 SIRT1 increases the transcriptional activity of PPARα by activating its deacetylated coactivator PPAR-γ coactivator 1-α (PGC-1α), which promotes liver fatty acid oxidation, increases lipid utilization, and improves fatty liver, thus playing a crucial role in regulating energy and hepatic lipid homeostasis.28 We propose targeting SIRT1 improves mitochondrial function and FAO, promoting hepatic lipid metabolism and ameliorating NAFLD phenotype (Figure 2).
Moreover, SIRT1 upregulates SIRT6 expression by forming a complex with FOXO3a and NRF1 on the SIRT6 promoter, which can reduce triglyceride synthesis, enhancing FAO and alleviating fatty liver.29
Oxidative Stress
Oxidative stress can cause or aggravate liver cell damage and contributes to mitochondrial dysfunction, lipid peroxidation, and cell apoptosis. Excessive reactive oxygen species (ROS) produced by the development of NAFLD and impaired FAO, may also stimulate additional mitochondrial DNA (mtDNA) damage and exacerbate mitochondrial damage.30 PGC-1α and forkhead box O (FOXO) proteins, which include FOXO1, FOXO3, FOXO4 and FOXO6 are key transcription factors in redox regulation. They can activate the transcription of antioxidant enzyme genes such as NADPH oxidase, superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX) to enhance the detoxification of oxidative stress caused by excess ROS.31,32 Moreover, PGC-1α also activates the expression of antioxidant proteins MnSOD and NRF1 to protect against long-term HFD-induced hepatic oxidative stress and metabolic damage. Moreover, PGC-1α also activates the expression of antioxidant proteins MnSOD and NRF1 to protect against long-term HFD-induced hepatic oxidative stress and metabolic damage.33 On the one hand, SIRT1 physically interacts with and deacetylates PGC-1α and FOXO proteins, which leads to the production of ROS-detoxifying enzymes, the decrease in hepatic malondialdehyde (MDA) levels and nitric oxide synthase, and enhancement of antioxidant enzyme activities. On the other hand, SIRT1 deacetylates the p53 tumor suppressor protein, inhibiting oxidative stress-induced apoptosis.34,35 SIRT1 also increases the expression of vascular nitric oxide synthase via endothelial nitric oxide synthetase (eNOS) and improves oxidative stress.36 Several clinical studies have shown that the activation of SIRT1 by resveratrol significantly reduces the ROS level of subjects (subjects in these clinical trials included healthy people, diabetics, obese patients and smokers) and enhances the antioxidant effect.37,38 In summary, SIRT1 activation increases antioxidant activity and reduces lipid peroxidation and liver cell damage (Figure 2).
Inflammation
Inflammation is associated with liver damage and the development of NAFLD. Adipose tissue-derived inflammation plays a crucial role in the pathogenesis of NAFLD. The M1 macrophages in visceral adipose tissue induce insulin resistance and inflammation, resulting in liver inflammation.39 Nuclear factor-κB (NF-κB) and its signaling pathway are considered to be central in the inflammatory process. Once the inflammatory response is triggered, NF-κB can be released by IκB kinase (IKK)‐mediated phosphorylation of IκB, translocated into the nucleus and then mediates recruitment of p300/CBP, and acetylated rela/p65 to activate transcription of NF-κB and enhance the expression of genes involved in inflammatory pathways.40 SIRT1 reduces macrophage infiltration and production of pro-inflammatory cytokines such as interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α) in liver and adipose tissue, mainly through deacetylating NF-κB and downregulating its transcriptional activity. As an inhibitor of NF-κB, AMPK plays a significant anti-inflammatory role, and PPARα interacts with P65 to inhibit the activation of NF-κB. Therefore, SIRT1 can also indirectly inhibit NF-κB signaling pathway by activating AMPK and PPARα.41 Furthermore, macrophage-activating protein-1 (AP-1), a transcription factor, is involved in the expression of genes involved in inflammatory and other stimulatory responses through the action of growth factors and cytokines. SIRT1 exerts anti-inflammatory activity by deacetylating PGC-1α and inhibiting AP-1 transcriptional activity and the expression of its downstream inflammatory mediator cyclooxygenase 2 (COX2).42 Thus, SIRT1, an essential negative inflammatory regulator, has an anti-inflammatory effect on the inflammatory mechanism in NAFLD (Figure 2).
Insulin Resistance
Studies have shown that NAFLD is closely related to type 2 diabetes mellitus (T2DM). T2DM is a significant dangerous factor for the progression of hepatic steatosis to steatohepatitis.43 Similarly, Patients with NAFLD have a significantly increased risk of T2DM.44 The relationship between T2DM and NAFLD is associated with Insulin resistance(IR) support and mediation. IR causes disturbance of fatty acid metabolism, leading to hepatic steatosis, inflammation, and fibrosis. Therefore, IR is a significant risk factor for NAFLD. Meanwhile, chronic hepatitis is also an important pathophysiological mechanism of the decrease of systemic insulin sensitivity.45 Fibroblast growth factor 21 (FGF21), a hepatocyte-derived hormone, restores glucose and lipid homeostasis and insulin sensitivity in obesity-induced diabetes. FGF21 down-regulates blood glucose, insulin, and lipid levels and alleviates hepatic steatosis, thereby exerting therapeutic effects on NAFLD and T2DM.46 SIRT1 plays a crucial role in glucose homeostasis. SIRT1 enhances insulin sensitivity and regulates energy homeostasis and hepatic lipid metabolism by activating FGF21.47 SIRT1 activation in the central arcuate nucleus of the hypothalamus also reduces hepatic glucose production and increases insulin sensitivity.48 Furthermore, Overexpression of SIRT1 inhibits the NF-κB signaling pathway and deacetylates FOXO1 as described above, so SIRT1 not only protects pancreatic beta-cells and insulin secretion from cytokine-mediated apoptosis and damage but also regulates the production of insulin-sensitizing adipokines such as adiponectin.49,50 SIRT1 activation improves insulin resistance by producing anti-inflammatory effects and enhancing insulin sensitivity, thereby improving glucose homeostasis and mitigating the development of NAFLD (Figure 3).
Other Regulation of SIRT1 Targets in NAFLD Development and Treatment
miR-34a
MiR-34a, as a microRNA (miRNA), induces senescence and apoptosis and plays a vital role in developing metabolic diseases, especially NAFLD. MiR-34a is a potential biomarker of NAFLD, whose expression increases in NAFLD patients.51 A previous study showed that miR-34a directly targets NAMPT and reduces hepatic NAD+ levels and SIRT1 deacetylase activity.52 Former research proves inhibition of miR-34 expression upregulates the expression of SIRT1 and its downstream genes, thereby improving lipid metabolism, hepatocyte steatosis, inflammation, and apoptosis in NAFLD.53–55
Furthermore, a study suggested that inhibition of p53 also induced the activation of SIRT1 and suppressed steatosis, oxidative stress, and apoptosis in NAFLD by preventing the HFD-induced upregulation of miR-34a.56
FLRL2
Fatty liver-related lncRNA2 (FLRL2), a potential master therapeutic target of NAFLD, can activate circadian gene aryl-hydrocarbon receptor nuclear translocator-like (Arntl) and SIRT1, thereby participating in NAFLD pathogenesis mediated. Chen Y et al57 showed that FLRL2 enhancement improved NAFLD by activating the Arntl-Sirt1 axis and inhibiting lipogenesis, endoplasmic reticulum (ER) stress, and inflammation in vivo and in vitro. FLRL2 and its downstream Arntl-Sirt1 axis may become a potential therapeutic target for NAFLD.
PLIN5
Perilipin 5 (PLIN5), a member of the perilipin family of lipid droplet (LD)-associated proteins, is highly expressed in the liver and protects against hepatic lipotoxicity. A previous study showed that activation of PLIN5 enhanced the expression of its downstream target gene, SIRT1 and protected against palmitate-induced hepatocyte lipoapoptosis and inflammatory responses.58
IGF2
Normal mitochondrial function regulates lipid metabolism in the liver and maintains its optimal state. Steatosis disrupts mitochondrial function and oxidative metabolism, forming and exacerbating a vicious circle. Gui W et al59 found that IGF2 knockdown in HepG2 and AML12 cells impaired mitochondrial respiration, decreased mitochondrial contents and mitochondrial membrane potential(MMP), promoted ROS production, and disrupted the balance between mitochondrial fission and fusion. In the liver tissues of obese mice, IGF2 upregulated the expression of SIRT1 and its downstream gene PGC-1α and improved mitochondrial functions by regulating the transcription of mitochondria and downstream ETC-related genes, and then prevent the occurrence and development of NAFLD.
Moreover, Zhang L et al60 showed that activation of IQGAP2 upregulated SIRT1 expression and regulated the expression of its downstream target genes, such as SREBP and PPARα, by promoting the phosphorylation of CREB to treat NAFLD.
Mst1 overexpression upregulated the expression of SIRT1 by inhibiting SIRT1 ubiquitination and regulated hepatic lipid metabolism in vivo and in vitro.61 Therefore, IQGAP2 and Mst1 are also potential targets for treating NAFLD by regulating SIRT1 and its downstream pathways.
Natural Products That Affect the Occurrence and Development of NAFLD by Acting on SIRT1
We reviewed a total of 36 natural products that have been shown to improve NAFLD or its related pathogenesis by activating SIRT1 and its associated pathways in experimental animal data or cell culture (Table 1).
 |
Table 1 Natural Products That Affect the Occurrence and Development of NAFLD by Acting on SIRT1 |
Natural Polyphenols
Polyphenols, found in various edible plants, can reduce the risk of various metabolic diseases, especially NAFLD. Polyphenols have anti-inflammatory and antioxidant properties and improve lipid metabolism and insulin resistance, thereby playing a role in preventing and treating NAFLD.101
Curcumin is a natural polyphenol compound extracted from turmeric that can protect the liver and prevent the development of NASH because of its anti-inflammatory and antioxidant properties.102 Lee DE et al62 found that Curcumin upregulated the protein expression of SIRT1 and its downstream antioxidant proteins, such as SOD1, by inhibiting O-GlcNAcylation in AML12 Cells, leading to antioxidant responses in the development of NAFLD.
Resveratrol (Res), a natural polyphenol found in the skins of grapes and nuts, has anti-inflammatory, anti-tumor, anti-diabetic and heart-protecting activities.103
Zhu W et al63 showed that Res upregulated the protein expression of SIRT1 and AMPK and subsequently attenuated fat deposition and ameliorated oxidative stress in a KKAy mouse model. Tian Y et al65 also found that Res enhanced the protein expression of SIRT1 and AMPK, thereby inhibiting the NF-κB inflammation pathway in mice with steatohepatitis.
Zhang Y et al66 found that Res increased the protein expression and activity of SIRT1 in HepG2 cells in dose- and time-dependent manners and mitigated the development of NAFLD by inducing autophagy via the cAMP-PRKA-AMPK-SIRT1 signaling pathway in vitro and in vivo.
Hajighasem A et al64 showed that Res significantly elevated mRNA expression of SIRT1 in the livers of NAFLD rats. Combined with physical activities it could enhance the effect of decreasing NAFLD-induced abnormalities. Andrade JM et al68 also showed that oral treatment with Res upregulated SIRT1 mRNA expression and improved liver inflammation and lipid metabolism in HFD mice.
Wang GL et al67 showed that Res notably increased the mRNA and protein expression of SIRT1 and then activated the SIRT1-FOXO1 pathway and inhibited the expression of SREBP1 in the cell model of steatosis.
Xu K et al69 found that Res significantly enhanced the mRNA and protein expression of SIRT1 and reversed hyperuricemia, improved insulin resistance, inhibited hepatic steatosis, and reduced oxidative stress and hepatic inflammation in a rat model of NAFLD with hyperuricemia.
These studies collectively show that Res can increase the protein and mRNA levels of SIRT1, activate its related pathways in vivo and in vitro, and alleviate the occurrence and development of NAFLD by reducing lipid accumulation, promoting lipid metabolism, improving oxidative stress and insulin resistance, and anti-inflammation. In addition, resveratrol can also play a role in improving other subtypes of metabolic syndrome, such as hyperuricemia.
However, in a randomized controlled clinical trial, Asghari S et al104 found that the serum levels SIRT1 of the study participants receiving 600 mg pure trans-resveratrol (2 × 300 mg) daily cannot be influenced at the end of three months of treatment. The treatment only had the effect of reducing the weight and BMI of the participants. In the future, we should further study resveratrol’s long-term and dose-dependent effects on SIRT1 transcription and physiological levels in clinical trials.
Green tea (GT) is among the world’s most popular beverages. Green tea has antioxidant, anti-inflammatory, anti-diabetic and liver protective activities because it contains a high content of polyphenols.105 Torres LF et al106 found that GT prevents NAFLD in a high-fat diet mouse model. Epigallocatechin-3-gallate (EGCG) is the most abundant and biologically active catechin (one of the polyphenol compounds in green tea) in green tea. It has anti-fibrotic, anti-inflammatory and antioxidant effects in the NAFLD rat model.107 Santamarina AB et al70 showed that EGCC improved SIRT1 protein expression and stimulated the activation of the AMPK via LKB1 through adiponectin in HFD-fed mice.
RadixSalvia miltiorrhiza(Danshen) is a traditional herb. Its extract has the effect of alleviating lipid accumulation in the liver, promoting fatty acid catabolism, and attenuating cellular oxidative stress-induced liver damage.108
Li S et al71 showed that Salvianolic acid A (SalA), a natural polyphenolic compound extracted from RadixSalvia miltiorrhiza, upregulated SIRT1 protein expression in a dose-dependent manner and activated the AMPK-SIRT1 signaling pathway to protect against hepatic lipotoxicity in vivo and in vitro.
Zeng W et al72 found that salvianolic acid B (SalB), a water-soluble phenolic acid extracted from Radix Salvia miltiorrhiza, also enhanced the protein expression of SIRT1 and had a protective effect against HFD/PA-induced NAFLD by reducing steatosis and anti-inflammation in vivo and in vitro.
Carnosic acid (CA), a phenolic compound possessing anti-steatosis and antioxidant activity, is present abundantly in the leaf of Rosmarinus officinalis L(Lamiaceae).109 Shan W et al55 showed that CA enhanced the protein expression of SIRT1 by inhibiting miR-34a expression in a dose-dependent manner, whether in vivo or in vitro, and then reducing the accumulation of lipids in the body, inhibiting apoptosis, and reducing the damage of NAFLD to the body of the rat.
Natural Flavonoid
Flavonoids can produce hepatoprotective effects through their anti-inflammatory, antioxidant, anticancer, and anti-fibrotic activities. Thus, it can alleviate the occurrence and development of various chronic liver diseases and their complications, such as hepatitis and cirrhosis.110,111 Previous studies have shown that several natural flavonoids can improve NAFLD by activating SIRT1.
Licorice, a commonly used traditional Chinese medicine, has anti-inflammatory and hepatoprotective activity. Licochalcone A (LicA) and Isoliquiritigenin (ISL) are two characteristic chalcone abundant in licorice, and both have anti-inflammatory, anti-tumor, and anti-oxidative properties.112
Chian-Jiun Liou et al73 discovered that LicA upregulated the mRNA and protein expression of SIRT1 and reduced lipid accumulation in the liver of HFD-induced obese mice. LicA also increased SIRT1 expression in oleic acid (OA)(0.5 mM)-induced HepG2 cells and decreased lipid accumulation dose-dependently.
Na AY et al74 showed that ISL obviously and dose-dependently upregulated protein expression of SIRT1 and protects against ethanol-induced hepatic steatosis in AML-12 cells.
Phloretin (PT) is a flavonoid of the chalcone class derived from apple trees and has a variety of biological functions, such as inhibiting inflammation and regulating lipid metabolism.113 Liou CJ et al75 showed that Phloretin enhanced the mRNA expression and protein levels of SIRT1 in HFD-induced obese mice and upregulated protein levels of SIRT1 in OA-induced HepG2 cells, thereby improving hepatic steatosis.
Nobiletin (Nob) is a polymethoxylated flavonoid abundant in citrus fruits and exhibits antioxidant and antitumor effects. Peng Z et al76 found that Nob inhibits the activation of inflammatory factors such as NLRP3 by upregulating SIRT1 protein expression and ameliorated palmitic acid-induced lipotoxicity in AML-12 cells.
Quercetin, a typical flavonol-type flavonoid, is abundant in various edible plants and has antioxidant, anti-inflammatory, hypolipidemic, anti-apoptosis and hepatoprotective activities.114 Tang Y et al77 showed that Quercetin upregulated SIRT1 protein expression and attenuated hepatic lipid accumulation and inflammatory responses in HFD-gerbils. Peng J et al78 found that Quercetin also could improve glucose and lipid metabolism disorders by upregulating the activity and protein level of SIRT1 in diabetic rats.
Silibinin, a flavonolignan, has been used for years as a nutraceutical with solid antioxidant activity for liver diseases. Salomone F et al79 showed that Silibinin upregulated the mRNA expression and activity of SIRT1 and AMPK by restoring NAD+ levels and improved lipid metabolism, reduced oxidative stress and inflammation in vitro and in vivo.
Natural Alkaloid
Tomatidine is a steroidal alkaloid extracted from a few plants in the Solanaceae family. Wu SJ et al80 showed that tomatidine significantly upregulated the mRNA expression of SIRT1 in the liver tissue of HFD mice and markedly enhanced the protein level of SIRT1 in oleic acid-induced FL83B cells, thus promoting the SIRT1/AMPK signaling pathway to increase lipolysis and β-oxidation in fatty liver cells.
Oxymatrine (OMT) is a monosomic alkaloid isolated from the root of Sophora flavescens Ait and has antioxidant, anti-inflammatory, and hepatoprotective effects.115 Xu H et al81 showed that OMT significantly increased in protein expression of SIRT1 and its critical downstream regulators of lipid metabolism in the liver of rats with steatosis.
Berberine(BBR) is an isoquinoline alkaloid derived from Rhizoma copies, which has been widely applied in Chinese medicine to treat diabetes. Recent studies showed that BBR could ameliorate hepatic steatosis and insulin resistance in NAFLD patients and animal models.116 Shan MY et al82 found that BBR upregulated the mRNA and protein levels of SIRT1 and activated the SIRT1-FoxO1-SREBP2 signal pathway, thereby reducing cholesterol synthesis in FFA-fed HepG2 cells.
Natural Polysaccharides
Polysaccharides are polymeric carbohydrate macromolecules composed of long chains of monosaccharide units linked by various glycosidic bonds. Many polysaccharides extracted from Chinese medicines have potential pharmacological activities and can effectively and efficiently attenuate NAFLD.84
Low molecular weight fucoidan (LMWF) has antioxidant and anti-inflammatory activities and regulates plasma triglycerides and total cholesterol. Zheng Y et al83 showed that LWWF markedly upregulated protein expression of SIRT1 and its downstream target genes, such as AMPK and PGC1α, thereby improving liver oxidative stress and inflammation in a SIRT1-dependent manner in db/db mice.
Wang K et al84 found that chronic administration of Angelica sinensis polysaccharide (ASP), a natural compound derived from the roots of Angelica sinensis with bioactivity that ameliorates liver damage and oxidative stress, effectively reduced liver lipid accumulation and steatosis in high-fat diet-fed mice. SIRT1 protein expression was also significantly increased by ASP.
Jia L et al85 showed that Lycium barbarum polysaccharide(LBP), a novel natural antioxidant, significantly increased SIRT1 protein expression and deacetylase activity and attenuated high-fat-induced hepatic steatosis in a dose- or time-dependent manner, both in vitro and in vivo.
Antrodan (Ant) is a β-glucan derived from Antrodia cinnamomea, which has been reported to possess anti-inflammatory, antioxidant, and hepatoprotective activities. Chyau CC et al86 showed that Ant enhanced protein expression of the SIRT1 and pAMPK and decreased PPARγ and SREBP-1c expression, thereby significantly suppressing lipid synthesis, hepatic steatosis, inflammation, and alleviating insulin resistance in HFD mice.
Other Natural Monomer
Bitter melon seed oil (BMSO) has anti-inflammatory properties and effectively improves hepatic steatosis. Chen GC et al87 found that α-Eleostearic acid (α-ESA), a special fatty acid present in abundance in BMSO, upregulated the protein expression of SIRT1 and played an active role in enhancing lipid metabolism and lowering lipid levels in rat hepatoma H4IIEC3 cells.
Zhang E et al58 showed that Glycycoumarin (GCM), a major coumarin compound isolated from licorice, upregulated protein expression of SIRT1 via targeting the PLIN5-SIRT1 pathway and protected palmitate-induced hepatocytes lipoapoptosis and inflammatory responses in vivo and in vitro.
Panax ginseng, one of the most famous traditional herbal tonics, has the effects of lowering blood fat and improving insulin resistance and hepatic steatosis in the treatment of metabolic diseases. Ginsenosides, the main active ingredient of ginseng, has anti-inflammatory effects and can prevent NAFLD.117
Huang Q et al88 showed that Ginsenoside Rb2 could restore autophagy via upregulating the expression of SIRT1 and AMPK, thereby alleviating hepatic lipid accumulation and improving NAFLD.
Cheng B et al89 found that Ginsenoside Rg2 treatment upregulated SIRT1 expression in vivo and in vitro and significantly increased the deacetylase activity of SIRT1, thus ameliorating high-fat diet-induced metabolic disease.
Dioscin, a steroidal saponin extracted from the roots of Dioscorea plants, has a significant role in anti-inflammatory, anti-tumor, and hypolipidemic. Tao X et al90 showed that dioscin upregulated SIRT1 protein levels and the levels of SIRT1 in plasma in mice and rats. In addition, dioscin significantly enhanced the expression levels of SIRT1 based on immunofluorescence staining and Western blotting assay in primary cultured hepatocytes, AML-12, and HepG-2 cells. Dioscin protects against NAFLD by targeting the SIRT1/AMPK pathway, reduces lipid accumulation in vivo and in vitro and lowers serum and liver lipid levels.
Acanthoic acid (AA), a pimaradiene diterpene, can protect the liver and improve liver fibrosis in mice. Han X et al91 showed that AA significantly upregulated the protein expression of SIRT1 in mice with NAFLD and enhanced fatty acid homeostasis in their liver.
Atractylenolide III (ATLIII) is one of the main active products in Atractylodes macrocephala Koidz (AMK) and has anti-inflammatory and antioxidant effects. Li Qet al92 found that ATLIII significantly upregulated the protein expression of SIRT1 and downstream signaling molecules by activating hepatic adiponectin receptor 1 in HFD-fed induced NAFLD mice and FFAs-induced HepG2 cells.
Zhang J et al93 showed that Pinolenic acid, a polyunsaturated fatty acid extracted from pine nuts oil, enhanced the protein expression of SIRT1 and AMPK in OA-induced HepG2 cells and decreased synthesis of the fatty acid chain for lipid metabolism.
(-)-Epicatechin (EC), a flavanol monomer in plant foods such as green tea, has anti-inflammatory and antioxidant activity.118 Cheng H et al94 showed that EC increased mRNA and protein expression of SIRT1 in a dose-dependent manner in rats fed a high-fat diet.
Li XX et al119 showed that Sodium tanshinone IIA sulfonate(STS), a water-soluble compound derived from traditional Chinese medicine Salvia miltiorrhiza (danshen), activated SIRT1 and p-PRKAA1 protein expression, thereby suppressing lipogenesis and inflammation.
Mangiferin(MGF), a natural xanthone extracted from the leaves of Mangifera indica or the root of Anemarrhena asphodeloides, regulates lipid metabolism. Li J et al95 found that MGF upregulated the protein expression of SIRT1 in vivo, and its metabolites M1 significantly enhanced the protein expression of SIRT1 in a dose-dependent manner in Sodium Oleate (SO)-induced HepG2 Cells.
Purple sweet potato color(PSPC), a class of natural anthocyanins, can significantly decrease some symptoms of HFD-induced NAFLD through its anti-inflammatory, antioxidant, anti-diabetic, and hepatoprotective activities.120 Su W et al96 showed that PSPC alleviated oxidative stress to restore NAD+level and upregulated the protein expression and activity of SIRT1, thereby inhibiting the p53-apoptotic pathway and enhancing the Akt survival pathway, ultimately protecting against HFD-induced hepatic apoptosis in vivo and in vitro.
Other Natural Plant Extracts
Tavares TB et al97 found that açaí seed extract (ASE) augmented the protein expression of SIRT1, improving oxidative stress and hepatic lipidosis in HFD-induced obesity in male mice.
Yu Y et al98 showed that Bitter melon extract enhanced the protein expression of SIRT1 and its downstream target genes in mice fed an HFD, thereby increasing insulin sensitivity and attenuating hepatic steatosis.
Cynanchum atratum, a medicinal herb in eastern Asia, is traditionally believed to have diuretic, detoxifying, antipyretic, and anti-inflammatory effects. Wang JH et al99 found that ethanol extract of Cynanchum atratum(CAE) significantly upregulated the mRNA expression of SIRT1 and other β-oxidation-related genes.
Kim YJ et al100 found that P. grandiflorus root ethanol extract (PGE) significantly upregulated mRNA expression levels of thermogenic genes such as SIRT1 and improved glucose and lipid homeostasis in high-fat diet mice and protected the occurrence and development of liver injury.
Conclusions and Prospects
Due to the rising prevalence of NAFLD worldwide and the lack of available therapeutic drugs, the potential limitations of current approaches to the prevention, diagnosis, and treatment of NAFLD are becoming increasingly apparent. The number of NAFLD cases in China, the United States, and others will still grow. Especially in China, the number of issues and disease burdens will increase significantly due to urbanization, ageing, and the prevalence of obesity and diabetes. This further increases advanced liver disease and liver-related mortality and disease burden. Therefore, improving the understanding, diagnosis and treatment of NAFLD is essential.121 In addition, since NAFLD is often caused by a combination of factors such as lipid accumulation, inflammation, oxidative stress and insulin resistance, and in some clinical trials, only no more than 40% of patients can be treated with a single therapy, most patients need to take weight-loss, blood-lipid-lowering, insulin-sensitizing drugs and liver-protecting drugs with anti-inflammatory and anti-oxidative effects at the same time to improve the development of NAFLD and prevent complications such as liver cirrhosis and liver cancer. Thus, it is indispensable to constantly explore new therapeutic targets for NAFLD and develop new multi-target medicinal drugs with few side effects. We summarized that SIRT1 maintain normal liver development and function by regulating lipid and cholesterol metabolism, insulin sensitivity, hepatic oxidative stress and hepatic inflammation. SIRT1 can deacetylate some transcriptional regulators associated with hepatic steatosis, showing potential as an important therapeutic target for NAFLD.122
Due to their excellent efficacy, safety, and low cost of use, a large amount of literature data shows that many natural products have the potential to become alternative therapies for NAFLD treatment or to be developed as anti-NAFLD drugs through different mechanisms and have received widespread attention from many clinicians to meet the needs for prevention and treatment of NAFLD.123 This review summarizes 36 natural products that can alleviate NAFLD in vivo and/or in vitro by activating SIRT1 and its related pathways. Some of these natural products, such as Resveratrol and Silibinin, have been used as dietary supplements or drugs to prevent and improve the occurrence of and development of NALFD, and these studies provide new ideas and research directions for preventing and treating NAFLD.
However, taking resveratrol as an example, the results of clinical trials related to treating fatty liver with natural products targeted by SIRT1 are inconsistent. Chen S et al124 showed that resveratrol benefits NAFLD patients by improving liver damage and insulin resistance, lowering total cholesterol, anti-inflammation and anti-fibrosis. Faghihzadeh F et al125 also found that resveratrol has hepatoprotective and anti-inflammatory activities, but their results suggest that resveratrol does not improve insulin resistance. Heebøll S et al126 showed that resveratrol not only does not improve insulin resistance but also may cause a few adverse reactions, such as fever and bicytopenia. Even studies point out that resveratrol can not benefit patients with NAFLD.127 There may be several reasons for the inconsistent findings. On the one hand, the daily dose and duration of resveratrol administration varied in these trials. For example, a previous study showed when resveratrol was administered at moderate levels (25 mM), SIRT1 might be activated first, and then AMPK is activated, while a high dose (50 mM) might activate AMPK directly.128 On the other hand, activation of SIRT1 in adipocytes stimulates lipolysis by inhibiting peroxisome proliferator-activated receptor gamma (PPARγ) and activating the FOXO1/adipose triglyceride lipase (ATGL) signaling pathway, releasing large amounts of free fatty acid(FFA) into circulation. It increases fatty acid flux from adipose tissue to the liver, which may cause or aggravate hepatic steatosis (Figure 3). Hence, it is necessary to further elucidate the different roles of SIRT1 at the multi-organ level, especially in the adipose tissue-liver axis associated with fatty liver.129 In addition, although the current research has proved that many natural products can prevent and treat diseases by activating multiple target genes, including SIRT1, in animal experiments or cell culture, it remains unclear whether they can improve the occurrence and development of NAFLD by activating SIRT1 and its related pathways in humans as in animal and in vitro cell experiments due to their rarity of clinical trials.
Based on many high-quality studies and reviews in recent years, we believe that SIRT1 may be able to be used as a promising target for the treatment of NAFLD and applied to the development of related drugs. Some natural agonists of SIRT1 have been studied in vivo and in vitro, showing good potential for the prevention and treatment of NAFLD. However, more clinical application research is still needed in the future. We must demonstrate the safety and efficacy of these natural products for disease prevention and treatment in clinical applications and further understand the optimal dosage of these natural products to ensure their proper biological activity. Although most of these natural agonists of SIRT1 summarized in this paper cannot be directly applied in the clinic, they provide a new idea for developing anti-NAFLD drugs with more favorable pharmacokinetic and pharmacodynamic properties.
Abbreviations
NAFLD, Non-alcoholic fatty liver disease; FFA, free fatty acids; MAFLD, metabolic dysfunction-associated fatty liver disease; SIRTs, sirtuins; HDACs, histone deacetylases; Sir2, silent information regulator2; DNL, De novo lipogenesis; TG, triglycerides; SREBP-1c, sterol regulatory element-binding protein-1c; ChREBP, carbohydrate response element-binding protein; ACC1, acetyl-CoA carboxylase 1; FAS, fatty acid synthase; SCD1, Stearoyl-CoA Desaturase 1; AMPK, Adenosine 5‘-monophosphate (AMP)-activated protein kinase; LKB1, Liver kinase B1; LXRs, liver X receptors; HDL, high-density lipoprotein; ABCA1, ATP-binding cassette transporter-A1; ABCG1, ATP-binding cassette transporter-G1; CYP7A1, cytochrome P450 7α-hydroxylase; FAO, Fatty Acid Oxidative; ROS, reactive oxygen species; PGC-1α, PPAR-γ coactivator 1-α; PPARα, Peroxisome proliferator-activated receptor alpha; FOXO, forkhead box O; SOD, superoxide dismutase; CAT, catalase; GPX, glutathione peroxidase; MDA, malondialdehyde; eNOS, endothelial nitric oxide synthetase; NF-κB, Nuclear factor-κB; IKK, IκB kinase; IL-6, interleukin 6; TNF-α, tumor necrosis factor-alpha; AP-1, macrophage-activating protein-1; COX2, cyclooxygenase 2; T2DM, type 2 diabetes mellitus; IR, Insulin resistance; FGF21, Fibroblast growth factor 21; miRNA, microRNA; LFD, lower-fat diet; HFD, high-fat diet; FLRL2, Fatty liver-related lncRNA2; Arntl, aryl-hydrocarbon receptor nuclear translocator-like; ER, endoplasmic reticulum; PLIN5, Perilipin 5; LD, lipid droplet; IGF2, Insulin-like growth factor 2 IGF2; MMP, mitochondrial membrane potential; BMSO, Bitter melon seed oil; α-ESA, α-Eleostearic acid; LicA, Licochalcone A; ISL, Isoliquiritigenin; GCM, Glycycoumarin; GT, Green tea; EC, (-)-Epicatechin; EGCG, Epigallocatechin-3-gallate; SalA, Salvianolic acid A; SalB, salvianolic acid B; LMWF, Low molecular weight fucoidan; ASP, Angelica sinensis polysaccharide; LBP, Lycium barbarum polysaccharide; Res, Resveratrol; PT, Phloretin; Nob, Nobiletin; Ant, Antrodan; CAE, Cynanchum atratum; AA, Acanthoic acid; ATLIII, Atractylenolide III; AMK, Atractylodes macrocephala Koidz; OMT, Oxymatrine; CA, Carnosic acid; STS, Sodium tanshinone IIA sulfonate; ASE, açaí seed extract; MGF, Mangiferin; SO, Sodium Oleate; PSPC, Purple sweet potato color; BBR, Berberine; PPARγ, peroxisome proliferator-activated receptor gamma; ATGL, adipose triglyceride lipase; FFA, free fatty acid.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi:10.1038/nrgastro.2017.109
2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi:10.1002/hep.28431
3. Björkström K, Widman L, Hagström H. Risk of hepatic and extrahepatic cancer in NAFLD: a population-based cohort study. Liver Int. 2022;42(4):820–828. doi:10.1111/liv.15195
4. Tarantino G, Crocetto F, Di Vito C, et al. Association of NAFLD and Insulin Resistance with Non Metastatic Bladder Cancer Patients: a Cross-Sectional Retrospective Study. J Clin Med. 2021;10(2):346. doi:10.3390/jcm10020346
5. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi:10.1016/j.jhep.2020.03.039
6. Gofton C, Upendran Y, Zheng MH, George J. MAFLD: how is it different from NAFLD. Clin Mol Hepatol. 2023;29(Suppl):S17–S31. doi:10.3350/cmh.2022.0367
7. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–922. doi:10.1038/s41591-018-0104-9
8. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65(8):1038–1048. doi:10.1016/j.metabol.2015.12.012
9. Nassir F, Ibdah JA. Sirtuins and nonalcoholic fatty liver disease. World J Gastroenterol. 2016;22(46):10084–10092. doi:10.3748/wjg.v22.i46.10084
10. Colak Y, Yesil A, Mutlu HH, et al. A potential treatment of non-alcoholic fatty liver disease with SIRT1 activators. J Gastrointestin Liver Dis. 2014;23(3):311–319. doi:10.15403/jgld.2014.1121.233.yck
11. Chalasani N, Vuppalanchi R, Rinella M, et al. Randomised clinical trial: a leucine-metformin-sildenafil combination (NS-0200) vs placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2018;47(12):1639–1651. doi:10.1111/apt.14674
12. Ameer F, Scandiuzzi L, Hasnain S, Kalbacher H, Zaidi N. De novo lipogenesis in health and disease. Metabolism. 2014;63(7):895–902. doi:10.1016/j.metabol.2014.04.003
13. Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(3):726–735. doi:10.1053/j.gastro.2013.11.049
14. Ferré P, Phan F, Foufelle F. SREBP-1c and lipogenesis in the liver: an update1. Biochem J. 2021;478(20):3723–3739. doi:10.1042/BCJ20210071
15. Filhoulaud G, Guilmeau S, Dentin R, Girard J, Postic C. Novel insights into ChREBP regulation and function. Trends Endocrinol Metab. 2013;24(5):257–268. doi:10.1016/j.tem.2013.01.003
16. Dentin R, Pégorier JP, Benhamed F, et al. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J Biol Chem. 2004;279(19):20314–20326. doi:10.1074/jbc.M312475200
17. Kemper JK, Choi SE, Kim DH. Sirtuin 1 deacetylase: a key regulator of hepatic lipid metabolism. Vitam Horm. 2013;91:385–404.
18. Wang RH, Li C, Deng CX. Liver steatosis and increased ChREBP expression in mice carrying a liver specific SIRT1 null mutation under a normal feeding condition. Int J Biol Sci. 2010;6(7):682–690. doi:10.7150/ijbs.6.682
19. Ponugoti B, Kim DH, Xiao Z, et al. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010;285(44):33959–33970. doi:10.1074/jbc.M110.122978
20. Bricambert J, Miranda J, Benhamed F, Girard J, Postic C, Dentin R. Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J Clin Invest. 2010;120(12):4316–4331. doi:10.1172/JCI41624
21. Zhang X, Coker OO, Chu ES, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70(4):761–774. doi:10.1136/gutjnl-2019-319664
22. Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol. 2006;68(1):159–191. doi:10.1146/annurev.physiol.68.033104.152158
23. Repa JJ, Liang G, Ou J, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14(22):2819–2830. doi:10.1101/gad.844900
24. Walker AK, Yang F, Jiang K, et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24(13):1403–1417. doi:10.1101/gad.1901210
25. Moore MP, Cunningham RP, Meers GM, et al. Compromised hepatic mitochondrial fatty acid oxidation and reduced markers of mitochondrial turnover in human NAFLD. Hepatology. 2022;76(5):1452–1465. doi:10.1002/hep.32324
26. Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813(7):1269–1278. doi:10.1016/j.bbamcr.2010.09.019
27. Montagner A, Polizzi A, Fouché E, et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 2016;65(7):1202–1214. doi:10.1136/gutjnl-2015-310798
28. Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9(4):327–338. doi:10.1016/j.cmet.2009.02.006
29. Kim HS, Xiao C, Wang RH, et al. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 2010;12(3):224–236. doi:10.1016/j.cmet.2010.06.009
30. Kojima H, Sakurai S, Uemura M, Fukui H, Morimoto H, Tamagawa Y. Mitochondrial abnormality and oxidative stress in nonalcoholic steatohepatitis. Alcohol Clin Exp Res. 2007;31(1 Suppl):S61–6. doi:10.1111/j.1530-0277.2006.00288.x
31. St-Pierre J, Drori S, Uldry M, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408. doi:10.1016/j.cell.2006.09.024
32. Klotz LO, Sánchez-Ramos C, Prieto-Arroyo I, Urbánek P, Steinbrenner H, Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51–72. doi:10.1016/j.redox.2015.06.019
33. Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105(28):9793–9798. doi:10.1073/pnas.0802917105
34. Zhao Y, Zhang J, Zheng Y, et al. NAD(+) improves cognitive function and reduces neuroinflammation by ameliorating mitochondrial damage and decreasing ROS production in chronic cerebral hypoperfusion models through Sirt1/PGC-1α pathway. J Neuroinflammation. 2021;18(1):207. doi:10.1186/s12974-021-02250-8
35. Hori YS, Kuno A, Hosoda R, Horio Y. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS One. 2013;8(9):e73875. doi:10.1371/journal.pone.0073875
36. Takizawa Y, Kosuge Y, Awaji H, et al. Up-regulation of endothelial nitric oxide synthase (eNOS), silent mating type information regulation 2 homologue 1 (SIRT1) and autophagy-related genes by repeated treatments with resveratrol in human umbilical vein endothelial cells. Br J Nutr. 2013;110(12):2150–2155. doi:10.1017/S0007114513001670
37. Carrizzo A, Forte M, Damato A, et al. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem Toxicol. 2013;61:215–226. doi:10.1016/j.fct.2013.07.021
38. Timmers S, Konings E, Bilet L, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14(5):612–622. doi:10.1016/j.cmet.2011.10.002
39. Machado MV, Diehl AM. Pathogenesis of Nonalcoholic Steatohepatitis. Gastroenterology. 2016;150(8):1769–1777. doi:10.1053/j.gastro.2016.02.066
40. Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. doi:10.1172/JCI11830
41. Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25(10):1939–1948. doi:10.1016/j.cellsig.2013.06.007
42. Zhang R, Chen HZ, Liu JJ, et al. SIRT1 suppresses activator protein-1 transcriptional activity and cyclooxygenase-2 expression in macrophages. J Biol Chem. 2010;285(10):7097–7110. doi:10.1074/jbc.M109.038604
43. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi:10.1016/j.jhep.2019.06.021
44. Ballestri S, Zona S, Targher G, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31(5):936–944. doi:10.1111/jgh.13264
45. Fujii H, Kawada N, Jsg-Nafld JSGON. The Role of Insulin Resistance and Diabetes in Nonalcoholic Fatty Liver Disease. Int J Mol Sci. 2020;21(11):3863. doi:10.3390/ijms21113863
46. Xu J, Lloyd DJ, Hale C, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58(1):250–259. doi:10.2337/db08-0392
47. Li Y, Wong K, Walsh K, Gao B, Zang M. Retinoic acid receptor β stimulates hepatic induction of fibroblast growth factor 21 to promote fatty acid oxidation and control whole-body energy homeostasis in mice. J Biol Chem. 2013;288(15):10490–10504. doi:10.1074/jbc.M112.429852
48. Knight CM, Gutierrez-Juarez R, Lam TK, et al. Mediobasal hypothalamic SIRT1 is essential for resveratrol’s effects on insulin action in rats. Diabetes. 2011;60(11):2691–2700. doi:10.2337/db10-0987
49. Lee JH, Song MY, Song EK, et al. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes. 2009;58(2):344–351. doi:10.2337/db07-1795
50. Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem. 2006;281(52):39915–39924. doi:10.1074/jbc.M607215200
51. Yamada H, Suzuki K, Ichino N, et al. Associations between circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and non-alcoholic fatty liver. Clin Chim Acta. 2013;424:99–103. doi:10.1016/j.cca.2013.05.021
52. Choi SE, Fu T, Seok S, et al. Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell. 2013;12(6):1062–1072. doi:10.1111/acel.12135
53. Wang L, Sun M, Cao Y, et al. miR-34a regulates lipid metabolism by targeting SIRT1 in non-alcoholic fatty liver disease with iron overload. Arch Biochem Biophys. 2020;695:108642. doi:10.1016/j.abb.2020.108642
54. Ding J, Li M, Wan X, et al. Effect of miR-34a in regulating steatosis by targeting PPARα expression in nonalcoholic fatty liver disease. Sci Rep. 2015;5:13729. doi:10.1038/srep13729
55. Shan W, Gao L, Zeng W, et al. Activation of the SIRT1/p66shc antiapoptosis pathway via carnosic acid-induced inhibition of miR-34a protects rats against nonalcoholic fatty liver disease. Cell Death Dis. 2015;6(7):e1833. doi:10.1038/cddis.2015.196
56. Derdak Z, Villegas KA, Harb R, Wu AM, Sousa A, Wands JR. Inhibition of p53 attenuates steatosis and liver injury in a mouse model of non-alcoholic fatty liver disease. J Hepatol. 2013;58(4):785–791. doi:10.1016/j.jhep.2012.11.042
57. Chen Y, Chen X, Gao J, et al. Long noncoding RNA FLRL2 alleviated nonalcoholic fatty liver disease through Arntl-Sirt1 pathway. FASEB J. 2019;33(10):11411–11419. doi:10.1096/fj.201900643RRR
58. Zhang E, Yin S, Zhao C, Fan L, Hu H. Involvement of activation of PLIN5-Sirt1 axis in protective effect of glycycoumarin on hepatic lipotoxicity. Biochem Biophys Res Commun. 2020;528(1):7–13. doi:10.1016/j.bbrc.2020.05.072
59. Gui W, Zhu Y, Sun S, et al. Knockdown of insulin-like growth factor 2 gene disrupts mitochondrial functions in the liver. J Mol Cell Biol. 2021;13(8):543–555. doi:10.1093/jmcb/mjab030
60. Zhang L, Yang SY, Qi-Li FR, et al. Administration of isoliquiritigenin prevents nonalcoholic fatty liver disease through a novel IQGAP2-CREB-SIRT1 axis. Phytother Res. 2021;35(7):3898–3915. doi:10.1002/ptr.7101
61. Geng C, Zhang Y, Gao Y, et al. Mst1 regulates hepatic lipid metabolism by inhibiting Sirt1 ubiquitination in mice. Biochem Biophys Res Commun. 2016;471(4):444–449. doi:10.1016/j.bbrc.2016.02.059
62. Lee DE, Lee SJ, Kim SJ, Lee HS, Kwon OS. Curcumin Ameliorates Nonalcoholic Fatty Liver Disease through Inhibition of O-GlcNAcylation. Nutrients. 2019;11(11):2702. doi:10.3390/nu11112702
63. Zhu W, Chen S, Li Z, et al. Effects and mechanisms of resveratrol on the amelioration of oxidative stress and hepatic steatosis in KKAy mice. Nutr Metab. 2014;11(1):35. doi:10.1186/1743-7075-11-35
64. Hajighasem A, Farzanegi P, Mazaheri Z, Naghizadeh M, Salehi G. Effects of resveratrol, exercises and their combination on Farnesoid X receptor, Liver X receptor and Sirtuin 1 gene expression and apoptosis in the liver of elderly rats with nonalcoholic fatty liver. PeerJ. 2018;6:e5522. doi:10.7717/peerj.5522
65. Tian Y, Ma J, Wang W, et al. Resveratrol supplement inhibited the NF-κB inflammation pathway through activating AMPKα-SIRT1 pathway in mice with fatty liver. Mol Cell Biochem. 2016;422(1–2):75–84. doi:10.1007/s11010-016-2807-x
66. Zhang Y, Chen ML, Zhou Y, et al. Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway. Mol Nutr Food Res. 2015;59(8):1443–1457. doi:10.1002/mnfr.201500016
67. Wang GL, Fu YC, Xu WC, Feng YQ, Fang SR, Zhou XH. Resveratrol inhibits the expression of SREBP1 in cell model of steatosis via Sirt1-FOXO1 signaling pathway. Biochem Biophys Res Commun. 2009;380(3):644–649. doi:10.1016/j.bbrc.2009.01.163
68. Andrade JM, Paraíso AF, de Oliveira MV, et al. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition. 2014;30(7–8):915–919. doi:10.1016/j.nut.2013.11.016
69. Xu K, Liu S, Zhao X, et al. Treating hyperuricemia related non-alcoholic fatty liver disease in rats with resveratrol. Biomed Pharmacother. 2019;110:844–849. doi:10.1016/j.biopha.2018.12.039
70. Santamarina AB, Oliveira JL, Silva FP, et al. Green Tea Extract Rich in Epigallocatechin-3-Gallate Prevents Fatty Liver by AMPK Activation via LKB1 in Mice Fed a High-Fat Diet. PLoS One. 2015;10(11):e0141227. doi:10.1371/journal.pone.0141227
71. Li S, Qian Q, Ying N, et al. Activation of the AMPK-SIRT1 pathway contributes to protective effects of Salvianolic acid A against lipotoxicity in hepatocytes and NAFLD in mice. Front Pharmacol. 2020;11:560905. doi:10.3389/fphar.2020.560905
72. Zeng W, Shan W, Gao L, et al. Inhibition of HMGB1 release via salvianolic acid B-mediated SIRT1 up-regulation protects rats against non-alcoholic fatty liver disease. Sci Rep. 2015;5(1):16013. doi:10.1038/srep16013
73. Liou CJ, Lee YK, Ting NC, et al. Protective Effects of Licochalcone A Ameliorates Obesity and Non-Alcoholic Fatty Liver Disease Via Promotion of the Sirt-1/AMPK Pathway in Mice Fed a High-Fat Diet. Cells. 2019;8(5):447. doi:10.3390/cells8050447
74. Na AY, Yang EJ, Jeon JM, Ki SH, Song KS, Lee S. Protective Effect of Isoliquiritigenin against Ethanol-Induced Hepatic Steatosis by Regulating the SIRT1-AMPK Pathway. Toxicol Res. 2018;34(1):23–29. doi:10.5487/TR.2018.34.1.023
75. Liou CJ, Wu SJ, Shen SC, Chen LC, Chen YL, Huang WC. Phloretin ameliorates hepatic steatosis through regulation of lipogenesis and Sirt1/AMPK signaling in obese mice. Cell Biosci. 2020;10:114. doi:10.1186/s13578-020-00477-1
76. Peng Z, Li X, Xing D, et al. Nobiletin alleviates palmitic acid‑induced NLRP3 inflammasome activation in a sirtuin 1‑dependent manner in AML‑12 cells. Mol Med Rep. 2018;18(6):5815–5822. doi:10.3892/mmr.2018.9615
77. Ying HZ, Liu YH, Yu B, Wang ZY, Zang JN, Yu CH. Dietary quercetin ameliorates nonalcoholic steatohepatitis induced by a high-fat diet in gerbils. Food Chem Toxicol. 2013;52:53–60. doi:10.1016/j.fct.2012.10.030
78. Peng J, Li Q, Li K, et al. Quercetin Improves Glucose and Lipid Metabolism of Diabetic Rats: involvement of Akt Signaling and SIRT1. J Diabetes Res. 2017;2017:3417306. doi:10.1155/2017/3417306
79. Salomone F, Barbagallo I, Godos J, et al. Silibinin Restores NAD⁺ Levels and Induces the SIRT1/AMPK Pathway in Non-Alcoholic Fatty Liver. Nutrients. 2017;9(10):1086. doi:10.3390/nu9101086
80. Wu SJ, Huang WC, Yu MC, et al. Tomatidine ameliorates obesity-induced nonalcoholic fatty liver disease in mice. J Nutr Biochem. 2021;91:108602. doi:10.1016/j.jnutbio.2021.108602
81. Xu H, Chen GF, Ma YS, et al. Hepatic Proteomic Changes and Sirt1/AMPK Signaling Activation by Oxymatrine Treatment in Rats With Non-alcoholic Steatosis. Front Pharmacol. 2020;11:216. doi:10.3389/fphar.2020.00216
82. Shan MY, Dai Y, Ren XD, et al. Berberine mitigates nonalcoholic hepatic steatosis by downregulating SIRT1-FoxO1-SREBP2 pathway for cholesterol synthesis. J Integr Med. 2021;19(6):545–554. doi:10.1016/j.joim.2021.09.003
83. Zheng Y, Liu T, Wang Z, Xu Y, Zhang Q, Luo D. Low molecular weight fucoidan attenuates liver injury via SIRT1/AMPK/PGC1α axis in db/db mice. Int J Biol Macromol. 2018;112:929–936. doi:10.1016/j.ijbiomac.2018.02.072
84. Wang K, Cao P, Wang H, et al. Chronic administration of Angelica sinensis polysaccharide effectively improves fatty liver and glucose homeostasis in high-fat diet-fed mice. Sci Rep. 2016;6(1):26229. doi:10.1038/srep26229
85. Jia L, Li W, Li J, et al. Lycium barbarum polysaccharide attenuates high-fat diet-induced hepatic steatosis by up-regulating SIRT1 expression and deacetylase activity. Sci Rep. 2016;6(1):36209. doi:10.1038/srep36209
86. Chyau CC, Wang HF, Zhang WJ, et al. Antrodan Alleviates High-Fat and High-Fructose Diet-Induced Fatty Liver Disease in C57BL/6 Mice Model via AMPK/Sirt1/SREBP-1c/PPARγ Pathway. Int J Mol Sci. 2020;21(1):360. doi:10.3390/ijms21010360
87. Chen GC, Su HM, Lin YS, Tsou PY, Chyuan JH, Chao PM. A conjugated fatty acid present at high levels in bitter melon seed favorably affects lipid metabolism in hepatocytes by increasing NAD(+)/NADH ratio and activating PPARα, AMPK and SIRT1 signaling pathway. J Nutr Biochem. 2016;33:28–35. doi:10.1016/j.jnutbio.2016.03.009
88. Huang Q, Wang T, Yang L, Wang HY. Ginsenoside Rb2 Alleviates Hepatic Lipid Accumulation by Restoring Autophagy via Induction of Sirt1 and Activation of AMPK. Int J Mol Sci. 2017;18(5):1063. doi:10.3390/ijms18051063
89. Cheng B, Gao W, Wu X, et al. Ginsenoside Rg2 Ameliorates High-Fat Diet-Induced Metabolic Disease through SIRT1. J Agric Food Chem. 2020;68(14):4215–4226. doi:10.1021/acs.jafc.0c00833
90. Yao H, Tao X, Xu L, et al. Dioscin alleviates non-alcoholic fatty liver disease through adjusting lipid metabolism via SIRT1/AMPK signaling pathway. Pharmacol Res. 2018;131:51–60. doi:10.1016/j.phrs.2018.03.017
91. Han X, Cui ZY, Song J, et al. Acanthoic acid modulates lipogenesis in nonalcoholic fatty liver disease via FXR/LXRs-dependent manner. Chem Biol Interact. 2019;311:108794. doi:10.1016/j.cbi.2019.108794
92. Zhou K, Chen J, Wu J, et al. Atractylenolide III ameliorates cerebral ischemic injury and neuroinflammation associated with inhibiting JAK2/STAT3/Drp1-dependent mitochondrial fission in microglia. Phytomedicine. 2019;59:152922. doi:10.1016/j.phymed.2019.152922
93. Li Q, Tan JX, He Y, et al. Atractylenolide III ameliorates Non-Alcoholic Fatty Liver Disease by activating Hepatic Adiponectin Receptor 1-Mediated AMPK Pathway. Int J Biol Sci. 2022;18(4):1594–1611. doi:10.7150/ijbs.68873
94. Cheng H, Xu N, Zhao W, et al. (-)-Epicatechin regulates blood lipids and attenuates hepatic steatosis in rats fed high-fat diet. Mol Nutr Food Res. 2017;61(11). doi:10.1002/mnfr.201700303
95. Li J, Liu M, Yu H, et al. Mangiferin Improves Hepatic Lipid Metabolism Mainly Through Its Metabolite-Norathyriol by Modulating SIRT-1/AMPK/SREBP-1c Signaling. Front Pharmacol. 2018;9:201. doi:10.3389/fphar.2018.00201
96. Su W, Zhang C, Chen F, et al. Purple sweet potato color protects against hepatocyte apoptosis through Sirt1 activation in high-fat-diet-treated mice. Food Nutr Res. 2020;64.
97. Tavares TB, Santos IB, de Bem GF, et al. Therapeutic effects of açaí seed extract on hepatic steatosis in high-fat diet-induced obesity in male mice: a comparative effect with rosuvastatin. J Pharm Pharmacol. 2020;72(12):1921–1932. doi:10.1111/jphp.13356
98. Yu Y, Zhang XH, Ebersole B, Ribnicky D, Wang ZQ. Bitter melon extract attenuating hepatic steatosis may be mediated by FGF21 and AMPK/Sirt1 signaling in mice. Sci Rep. 2013;3(1):3142. doi:10.1038/srep03142
99. Wang JH, Hwang SJ, Lim DW, Son CG. Cynanchum atratum Alleviates Non-Alcoholic Fatty Liver by Balancing Lipogenesis and Fatty Acid Oxidation in a High-Fat, High-Fructose Diet Mice Model. Cells. 2021;11(1):23. doi:10.3390/cells11010023
100. Kim YJ, Choi JY, Ryu R, et al. Platycodon grandiflorus Root Extract Attenuates Body Fat Mass, Hepatic Steatosis and Insulin Resistance through the Interplay between the Liver and Adipose Tissue. Nutrients. 2016;8(9):532. doi:10.3390/nu8090532
101. Rodriguez-Ramiro I, Vauzour D, Minihane AM. Polyphenols and non-alcoholic fatty liver disease: impact and mechanisms. Proc Nutr Soc. 2016;75(1):47–60. doi:10.1017/S0029665115004218
102. Yarru LP, Settivari RS, Gowda NK, Antoniou E, Ledoux DR, Rottinghaus GE. Effects of turmeric (Curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and immune systems in broiler chicks fed aflatoxin. Poult Sci. 2009;88(12):2620–2627. doi:10.3382/ps.2009-00204
103. Meng X, Zhou J, Zhao CN, Gan RY, Li HB. Health Benefits and Molecular Mechanisms of Resveratrol: a Narrative Review. Foods. 2020;9(3):340. doi:10.3390/foods9030340
104. Asghari S, Asghari-Jafarabadi M, Somi MH, Ghavami SM, Rafraf M. Comparison of Calorie-Restricted Diet and Resveratrol Supplementation on Anthropometric Indices, Metabolic Parameters, and Serum Sirtuin-1 Levels in Patients With Nonalcoholic Fatty Liver Disease: a Randomized Controlled Clinical Trial. J Am Coll Nutr. 2018;37(3):223–233. doi:10.1080/07315724.2017.1392264
105. Saeed M, Naveed M, Arif M, et al. Green tea (Camellia sinensis) and l-theanine: medicinal values and beneficial applications in humans-A comprehensive review. Biomed Pharmacother. 2017;95:1260–1275. doi:10.1016/j.biopha.2017.09.024
106. Torres LF, Cogliati B, Otton R. Green Tea Prevents NAFLD by Modulation of miR-34a and miR-194 Expression in a High-Fat Diet Mouse Model. Oxid Med Cell Longev. 2019;2019:4168380. doi:10.1155/2019/4168380
107. Xiao J, Ho CT, Liong EC, et al. Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur J Nutr. 2014;53(1):187–199. doi:10.1007/s00394-013-0516-8
108. Ding C, Zhao Y, Shi X, et al. New insights into salvianolic acid A action: regulation of the TXNIP/NLRP3 and TXNIP/ChREBP pathways ameliorates HFD-induced NAFLD in rats. Sci Rep. 2016;6(1):28734. doi:10.1038/srep28734
109. Park MY, Mun ST. Dietary carnosic acid suppresses hepatic steatosis formation via regulation of hepatic fatty acid metabolism in high-fat diet-fed mice. Nutr Res Pract. 2013;7(4):294–301. doi:10.4162/nrp.2013.7.4.294
110. Sahu R, Goswami S, Narahari Sastry G, Rawal RK. The Preventive and Therapeutic Potential of the Flavonoids in Liver Cirrhosis: current and Future Perspectives. Chem Biodivers. 2023;20(2):e202201029. doi:10.1002/cbdv.202201029
111. Lemmens KJ, van de Wier B, Koek GH, et al. The flavonoid monoHER promotes the adaption to oxidative stress during the onset of NAFLD. Biochem Biophys Res Commun. 2015;456(1):179–182. doi:10.1016/j.bbrc.2014.11.055
112. Chen M, Zhu J, Kang J, et al. Exploration in the Mechanism of Action of Licorice by Network Pharmacology. Molecules. 2019;24(16):2959. doi:10.3390/molecules24162959
113. Huang WC, Chang WT, Wu SJ, Xu PY, Ting NC, Liou CJ. Phloretin and phlorizin promote lipolysis and inhibit inflammation in mouse 3T3-L1 cells and in macrophage-adipocyte co-cultures. Mol Nutr Food Res. 2013;57(10):1803–1813. doi:10.1002/mnfr.201300001
114. Roslan J, Giribabu N, Karim K, Salleh N. Quercetin ameliorates oxidative stress, inflammation and apoptosis in the heart of streptozotocin-nicotinamide-induced adult male diabetic rats. Biomed Pharmacother. 2017;86:570–582. doi:10.1016/j.biopha.2016.12.044
115. Lu ML, Xiang XH, Xia SH. Potential Signaling Pathways Involved in the Clinical Application of Oxymatrine. Phytother Res. 2016;30(7):1104–1112. doi:10.1002/ptr.5632
116. Yuan X, Wang J, Tang X, Li Y, Xia P, Gao X. Berberine ameliorates nonalcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J Transl Med. 2015;13(1):24. doi:10.1186/s12967-015-0383-6
117. Lü JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7(3):293–302. doi:10.2174/157016109788340767
118. Prince PD, Fischerman L, Toblli JE, Fraga CG, Galleano M. LPS-induced renal inflammation is prevented by (-)-epicatechin in rats. Redox Biol. 2017;11:342–349. doi:10.1016/j.redox.2016.12.023
119. Li XX, Lu XY, Zhang SJ, et al. Sodium tanshinone IIA sulfonate ameliorates hepatic steatosis by inhibiting lipogenesis and inflammation. Biomed Pharmacother. 2019;111:68–75. doi:10.1016/j.biopha.2018.12.019
120. Wang X, Zhang ZF, Zheng GH, et al. The Inhibitory Effects of Purple Sweet Potato Color on Hepatic Inflammation Is Associated with Restoration of NAD⁺ Levels and Attenuation of NLRP3 Inflammasome Activation in High-Fat-Diet-Treated Mice. Molecules. 2017;22(8):1315. doi:10.3390/molecules22081315
121. Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69(4):896–904. doi:10.1016/j.jhep.2018.05.036
122. Ding RB, Bao J, Deng CX. Emerging roles of SIRT1 in fatty liver diseases. Int J Biol Sci. 2017;13(7):852–867. doi:10.7150/ijbs.19370
123. Tarantino G, Balsano C, Santini SJ, et al. It Is High Time Physicians Thought of Natural Products for Alleviating NAFLD. Is There Sufficient Evidence to Use Them. Int J Mol Sci. 2021;22(24):13424. doi:10.3390/ijms222413424
124. Chen S, Zhao X, Ran L, et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Dig Liver Dis. 2015;47(3):226–232. doi:10.1016/j.dld.2014.11.015
125. Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res. 2014;34(10):837–843. doi:10.1016/j.nutres.2014.09.005
126. Heebøll S, Kreuzfeldt M, Hamilton-Dutoit S, et al. Placebo-controlled, randomised clinical trial: high-dose resveratrol treatment for non-alcoholic fatty liver disease. Scand J Gastroenterol. 2016;51(4):456–464. doi:10.3109/00365521.2015.1107620
127. Chachay VS, Macdonald GA, Martin JH, et al. Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12(12):2092–103.e1–6. doi:10.1016/j.cgh.2014.02.024
128. Price NL, Gomes AP, Ling AJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15(5):675–690. doi:10.1016/j.cmet.2012.04.003
129. Chakrabarti P, English T, Karki S, et al. SIRT1 controls lipolysis in adipocytes via FOXO1-mediated expression of ATGL. J Lipid Res. 2011;52(9):1693–1701. doi:10.1194/jlr.M014647
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.