Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 14
Biologic Initiation Rate in Systemic-Naïve Psoriatic Arthritis Patients Starting Treatment with Apremilast vs Methotrexate: 1-Year Retrospective Analysis of a US Claims Database
Authors Husni ME , Chang E , Broder MS , Paydar C , Bognar K , Desai P, Klyachkin Y, Khilfeh I
Received 1 December 2021
Accepted for publication 3 May 2022
Published 15 June 2022 Volume 2022:14 Pages 123—132
DOI https://doi.org/10.2147/OARRR.S342123
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Daniel Furst
M Elaine Husni,1 Eunice Chang,2 Michael S Broder,2 Caleb Paydar,2 Katalin Bognar,2 Pooja Desai,3 Yuri Klyachkin,3 Ibrahim Khilfeh3
1Cleveland Clinic, Cleveland, OH, USA; 2Partnership for Health Analytic Research, LLC, Beverly Hills, CA, USA; 3Amgen Inc., Thousand Oaks, CA, USA
Correspondence: Michael S Broder, Partnership for Health Analytic Research, LLC, 280 S. Beverly Drive, Suite 404, Beverly Hills, CA, 90212, USA, Tel +1 310 858 9555, Fax +1 310 858 9552, Email [email protected]
Purpose: To compare the rate of biologic initiation after commencing treatment with apremilast (APR) vs methotrexate (MTX), in systemic-naïve patients with psoriatic arthritis (PsA).
Patients and Methods: Systemic-naïve patients with PsA who started treatment with either APR or MTX between 01/01/2015 and 12/31/2018 were analyzed using claims data from the IBM® MarketScan® Commercial and Medicare Supplemental databases (2014– 2019). PsA patients were identified via diagnosis codes; the first prescription date for APR or MTX was the index date. Patient demographics, clinical characteristics, healthcare utilization during the year pre-index (baseline) and the year post-index (follow-up), and median time to biologic initiation were reported descriptively. The rates and risk of biologic initiation during follow-up were compared between APR and MTX users by logistic and Cox regressions, respectively. Models were adjusted for demographics, clinical and utilization measures during the baseline period.
Results: A total of 2116 patients with PsA newly treated with APR (n = 534) or MTX (n = 1582) were identified. Mean age was similar (50.5 vs 50.4; P = 0.938), and proportion of females was higher for APR vs MTX users (59.4% vs 54.0%; P = 0.031). Mean time to biologic initiation among patients who initiated during follow-up was 194.1 vs 138.7 days between APR vs MTX users (P < 0.001). After adjusting for confounders, the likelihood of biologic initiation was 58% lower (OR, 0.42 [95% CI, 0.32– 0.54]; P < 0.001) with APR, with a significantly lower predicted rate of biologic initiation among APR users when compared to MTX users during follow-up (20.0% [95% CI, 16.6– 23.9%] vs 37.5% [95% CI, 35.0– 40.1%]). Additionally, APR users had a lower risk of biologic initiation than MTX users (HR, 0.46 [95% CI, 0.37– 0.57]; P < 0.001) during the 1-year follow-up.
Conclusion: Systemic-naïve patients with PsA have a lower rate of, and longer time to, biologic initiation over one-year following APR initiation, compared to those initiating MTX.
Keywords: psoriatic arthritis, oral small molecules, biologics, systemic treatment initiation, administrative claims analysis
Introduction
Psoriatic arthritis (PsA) is a systemic inflammatory musculoskeletal disease that can lead to permanent joint damage and disability. Prior studies have shown PsA affects approximately 1–2 per 1000 persons, with annual incidence rates estimated to be six per 100,000 persons-per-year in the general population.1–6 Due to its systemic impact and the burden of comorbidities, the choice of the therapy reflects a global evaluation of the concomitant pathological conditions.7–9 Choosing an effective therapy to manage PsA is complex due to differences in patient profiles (ie, disease severity, presence of psoriasis [PsO], contraindications of comorbidities), varied routes of administration, insurance coverage, cost, and side effect profiles of the different therapies.10–12 PsA is a lifelong disease requiring continued treatment, and for many patients, a sequence of consecutive pharmacological agents as indicated by disease progression or treatment failure.
Systemic treatments available for PsA include oral small molecule (OSM) therapies and biologics.13 Nonsteroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids are used as symptomatic therapies. Guidelines from the European League Against Rheumatism (EULAR)14 and from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA)15 recommend OSMs, particularly methotrexate (MTX) as first-line therapy and endorse apremilast (APR) for mild disease after failure of initial therapy. While in the US APR is approved as first-line treatment, the 2018 American College of Rheumatology (ACR)/National Psoriasis Foundation treatment guidelines recommend that treatment-naïve patients with active PsA use biologics over OSMs. However, the level of evidence supporting this recommendation is low.6 Biologic therapies can be effective, but require laboratory monitoring, have less convenient routes of administration, and tend to be more expensive.16 Physicians and patients considering these factors may prefer to start treatment with OSM therapy and postpone the use of biologics as long as clinically possible. Real-life treatment pattern studies document that the majority of treatment-naïve patients initiate OSM therapies.17,18
Evidence comparing APR with MTX - two commonly used OSMs (note, however, that MTX can be dosed subcutaneously) - in treatment of PsA is limited.19 While a prior indirect comparison did not find a statistically significant difference in efficacy, its point estimate favored APR when compared with MTX.19,20 The objective of this study was to assess time to biologic initiation after APR versus MTX treatment in a real-world setting among patients with PsA. Particularly, it aimed to compare biologic initiation rates, time to biologic initiation, and index medication adherence and discontinuation between PsA patients who were newly initiating APR or MTX and had not previously been treated with OSMs or biologics.
Materials and Methods
This study was a retrospective cohort study using the six most recent years (2014–2019) of administrative claims data from the IBM® MarketScan® Commercial and Medicare Supplemental databases to examine biologic initiation rates in patients with PsA who newly initiated APR and MTX. The MarketScan® data comprise health services for over 37.9 million patients through privately insured fee-for-service, point-of-service, or capitated health plans. This database contains enrollment information and administrative claims data with healthcare utilization information (eg, inpatient and outpatient services, prescription drug claims). Diagnosis and procedure codes are validated. This study used deidentified patient records and did not involve the collection, use, or transmittal of individually identifiable data, therefore, Institutional Review Board approval to conduct this study was not necessary.
Patients diagnosed with PsA were identified based on the presence of 1) at least two medical claims with an International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification (ICD-9/10-CM) diagnosis code for PsA (ICD-9-CM: 696.0x; ICD-10-CM: L40.50, L40.51, L40.52, L40.53, L40.54, L40.59) with at least one diagnosis by a rheumatologist or, 2) the combination of at least one diagnosis for PsA recorded by a rheumatologist and at least one diagnosis for PsO recorded by a dermatologist during the entire study period (01/01/2014 to 12/31/2019).21 Patients were included if they initiated APR or MTX during the identification (ID) period (01/01/2015 to 12/31/2018). The date of the first claim for APR or MTX during the ID period was assigned as the index date. Patients were required to be at least 18 years of age on the index date, have continuous enrollment for at least one year prior to (baseline period) and one year after (follow-up period) the index date, and have at least one of the diagnosis claims for PsA in the baseline period or on the index date. Patients were excluded if they had claims for any systemic treatment agents for PsA in the baseline period, biologic-indicated autoimmune conditions (eg, ulcerative colitis, Crohn’s disease, rheumatoid arthritis and other inflammatory polyarthropathies, ankylosing spondylitis, juvenile idiopathic arthritis), or cancer (malignant neoplasms excluding non-melanoma skin cancer)22 in the baseline and follow-up periods, or had multiple systemic medications administered on the index date. A subset of patients with two years of follow-up was also identified for a subgroup analysis.
Analyses were based on intention-to-treat, with individuals analyzed as part of their index treatment group regardless of subsequent changes in therapy. Demographic characteristics, prescriber specialty (defined as the specialty on the medical claim closest in time to the index date), and comorbidities, including the Charlson Comorbidity Index, as well as healthcare utilization and costs were measured in the baseline period.23–25 The primary outcomes were biologic initiation rate and time to biologic initiation during the one-year follow-up period in the main analysis, and for two years follow-up period in the subgroup analysis. Biologic initiation was defined as having a claim for a biologic therapy during the follow-up period, regardless of if it was in addition to (add-on) or instead of (switch from) the index therapy. The secondary outcomes were treatment patterns. Particularly, index therapy adherence was measured as the proportion of days covered (PDC) during the follow-up period, defined as the number of days with index therapy available divided by the length of the follow-up period. Index treatment discontinuation was also reported for the follow-up period, defined as ≥60-day gap in index therapy days’ supply. Restart of the index therapy was also measured (defined as re-initiating the index treatment following discontinuation of the same treatment). Descriptive statistics including means, standard deviations (SD), and relative frequencies and percentages were reported for continuous and categorical data.
In addition to descriptive analyses, modeling was performed to control for differences in observed characteristics of the two cohorts that may confound the findings. Logistic regression models were conducted to estimate the likelihood of biologic initiation during the one-year follow-up period. Cox regression models were used to evaluate the risk of biologic initiation at any point in time. All models were adjusted for the following: age group, gender, region, prescriber specialty, comorbid PsO, Charlson Comorbidity Index, index year, comorbid non-alcoholic fatty liver disease, presence of serious infection, pain medication and glucocorticoid utilization, X-ray and MRI utilization (separately), baseline healthcare utilization (in both inpatient and outpatient settings) and baseline healthcare costs (per $1000). Odds Ratio (OR) and 95% confidence intervals (CI), as well as adjusted rates and 95% CI, were reported for the logistic regression model, while hazard ratio (HR) and 95% CI were reported for the Cox regression model. In a subgroup analysis, estimations were replicated for a sub-cohort with two years of follow-up.
All data transformations and statistical analyses were performed using SAS© version 9.4.
Results
Among the total of 2116 systemic-naïve patients with PsA who were identified and met the study criteria between 01/01/2015 and 12/31/2018, 534 initiated APR and 1582 initiated MTX. Table 1 and Supplemental Table 1 provide baseline characteristics for the study cohort. The mean age of APR initiators was 50.5 years versus 50.4 years for MTX initiators (P=0.938). The percentage of females among APR initiators was higher (59.4%) than among MTX initiators (54.0%) (P=0.031). Approximately 90% of each group was commercially insured (P=0.767). The prescriber specialty was significantly different, with 73.5% of the MTX initiators receiving the index prescription from a rheumatologist compared to 30.9% of the APR initiators (P<0.001). The APR cohort had a higher mean number of comorbidities (4.6 vs 4.2, P<0.001), and 71.9% of this group had a concurrent PsO diagnosis compared to 59.4% of the MTX group (P<0.001). APR users were more likely than MTX users to have non-alcoholic fatty liver disease (4.9% vs 2.3%, P=0.003) and history of serious infection (3.6% vs 1.4%, P=0.002); the differences between the two groups were not statistically significant for every other comorbidity of interest (Supplemental Table 2).
 |
Table 1 Baseline Patient Characteristics, Utilization, and Costs |
APR users were less likely than MTX users to be on pain medications (61.2% vs 66.3%, P=0.034) and glucocorticoids (35.2% vs 44.2%, P<0.001) at baseline (Supplemental Table 3). The use of diagnostic tests was higher among MTX users than APR users (72.5% vs 64.0%, P<0.001; Supplemental Table 4). Finally, the mean baseline healthcare costs were higher among APR users than MTX users ($17,871 vs $10,683, P<0.001; Supplemental Table 4).
Fewer APR users initiated biologic treatment than MTX users throughout the follow-up period (Figure 1). During the first three months of follow-up, the unadjusted biologic initiation rates in the APR and MTX cohorts were 3.2% versus 14.2%, respectively, and 8.8% versus 27.0% in the first six months of follow-up, respectively (P<0.001 for both). At the end of the one-year follow-up, fewer APR patients (19.5%) than MTX patients (38.9%) initiated biologic treatment (P<0.001) (Table 2, unadjusted result). The number of index medication fills prior to biologic initiation are shown in Supplemental Table 5.
 |
Table 2 Biologic Initiation During the 1-Year Follow-Up Period (Unadjusted) |
 |
Figure 1 Time to biologic initiation during the 1-year follow-up period. |
The mean time to biologic initiation among patients who initiated a biologic during the one-year follow-up was 194.1 days in the APR cohort compared to 138.7 days in the MTX cohort (P<0.001) (Table 2). The most commonly used first biologic treatment was adalimumab for both the APR and MTX groups (Supplemental Tables 6 and 7).
APR users were more compliant to their index therapies. The mean PDC for the index therapy was 0.62 for the APR cohort and 0.57 for the MTX cohort (P=0.007). The discontinuation rate of the index therapy was 52.1% for APR users and 57.6% for MTX users during the one-year follow-up period (P=0.024). Among patients who discontinued their index therapy, 24.1% of APR users and 14.5% of MTX users restarted after more than 60 days of gap in treatment with the index therapy within the 1-year follow-up period (P<0.001) (Table 3).
 |
Table 3 Adherence to Index Therapy Among Patients with 1-Year Follow-Up |
After adjusting for potential confounders, patients treated with APR still had lower risk of biologic initiation when compared with patients treated with MTX (HR, 0.46 [95% CI, 0.37–0.57]; P<0.001) (Table 4 and Supplemental Table 8).
 |
Table 4 Biologic Initiation Adjusted Results in Cohorts with 1-Year and 2-Years of Follow-Up |
The logistic model showed that the likelihood of biologic initiation was statistically significantly lower with APR treatment even after adjusting for potential confounders (OR, 0.42 [95% CI, 0.32–0.54]; P<0.001) (Table 4) with estimated adjusted rates of biologic initiation being 20.0% (95% CI, 16.6–23.9%) for the APR cohort compared to 37.5% (95% CI, 35.0–40.1%) for the MTX cohort (Figure 2). In both the Cox and logistic regression models, the only additional covariates with a statistically significant relationship with biologic initiation were age and baseline X-ray utilization.
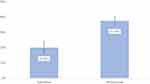 |
Figure 2 Predicted rates of biologic initiation. |
Results for Patients with Two Years of Follow-Up
Out of a total of 1226 systemic-naïve patients with PsA and two years of follow-up, 282 started APR and 944 started MTX at index. Supplemental Table 9 provides baseline characteristics for this cohort, which were similar to the characteristics of the main study cohort. APR users with two years of follow-up had higher mean number of comorbidities (4.6 vs 4.1, P<0.001) and were more likely to have a concurrent PsO diagnosis (72.3% vs 59.3%, P<0.001) than MTX users. Similar to the main study cohort, APR users were more likely than MTX users to have non-alcoholic fatty liver disease and serious infections (Supplemental Table 10). Baseline medication and healthcare utilization are shown in Supplemental Tables 11 and 12.
In the cohort with two years of follow-up, APR users were less likely to initiate a biologic treatment throughout the follow-up period (31.2% vs 49.6%, P<0.001) (unadjusted; Supplemental Table 13). The difference in biologic initiation rate was statistically significant between the two treatment groups as early as three months after index therapy initiation. The mean time to biologic initiation among patients who initiated during the two-year follow-up was 297 days among APR users compared to 212 days among MTX users (P<0.001) (Supplemental Table 13 and Supplemental Figure 1).
After adjusting for potential confounders, patients in the subgroup with two years of follow-up treated with APR had a lower risk of biologic initiation at any point in time during the follow-up period when compared to patients treated with MTX (HR, 0.53 [95% CI, 0.41–0.67]; P<0.001) (Table 4 and Supplemental Table 14). The logistic model showed that the likelihood of biologic initiation was almost twice as high after MTX treatment than after APR treatment once adjusted for potential confounders (OR, 0.46 [95% CI, 0.34–0.64]; P<0.001) (Table 4) with estimated adjusted rates of biologic initiation of 31.0% (95% CI, 25.5–37.2%) for APR users and 49.2% (95% CI, 45.9–52.5%) for MTX users.
Discussion
In this study of systemic-naïve PsA adult patients, we found that patients treated with APR had a lower likelihood of, as well as longer time to, biologic initiation compared with patients treated with MTX. These results were consistent in patients with one and two years of follow-up after APR or MTX initiation, with differences being observed as early as three-month post-index.
Treatment guidelines recommend OSMs as first-line treatment in PsA.14,15 Methotrexate (MTX) is the most commonly used first-line OSM for the condition,17,18 although not indicated for PsA. The effectiveness of MTX in PsA is reported based on clinical experience and observational studies rather than evidence from randomized clinical trials (RCTs).26–29 Moreover, MTX may lead to severe adverse effects such as hepatotoxicity, and myelosuppression.30 Apremilast (APR), an OSM phosphodiesterase 4 (PDE4) inhibitor approved by the United States Food and Drug Administration in 2014, is indicated for adult patients with active PsA.31 Unlike MTX and biologics, APR does not require frequent laboratory monitoring, potentially making it more convenient to use.
There are no large RCTs directly comparing APR to MTX to treat PsA. An indirect comparison based on data from clinical trials19 An indirect comparison based on data from clinical trials32 found no statistically significant difference between APR and MTX. Thus, there is a need for more evidence on MTX versus APR in the treatment of PsA. In the absence of RCTs, real-world evidence into treatment progression can be informative for clinical decision-making.
Biologic initiation as an outcome may serve as a suitable proxy for suboptimal disease control in PsA, and it can easily be assessed from administrative claims databases. Previous studies demonstrated that higher disease activity is a predictor for biologic therapy initiation in various autoimmune diseases such as rheumatoid arthritis33–35 and psoriasis.36
Use of biologic initiation in PsA can be particularly useful as other traditional treatment pattern measures used in claims studies, such as adherence or persistence, may be harder to interpret in this condition due to fluctuating symptoms, frequent-dose adjustments, and medication hoarding. While low adherence is usually negatively associated with adequate control of disease; it is possible that patients with PsA experience periods of symptom control and temporarily pause treatment. This is consistent with the higher restart rate observed with APR.
In a cohort of patients with PsA, this study found that treatment with APR was associated with extended time to biologic initiation—the next line of treatment—when compared with treatment with MTX. Future research using clinical measures should confirm our results and investigate whether biologic initiation is associated with disease progression, suboptimal disease control, or toxicity in PsA. Such association could help with a more personalized treatment algorithm for patients with PsA. Research should also examine patient-specific and clinical factors associated with progression to biologic therapies. PsA as a systemic disease, has a complex pathogenesis and affects multiple organ systems. Given the nature of the disease, a multidisciplinary research approach is especially critical to improve outcomes in this disease.
Limitations
This study was a retrospective, observational analysis using large administrative claims data. These data lack important clinical details on disease severity, activity, and symptoms as well as other patient socioeconomic and physician-specific information that may influence treatment choice, and thus make it difficult to control for potentially confounding variables. However, the modeling analyses included several measurable proxies of disease severity such as baseline comorbidities, medication use and healthcare utilization. Additionally, administrative claims data do not reflect whether medications are taken as prescribed, and the analysis relied instead on medication fills. Furthermore, we have not analyzed initial prescribed dose, fluctuation in dose, or route of administration for MTX, which can be associated with discontinuation of index drug. Finally, this study is limited to patients with commercial and Medicare supplemental insurance. Results may not be generalizable to other populations.
Conclusion
In a population of systemic-naïve adult patients with PsA, patients who initiated APR had a lower likelihood of biologic initiation when compared with patients initiating MTX. Additionally, among patients who initiated biologics during the follow-up period, time to biologic initiation was longer in APR users than in MTX users. APR use may delay initiation of the next line of treatment in patients with PsA to a greater extent than MTX.
Acknowledgments
Writing support was provided by Catherine Le Dellovo, MS, AMFT and funded by Amgen Inc.
Funding
The study was funded by Amgen Inc.
Disclosure
Dr. Michael S. Broder, Dr. Eunice Chang, Mr. Caleb Paydar, and Dr. Katalin Bognar are employees of Partnership for Health Analytic Research (PHAR), LLC, which was paid by Amgen to conduct this research, and reports relevant financial activities with AbbVie, AstraZeneca, BioMarin Pharmaceuticals, BMS, Celgene, Dompe, Eisai, Genentech, Mirum Pharmaceuticals, Novartis, Otsuka, Prothena, Regeneron, Sanofi US Services, and Takeda Pharmaceuticals USA, outside the submitted work. Dr M Elaine Husni reports personal fees for advisory board from AbbVie, BMS, Lilly, Novartis, Janssen, and UCB, outside the submitted work. Dr Pooja Desai and Dr Ibrahim Khilfeh were previously employed at Amgen at the time of conduct of the study and owns Amgen stock. They are currently employees at Janssen Pharmaceuticals. Dr Yuri Klyachkin is an employee of Amgen Inc. The authors report no other conflicts of interest in this work.
References
1. Shbeeb M, Uramoto KM, Gibson LE, O’Fallon WM, Gabriel SE. The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, 1982–1991. J Rheumatol. 2000;27(5):1247–1250.
2. Ogdie A, Langan S, Love T, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology. 2013;52(3):568–575. doi:10.1093/rheumatology/kes324
3. Love TJ, Gudbjornsson B, Gudjonsson JE, Valdimarsson H. Psoriatic arthritis in Reykjavik, Iceland: prevalence, demographics, and disease course. J Rheumatol. 2007;34(10):2082–2088.
4. Madland TM, Apalset EM, Johannessen AE, Rossebö B, Brun JG. Prevalence, disease manifestations, and treatment of psoriatic arthritis in Western Norway. J Rheumatol. 2005;32(10):1918–1922.
5. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic Arthritis. N Engl J Med. 2017;376(21):2094–2096. doi:10.1056/NEJMc1704342
6. Singh JA, Guyatt G, Ogdie A, et al. 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol. 2019;71(1):5–32. doi:10.1002/art.40726
7. Dehpouri T, Rahmatpour Rokni G, Ahangar Narenjbon N, et al. Evaluation of the glycemic effect of methotrexate in psoriatic arthritis patients with metabolic syndrome: a pilot study. Dermatol Rep. 2019;11(1). doi:10.4081/dr.2019.7965
8. Conic RR, Damiani G, Schrom KP, et al. Psoriasis and psoriatic arthritis cardiovascular disease endotypes identified by red blood cell distribution width and mean platelet volume. J Clin Med. 2020;9(1):186. doi:10.3390/jcm9010186
9. Damiani G, Pacifico A, Rizzi M, et al. Patients with psoriatic arthritis have higher levels of FeNO than those with only psoriasis, which may reflect a higher prevalence of a subclinical respiratory involvement. Clin Rheumatol. 2020;39(10):2981–2988. doi:10.1007/s10067-020-05050-2
10. Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135(12):2955–2963. doi:10.1038/jid.2015.296
11. Higa S, Devine B, Patel V, Baradaran S, Wang D, Bansal A. Psoriasis treatment patterns: a retrospective claims study. Curr Med Res Opin. 2019;35(10):1727–1733. doi:10.1080/03007995.2019.1618805
12. Xu Y, Sudharshan L, Hsu MA, et al. Patient preferences associated with therapies for psoriatic arthritis: a conjoint analysis. Am Health Drug Benefits. 2018;11(8):408–417.
13. Ogdie A, Coates LC, Gladman DD. Treatment guidelines in psoriatic arthritis. Rheumatology. 2020;59(Supplement_1):i37–i46. doi:10.1093/rheumatology/kez383
14. Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):700–712. doi:10.1136/annrheumdis-2020-217159
15. Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis: Grappa treatment recommendations for PsA. Arthritis Rheumatol. 2016. doi:10.1002/art.39573
16. Feldman SR, Pelletier CL, Wilson KL, et al. Treatment patterns and costs among biologic-naive patients initiating apremilast or biologics for psoriatic arthritis. J Comp Eff Res. 2019;8(9):699–709. doi:10.2217/cer-2019-0034
17. Lee MP, Lii J, Jin Y, et al. Patterns of systemic treatment for psoriatic arthritis in the US: 2004–2015. Arthritis Care Res. 2018;70(5):791–796. doi:10.1002/acr.23337
18. Hernandez EJM, Tkacz J, Lopez-Gonzalez L, Higgins K, Ogdie A, Stolshek BS. Psoriatic arthritis treatment patterns and costs among pharmacologic treatment–naïve patients. Am J Manag Care. 2020;26(8):e252–e257. doi:10.37765/ajmc.2020.44075
19. Betts KA, Griffith J, Friedman A, Zhou ZY, Signorovitch JE, Ganguli A. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naïve patients with psoriatic arthritis. Curr Med Res Opin. 2016;32(4):721–729. doi:10.1185/03007995.2016.1140026
20. Reed M, Crosbie D. Apremilast in the treatment of psoriatic arthritis: a perspective review. Ther Adv Musculoskelet Dis. 2017;9(2):45–53. doi:10.1177/1759720X16673786
21. Asgari MM, Wu JJ, Gelfand JM, et al. Validity of diagnostic codes and prevalence of psoriasis and psoriatic arthritis in a managed care population, 1996–2009: occurrence of psoriasis and psoriatic arthritis. Pharmacoepidemiol Drug Saf. 2013;22(8):842–849. doi:10.1002/pds.3447
22. Wu JJ, Pelletier C, Ung B, Tian M. Real-world treatment patterns and healthcare costs among biologic-naive patients initiating apremilast or biologics for the treatment of psoriasis. J Med Econ. 2019;22(4):365–371. doi:10.1080/13696998.2019.1571500
23. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
24. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi:10.1016/0895-4356(92)90133-8
25. Agency for Healthcare Research and Quality. HCUP chronic condition indicator. Healthcare Cost and Utilization Project (HCUP); 2015. Available from: www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp.
26. Elmamoun M, Chandran V. Role of methotrexate in the management of psoriatic arthritis. Drugs. 2018;78(6):611–619. doi:10.1007/s40265-018-0898-2
27. Chandran V, Schentag CT, Gladman DD. Reappraisal of the effectiveness of methotrexate in psoriatic arthritis: results from a longitudinal observational cohort. J Rheumatol. 2008;35(3):469–471.
28. Kingsley GH, Kowalczyk A, Taylor H, et al. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatol Oxf Engl. 2012;51(8):1368–1377. doi:10.1093/rheumatology/kes001
29. Lie E, van der Heijde D, Uhlig T, et al. Effectiveness and retention rates of methotrexate in psoriatic arthritis in comparison with methotrexate-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2010;69(4):671–676. doi:10.1136/ard.2009.113308
30. Kremer JM. Major side effects of low-dose methotrexate. UpToDate; 2020. Available from: https://www.uptodate.com/contents/major-side-effects-of-low-dose-methotrexate.
31. Food and Drug Administration. Methotrexate drug label; 2019. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210737s000lbl.pdf.
32. Samanta J, Naidu G, Chattopadhyay A, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80(Suppl1):1305. doi:10.1136/annrheumdis-2021-eular.1850
33. DeWitt EM, Lin L, Glick HA, Anstrom KJ, Schulman KA, Reed SD. Pattern and predictors of the initiation of biologic agents for the treatment of rheumatoid arthritis in the United States: an analysis using a large observational data bank. Clin Ther. 2009;31(8):
34. Verstappen SMM, Lunt M, Bunn DK, Scott DGI, Symmons DPM. In patients with early inflammatory polyarthritis, ACPA positivity, younger age and inefficacy of the first non-biological DMARD are predictors for receiving biological therapy: results from the Norfolk Arthritis Register. Ann Rheum Dis. 2011;70(8):1428–1432. doi:10.1136/ard.2010.148106
35. Png WY, Kwan YH, Lim KK, et al. A systematic review of the factors associated with the initiation of biologicals in patients with rheumatological conditions. Eur J Hosp Pharm Sci Pract. 2019;26(3):163–169. doi:10.1136/ejhpharm-2017-001431
36. Geale K, Henriksson M, Schmitt-Egenolf M. Evaluating equality in psoriasis healthcare: a cohort study of the impact of age on prescription of biologics. Br J Dermatol. 2016;174(3):579–587. doi:10.1111/bjd.14331
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
