Back to Journals » OncoTargets and Therapy » Volume 12
Bioinformatic analysis of miR-4792 regulates Radix Tetrastigma hemsleyani flavone to inhibit proliferation, invasion, and induce apoptosis of A549 cells
Authors Liu P , Pu J, Zhang J, Chen Z, Wei K, Shi L
Received 3 August 2018
Accepted for publication 27 December 2018
Published 20 February 2019 Volume 2019:12 Pages 1401—1412
DOI https://doi.org/10.2147/OTT.S182525
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Peigang Liu,1,2 Jinbao Pu,2 Junhui Zhang,3 Zhilu Chen,4 Kemin Wei,2,4,* Lian’gen Shi1,*
1College of Animal Sciences, Zhejiang University, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Chinese Medicine, Zhejiang Academy of Traditional Chinese Medicine, Hangzhou, Zhejiang, People’s Republic of China; 3RuoHeng Family Farm, Zhejiang Dou Dou Bao Traditional Chinese Medicine Research Co., Ltd, Taizhou, Zhejiang, People’s Republic of China; 4Department of Hematology, Zhejiang Provincial Tongde Hospital, Hangzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Background: Radix Tetrastigma hemsleyani, a kind of Chinese medicinal herb, contains multiple medicinal ingredients and can exert a variety of pharmacological activities. Our previous study revealed that miR-4792 was significantly upregulated in Radix Tetrastigma hemsleyani flavone (RTHF)-treated A549 cells; however, the regulatory mechanism of RTHF-treated A549 cells remains unclear.
Materials and methods: In this study, we investigated the antitumor mechanism and regulatory pathway of miR-4792 in RTHF-treated A549 cells, and the target genes were predicted and pathway enrichment of miR-4792 was performed using bioinformatic analysis.
Results: Our results confirmed that the upregulated expression of miR-4792 could inhibit cell proliferation and invasion, provoke cell cycle arrest, and induce apoptosis in A549 cells. Gene Ontology analysis showed that target genes of miR-4792 were enriched in protein binding, cytosol, cytoplasm, plasma membrane, and metal ion binding. Kyoto Encyclopedia of Genes and Genomes analysis showed that target genes of miR-4792 were enriched in aminoacyl-tRNA biosynthesis, AGE–RAGE signaling pathway in diabetic complications, sphingolipid signaling pathway, neuroactive ligand–receptor interaction, glycosaminoglycan degradation, and regulation of lipolysis in adipocytes. Additionally, FOXC1 was identified as an important target gene of miR-4792 in RTHF-treated A549 cells, and miR-4792 may be the target of some apoptotic-related proteins involved in induction of apoptosis in A549 cells by RTHF. Moreover, the intracellular Ca2+ levels of A549 cells were increased after RTHF treatment, which may be involved in the anticancer regulatory process of miR-4792 in RTHF-treated A549 cells.
Conclusion: These findings suggest a novel therapeutic approach for lung cancer that will be investigated in future studies.
Keywords: Radix Tetrastigma hemsleyani, flavone, miR-4792, GO, KEGG, FOXC1, potential therapeutic agents
Introduction
As one of the most common cancers in the world, lung cancer causes over 1.4 million deaths per year.1 In People’s Republic of China, lung cancer replaced liver cancer as the number one cause of death among people with malignant tumors in 2008.2 Although advances in surgery, radiotherapy, and chemotherapy have been made, the mean age-adjusted 5-year survival rate for lung cancer remains very low at 12.5%.3 Therefore, novel anti-lung cancer drugs with remarkable effects are very important and necessary.
Chinese herbs have good effects on cancer treatment with little side effects, and have been wildly used for treating esophageal cancer in combination with radiotherapy.4 Radix Tetrastigma hemsleyani (RTH) is an important folk medicinal plant in People’s Republic of China that has been used as an anticancer drug in various cancers.5 Clinical studies have shown that flavonoids may have great implications in the prevention and treatment of cancer in humans.6,7 Component analyses showed that RTH is rich in flavones, and RTH flavone (RTHF) has good in vivo and in vitro effects on various cancers, including lung cancer.8,9 However, its antitumor mechanism and related regulatory pathway are still uncertain.
miRNAs are non-coding RNAs of length 20–22 nucleotides that bind to the 3′-UTRs of cognate mRNAs to negatively regulate them.10,11 miRNAs have been found to modulate cell growth and death.12 Depending upon the nature of their target gene(s), miRNAs may function as tumor suppressors or oncogenes by downregulating target mRNAs. In our previous studies, expression changes of miRNAs in RTHF-treated A549 cells were analyzed using miRNA-seq techniques, and 162 miRNAs were found to be differentially expressed after RTHF treatment.13 Among the differentially expressed miRNAs (DE-miRNAs), miR-4792 was found to be upregulated 6.65-fold, and previous studies have found its participation in numerous biological processes of many cancers by targeting a broad set of cell factors.13 Therefore, miR-4792 has the potential to become one of the important potential therapeutic agents for lung cancer by many drugs such as RTHF.
In this study, to further explore whether the upregulated expression of miR-4792 was related to the anticancer effects of RTHF on A549 cells, we analyzed the effect of RTHF treatment involved in inhibition of cell proliferation and invasion, cell cycle arrest, and apoptosis induction. Moreover, target genes of miR-4792 were predicted and Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of those target genes were performed. Additionally, we determined whether FOXC1 was a target gene of miR-4792 in RTHF-treated A549 cells and the relation between miR-4792 and some apoptotic-related proteins. Finally, changes in Ca2+ levels of different treatment groups of A549 cells were analyzed.
Materials and methods
Reagents and antibodies
RTH is a triennial artificial plant obtained from Zhejiang Dou Dou Bao traditional Chinese Medicine Research Co., Ltd (Taizhou, People’s Republic of China), and was authenticated by Professor Jinbao Pu (Zhejiang Academy of Traditional Chinese Medicine). Three hundred grams of dried RTH was extracted with 75% ethanol (4.5 L) at 80°C for 1.5 hours twice and filtered. All the resulting extract filtrations were freeze-dried to power and the power was diluted with distilled water. Then the water solution was purified by refining through water-saturated n-butanol and D101 microporous adsorption resin. The purified extract was freeze-dried to RTHF power and then dissolved in Roswell Park Memorial Institute 1640 culture medium to 10 mg/mL and finally filtered through a 0.45 μm filter for use. In our previous experiments, through chemical composition analysis by HPLC–mass spectrometry (MS) and HPLC–MS/MS, we found that RTHF used in this study mainly included six foregone active substances, including kaempferol (PubChem CID: 5280823, 12.57%), kaempferol-3-O-neohesperidoside (PubChem CID: 44575467, 19.11%), malvidin-3-glucoside (PubChem CID: 443652, 10.23%), myricitrin (PubChem CID: 5281673, 8.56%), baohuoside (PubChem CID: 5488822, 9.98%), and isoschaftoside (PubChem CID: 3084995, 13.28%).13
FBS (10099), DMEM (31966), antibiotic–antimycotic (15240-112), PBS (10010-049, pH 7.4), trypsin-EDTA (25300-054, 0.05%), Lipofectamine® 2000 Transfection Reagent (11668-019), and Opti-MEM® I Reduced Serum Medium (31985-062) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Cell Counting Kit-8 (CCK-8; 20140419) was purchased from 7 Sea Biotech (Shanghai, People’s Republic of China), and Transwell invasion chamber (3422) and Matrigel gel (356234) were purchased from Corning Incorporated (Corning, NY, USA). Calcium colorimetric assay kit (C004-2) was purchased from Jiancheng Biotech (Nanjing, People’s Republic of China). The miR-4792 mimics and the miR-4792 inhibitor were purchased from GenePharma Company (Shanghai, People’s Republic of China).
Cell lines and culture
Human lung cancer A549 cells were obtained from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Science, and ethical permission was not required to use these cell lines in this study. A549 cells were cultured in low-sugar DMEM medium supplemented with 10% (v/v) heat-inactivated FBS and 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in a humidified incubator with 5% CO2. Cells were passaged every 2–3 days and used at 80%–90% confluency.
Transfection and treatment of cell lines
Cells were seeded at a density of 5×103 cells/well in a 96-well plate. Cells were then subjected to various methods to establish the different groups, including miRNA negative control (NC) group (cells transfected with NC miRNA and used as the control group), miR-4792 mimics group (cells transfected with miR-4792 mimics), RTHF + miRNA NC group (cells treated with RTHF after transfection with miRNA NC), and RTHF + miR-4792 inhibitor group (cells treated with RTHF after transfection with miR-4792 inhibitor). The miRNA alignment of the different transfection groups is shown in Table 1.
  | Table 1 miRNA alignment of differential transfection groups |
A549 cells transfected with miRNA mimics, inhibitor, or miRNA NC were used at 50 nM concentration, and Lipofectamine 2000 (Thermo Fisher Scientific) was used for all transfections following the manufacturer’s suggestions. After transfection, A549 cells of RTHF + miRNA NC group and RTHF + miR-4792 inhibitor group were treated with 2 μg/mL RTHF for 48 hours. Each treatment was repeated six times.
Cell viability assay
The effect of RTHF on A549 cell growth was examined using CCK-8 assay (7 Sea Biotech) following the manufacturer’s protocol. After differential exposure for 48 hours, 10 μL of WST-8 was added to each well and allowed to incubate for 4 hours at 37°C. Finally, the absorbance reading of each well was detected with microplate reader at 450 nm (Sigma 1-15K; Thermo Fisher Scientific). Decrease in the absorbance indicates increased cytotoxicity. The percentage of viable cells was estimated by comparison with untreated control cells.
Cell invasion assays
The cell invasion assay was performed in 24-Transwell chambers (Corning Incorporated). Chambers with 8 μm membrane filter pore size were coated with 80 μL Matrigel (0.8 mg/mL; BD Biosciences, San Jose, CA, USA) Matrigel. After transfection for 48 hours, cells were digested with trypsin and resuspended and adjusted to a density of 1×105/mL using DMEM medium. After that, 0.2 mL of the suspended cells was inoculated into Transwell. In addition, cells of the RTHF + miRNA NC group and RTHF + miR-4792 inhibitor group were also treated with 2 μg/mL of RTHF. The lower chamber was filled with 0.6 mL of complete culture medium and then cultured at 37°C for 12 hours. After incubation for 12 hours, cells were fixed with 4% paraformaldehyde for 15 minutes at room temperature, washed twice using PBS, and stained with 1 mL of 0.5% crystal violet for 1 hour. Finally, cells in the upper chamber were removed with a cotton swab, and the stained invasive cells were photographed and quantified from randomly chosen fields in an inverted microscope (CX23; Olympus Corporation, Tokyo, Japan). Each experiment was carried out in triplicate.
Cell cycle distribution and apoptosis assays
After treatment, A549 cells of each group were digested with trypsin to prepare single-cell suspensions and the cell density was adjusted to 1×106/mL. Cells were then washed twice with PBS and fixed by the slow addition of cold 70% ethanol to a total volume of 1 mL, and stored at 4°C overnight. After a gentle wash with cold 1× PBS, cells were resuspended in 1 mL propidium iodide (PI) staining solution (50 μg/mL PI, 0.2 mg/mL RNase A, and 0.1% Triton X-100) and allowed to react for 30 minutes at room temperature in the dark, before detection of cell cycle using flow cytometry (FACSCalibur; BD Biosciences).
After treatment, each group of A549 cells was centrifuged at 1,000 rpm for 5 minutes and the cell density was adjusted to 1×106/mL with 1× Annexin V binding solution. One hundred microliters of cell suspensions were mixed with 5 μL of Annexin V-fluorescein isothiocyanate (FITC) and 5 μL PI solutions, and then allowed to react for 15 minutes at room temperature in the dark. Finally, 400 μL of 1× Annexin V binding solution was added before flow cytometry (FACSCalibur; BD Biosciences) within 1 hour to detect cellular apoptosis.
Detection of Ca2+ concentration
After treatment, the media were removed and cells were rinsed with calcium- and magnesium-free PBS. Cell solutions were then collected into 1.5 mL tubes and centrifuged at 1,000 rpm for 3 minutes. The cell density was adjusted to 5×106/mL using calcium- and magnesium-free PBS and then incubated with 0.1% Triton X-100 for 15 minutes at room temperature. Ca2+ concentration of four samples was detected using Ca2+ test kits.
Prediction of target gene for miR-4792
The potential target genes of miR-4792 were predicted by three online tools, including miRDB (http://www.mirdb.org/), miRanda (http://www.microrna.org/microrna/home.do), and miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/php/index.php).14–16 Only the target genes identified by the three online analysis tools were considered as target genes of miR-4792 and were chosen for further analysis.
GO and KEGG enrichment analysis of target genes of miR-4792
For GO mapping, the GO terms for miR-4792 target genes based on homologies were extracted (http://www.geneontology.org). The GO enrichment analysis of miR-4792 target genes was performed using the GO seq R package, which corrected for miRNA length bias. GO terms with corrected P-values <0.05 were considered to be significantly enriched. REVIGO was used for analysis of the enriched GO terms (http://revigo.irb.hr/); this program removes redundant GO terms and attempts to reflect the similarity of given terms based on semantic space34. The GO biological process category terms with the lowest P-values for enrichment in miR-4792 in the RTH-treated A549 cells were analyzed by REVIGO.
KEGG pathway terms for the miR-4792-predicted target genes were retrieved from the KEGG database (http://www.genome.jp/kegg/), and KOBAS software was used to test the statistical significance of the enrichment of miR-4792 target genes in KEGG pathways. A pathway with an false discovery rate (FDR) <0.05 was defined as an enrichment pathway.
Western blotting analysis of FOXC1 and apoptotic-related proteins
After extraction, the protein concentration was determined by the Lowry method. Protein lysates (40 μg) from each sample were subjected to SDS-PAGE on 12% acrylamide separating gels (25 mA) with a 4% acrylamide stacking gels (30 mA). The separated proteins were transferred to polyvinylidene fluoride membranes (150 mA for 2 hours). After transfer, the membranes were blocked with 5% non-fat dry milk in tris-buffered saline for 1 hour at room temperature, followed by incubation with primary antibodies (detection proteins 1:1,000; GAPDH 1:2,000) at 4°C overnight. Then they were incubated with secondary IRDye® 800CW goat anti-rabbit IgG (H+L) (1:2,000; Santa Cruz Biotechnology Inc., Dallas, TX, USA) at 37°C for 1 hour. The membranes were finally washed three times with PBS before the target proteins on them were analyzed using Lipofectamine 2000 Transfection Reagent. GAPDH was chosen as an internal control, and the result of Western blot was measured as levels of grayscale intensity.
Statistical analysis
SPSS 16.0 software was used to input and analyze the data. All measurement data from each group were expressed as mean ± SD (x_ ± SD). The measurement data of multiple groups were compared using the least significant difference method of one-way ANOVA, and P<0.05 indicated statistically significant difference.
Results
Effects of RTHF on the viability of A549 cells
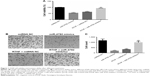
A549 cell proliferation in the different treatment groups is shown in Figure 1A. There was a significant decrease in cell proliferation in the miR-4792 mimics and RTHF + miRNA NC groups compared to the control group (miRNA NC group), indicating that RTHF inhibited A549 cell proliferation by upregulating the expression of miR-4792, and the inhibition effect was similar to that of miR-4792 mimics. Compared to the RTHF + miRNA NC group in which only RTHF played a role in cell proliferation inhibition, cell proliferation in the RTHF + miR-4792 inhibitor group was significantly increased, suggesting that miR-4792 played a more important role than other DE-miRNAs found in our previous studies in the cell proliferation inhibition by RTHF.13 The cell viability assays revealed that miR-4792 had effects on inhibiting cell proliferation, and suggested it might have played an important regulatory role in RTHF-mediated inhibition of A549 cell proliferation.
Changes in A549 cell invasion ability after differential treatments
As shown in Figure 2B, it is obvious that there were large differences in A549 cell invasion abilities of the different treatment groups. Cell count result revealed significant decrease in cell number of miR-4792 mimics and RTHF + miRNA NC groups compared to the miRNA NC group (P<0.01; Figure 1B and C); however, in comparison to RTHF + miRNA NC group, cell invasion ability of RTHF + miR-4792 inhibitor group was significantly increased (P<0.01), demonstrating that the effect of RTHF on inhibiting cell invasion ability was significantly decreased after inhibition of miR-4792 in A549 cells. Hence, the invasion ability test results showed that upregulation of miR-4792 could inhibit cell invasion ability, and it might be involved in the RTHF-mediated inhibition of A549 cell invasion.
Cell cycle distribution and apoptosis of A549 cells after differential treatments
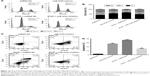
Cell cycle of A549 cells in the different treatment groups was analyzed using flow cytometry (Figure 2A). The proportion of cells in G1 phase of the cell cycle was significantly increased in miR-4792 mimics and RTHF + miRNA NC groups than miRNA NC group (P<0.01). The proportion of cells in G1 phase of RTHF + miR-4792 inhibitor treatment group was more than in RTHF + miRNA NC group (Figure 2B). Therefore, the result indicated that miR-4792 played a role in cell cycle arrest in A549 cells and could arrest A549 cells in the G1 phase. We conclude this might explain RTHF-induced cell cycle arrest at the G1 phase.
Further cytometric analysis using FITC-labeled Annexin V showed that apoptosis of A549 cells in the different treatment groups exhibited significant differences (Figure 2C). As shown in Figure 2D, the proportion of apoptotic cells was significantly increased in miR-4792 mimics, RTHF + miRNA NC, and RTHF + miR-4792 inhibitor groups than in miRNA NC group (P<0.01). The proportion of apoptotic cells in the RTHF + miR-4792 inhibitor treatment group was less than in miR-4792 mimics and RTHF + miRNA NC groups; therefore, this apoptotic result indicated that upregulation of miR-4792 expression could enhance the apoptosis of A549 cells and miR-4792. Because the proportion of apoptotic cells in RTHF + miRNA NC group was higher than in miR-4792 mimics and RTHF + miR-4792 inhibitor groups, we conclude that miR-4792 potentially plays a role in lung cancer apoptosis and could be partly a reason for RTHF-induced apoptosis of A549 cells.
Target gene prediction of miR-4792
The potential target genes of miR-4792 predicted using miRanda, miRDB, and miRTarBase online analysis tools are 343, 167, and 100. The genes identified as targets by the three analysis tools were selected. Finally, a total of 92 target genes were predicted. Detailed information on miR-4792 target genes is shown in Table S1.
GO and KEGG enrichment analysis of target genes of miR-4792
The results of the GO analysis of miR-4792 target genes are shown in Figure 3A (top ten terms of each categories), and we found that the significantly enriched GO terms were mainly distributed in the cellular component and molecular function categories, and these functional processes were mainly involved in cytosol, cytoplasm, plasma membrane, protein binding, metal ion binding, ATP binding, and extracellular exosome. Data of all the enrichment GO terms are shown in Table S2.
By the analysis of KEGG pathways of the predicted target genes, we found that the predicted target genes of miR-4792 were significantly enriched in 31 pathways (P<0.05). The top 15 statistically significant pathways are shown in Figure 3B, and data of all the enrichment pathways are shown in Table S3.
Among the significantly enriched pathways, some play a pivotal role in cancer development and maintenance, such as vascular endothelial growth factor (VEGF) signaling pathway, calcium signaling pathway, p53 signaling pathway, chemokine signaling pathway, cAMP signaling pathway, and protein processing in the endoplasmic reticulum (ER).
Change in Ca2+ concentration of A549 cells
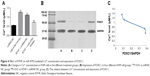
Calcium signaling plays an important role in cancer progression by changing the Ca2+ levels in cells. To reveal the role of calcium signaling in A549 cells, we analyzed the changes of Ca2+ concentration after different treatments in A549 cells. As can be seen in Figure 4A, Ca2+ concentration was significantly increased in A549 cells of miR-4792 mimics and RTHF + miRNA NC groups in contrast to miRNA NC group (P<0.01), indicating that upregulation of miR-4792 may be the main target factor for RTHF to increase the Ca2+ concentration in A549 cells. In addition, Ca2+ concentration was significantly increased in RTHF + miRNA NC group than in RTHF + miR-4792 inhibitor group (P<0.01). Therefore, we concluded that the upregulation of miR-4792 is one of the most important reasons for the increased Ca2+ levels in A549 cells, and RTHF could elevate Ca2+ levels in cells partly through upregulation of miR-4792.
Effect of RTHF on miR-4792-mediated expression of FOXC1 and apoptotic-related proteins
Although there were many studies about changes in miR-4792 expression in some cancers, a few studies have focused on its target genes. An important predicted target gene of miR-4792 with a high prediction score, FOXC1 was found to play important roles in cell proliferation and in vitro migration of human non-small-cell lung carcinoma (NSCLC) cells by siRNA analysis. It was the only verified target gene of miR-4792 by reporter assay, quantitative PCR, and Western blot validation and indicated that miR-4792 targeted the gene in inhibition of epithelial–mesenchymal transition and invasion in nasopharyngeal carcinoma as reported in previous studies.17,18 Therefore, we evaluated the expression of FOXC1 protein level after RTHF treatment (Figure 4B) by Western blot assay (Table 2) to examine whether the regulatory effect of miR-4792 was related to suppression of FOXC1 in RTHF-treated A549 cells. The results showed that the expression of FOXC1 in A549 cells of miR-4792 mimics and RTHF + miRNA NC groups was significant it lower than in A549 cells of miRNA NC and RTHF + miR-4792 inhibitor groups. These results suggested that miR-4792 could also downregulate the expression of FOXC1 in lung cancer A549 cells, which is consistent with the results of previous studies.17 It is also clear that RTHF-induced downregulation of FOXC1 expression was through upregulating miR-4792 expression.
In Figure 4C, we can see that the Ca2+ level has a certain negative correlation with the expression of FOXC1, and previous studies reported that knockdown of FOXC1 in TM1 cells changed the levels of key proteins in the Ca2+-directed exocytosis pathway, and FOXC1 regulates early cardiomyogenesis of embryonic stem cells by some pathways including calcium signaling pathway.19–21 Our present result indicates that miR-4792 targets downregulation of FOXC1 in lung cancer A549 cells via increase of A549 cell Ca2+ levels, which are closely related to Ca2+ channels and signal transduction.
In previous studies, Zhong et al have reported that RFHT induces apoptosis in A549 cells by modulating the mitogen-activated protein kinase (MAPK) pathway.8 Therefore, the expression of some apoptotic-related proteins and important proteins in the MAPK pathway was analyzed in the present study to validate whether miR-4792 participates in the apoptotic induction of A549 cells by RTHF via MAPK pathway and determine the potential apoptotic mechanism. In Figure 5, we see that compared to miRNA NC group, the expression of B-cell lymphoma-2 (Bcl2), caspase-3, caspase-9, phosphorylated c-Jun N-terminal kinase (p-JNK), and phosphorylated PKR-like endoplasmic reticulum kinase (p-pERK) decreased in miR-4792 mimics and RTHF + miRNA NC groups, while Bcl-2 Associated X Protein (Bax) and phosphorylated p38 (p-p38) showed the opposite trend, that is, they were increased in miR-4792 mimics and RTHF + miRNA NC groups. This Western blot result indicated that RTHF induces apoptosis in A549 cells by modulating some apoptotic-related proteins, and miR-4792 plays a significant role in RTHF-induced apoptosis of A549 cells.
Discussion
RTH is an important Chinese traditional herb with detoxification and anti-inflammatory effects. In Chinese folk medicine, it is boiled/squeezed/extracted and used as functional soup/juice/nutraceutical for its health benefits including prevention of some chronic diseases, immunity enhancement, antioxidant ability, and protection of the liver from chemical injury.5,22 RTHF has been considered the most significant bioactive constituent and found to have good anticancer effects in different types of cancer.
In our previous studies, RTHF was found to have good effects on inhibiting proliferation and invasion, cell cycle arrest, and inducing apoptosis of A549 cells. miRNA expression analysis using miRNA-seq techniques revealed significant differential expression of 162 miRNAs after RTHF treatment. Among the differentially expressed miRNAs, miR-4792 was upregulated 6.65 times in RTHF-treated A549 cells, suggesting that miR-4792 can become an important anticancer agent for lung cancer therapy, similar to other miRNAs that are candidates for being anticancer agents.13,21 In this study, the mechanism by which miR-4792 regulates RTHF to inhibit proliferation, invasion, and induce apoptosis of A549 cells and its main targeting genes were further explored.
Numerous studies have shown that miR-4792 is involved in different cellular processes such as differentiation, apoptosis, senescence, and metabolism.23,24 In our study, miRNA mimic and inhibitor experiments indicated miR-4792 plays important roles in inhibiting cell proliferation and invasion and inducing cell cycle arrest and apoptosis in RTHF-treated A549 cells, suggesting that miR-4792 is a key regulatory factor of RTHF-mediated inhibition of cell proliferation and invasion, cell cycle arrest, and induction of apoptosis of A549 cells.
miRNAs are a large class of small regulatory RNAs known to directly and negatively regulate the expression of a large fraction of all protein-encoding genes.25,26 As one important type of potential regulators of gene expression, miRNAs play a critical role in cancer development and progression by regulating functionally related gene networks.27 In this study, target genes of miR-4792 were predicted using three target data sets, miRDB, miRTarBase, and miRanda, and then all target genes were analyzed further by GO term analysis and KEGG pathway analysis.
KEGG analysis result showed that target genes of miR-4792 were enriched in 31 pathways. Also, some of them have been found to play a critical role in the development of cancer, including VEGF signaling pathway, calcium signaling pathway, p53 signaling pathway, chemokine signaling pathway, and protein processing in ER pathway.
VEGF signaling pathway has been associated with tumor development in many tumors and plays pivotal role in angiogenesis and metastasis in various malignant tumors, including lung cancer.28,29 p53 signaling pathway has been reported to be involved in the pathogenesis of cancer and in drug development programs, and p53 is an important nuclear transcription factor in this pathway, regulating the expression of >100 target genes to initiate apoptosis, cell cycle arrest, DNA repair, cellular senescence, as well as differentiation.30,31 The dysregulation of Ca2+ homeostasis has been suggested as an important event in driving the expression of the malignant phenotypes such as proliferation, migration, invasion, and metastasis.32 Changes in Ca2+ levels in RTHF-treated A549 cells may be related to regulation of calcium signaling pathway for signal transduction of some cancer development factors. De-regulated expression and activity of several chemokine signaling pathways have been implicated in cancer development and progression.33 Chronic ER stress is increasingly being recognized as a factor in many human diseases such as diabetes, neurodegenerative disorders, and cancer.34 The ER is an essential organelle in eukaryotic cells for the storage and regulated release of calcium and acts as the entrance to the secretory pathway.35
miR-4792 expression was found to be dysregulated in many kinds of cancers, including lung cancer, and little research has been done about its target genes related to cancer development regulation.13,17,36,37 Especially, FOXC1 was found to play important roles in cell proliferation and in vitro migration of human NSCLC cells by siRNA analysis,18 and FOXC1 is the only verified target gene of miR-4792 which has been reported in nasopharyngeal carcinoma and found to have a high prediction score in our bioinformatic analysis, therefore, FOXC1 may be one important target gene of miR-4792 for regulating RTHF-treated A549 cells progress. FOXC1, as a member of the FOX protein family, is dysregulated in a number of human cancers, including lung cancer.18 Recently, numerous types of miRNAs, including miR-204 in endometrial cancer, miR-133 in pituitary cancer, miR-495 in endometrial cancer, and miR-4792 in nasopharyngeal cancer, have been demonstrated to serve suppressive roles through inhibiting FOXC1 expression.38–40 In regard to control of FOXC1 by miR-4792, Li et al found that miR-4792 inhibited epithelial–mesenchymal transition and invasion of carcinoma cells through direct interaction with the 3′-UTR of FOXC1 miRNA to repress its expression in nasopharyngeal carcinoma.17 In our studies, FOXC1 also appeared as an important direct target of miR-4792 in lung cancer A549 cells, and Western blot analysis showed that miR-4792 negatively regulated FOXC1 expression, which is consistent with the findings of other studies.41,42 Therefore, we conclude that FOXC1 plays important roles in the process of miR-4792 regulating RTHF-mediated inhibition of cell proliferation and invasion, cell cycle arrest, and induction of apoptosis in A549 cells.
The ubiquitous second messenger, Ca2+, has been demonstrated to play an important role in cancer progression. Store-operated Ca2+ entry (SOCE) is the main Ca2+ entry pathway that regulates intracellular Ca2+ concentration in a variety of cancers.43 Intracellular Ca2+ regulation is important for normal cellular homeostasis. Some studies have indicated that miRNAs could modulate intracellular Ca2+ levels in cancer cells by targeting many cellular factors.43,44 In our KEGG analysis of predicted target genes, calcium signaling pathway was a significantly enriched pathway of miR-4792-predicted target genes. Calcium signaling pathway was deciphered as a crucial mediator in development and it has been indicated can mediate cell development via regulated Ca2+ channels, including cancer development.45,46 Changes in Ca2+ levels was used for regulated Ca2+ channels to activate or inhibit some proteins played its role in cell development and miRNAs regulation of cell development, also including cancer cell development.48,49 Our finding is the first to uncover that miR-4792 could regulate intracellular Ca2+ levels in A549 cells in vitro, and Ca2+ levels’ negative correlation with FOXC1 suggests miR-4792 may be involved in regulating the expression of FOXC1 via calcium signaling pathway by controlling the intracellular Ca2+ levels in A549 cells. RTHF increased intracellular Ca2+ levels in A549 cells by upregulating miR-4792; therefore, our results suggest that increased Ca2+ levels in A549 cells treated with RTHF are related to FOXC1 targeting by miR-4792.
It is obvious that miR-4792 played a more significant role in RTHF-induced A549 cells apoptosis, and previous studies reported that RTHF induced A549 cells apoptosis by modulating the MAPK pathway.8 In our present study, Western blot analysis results showed that overexpressed miR-4792 promoted cell apoptosis probably by negatively regulating Bcl2, caspase-3, caspase-9, p-JNK, and p-pERK and positively regulating Bax and p-p38. Caspase-3 is the key point for cell apoptosis and its activity may thus serve as a good marker to monitor cell death. Caspase-9 is a critical regulator of mitochondria-mediated apoptosis.49,50 Bcl-2 protein is able to repress a number of apoptotic death programs, and the ratio of Bax/Bcl-2 plays a critical role in apoptosis of cells.51
The MAPK signaling pathway can induce cell proliferation, differentiation, and apoptosis. p-p38, p-JNK, and p-pERK are the three important proteins of the MAPK signaling pathway. They have been proven to play a crucial role in the cell survival and cellular apoptosis pathways.52 Activation of the JNK/p38 signaling pathway has been confirmed to induce cells apoptosis by activating Bax,53 and PERK is a kinase that can phosphorylate eukaryotic initiation factor 2a, thereby preventing general mRNA translation; nevertheless, translation of a small number of mRNAs will be preferentially induced under stress conditions.54,55 Therefore, upregulation of the expression of miR-4792 would result in modifications in the phosphorylation state of MAPKs; especially, p-p38, p-JNK, and p-pERK may play a main role in the RTHF-induced apoptotic effect in A549 cells.
Taken together, our findings suggest miR-4792 functions as a tumor suppressor in A549 cells by targeting FOXC1 and some apoptotic-related proteins, and could be one of the molecular mechanisms by which RTHF inhibited A549 cell proliferation and invasion and induced cell cycle arrest and apoptosis. Additionally, changes in intracellular Ca2+ levels may be related to regulation of FOXC1 and some apoptotic-related proteins by miR-4792 in A549 cells treated with RTHF. miR-4792 and miR-4792/FOXC1 pathway could, therefore, be novel potential therapeutic targets for lung cancer A549 cells.
Acknowledgments
This work was supported by Analysis and Testing Science and Technology Planning Project of Zhejiang Province (No 2018C37009), the National Natural Science Foundation of China (No 81541084), the Wei KeMin Famous Old Chinese Medicine Experts Inheritance Studio Project held by the State Administration of Traditional Chinese Medicine, and the special funder for super experts of Zhejiang Province, People’s Republic of China.
Disclosure
The authors report no conflicts of interest in this work.
References
Chen J, Ye L, Xie F, Yang Y, Zhang L, Jiang WG. Expression of bone morphogenetic protein 7 in lung cancer and its biological impact on lung cancer cells. Anticancer Res. 2010;30(4):1113–1120. | ||
Zhao P, Dai M, Chen W, Li N. Cancer trends in China. Jpn J Clin Oncol. 2010;40(4):281–285. | ||
Kosacka M, Piesiak P, Kowal A, Gołecki M, Jankowska R. Galectin-3 and cyclin D1 expression in non-small cell lung cancer. J Exp Clin Cancer Res. 2011;30(1):101. | ||
Hong M, Wang N, Tan HY, Tsao SW, Feng Y. MicroRNAs and Chinese medicinal herbs: new possibilities in cancer therapy. Cancers. 2015;7(3):1643–1657. | ||
Qian LH, Dai DL, Jiang HY, Lin WH. Research progresses of the endangered medicinal plant Tetrastigma hemsleyanum Diels et Gilg. Zhejiang Nong Ye Xue Bao. 2015;27(7):1301–1308. | ||
Knekt P, Järvinen R, Seppänen R, et al. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am J Epidemiol. 1997;146(3):223–230. | ||
Le Marchand L. Cancer preventive effects of flavonoids – a review. Biomed Pharmacother. 2002;56(6):296–301. | ||
Zhong LR, Chen X, Wei KM. Radix Tetrastigma hemsleyani flavone induces apoptosis in human lung carcinoma A549 cells by modulating the MAPK pathway. Asian Pac J Cancer Prev. 2013;14(10):5983–5987. | ||
Zhong LR, Zheng J, Sun Q, Wei K, Hu YJ. Radix Tetrastigma hemsleyani flavone inhibits proliferation, migration, and invasion of human lung carcinoma A549 cells. Onco Targets Ther. 2016;9(1):635–641. | ||
Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Stress-induced reversal of microRNA repression and mRNA P-body localization in human cells. Cold Spring Harb Symp Quant Biol. 2006;71:513–521. | ||
Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113(6):673–676. | ||
Wang PY, Li YJ, Zhang S, et al. Regulating A549 cells growth by ASO inhibiting miRNA expression. Mol Cell Biochem. 2010;339(1–2):163–171. | ||
Liu P, Yang X, Zhang H, Pu J, Wei K. Analysis of change in microRNA expression profiles of lung cancer A549 cells treated with Radix Tetrastigma hemsleyani flavonoids. Onco Targets Ther. 2018;11:4283–4300. | ||
Wong N, Wang XW. miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015;43(D1):D146–D152. | ||
John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. miRanda application: Human MicroRNA targets. PLoS Biol. 2005;3(7):e264. | ||
Hsu SD, Lin FM, Wu WY, et al. miRTarBase: a database curates experimentally validated microRNA–target interactions. Nucleic Acids Res. 2011;39(D):D163–169. | ||
Li Y, Chen X. miR-4792 inhibits epithelial-mesenchymal transition and invasion in nasopharyngeal carcinoma by targeting FOXC1. Biochem Biophys Res Commun. 2015;468(4):863–869. | ||
Chen S, Jiao S, Jia Y, Li Y. Effects of targeted silencing of FOXC1 gene on proliferation and in vitro migration of human non-small-cell lung carcinoma cells. Am J Transl Res. 2016;8(8):3309–3318. | ||
An X, Sarmiento C, Tan T, Zhu H. Regulation of multidrug resistance by microRNAs in anti-cancer therapy. Acta Pharm Sin B. 2017;7(1):38–51. | ||
Rasnitsyn A, Footz T, Doucette LP, Yu M, Walter MA. Analysis of exocytosis regulation by transcription factor FOXC1 and its role in Axenfeld–Rieger syndrome pathogenesis. Invest Ophthalmol Vis Sci. 2014;55(13):3802. | ||
Lambers E, Arnone B, Fatima A, Qin G, Wasserstrom JA, Kume T. FOXC1 regulates early cardiomyogenesis and functional properties of embryonic stem cell derived cardiomyocytes. Stem Cells. 2016;34(6):1487–1500. | ||
Ding FJ, Li HF, Cui WJ, Jiang HQ, Liu JT. Discrimination of name and nature of Tetrastigma hemsleyanum. Chin J Exp Tradit Med Formulae. 2018;24(9):208–212. | ||
Han B, Qu Y, Jin Y, et al. FOXC1 activates smoothened-independent hedgehog signaling in basal-like breast cancer. Cell Rep. 2015;13(5):1046–1058. | ||
Huang W, Chen Z, Zhang L, et al. Interleukin-8 induces expression of FOXC1 to promote transactivation of CXCR1 and CCL2 in hepatocellular carcinoma cell lines and formation of metastases in mice. Gastroenterology. 2015;149(4):1053–1067.e14. | ||
Fang Z, Rajewsky N. The impact of miRNA target sites in coding sequences and in 3′UTRs. PLoS One. 2011;6(3):e18067. | ||
Heeyoung S, Juyoung H, Eun-Sook J, Chi SW. microRNA target recognition: insights from transcriptome-wide non-canonical interactions. Mol Cells. 2016;39(5):375–381. | ||
Reddy KB. microRNA (miRNA) in cancer. Cancer Cell Int. 2015;15(1):38. | ||
Stacker SA, Achen MG. The VEGF signaling pathway in cancer: the road ahead. Chin J Cancer. 2013;32(6):297–302. | ||
Lei Z, Duan H, Zhao T, et al. PARK2 inhibits osteosarcoma cell growth through the JAK2/STAT3/VEGF signaling pathway. Cell Death Dis. 2018;9(3):375. | ||
Stegh AH. Targeting the p53 signaling pathway in cancer therapy – the promises, challenges and perils. Expert Opin Ther Targets. 2012;16(1):67–83. | ||
Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8(4):275–283. | ||
Chen YF, Chen YT, Chiu WT, Shen MR. Remodeling of calcium signaling in tumor progression. J Biomed Sci. 2013;20:23. | ||
Hembruff SL, Cheng N. Chemokine signaling in cancer: implications on the tumor microenvironment and therapeutic targeting. Cancer Ther. 2009;7(A):254–267. | ||
Tsai YC, Weissman AM. The unfolded protein response, degradation from endoplasmic reticulum and cancer. Genes Cancer. 2010;1(7):764–778. | ||
Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer. 2014;14(9):581–597. | ||
Georgieva B, Milev I, Minkov I, Dimitrova I, Bradford AP, Baev V. Characterization of the uterine leiomyoma microRNAome by deep sequencing. Genomics. 2012;99(5):275–281. | ||
Shi Y, Yang F, Sun Z, Zhang W, Gu J, Guan X. Differential microRNA expression is associated with androgen receptor expression in breast cancer. Mol Med Rep. 2017;15(1):29–36. | ||
Wang DS, Zhang HQ, Zhang B, et al. miR-133 inhibits pituitary tumor cell migration and invasion via down-regulating FOXC1 expression. Genet Mol Res. 2016;15(1):1–10. | ||
Chung TK, Lau TS, Cheung TH, et al. Dysregulation of microRNA-204 mediates migration and invasion of endometrial cancer by regulating FOXC1. Int J Cancer. 2012;130(5):1036–1045. | ||
Xu YY, Tian J, Hao Q, Yin LR. MicroRNA-495 downregulates FOXC1 expression to suppress cell growth and migration in endometrial cancer. Tumour Biol. 2016;37(1):239–251. | ||
Zhu M, Chen L, Zhao P, et al. Store-operated Ca2+ entry regulates glioma cell migration and invasion via modulation of Pyk2 phosphorylation. J Exp Clin Cancer Res. 2014;33(1):98. | ||
Marchi S, Lupini L, Patergnani S, et al. Downregulation of the mitochondrial calcium uniporter by cancer-related miR-25. Curr Biol. 2013;23(1):58–63. | ||
Zhou X, Zhang L, Zheng B, et al. MicroRNA-761 is upregulated in hepatocellular carcinoma and regulates tumorigenesis by targeting mitofusin-2. Cancer Sci. 2016;107(4):424–432. | ||
Ryu S, McDonnell K, Choi H, et al. Suppression of miRNA-708 by polycomb group promotes metastases by calcium-induced cell migration. Cancer Cell. 2013;23(1):63–76. | ||
De A. Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin (Shanghai). 2011;43(10):745–756. | ||
Berridge MJ. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev. 2016;96(4):1261–1296. | ||
Toft-Bertelsen TL, Ziomkiewicz I, Houy S, Pinheiro PS, Sørensen JB. Regulation of Ca2+ channels by SNAP-25 via recruitment of syntaxin-1 from plasma membrane clusters. Mol Biol Cell. 2016;27(21):3329–3341. | ||
Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annu Rev Plant Biol. 2010;61:593–620. | ||
Gregoraszczuk EL, Rak-Mardyła A, Ryś J, Jakubowicz J, Urbański K. Effect of chemotherapeutic drugs on caspase-3 activity, as a key biomarker for apoptosis in ovarian tumor cell cultured as monolayer. A pilot study. Iran J Pharm Res. 2015;14(4):1153–1161. | ||
Xu CQ, Lu YJ, Pan ZW, et al. The muscle-specific microRNAs miR-1 and miR-133 produce opposing effects on apoptosis by targeting hsp60, Hsp70 and caspase-9 in cardiomyocytes. J Cell Sci. 2011;120(Pt 17):3045–3052. | ||
Renault TT, Floros KV, Elkholi R, et al. Mitochondrial shape governs Bax-induced membrane permeabilization and apoptosis. Mol Cells. 2015;57(1):69–82. | ||
Leelahavanichkul K, Amornphimoltham P, Molinolo AA, Basile JR, Koontongkaew S, Gutkind JS. A role for p38 MAPK in head and neck cancer cell growth and tumor-induced angiogenesis and lymphangiogenesis. Mol Oncol. 2014;8(1):105–118. | ||
Li J, Xu B, Chen Z, et al. PI3K/AKT/JNK/p38 signalling pathway-mediated neural apoptosis in the prefrontal cortex of mice is involved in the antidepressant-like effect of pioglitazone. Clin Exp Pharmacol Physiol. 2018;45(6):525–535. | ||
Koumenis C, Naczki C, Koritzinsky M, et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2 alpha. Mol Cell Biol. 2002;22(21):7405–7416. | ||
Nagelkerke A, Sweep FC, Stegeman H, et al. Hypoxic regulation of the PERK/ATF4/LAMP3-arm of the unfolded protein response in head and neck squamous cell carcinoma. Head Neck. 2015;37(6):896–905. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.