Back to Journals » International Journal of Nanomedicine » Volume 18
Biogenesis, Composition and Potential Therapeutic Applications of Mesenchymal Stem Cells Derived Exosomes in Various Diseases
Authors Yuan YG, Wang JL, Zhang YX, Li L, Reza AMMT, Gurunathan S
Received 6 March 2023
Accepted for publication 31 May 2023
Published 14 June 2023 Volume 2023:18 Pages 3177—3210
DOI https://doi.org/10.2147/IJN.S407029
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Yu-Guo Yuan,1,2 Jia-Lin Wang,1,2 Ya-Xin Zhang,1,2 Ling Li,1,2 Abu Musa Md Talimur Reza,3 Sangiliyandi Gurunathan4
1Department of Clinical Veterinary Medicine, College of Veterinary Medicine, Yangzhou University, Yangzhou, Jiangsu, People’s Republic of China; 2Jiangsu Co-Innovation Center of Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou University, Yangzhou, Jiangsu, People’s Republic of China; 3Department of Molecular Biology and Genetics, Faculty of Science, Gebze Technical University, Gebze, Kocaeli, Türkiye; 4Department of Biotechnology, Rathinam College of Arts and Science, Coimbatore, TN, India
Correspondence: Yu-Guo Yuan; Sangiliyandi Gurunathan, Email [email protected]; [email protected]
Abstract: Exosomes are nanovesicles with a wide range of chemical compositions used in many different applications. Mesenchymal stem cell-derived exosomes (MSCs-EXOs) are spherical vesicles that have been shown to mediate tissue regeneration in a variety of diseases, including neurological, autoimmune and inflammatory, cancer, ischemic heart disease, lung injury, and liver fibrosis. They can modulate the immune response by interacting with immune effector cells due to the presence of anti-inflammatory compounds and are involved in intercellular communication through various types of cargo. MSCs-EXOs exhibit cytokine storm-mitigating properties in response to COVID-19. This review discussed the potential function of MSCs-EXOs in a variety of diseases including neurological, notably epileptic encephalopathy and Parkinson’s disease, cancer, angiogenesis, autoimmune and inflammatory diseases. We provided an overview of exosome biogenesis and factors that regulate exosome biogenesis. Additionally, we highlight the functions and potential use of MSCs-EXOs in the treatment of the inflammatory disease COVID-19. Finally, we covered a strategies and challenges of MSCs-EXOs. Finally, we discuss conclusion and future perspectives of MSCs-EXOs.
Keywords: extracellular vesicle, biogenesis, mesenchymal stem cells, exosomes, autoimmune disease, angiogenesis
Introduction
Generally, extracellular vesicles (EVs) are a heterogeneous group of cell-derived membranous nanovesicles generated from cells that range in size from 30 to 1000 nm. Based on size and origin, EVs are classified into exosomes, microvesicles (MVs), and apoptotic bodies (ABs).1,2 They are released from most cell types and bio-fluids such as blood, saliva, breast milk, semen, urine, cerebrospinal fluid (CSF), colostrum,3 tears,4 bronchoalveolar fluid,5 epididymal fluid,6 amniotic fluid,7 bile,8 blastocoel fluid,9 middle ear effusion,10 and ascites.11 Exosomes are having with an average size between 30 and 150 nm, which are a subset of extracellular vesicles and are important messengers of paracrine activity. Tetraspanins (CD9, CD63, and CD81), heat shock proteins (HSPs), membrane transporters and fusion proteins, multivesicular bodies (MVBs) proteins, phospholipases, and lipid-related proteins are considered to be biomarkers found in exosomes.12,13 The biogenesis and secretion of exosomes occurs by the formation of MVBs and fuse with the plasma membrane and eventually leads to secretion of cargoes in the extracellular milieu.14 Exosomes are subset of EVs, which are playing vital role in physiological and pathological conditions, such as cellular junctions, integrins, and selectins.15
Exosomes are found abundantly in the secretome of many cell types such as embryonic stem cells, and induced pluripotent stem cells (iPSCs).16–18 Exosomes are vesicles that carry a variety of bioactive substances, including proteins, DNA, soluble and membrane proteins, microRNAs (miRNAs), and nucleic acids. These exosomes are a reflection of the cell from which they were produced as well as of the biogenesis and release routes.19,20 Exosomes are produced by nearly all forms of life and are present in a variety of bodily fluids. They have a variety of roles in cellular processes such as intercellular communication, immunological regulation, senescence, proliferation, and differentiation.21–24 Exosomes produced from iPSCs are believed to be therapeutic agents as targeted delivery agents and are safer than parental cells. They can promote a variety of functions, including angiogenesis, extracellular matrix (ECM) remodelling, and immune response regulation.25–29 Exosomes can transport exogenous bioactive substances and can pass the blood-brain barrier. Exosomes, for instance, are employed as indicators or therapy options for a number of diseases, such as cancer, neurological disorders, and immune-related illnesses.30
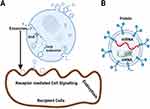
MSC-derived exosomes (MSC-EXOs) are nanosized vesicles that have demonstrated therapeutic efficacy in the treatment of a number of diseases, including liver diseases,31 kidney diseases,32 brain diseases,33 and cardio vascular diseases (CVDs),34 through interaction with their target cells through a variety of mechanisms, such as interaction, fusion, and internalisation of exosomes with the plasma membrane of recipient cells and these vesicles are necessary for cell-cell communication. The exosome cargoes that are released into physiological fluids mediate cell-cell contacts and regulate a variety of cellular processes. These mediators are crucial for the regulation of a variety of vital functions of their target cells to maintain homeostasis.35,36
Recent research suggests that active exosomes produced by stem cells can prevent heart and blood vessel illnesses by carrying a variety of payloads, including as proteins, miRNAs, and lncRNAs, to nearby or distant target cells. According to studies, the payloads and functions of exosomes produced by various types of cells varies greatly.37 For instance, EVs produced from cancer stem cells (CSCs) carry out a variety of biological activities, such as metastasis, angiogenesis, immunosuppression, and resistance to treatment.38,39 In order to maintain tumour heterogeneity, CSCs release EVs that communicate certain proteins and transcription factors to nearby cells.40 Recent research suggests that MSC-EXOs could be used to treat inflammatory and autoimmune illnesses since MSCs have immunosuppressive qualities. Through the transport of parental cell cargo, MSCs’ paracrine characteristics play a crucial role in cell-to-cell communication and are also implicated in the innate and adaptive immune response.41 MSC-EXOs are capable of triggering the necessary response in the event of injury, inflammation, or repair due to their dynamic immuno-modulatory function.42 According to numerous research, MSC-EXO’s bioactive chemicals and anti-inflammatory molecules interact with immune effector cells to play a vital role in immune response and control. These properties can be used to treat autoimmune illnesses.43 Exosomes produced by neurons, astrocytes, oligodendrocytes, and microglia and found in serum are thought to be early indicators of CNS illnesses, particularly neurodevelopmental disorders.44–46 Exosomes may be able to stop the loss of neurons by specifically attaching to target cells in the central nervous system.47 Pilocarpine-induced epilepsy was shown to reduce inflammation and memory impairment by MSC-EXOs by targeting hippocampus astrocytes in a mouse in vivo investigation.48 Particularly, Parkinson’s disease (PD), which affects about 1% of persons over,49 is the second most common neurodegenerative disease in the world. Among these paracrine effectors, MSC-EVs are a significant contributor and have the property of becoming a directed anti-tumor drug delivery platform or agent.50,51 The MSC-EXOs cargo has positive cardioprotective effects as a result of transporting and distributing a range of substances to the wounded cells.18,19 MSC-EXOs have demonstrated efficacy as minimally invasive agents for the repair, regeneration, and protection of human organs against a variety of bodily injuries. Stem cell secretome (SCS) released exosomes shows significant anti-fibrotic, anti-inflammatory, immunomodulatory, and anti-angiogenic actions.52–55
Considering all the literatures into account, in this review, we discuss general aspects of biogenesis and factors involved in promotion of biogenesis and secretion of exosomes and we also discuss in detail account about the role of MSC-EXOs in various diseases including neurological diseases, epileptic encephalopathy and Parkinson’s. Further, we discuss the involvement of MSC-EXOs in cardiovascular, cancer, angiogenesis and autoimmune and inflammatory diseases and specifically multiple sclerosis, rheumatoid arthritis, type 1 diabetes mellitus, uveitis, systemic lupus erythematosus, inflammatory bowel disease and COVID-19. Besides, this review summarizes the current advances of potential therapeutic benefits, underlying mechanisms, diagnosis, treatment, challenge and strategies of use of MSC-EXOs and limitations of mesenchymal stem cell-derived exosomes in various types of diseases.
Biogenesis of Exosomes
Exosomes are a subset of EVs that typically range in size from 30 to 180 nm and are important for intercellular communication between cells and organs.56 Almost all types of cells secrete exosomes, which contain a variety of biomolecules including DNA, RNA, and proteins.57–59 Exosomes are created by the double invagination of the plasma membrane, which also produces early endosomes that develop into intraluminal vesicles (ILVs) before maturing gradually and being delivered into multivesicular bodies (MVBs) by inward budding, preventing cytoplasmic lysosomes from degrading them.60–62 (Figure 1) The endosomal sorting complex required for transport (ESCRT), a separate mechanism made up of roughly 30 proteins that assemble into four complexes (ESCRT-0, -I, -II, and -III), controls the development of ILVs. In addition, two significant proteins, TSG101 and ALIX, are involved in the synthesis of exosomes, and syndecan and syntenin are typically found as a biomarker in exosomes produced from tumour cells. Exosome secretion can also take place by means of an ESCRT-independent mechanism. Rab GTPase family, cytoskeleton, molecular motors, and membrane fusion devices (SNARE complex) all play a role in controlling MVBs transports.63–65 Exosomes releases are influenced by several physiological factors and cellular conditions such as lipopolysaccharide, tumor necrosis factor-α, interferon comma and hypoxia. Some others, which are secreted from injured organs, contain healing RNAs and protein-containing exosomes and induce stem cells, facilitate the maintenance of tissue homeostasis.49
Exosome secretion is affected by a number of variables, such as intracellular calcium concentrations, cellular energy levels, composition of membrane phospholipids, membrane-acting enzymes, cytoskeleton-membrane interactions, other exocytosis effectors, hypoxia, and oxidative stress.66,67 Exosomes in circulation are picked up by recipient cells by three separate methods, including endocytosis, ligand-receptor uptake, and fusion. Exosomes were carefully targeted and internalised by ligand-receptors to deliver bioactive substances to the cells.68–70 Exosomes are internalized into target cells through particular receptors such as integrins, CD9, CD63, and CD81.71 The fusion of the exosome with the cell membrane releases the cargos produced from exosomes into the cytoplasm of the target cells.72 For example, MSC-EXOs express markers CD9, CD63, and CD81 which are involved in accelerate skeletal muscle regeneration.73 The structure, functions, delivery potential of cargos into target cells and protective nature of exosomes from cytosolic enzymes and cargos depends on composition of high levels of lipid species including ceramides, sphingomyelins (SM), cholesterol, gangliosidesphosphatidylserine, phosphatidylethanolamines, phosphatidylcholines, lyso-phosphatidylcholines, and phosphatidylinositols.13,74 There are no proteins from the nucleus, mitochondria, endoplasmic reticulum, or Golgi complex in exosomes that are derived from endosomes. Tetraspanins, adhesion proteins, antigen presentation proteins, membrane transport proteins, and fusion proteins are four different types of proteins that make up exosomes and act as biomarkers for exosome characterization.75 Adhesion proteins are playing a role in exosome maturation and target cell binding. Major Histocompatibility Complex (MHC) classes I and II and other antigen presentation proteins are involved in immune regulation, energy, and priming. Synaptosomal associated protein (SNAP), annexins, and Ran5b are examples of membrane transport and fusion proteins that are involved in exosome synthesis, secretion, and downstream cell fusion.76 Furthermore, exosomes are characterized by the expression of various surface markers including CD9, CD63, CD81, ALIX, TSG101, Hsc70, and MHC class II.77 Exosomes contain various types of cargoes with various sizes such as mRNA and miRNA. For example, dendritic cell-derived exosomes contain miR-451 are involved in an attenuation of the innate immune response to whole virus vaccines and monocyte-derived EVs contain miR-223 play essential role in suppressing macrophage activation and lung inflammation.78 EVs derived from MSC were found to be enriched with miR-16 that targets expression of vascular endothelial growth factor (VEGF).79 The functions of EVs are regulated by the compositions of EVs such as lipopolysaccharide (LPS), tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and hypoxia. According to Nakamura et al, adiponectin increases exosome release to improve MSCs-driven heart failure therapy. This increased exosome synthesis was strongly correlated with an increased amount of circulating hMSC-derived exosomes.80 The spreading areas of BMSCs on the titanium surface are enhanced by physicochemical characteristics like roughness and hydrophilicity of micro/nanotextured hierarchical titanium topographies, which facilitate a stronger promotion of BMSC proliferation. It also promoted the synthesis, transport, and secretion of BMSCs-derived exosomes into the extracellular milieu.81 Cortical spheroids made from human stem cells were subjected to iron oxide nanoparticles, which not only caused cytotoxicity but also greatly increased the number of extracellular vesicles.82 Peng et al investigated the processes behind the regulation of multivesicular body (MVB) trafficking, exosome secretion, invadopodia formation, and tumour invasion by long noncoding RNA LINC00511 (LINC00511).83 According to the findings of these studies, abnormal LINC00511 causes the formation of invadopodia in HCC cells by controlling the colocalization of two proteins, vesicle associated membrane protein 7 (VAMP7) and synaptosome associated protein 23 (SNAP23). Invadopodia are important secretion sites for MVBs and regulate the release of exosomes. Tetraspanin proteins found in MSC-EXOs, including CD63, CD9, CD81, CD29, CD73, CD90, CD44, and CD105, are MSC-specific and have a significant impact on exosome formation and secretion.84 Collectively, these investigations offered proof of the molecular process underlying exosome formation.
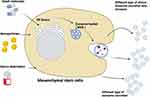
Factors Influences Biogenesis and Secretion of Exosomes from Stem Cells
The biogenesis of the exosomes are complex process and regulated by various conditions may lead to discrepant therapeutic outcomes.85–87 Biogenesis and secretion of exosomes are regulated by various factors including cell type, cell confluency, serum conditions, soluble factors, and the presence and absence of cytokines and growth factors and also sites of exosomes, protein sorting, physico-chemical aspects, and transacting mediators are playing significant role in biogenesis and secretion.88 Biogenesis and secretion of exosomes of stem cells can be increased by addition of certain soluble cytokines directly into the culture medium. Bioactive molecules such as lipopolysaccharide,89 N-methyldopamine,90 noradrenaline,90 and adiponectin91 shows SuxiaoJiuxin pills increased the level of exosome secretion by stem cells.92 Exosomes biogenesis and secretion can be increased by serum starvation, exogenous stress, nanoparticles and small molecules (Figure 2). Platelet-derived growth factor (PDGF) is an important factor for the selective expansion and recruitment of undifferentiated mesenchymal stem cells.93–95 PDGF induces the migration and proliferation of mural progenitor cells during vascular development,95 stimulates endothelial cells96 and induces mesenchymal cell transdifferentiation into vessel cells.97,98 The vascular niche in the tumor microenvironment (TME) also releases growth factors through adjacent and paracrine pathways to support the growth of cancer stem cells (CSCs) and maintain its stemness.99,100 Exosomes were produced when human endothelial cells were exposed to low-level laser irradiation (LLLI) at an energy density of 80 J/cm2. Irradiation of the cells causes an increase in exosome release as well as enhanced gene expression, including CD63, Alix, Rab27a, and Rab27b. The increased levels of exosomes were associated with an enhanced acetylcholine esterase activity, pseudopodia formation, and reduced zeta potential value 24 h post-irradiation.101 C1q-TNF-α related protein-9 (CTRP9) polypeptide that upregulates SOD2/SOD3 expression and improves cortical bone-derived mesenchymal stem cell (CBSC) CBSC survival/retention, similar to gCTRP9. Moreover, CTRP9-281 stimulates VEGFA-rich exosome production by CBSC, exerting superior pro-angiogenic, anti-fibrotic, and cardioprotective actions.102 Lopatina et al reported that adipose mesenchymal stem cells (ASCs) derived EVs induced formation of vessel-like structure in human microvascular endothelial cells (HMEC).103 Treatment of ASCs with platelet-derived growth factor (PDGF) stimulated the secretion of EVs, changed their protein composition and enhanced the angiogenic potential. Further, the authors described that PDGF treatment of ASC changed protein and RNA composition of released EVs by enhancing the expression of anti-inflammatory and immunomodulatory factors.103 Mesenchymal stem cell-derived exosomes (MSC-EXOs) improves osteoarthritis through long non-coding RNA KLF3-AS1. Chondrocytes were treated with IL-1β to induce chondrocyte injury, followed by MSC-EXOs treatment enhanced KLF3-AS1 expression. MSC-EXOs-derived exosomes containsKLF3-AS1 promotes cell viability and inhibits apoptosis of IL-1β-treated chondrocytes. MSC-EXOs-mediated KLF3-AS1 inhibits autophagy and apoptosis of IL-1β-treated chondrocyte through PI3K/Akt/mTOR signaling pathway.104 TNF-α-stimulated gingival MSC (GMSC)-exosomes (TG-EXOs), modulates inflammatory microglia and alleviates apoptosis.105 A study using a mouse model of acute graft-versus-host disease (aGVHD) suggested that epidermal growth factor (EGF)-stimulated microRNA-21 (miR-21) in bone marrow-derived mesenchymal stem cells (BMSCs). As a result, the stimulated miR-21 promoted the proliferation, invasion, and migration of BMSCs. Furthermore, miR-21 in BMSC-EXOs inhibited phosphatase and tensin homolog (PTEN), but enhanced AKT phosphorylation and Foxp3 expression in Tregs.106 The treatment of MSCs with a combination of N-methyldopamine and norepinephrine robustly increased exosome production by three-fold without altering the ability of the MSC exosomes.90
Physicochemical factors alter environment and eventually influences the biogenesis and secretion of exosomes. For example, stem cells exposed to chemical stimulations such as hypoxia can influence the secretion of exosomes. Hypoxia condition enhances of MSCs and also the derived exosomes enhanced therapeutic effect.11,107,108 Serum deprivation stimulated the secretion of exosomes by the stem cells.109,110 Furthermore, mechanical forces such as flow and stretching factors enhance the secretion of stem cells up to 37 folds via bioreactors.111,112 Similarly, other mechanical factor such as high-frequency ultrasound increases biogenesis and secretion of exosomes up to 10 folds.113 Interestingly, stimulations of exosomes secretion were observed and dependent on type of cells.114 The 3D culture plays significant role in biogenesis and secretion of exosomes and increases surface area and continuous supply of shear force.115 While the level of secretion increases 100 fold when cells are cultured on a hollow fibrillar scaffold that was 3D printed.116 Studies showed that compared to BMSCs and adipose tissue-derived stem cells (ADSCs), human umbilical cord mesenchymal stem cells (HUMSCs) generated from Wharton’s jelly had the highest exosome output. Biomaterials including ferroferric oxide-coated PLGA nanoparticles, NO-releasing polymer, lithium-incorporated bioactive glass ceramic, and bioglass enhance exosome secretion and biogenesis.116–120 In mesenchymal stem/stromal cells (MSCs), adiponectin enhanced exosome formation and secretion through binding to T-cadherin.80 Mice model showed that internalization of exosomes contained small RNA isolated from neuron-CMinto astrocytes improved its signalling in amyotrophic lateral sclerosis.121 MSCs exposed to ischemic brain extracts had increased levels of miR-133b in its released exosomes.122 Scaffold contains nitric oxide (NO) and chitosan induces biogenesis of exosomes from placenta with pro-angiogenic and pro-migratory factors compared to the normal human placenta MSCs.117 Endothelial cells exposed to hypoxia-mimetic agent desferrioxamine (1% O2) produced higher levels of EVs compared to cells exposed to normoxia. Conversely, treatment of cells with sodium nitrite (NaNO2) reduced the hypoxic enhancement of EVs production.123 TNF-α can promote the release of EVs in astrocytes through upregulation of glutaminase expression. TNF-α treatment significantly upregulated protein levels of glutaminase and increased the production of glutamate, suggesting that glutaminase activity is responsible for biogenesis and secretion of exosomes. Glutaminase inhibitor blocked TNF-α-mediated generation of reactive oxygen species in astrocytes. TNF-α-mediated increased release of EVs can be blocked by either the glutaminase inhibitor, antioxidant N-acetyl-L-cysteine, or genetic knockout of glutaminase, suggesting that glutaminase plays an important role in astrocyte EVs release during neuroinflammation.124 MSCs treated with small molecules such as combination of N-methyldopamine and norepinephrine strongly increased exosome production up to three-fold without altering the ability of the MSC exosomes to induce angiogenesis, polarize macrophages to an anti-inflammatory phenotype, or downregulate collagen expression.90
Recently, several studies reported that the effect of various types of nanoparticles on biogenesis and secretion of exosomes. For example, Gurunathan et al reported that platinum nanoparticles (PtNPs) enhance biogenesis and secretion of exosomes in A549 cells by generation of oxidative stress.125 While culturing A549 cells with PtNPs enhance exosome secretion by altering various cellular and physiological processes. These studies found that A549 cells treated with PtNPs increase total protein concentration, which is associated with PtNPs-induced oxidative stress. The molecular mechanism of enhanced secretion of exosomes were substantiated with acetylcholinesterase (AChE) inhibitor such as GW4869, which inhibits PtNPs induced biogenesis and release of exosomes and alsoacetylcholinesterase (AChE), neutral sphingomyelinase activity (n-SMase), and exosome counts. Conversely, A549 cells pre-treated with N-acetylcysteine significantly inhibited PtNPs induced exosome biogenesis and release. These findings confirmed that PtNPs-induced exosome release was due to the induction of oxidative stress and the ceramide pathway. The same group further investigated that how PdNPs enhance exosome release in human leukemia monocytic cells (THP-1). Exosome production was associated with increased level of total protein concentration, exosome counts, acetylcholinesterase activity, and neutral sphingomyelinase activity. The exosomes were spherical in shape and had an average diameter of 50–80 nm. The expression levels of TSG101, CD9, CD63, and CD81 were significantly higher in PdNPs-treated cells than in control cells. Further, cytokine and chemokine levels were significantly higher in exosomes isolated from PdNPs-treated THP-1 cells than in those isolated from control cells. THP-1 cells pre-treated with N-acetylcysteine or GW4869 showed significant decreases in PdNPs-induced exosome biogenesis and release.
The endoplasmic reticulum stress, oxidative stress, apoptosis, and immunomodulation are all involved in the process of PdNPs increased exosome secretion. Exosome release and biogenesis in SH-SY5Y cells treated with silver nanoparticles.125 While cells treated with N-acetylcysteine in the presence of AgNPs reduced the level of oxidative stress-induced exosome biogenesis and release, the expression levels of some key exosome biomarkers were significantly increased as a result ofAgNPs-induced oxidative stress. The authors also discovered that the ceramide pathway was involved in the exosome release caused by AgNPs. Exosome secretion and enhanced biogenesis can both be used therapeutically to treat a variety of illnesses.
Role of MSC-EXOs in Neurological Disease
Human pluripotent stem cell-derived EVs are essential for several biological functions, including the protection of tissue homeostasis.126 The recipient cells experience protective benefits, reduced oxidative stress, and apoptosis after receiving EVs produced from stem cells. In addition to forming myelin, releasing neurotransmitters, and providing glia-mediated trophic support to axons, the EVs released by neuronal cells like oligodendrocytes are also capable of communicating with other cells via miR-219.127,128 In AD mice, EVs from hippocampal neuronal cells reduce amyloid beta (Ab) load and restore synaptic activity.129,130 According to Haney et al, EVs produced from mouse macrophages boost the survival of brain cells while lowering ROS levels.131 EVs carrying active neprilysin are also able to degrade Ab and eventually reduce the burden of Ab plaques and the amounts of dystrophic neurites in both the cortex and hippocampus of AD mice. EVs carrying active neprilysin are derived from MSCs and exhibit neuroprotective properties and suppressed 6-hydroxydopamine-induced apoptosis in dopaminergic neurons.132,133 Exosomes produced by BM-MSCs demonstrated therapeutic effects in a mouse stroke model, including the enhancement of angio-neurogenesis and long-term neuro-protective properties.134 Exosomes also contain miR-133b, which, when delivered to astrocytes and neurons, aids in neurite remodelling and, in turn, stroke recovery.135 The miR-17-92 cluster is carried by exosomes and is involved in oligodendrogenesis, neurogenesis, and other neurological processes in the ischemia boundary zone.136 According to Drommelschmidt et al, exosomes produced by bone marrow-derived mesenchymal stem cells (BM-MSCs) prevent reactive astrogliosis, microgliosis, and brain degeneration.137 Exosomes from BM-MSCs demonstrated neuroprotective properties, including a decrease in neurological aftereffects and restoration of brain function.138 Signal transducer and activator of transcription 3 (STAT3), c-Jun N-terminal kinase, and p53 are only a few of the signalling pathways that are modulated by exosomes generated from mice, which include different angiogenesis-related proteins, to improve angiogenesis and directly boost endothelial cell growth.139 Exosomes from BM-MSCs demonstrated neuroprotective effects by lowering the extent of the lesion, restoring neurobehavioral function, upregulating the anti-apoptotic protein Bcl-2 expression, and modifying microglia/macrophage polarisation.140 Exosomes made from BM-MSCs shown anti-inflammatory characteristics by repairing injured cord tissue and enhancing locomotor function by disorganizing astrocytes and microglia.141 In an APP/PS1 mouse model of Alzheimer’s disease (AD), hypoxia-preconditioned BM-MSCs generated exosomes restored synaptic dysfunction and encouraged anti-inflammatory actions.142 Exosomes containing the enzyme neprilysin, which can break down -amyloid peptides in the brain tissue, were secreted by AD-MSCs. By generating 6-hydroxy-dopamine in a 3D culture, exosomes produced from dental pulp MSCs (DP-MSCs) protected dopaminergic neurons from apoptosis.132 Exosomes of bone marrow stromal cells from Type 1 diabetes mellitus rats and normal rats were compared in terms of their impacts.143 According to the study, when compared to Nor-BMSCs, DM-BMSCs and their derived exosomes boosted survival and angiogenesis processes while decreasing miR-145 expression. Additionally, in vivo research revealed that DM-BMSC treatment enhanced functional outcomes and white matter and vascular remodelling, which in turn reduced serum miR-145 expression and raised expression of miR-145 target genes ABCA1 and IGFR1. The therapeutic effects of extracellular vesicles produced from differentiated PC12 cells and MSCs on the treatment of spinal cord injury (SCI) were the subject of a study. According to the findings, miR-21 and miR-19b expression was elevated, PTEN mRNA expression was downregulated, and cell apoptosis was reduced. When miR-21/miR-19b precursors were transfected into the cells, cell apoptosis was inhibited.144 According to Zhao et al, delivery of MSC-EXOs to rats through the tail vein had an anti-inflammatory impact and may have a role in the treatment of acute cerebral ischemia.145 The findings demonstrated that MSC-EXOs treatment greatly enhanced the motor, learning, and memory abilities of MCAO/R rats seven days after treatment. Pro-inflammatory factors may be reduced by the treatment, whereas anti-inflammatory cytokines and neurotrophic factors may be increased in the cortex and hippocampus of the ischemic hemisphere that has received OGD and NMLTC4. Treatment with MSC-EXOs also significantly reduced the polarisation of M1 microglia and increased M2 microglia cells, while downregulating the expression of CysLT2R and ERK1/2 phosphorylation. By reversing CysLT2R-ERK1/2 driven microglia M1 polarisation, MSC-EXOs reduced brain damage and suppressed microglial inflammation. It’s interesting to note that a study discovered down-regulation of miR-133b expression and up-regulation of EZH2 in glioma tissues and cells. It was revealed that miR-133b targets and inhibits the expression of EZH2. Furthermore, glioma cell proliferation, invasion, and migration were decreased by EZH2 knockdown via the Wnt/-catenin signalling pathway.146 Lee et al observed that at how ADSC-EXOs prevented the disease phenotypes brought on by the A cascade in an AD.147 The study found that treatment with ADSC-EXOs decreased the levels of A-42, A-40, and the A-42/40 ratio in AD cells and enhanced apoptotic molecules such p53, Bax, pro-caspase-3, and cleaved-caspase-3 while decreasing the level of Bcl-2 protein. In addition, Aβ impaired neurite development in the brains of AD patients, which was improved by ADSC-EXOs therapy. When combined, ADSC-EXOs may act as a therapeutic drug to slow the development of AD and Aβ-induced neuronal death. According to Sun et al, human umbilical cord mesenchymal stem cells (hucMSC) efficiently cause bone marrow-derived macrophages (BMDM) to polarise from an M1 to an M2 phenotype, which enhances functional recovery following spinal cord injury by suppressing inflammatory cytokines like TNF-α, MIP-1, IL-6, and IFN-γ.148 These results show that hucMSC-derived exosomes promote SCI repair by reducing inflammation in the area of the lesion. The effect of cerebral ischemia/reperfusion (I/R) on exosomal miR-150-5p from bone marrow-derived mesenchymal stromal cells (BMSCs) was investigated. Neurological performance was enhanced by exosomal microRNA-150-5p from mesenchymal stromal cells in bone marrow. In rats with middle cerebral artery occlusion (MCAO), the pathogenic modifications slow down neuronal death and lessen inflammatory factors. By suppressing TLR5, enriched miR-150-5p strengthens BMSC-EXOs’ ability to protect against cerebral I/R damage. New therapeutic targets for the treatment of cerebral I/R injury were presented by this study.149 By encouraging autophagy and preventing neuronal apoptosis, exosomes with miR-455-5p found in bone marrow mesenchymal stem cells demonstrated neuroprotective effects against spinal cord ischemia reperfusion injury.150 In both normal and oxygen-glucose deprivation (OGD) culture conditions, exosomes isolated from NSCs (NSCs-EXOs) inhibited apoptosis while promoting the proliferation of SH-SY5Y cells. Additionally, NSC-EXOs facilitate the neuroprotective effects via transfer of miR-150-3p, which targets caspase-2, thereby suppressing neuronal apoptosis after brain injury.151 An investigation was made into the mechanisms by which TNFα-stimulated gingival MSC (GMSC)-exosomes (TG-EXOs) modulate inflammatory microglia and reduce apoptosis. As compared to G-EXOs (GMSC-exosomes), the results demonstrated that intraocular injection of TG-EXOs into animals with IRI significantly reduced inflammation and cell loss. miR-21-5p-containing exosomes were an essential component of TG-EXOs for neuroprotection and anti-inflammation.152 Exosomes derived from BMSCs contain microRNA-124-3p, which reduces tumour necrosis factor receptor associated factor 6 in newborn rats to lessen hypoxic-ischemic brain damage (HIBD). These exosomes improved neurological functions, reduced pathological and structural damage to neurons, slowed down oxidative stress, and decreased neuronal apoptosis in newborn HIBD rats.153 Exosome release from BM-MSCs is increased in cells treated under hypoxia/reoxygenation (H/R) conditions, and these exosomes contain high levels of miR-29c, which reduces cardiac ischemia/reperfusion injury by inhibiting excessive autophagy via the PTEN/Akt/mTOR signalling pathway.154 Exosomes from myocardial ischemia patients stimulate angiogenesis through the miR-939-iNOS-NO pathway.155 According to Zhao et al, human umbilical cord derived mesenchymal stem cells (HucM-SCs)-derived miR-206-knockdown exosomes significantly reduce neurological deficit and brain edema in subarachnoid haemorrhage induced early brain injury and suppress neuronal apoptosis targeting via BDNF/TrkB/CREB signalling.156 When compared to therapy with ordinary exosomes, miR-206-knockdown exosomes produced from hucMSCs significantly protect against early brain damage (EBI) brought on by subarachnoid haemorrhage (SAH). The exosomes with miR-206 knockdown drastically reduced brain edema and neurological deficits while also suppressing neuronal death by targeting BDNF.156
Exosomes from hepatocytes have been shown in in vivo experiments to efficiently treat hepatic I/R damage, reduce hepatocyte apoptosis, lower levels of liver enzymes, and promote hepatocyte regeneration. In vitro and in vivo findings suggested that exosomes effectively enhanced hepatocyte tolerance to ischemia and decreased hepatocyte death.157 The autophagy-related proteins LC3IIB and Beclin-1 are expressed more often by BMSC-EXOs, which also helped autophagosome formation. Pro-apoptotic protein cleaved caspase-3 expression was significantly reduced after treatment with BMSC-EXOs, although Bcl-2 expression was increased.158 The function of MSC-EXOs in cerebral ischemia-reperfusion (I/R) injury was the subject of a study. The findings demonstrated that miR-26a-5p-carrying MSC-EXOs reduced I/R damage in mice by preventing microglia from apoptosizing by suppressing CDK6. According to this study, MSC-EXOs are being investigated as a potential therapeutic option for cerebral I/R injury.159 A study was carried out to determine how hMSC-EXOs affected the suppression of I/R-induced apoptosis and autophagy. By upregulating Bcl-2, downregulating Traf6, and activating mTORC1, hMSC-EXOs dramatically reduced H/R damage as seen by enhanced cell survival, decreased lactate dehydrogenase (LDH), and decreased apoptosis.160 Another study looked into the molecular mechanisms underpinning oridonin’s role in the prevention and treatment of ischaemia/reperfusion (IR)-induced damage as well as the function of BM-MSC-EXOs in IR-induced damage. BM-MSC-EXOs inhibited the progression of IR-induced myocardial damage by down regulation of Beclin-1, ATG13 and Bcl-2, conversely Apaf1 and Bax were significantly up-regulated in IR rats.161 Exosomes derived from cochlear spiral ganglion progenitor cells prevent cochlea damage from ischemia-reperfusion injury via inhibiting the inflammatory process.162 An in vitro study showed that exosomes from BM-MSCs prevented acute SCI’s rupture of the blood-spinal cord barrier by inhibiting the TIMP2/MMP pathway.163 Ke et al studied the effect and potential mechanism on retinal Müller cells and retinal function using rat retinal tissues. Human embryonic stem extracellular vesicles (hESEVs) were injected into the vitreous cavity of RCS rats. The findings showed that RCS rats had more dedifferentiated Müller cell-like retinal progenitor cells at the postnatal 30-day.164 The presence of many CHX10-positive cells in the retinal inner layer of RCS rats after hESEV injection is proof that hESEVs encouraged Müller cells to dedifferentiate and retrodifferentiate into retinal progenitor cells. By controlling the expression of Oct4 in Müller cells through HSP90 mediation in MVs, hESEVs also facilitated the retrodifferentiation of Müller cells into retinal progenitor cells. Exosomes containing hypoxia-inducible factor 1-alpha were implanted into the damaged spinal cord by encapsulation in a peptide-modified adhesive hydrogel to create a pro-angiogenic treatment for SCI models. The local delivery of exosomes was made possible by the sticky peptide PPFLMLLKGSTR-modified hyaluronic acid hydrogel, which also supplied the spinal cavity left vacant by spinal cord injury.165 The function of hBMSCs-exosomes in controlling immune response and neuronal function.166 Human MSCs-EXOs were discovered to protect against nerve injury by modulating the immune microenvironment in a model of neonatal hypoxic-ischemic brain damage. Exosomal miR-145 generated from MSCs was discovered to be able to change the polarisation of microglia towards the anti-inflammatory M2 phenotype in OGD/R-stimulated BV2 cells. Additionally, exosomal miR-145 significantly reduced the expression of FOXO1 while significantly suppressing apoptosis, cell cycle arrest, and oxidative stress in OGD/R-treated BV2 cells.Through the downregulation of FOXO1, MSCs-EXOs enriched with miR-145 were able to have neuroprotective effects in brain damage following ischemic stroke (IS).167 Moreover, exosomal miR-145 markedly suppressed the apoptosis, cell cycle arrest and oxidative stress in OGD/R-treated BV2 cells and decreased the expression of FOXO1 in BV2 cell exposed to OGD/R and in brain tissues of middle cerebral artery occlusion (MCAO) rats.167 Exosomes derived from umbilical cord mesenchymal stem cells (UCMSCs) have been shown in rat models of traumatic brain injury (TBI) to promote functional recovery, reduce neuronal apoptosis, and increase functional recovery of brain injury by inactivating microglia and astrocytes.168 All of these findings provide a fresh viewpoint and treatment plan for the application of MSC-EXOs in regenerative medicine.
Role of MSC-EXOs in Epileptic Encephalopathy
Multiple factors contribute to epilepsy, which is a highly synchronised aberrant discharge of brain neurons that affects both health and behaviour. Epilepsy affects over 65 million people worldwide.169,170 Exosomes were often isolated from a variety of physiological fluids, including cerebrospinal fluid, blood, urine, and other biological fluids.48,171 MSC-EXOs have demonstrated positive effects on several neurological conditions, including stroke,137 multiple sclerosis,172 Alzheimer’s disease (AD),173 SCI,174 ischemia,134 traumatic brain injury,175 hypoxic-ischemia produced prenatal brain injury,138,176 and preterm brain injury. The microRNAs found in exosomes from patients with epilepsy and depression’s CSF fluid may be able to traverse the blood-brain barrier.177,178 By specifically connecting with target nervous system cells, exosomes control neuroinflammation of the central nervous system. Targeting hippocampal astrocytes, stem cell-derived exosomes reduced inflammation and corrected status epilepticus-induced learning and memory deficits in mice.48 Micro RNAs including miR-27a-3p, miR-328-3p, and miR-654-3p are found in exosomes produced from plasma, which are thought to be possible indicators for people with epilepsy.179 According to an in vitro study, overexpression of miR-132 in hippocampal neuronal culture dramatically increases the frequency of epileptic discharge in epileptic neurons and is associated with the onset of epilepsy.180 The epileptogenic factors generated by transforming growth factor beta 1 (TGF-1) and interleukin-1 beta (IL-I) are thought to be negatively regulated by miR-132.181 MicroRNAs found in exosomes, namely miR-219 and miR-338, have been shown to be resistant to demyelination.182,183 Exosome-mediated transfer of microRNAs like miR-219 and miR-338 into oligodendrocytes was demonstrated in an in vivo investigation to result in a larger number of Olig2 than in the control group. According to this study, administration of miR-219/miR-338 improves myelination following spinal cord injury (SCI) and also improves axonal remyelination following nerve damage in the central nervous system to cure epileptic depression.184 Inflammation in the brain plays a role in the complex process of epilepsy, and the activity of the seizure can encourage the synthesis of inflammatory molecules, affecting the severity of the epilepsy and the frequency of recurrence.185,186 For instance, the patients cerebrospinal fluid had much greater levels of the pro-inflammatory cytokine IL-1.187,188 The elevated level of IL-1 exhibits a favourable connection with depression.189 Furthermore, according to another study, IL-1 knock-down in the hippocampus can significantly improve memory deficits, anxiety, and depressive-like behaviour in mice induced by lipopolysaccharide (LPS), which is caused by downregulation of the neuropeptides brain-derived neurotrophic factor (BDNF) and LPS-induced neuropeptide (VEGF).190 According to a therapeutic investigation, the expression of IL-1 was dramatically elevated after therapy with MSC-EXOs for retinal detachment.191 According to an animal study, MSC-EXOs revealed anti-inflammatory action by reducing the amount of proinflammatory cytokines and inflammatory indicators in cerebrospinal fluid from sepsis syndrome (SS) animals as compared to the control group. It is well known that IL-1β disrupts blood brain barrier and this dysfunction leads to epilepsy. This theory is supported by an in vitro model study that found that overexpressing miR-132 lowers IL-I production, which in turn lowers blood-brain barrier disruption.192 After the seizure and expression of miR-132 reduce the expression of MMP-9 to protect the integrity of the blood-brain barrier by lowering tight junction protein degradation, blood-brain barrier malfunction results in an elevated level of MMP-9 concentration.193–197 All of these research came to the conclusion that miR-132 is important in the treatment of epilepsy because it increases anti-inflammatory effects and safeguards the blood-brain barrier’s integrity. An in vivo investigation utilising a mouse model showed that intranasal (IN) delivery of hMSC-derived EVs twice within 24 hours effectively suppressed “cytokine storm” in the hippocampus. Reduced levels of pro-inflammatory proteins were linked to cytokine storm blockage. Reduced levels of pro-inflammatory cytokines including TNF- and IL-1 and elevated levels of the anti-inflammatory protein IL-10 were seen in the hippocampus as a result of cytokine storm blockade.198 The advancement of epileptogenic alterations is slowed down by the administration of hMSC-EVs, as is the case with chronic epilepsy. Intra nasal delivery of EVs modifying abnormal long-term plasticity, such as aberrant mossy fibre sprouting, the activation of mTOR pathway, or psychiatric comorbidities, linked to epilepsy.199–206
Role of MSC-EXOs in Parkinson’s Disease
The second most prevalent neurological disease in the world is Parkinson’s disease (PD).207 Nearly 0.3% of the population has PD, and those over the age of 65 make up 1% of those who are affected.208,209 Clinical signs of Parkinson’s disease (PD) patients include bradykinesia, postural instability, and resting tremor. Dopaminergic neurons in the SNpc are still dying, which is one of many mechanisms that contribute to the pathophysiology of PD.210 The histological development of Lewy bodies, which predominately contain misfolded α-synuclein, is a hallmark of Parkinson’s disease.211 The misfolding, aggregation, and lewybody-induced deposition of α-syn cause neurotoxicity in PD. The neurotoxicity caused by non-cell autonomous mechanisms and the transmission of α-synuclein from cell to cell are the fundamental causes of Parkinson’s disease (PD).212 Parkinson’s disease aetiology significantly depends on the level of α-synuclein. A key factor in the development of Parkinson’s disease is cell dysfunction in those that contain α-synuclein.213 By delivering essential biological molecules to neurons via exosomes, astrocytes shield dopaminergic neurons from the oxidative stress and iron-mediated toxicity brought on by dopamine metabolic products in PD patients. Therefore, protection of astrocyte-neuron contact is a crucial step in eliminating the PD’s root cause.214 MiR-200a-3p, which is found in exosomes produced from astrocytes, inhibits the mitogen-activated protein kinase kinase 4 (MKK4) pathway to stop MPP(+)-induced apoptotic cell death.215 There are currently no proven and viable treatments for PD. Therefore, the development of novel medications or therapeutic techniques to treat PD is imperative. The majority of medications on the market are unable to pass through the blood-brain barrier (BBB).216 Exosomes, on the other hand, can act as natural nanoscale vesicles that can carry drugs over the BBB.217,218 According to rat models of Parkinson’s disease (PD), exosomes made from MSCs appear to be a viable therapeutic approach for the treatment of a number of diseases including Parkinson’s disease.219,220 The motor and histological symptoms of 6-hydroxydopamine (6-OHDA) may be partially reversed by injection in the SNpc-STR route.221 Exosomes containing dopamine, for instance, showed potential therapeutic effects and decreased toxicity in the PD mouse model.222 MSC-EXOs may be able to restore dopaminergic neurons in the 6-OHDA animal model of PD, suggesting that this may be a possible treatment for the disease.223 Studies using animal models showed that MSC-EXOs carry miRNAs that enhance neurogenesis and neuroinflammation. In MSC-EXOs, miR-21, miR-143, and miR133 are significantly influencing immunological regulation, neuronal apoptosis, and neuerite outgrowth.224 According to Xin et al, MSC-EXOs administered intravenously increased neurogenesis, neurite remodelling, and angiogenesis while reducing neuroinflammation, oxidative stress, and cell death (Figure 3). MSC-EXOs administrations in a brain model diminish inflammation.225 Additionally, MSC-EXO injection demonstrated effective treatment for SCI by lowering inflammation and encouraging neuro-regeneration in injured rats.226,227 In in vitro (6-OHDA) models of PD, MSC-EXOs were discovered to be able to rescue DAn, offering a possible regenerative treatment for this condition.132 According to a study, exosomes from neurons, astrocytes, and oligodendrocytes may be beneficial as a biomarker for Parkinson’s disease (PD).228 MSCs-EXOs expressing miRNAs work in concert with neuronal cells to reduce neuroinflammation and promote neurogenesis in PD animal models, and they also offer a potential treatment for PD.
Role of MSC-EXOs in Cardiovascular Diseases
Cardiovascular diseases (CVDs) are a major cause of mortality worldwide and have become a public health priority. The main causes of CVDs are low levels of cardiac progenitor cell proliferation and insufficient myocyte proliferation in existing myocytes.229 Recent research has shown that MSC-EXOs are important in cardio-protective functions. Stem/progenitor cell transplantation is regarded as an effective therapeutic approach that can replace missing cardiomyocytes and enhance contractility.230 A rat myocardial infarction (MI) model research that used vesicles from hBM-MSCs showed that these substances had cardioprotective properties.231 Signal transducer and activator of transcription 3 (STAT3) pathways are inactivated by MSC-EXOs, and miR-204 is increased in the lung cells, which reduces vascular remodelling and hypoxic pulmonary hypertension in mice.232 Tube formation, T cell inhibition, the reduction of infarct size, and the restoration of cardiac systolic and diastolic performance were all facilitated by BM-MSC-EXOs.233,234 BM-MSC-EXOs harbouring miR-221 demonstrated anti-apoptotic and cardioprotective effects by suppressing PUMA expression.235 Exosomes from BM-MSC containing miR-19a decreased the infarct size and restored cardiac function in a rat model of acute MI by downregulating PTEN and activating the Akt and ERK signalling pathways.236 When cardiac MSC-EXOs are delivered, cardiac function is improved by encouraging cardiac angiogenesis and triggering cardiomyocyte (CMC) proliferation through the production of chemokines and growth factors.237 Different miRNAs enriched in MSC-EXOs, such as miR-221 and miR-19a, are capable of producing stronger protective effects on CMC and also the regeneration of ischemia injury by boosting the survival of myocytes in ischemic injury by lowering the expression of the p53-upregulated apoptosis modulator (PUMA).238,239 The survival and bioenergetics of cardiac cells were directly impacted by MSC-EXOs. The findings showed a decrease in oxidative stress and an increase in the creation of molecules high in energy, such ATP and NADH.Additionally, the modification of pro- and anti-apoptotic pathways is a function of exosomes.240
Exosomal miR-210 was shown to stimulate angiogenesis and maintain heart function in an in vitro and in vivo model study.241 By inhibiting PTEN, activating Akt, and upregulating Bcl-2 and VEGF, endometrium-derived MSC exosomes have demonstrated cardioprotective actions, which ultimately result in the recovery of cardiac function. Exosomes produced by stem cells have been proven in studies to have cardioprotective benefits by delivering a range of cargos to damaged cells.242 Stem cell-based therapies have been shown in both animal and human studies to be effective in treating CVDs by replacing lost cardiomyocytes and enhancing contractility.243 Exosomes made from stem cells were able to repair and protect after transplantation.244 Human umbilical vein endothelial cells ability to form tubes was considerably improved by MSC-EXOs. They also reduced infarct size; degrade T-cell activity by preventing cell proliferation.233 In vivo murine auricle ischemic injury model revealed that exosomes produced from human placenta-derived mesenchymal stem cells may induce angiogenesis by factors of both angiogenic and angiostatic factors, which improved endothelial tube formation.245 Comparing hucMSCs to hucMSCs with Akt transfection, angiogenesis is dramatically increased. Additionally, Akt-Exo showed dramatically increased levels of platelet-derived growth factor D (PDGF-D) expression.246 Exosomes from mouse bone marrow mesenchymal stem cells (BMSCs) expressing Mir9-3hg were used to treat HL-1 mouse cardiomyocytes with hypoxia/reoxygenation (H/R), which promoted cell proliferation, increased glutathione (GSH) content, and decreased iron ion concentration, reactive oxygen species (ROS) level, and ferroptosis marker protein levels. Additionally, the administration of BMSC-EXOs to animals receiving I/R therapy improved their cardiac function by preventing cardiomyocyte ferroptosis by altering the Pum2/PRDX6 axis.247 According to Gong et al angiogenesis is boosted by nano-sized EVs generated from genetically altered MSCs that overexpress GATA-4.248 When compared to exosomes from the cells of origin, those produced from multiple myeloma BM mesenchymal stromal cells (BM-MSCs) had larger concentrations of oncogenic proteins, cytokines, and adhesion molecules, which hindered the proliferation of MM cells.249 Exosomes from FNDC5-preconditioned BM-MSCs have been shown to have anti-inflammatory properties and to enhance M2 macrophage polarisation through the NF-B signalling pathway and Nrf2/HO-1 Axis.250 Exosomes from BM-MSCs, has been shown to have protective effects against myocardial ischemic injury. It also plays a significant role in the regulation of the process by inhibiting the expression of PTEN, activating the PI3K/AKT signalling pathway, and subsequently preventing the apoptosis of injured cardiomyocytes. Exosomes generated by human trophoblast stem cells (TSC) and miR-200b inhibitor both reduced primary cardiomyocyte death. Similar improvements in cardiac function were shown in mice treated with TSC-EXOs and AAV-miR-200b inhibitor, together with a decrease in inflammation and apoptosis. TSC-EXOs reduced the heart damage caused by doxorubicin by acting as antiapoptotic and anti-inflammatory agents.251 Hypoxia-conditioned and normoxic-conditioned generated exosomes were injected intramyocardially in a study to determine the mechanism of cardiomyocyte survival. The study discovered that the presence of encapsulated lncRNA-UCA1 in exosomes protected cardiomyocytes and elevated levels of the anti-apoptotic protein BCL2.252 The processes attenuating mitochondrial fission and cellular senescence of cardiomyocytes generated by SD/H were attenuated by exosomes obtained from Hemin-pretreated MSCs, which markedly enhanced cardiac function and decreased fibrosis. By controlling the HMGB1/ERK pathway, Hemin-MSC-EXOs prevented SD/H-induced cardiomyocyte senescence in recipient cardiomyocytes.253 The impact of Nrf2-overexpressing BMSC-EXOs on rats with atrial fibrillation (AF) was investigated. According to the study’s findings, injecting Lv-Nrf2 exosomes into AF-affected mice significantly reduced AF durations, decreased cardiomyocyte apoptosis, decreased AF-driven atrial fibrosis, and inhibited inflammatory responses. By activating the Nrf2/HO-1 pathway, exosomes containing overexpressed Nrf2 prevented AF-induced arrhythmias, myocardial fibrosis, apoptosis, and inflammation.254 Bax and caspase expression were shown to be downregulated in exosome-overexpressing cells, whilst Bcl-2 expression was found to be upregulated. After intramyocardial injection of exosomes overexpressing miR-338 in rats, cardiac function was noticeably improved.255 A study using a rat model showed that exosomes containing miR-146a greatly reduced AMI-induced apoptosis, an inflammatory response, and fibrosis by suppressing EGR1 expression, which in turn reversed the activation of TLR4 or the NF-B signal caused by hypoxia or AMI.256 Apoptosis and fibrosis levels were dramatically lowered in mice treated with differentiated cardiomyocytes from induced pluripotent stem cells (iCM-Ex), suggesting a considerable improvement in the heart’s function following myocardial infarction.257 Circulating exosomal lncRNA-UCA1 may be a promising new biomarker for the detection of AMI, as shown by intramyocardial injection of lncRNA-UCA1-knockdown-Hypo-Exo in a rat model. Hypo-Exo lncRNA-UCA1 has a cardioprotective effect via the miR-873-5p/XIAP axis VEGF-targeting miR-16, miR-24, miR-29, miR-146 that binds to and inhibits EGFR mRNA-27, miR-294, and miR-494 are all present in enhanced levels.73,79,252,258 According to in vitro and in vivo research, BMSC-EXOs efficiently reduced intestinal pathological injury, decreased intestinal cell apoptosis, relieved oxidative stress, and controlled the PTEN/Akt/Nrf2 pathway. Additionally, the oxygen and glucose deprivation/reperfusion drastically decreased the exosomes carrying miR-144-3p, and miR-144-3p directly targets PTEN to control its expression.259 BMSC-derived exosomes containing lncRNA were cultured with HL-1 mouse cardiomyocytes that had been subjected to hypoxia/reoxygenation (H/R). By regulating cell proliferation, elevating glutathione (GSH) content, and lowering iron ion concentration, reactive oxygen species level, and ferroptosis marker protein levels, Mir9-3hg inhibits cardiomyocyte ferroptosis in ischemia-reperfusion mice via the Pum2/PRDX6 axis.260 A study was created to look at the cardioprotective effects of MSCs-EXOs in a model of cardiotoxicity caused by DOX/Trz. By altering the NRG-1/HER2, MAPK, PI3K/AKT, PJNK/JNK, and PSTAT/STAT signalling pathways, the intraperitoneal delivery of MSCs-EXOs reduced cardiac damage in both protective and curative conditions.247 Treatment of Exo/NC, Exo/miR-183-5p-Exo/anti-miR-183-5p-Exo increased left ventricular end-diastolic pressure (LVEDP), myocardial infarct size, and apoptosis index (AI) but decreased left ventricular ejection fraction (LVEF), left ventricular fraction shortening (LVFS), and left ventricular systolic pressure (LVSP) by altering the expression of FOXO1 and through the miR-15a/15b/16/NFATc3/OCN axis and osteogenic transdifferentiation.261 BM-MSC-EXOs prevent the calcification of human aortic vascular smooth muscle cells.261 According to the available research, MSC-EXOs can potentially protect against cardiovascular illnesses by reducing oxidative stress, inflammation, and apoptosis (Figure 4).
Role of MSC-EXOs in Angiogenesis
MSC-EXOs are important in the process of angiogenesis. Studies have shown that COVID-19 and other intractable illnesses can be effectively treated using stem cell-based therapies.262,263 Exosomes may be helpful in reducing damage, enhancing neurological recovery, and promoting wound healing.264 The efficacy of iMSC-EXOs to lessen limb ischemia and increase angiogenesis after being transplanted into mice with femoral artery excision. In rat ischemic limbs, intramuscular injection of iMSC-EXOs markedly improved microvessel density and blood perfusion. Additionally, iMSC-EXOs induced the expression of angiogenesis-related molecules and angiogenic parameters, which are thought to be potential therapeutic strategies for the treatment of ischemic disorders.265 MiR-185-enriched MSC-EXOs decreased inflammation, blocked angiogenesis and cell growth, and induced cell death.266 Through the release of anti-apoptotic and pro-angiogenic proteins, extracellular vesicles generated from endometrial mesenchymal stromal cells (endMSCs) demonstrated cardioprotective activity. The results of these investigations showed a marked rise in the overall number of blastomeres in enlarged murine blastocysts. Additionally, EV-endMSCs caused embryos to secrete pro-angiogenic molecules, showing a concentration-dependent rise in VEGF and PDGF-AA. The overall number of cells in the blastocyst rises as a result of pro-angiogenic substances, which also promote endometrial angiogenesis, vascularization, differentiation, and tissue remodelling.267 According to Gong et al, MSCs produced from mouse embryos carry vascular precursor receptors (such as miR-30b) that are transferred to human umbilical vein endothelial cells (hUVECs), which encouraged the development of the hUVECs’ tube-like structure.268 Human induced pluripotent stem cell-derived endothelial cell exosomes (hiPS-EC-Exos) enriched with miR-199b-5p were shown to dramatically promote neovascularization in a mouse model of hind limb ischemia. A further in vitro study demonstrates how miR-199b-5p induces overexpression of the vascular endothelial growth factor receptor 2 (VEGFR-2) by altering the Jagged1/Notch1 signalling pathway. This transcriptional upregulation of VEGFR-2 positively regulates HUVEC migration and proliferation.269 Exosomes are said to have anti-inflammatory and pro-angiogenic effects by targeting the ROCK1/PTEN pathway and delivering miR-132 and miR-146a.270 These results imply that exosomes are thought to be interesting treatment candidates for conditions linked to angiogenesis and inflammation. HuR’s impact on VEGF expression and angiogenesis in human umbilical vein endothelial cells (HUVECs) grown with exosomes produced from ADSCs. Through the stabilisation and overexpression of VEGF, human antigen R (HuR) encourages the angiogenesis of HUVECs co-cultured with exosomes produced from ADSCs.271 Exosomes produced by cancer stem cells were examined to determine their contribution to the growth of tumours.272 In order to achieve this, fibroblasts (FBs) were used as recipient cells and Piwil2 induced cancer stem cells (Piwil2 iCSCs) as exosome producing cells. The exosomes produced by Piwil2 iCSC were found to be uniformly sized (30–100 nm in diameter), oval or spherical, membrane-coated vesicles. The findings showed that MMP2 and MMP9 expression levels were elevated in Piwil2 iCSC EXOs, which boosted angiogenic capabilities. By down-regulating VEGFA, MMP-9, and ANGPT1, human deciduous exfoliated teeth (SHED-EXOs) prevent cell proliferation and migration and cause death in HUVECs. When miR-100-5p and miR-1246 are delivered from SHED-EXOs to endothelial cells, tube formation is reduced due to the down-regulation of VEGFA expression.273 While VEGFR signaling, inhibitors reduce endothelial cell permeability, glioblastoma stem-like cell-derived EVs enriched in VEGF-A showed improved angiogenesis and vascular permeability. In a rat model, human induced pluripotent stem cell-derived exosomes (hiPSC-MSC-EXOs) were administered subcutaneously near wound sites.274 Histological and immunofluorescence tests were performed to determine the effectiveness of the hiPSC-MSC-EXOs. The findings showed that hiPSC-MSC-EXO transplantation to wound sites led to rapid re-epithelialization, decreased scar widths, and the promotion of collagen maturity, as well as promoted numerous angiogenic processes.225 The function and probable mechanism of MALAT1-201 in preeclampsia (PE) model demonstrated that exosomes from MSCs that overexpressed MALAT1-201 were collected, and a number of angiogenic experiments were subsequently carried out. The findings suggested that MSC-EXOs expressing MALAT1-201 increased proliferation and migration while inhibiting trophoblast death. MSC-EXOs work as an anti-angiogenic agent in addition to being involved in angiogenic processes as a whole.
Role MSC-EXOs in Cancer
Exosomes produced by MSCs are crucial for both drug resistance and pro-angiogenesis. MSCs have the power to trigger immunological responses, tissue healing, cell proliferation, and tumour progression control.275 As regulators of the tumour niche, MSCs release exosomes, and their involvement in tumorigenesis and metastasis is controlled by a number of growth factor receptors, including EGFR and PDGFR. Through the AKT (protein kinase B), PKC, and MAP kinase pathways, the activation of these receptors by intracellular kinases causes downstream pro-growth signals to be released, which promotes the growth of tumour cells by activating the AKT/ERK1/2 signalling pathway.276 Through the activation of pathways like p53, AKT, and c-Jun N-terminal kinase (JNK), BM-MSC-EXOs promoted proliferation and migration.277 BM-MSC-EXOs contains miR-221 potentially accelerating their growth and increasing their invasive capacity.278 MSC-EXOs promote the growth and spread of breast cancer by activating Wnt signalling and establishing a favourable microenvironment.279 MSC-EXOs promote VEGF production in tumour cells, promoting the development of the tumour by stimulating the ERK1/2 pathway. Exosomes from AT-MSCs that contained platelet-derived growth factor encouraged angiogenesis.103 MSC-EXOs are involved in angiogenesis and promote it by transferring miR-NA-30b to endothelial cells, among other chemicals like miRNAs, IL-6, IL-8, and TGFB. Through the stimulation of the AKT/eNOS pathway and upregulation of miR-221-3p expression, MSC-EXOs promote angiogenesis.268,280,281 According to Liang et al, increasing expression of miR-144 reduced the amount of S phase-arrested cells, colony formation, and cell proliferation in NSCLC via down-regulating CCNE1 and CCNE2.282 Exosomal miR-144 produced by BM-MSCs also resulted in limited NSCLC cell growth and colony formation. MiR-21-5p transfected MSC-EXOs could greatly increase osteosarcoma cell proliferation and invasion and significant levels of human bone marrow MSCs produced miR-21-5p-exosomes stimulated the PI3K/Akt/mTOR pathway by inhibiting the expression of PIK3R1 in osteosarcoma cells.283 By transporting several microRNAs, the exosomes released by BM-MSC-EXOs encourage the growth of lung cancer and osteosarcoma cells.284,285 Human umbilical cord MSC-EXOs carrying microRNA-375 down regulated ENAH to retard esophageal squamous cell carcinoma progression, and AD-MSC-EXOs promote the differentiation of Th17 and Treg from naive CD4+ T cell to exert anti-tumor ability by carrying miR-10a.286,287
Cancer stem cells, also known as tumor-initiating cells, are the small population of cells in a tumor bulk, which represent a critical subset of the tumor population.288 EVs actively participate in cell-to-cell interactions by shutting cellular components and these EVs are promoted non-CSCs to acquire stem-like properties, leading to the enhanced tumorigenicity.289–291 EVs derived from CSCs carry the stemness markers of parent cells, which possess the ability to reprogram non-CSCs to obtain a stem-like phenotype.292 Exosomes derived from CSCs are wrapped with proteins and related to activation of tumor stemness signaling pathways, which may directly activate the stemness-related signaling pathways on non-CSCs, thereby facilitating their stem-like phenotype.290 Exosomes derived from clear cell renal cell carcinoma (CCRCC) stem cells accelerated the process of EMT and promoted lung metastasis and these exosomes containing miR-19b-3p strongly promoted tumor cell EMT through targeting PTEN signaling pathway.292 Similarly, a microvesicle shed by renal CSCs significantly increases the lung metastasis of renal cancer cells in mice.293 CSCs derived EVs carrying pro-angiogenic molecules, which promoted tumor angiogenesis through cross talk with endothelial cells and other stromal cells in the microenvironment.38,274,294 Exosomes derived from CSCs contains miR-26a significantly enhanced tumor angiogenesis and increased the expression levels of VEGF, MMP-2, and MMP-9.294 Collectively, multiple lines of evidence suggest that MSC-EXOs can modulate the tumour microenvironment and play important roles in tumour development, particularly, cancer resistance to chemotherapy agents, radiotherapy and immunotherapy.
Role of MSC-EXOs in Autoimmune and Inflammatory Diseases
Studies have been focused on EVs, which are utilized as a cell-free therapy and elucidate its potential significance of MSC-EXOs in the treatment of autoimmune diseases. Autoimmune diseases are potentially influencing multiple organs and systems, such as circulatory, respiratory, the motor and digestive system. Compared to men, women are more affected. Glucocorticoids and immunosuppressive medications are the only therapy for autoimmune illness that are currently accessible. However, using steroids frequently results in undesirable side effects.295 Increasing evidence indicates that MSC-EXOs play a crucial role in autoimmune disorders by decreasing immunological responses that are driven by immune cells. Exosomes from MSCs have a paracrine route that can modify innate and adaptive immune cells.296,297 MSC-EVs encourage M1 macrophage polarisation to M2 macrophages, which are associated with lower levels of VEGF-A, IFN-g, IL-12, and TNF-alpha and increased levels of IL-10.298–302 By establishing an immunosuppressive M2 phenotype shown by colonic macrophage polarisation, MSC-EXOs reduces dextran sodium sulphate (DSS)-induced colitis. Mice treated with exosomes produced more IL-10-producing M2 macrophages than those in the control group.302 Mice treated with exosomes containing miR-146a from human umbilical cord-derived MSCs (hUC-MSCs) successfully promote macrophage polarisation into an anti-inflammatory M2 phenotype, increasing survival.303 According to Zhao et al, AD-MSC-EXOs increased the production of IL-10 and Arg-1 in macrophages while also promoting an M2 macrophage polarisation by activating STAT3 transcription.304 Olfactory ectomesenchymal stem cell-derived exosomes (OE-MSC-EXOs) activate the JAK2/STAT3 pathway in myeloid-derived suppressor cells (MDSCs) and enhance their inhibitory function by increasing the levels of arginase, ROS, and NO. Additionally, these exosomes produced IL-6, which promoted the immunosuppressive function of MDSC.NK cells experience an immunosuppressive effect from MSC-EVs, which inhibits their growth, activation, and cytotoxicity.305 Through the surface expression of TGF-β, human foetal liver (FL) MSC-derived exosomes (hFL-MSCEXOs) mediated downstream TGF-β/Smad signal transduction to prevent the proliferation and activation of NK cells.306 Internalization of MSC-EVs through CD19+/CD86+ B cells inhibited B cell proliferation, differentiation, and antibody production, as well as inhibited memory B cell maturation.307 MSC-EVs exert dose-dependent anti-inflammatory effects by inhibiting B cell maturation and inducing Bregs in lymph nodes in a murine model of collagen induced arthritis (CIA) and delayed-type hypersensitivity (DTH). A study suggests that MSC-EVs carrying miR-155-5p inhibited B cell proliferation and reduced the activation capacity through downregulate the PI3K/Akt signaling pathway.308 Tavasolian et al investigated the therapeutic effect of miR-146a/miR-155 transduced MSC-EXOs on the immune response in collagen-induced arthritis (CIA) mice.309 MiR-146a-transduced MSC-EXOs increased expression of forkhead box P3 (Fox- P3), TGF-β and IL-10 and miR-155 further increased the gene expressions of RORγt, IL-17, and IL-6 in these mice.
Multiple Sclerosis (MS)
The central nervous system (CNS) is affected by the chronic autoimmune and inflammatory disease known as multiple sclerosis.310 The most widely utilised animal model of MS is called experimental autoimmune encephalomyelitis (EAE).311 The CNS is the primary pathogenic component in MS, and it is activated and recruited by auto-aggressive CD4+ and CD8+ T cells. Therapies that treat diseases might have undesirable side effects. MSC-EXOs were used to create an alternate manner of treatment.312–314 By delivering the tolerogenic molecules to the autoreactive immune cells, MSC-EXOs establish peripheral tolerance towards autoreactive T cells.315,316 MSC-derived extracellular vesicles have immunomodulatory effects on splenic mononuclear cells (MNCs) of EAE mice, such as reducing proliferation and increasing the quantity of IL-10, and these immunomodulatory effects are controlled by PD-L1, galectin-1, and TGF-β.317 Intravenous administration of MSC-EXOs recovered Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease.172,318 MSC-EXOs polarize microglia cells mainly into the M2 phenotype and consequently improve clinical scores of EAE. MSC-EXOs improved proliferation of oligodendroglia cell line (OLN93) through reduction of both inflammatory responses and demyelinated lesions in the CNS of EAE mice.319,320 Microglia are prevented from acquiring an M1 phenotype by BM-MSC-EXOs-mediated treatment, which also encourages M2 phenotypic polarisation and the release of anti-inflammatory cytokines. Treatment with MSC-EXOs and BM-MSC-EXOs dramatically reduced the neurobehavioral symptoms of EAE rats, as well as inflammation, demyelination, and polarisation of CNS microglia.321 MSC-EXOs can transport mRNA, miRNA, and proteins and control the polarisation of microglia. MSC-EXOs can transport mRNA, miRNA, and proteins and control the polarisation of microglia and significantly encouraged the establishment of Tregs and slowed the progression of EAE.322 MSC-EXOs therefore exhibit a lot of promise for the treatment of MS.
Rheumatoid Arthritis (RA)
RA is a long-lasting, inflammatory, and systemic autoimmune condition characterised by symptoms of joint degeneration and eventual loss of function brought on by both genetic and environmental causes.323,324 Disorders of innate and adaptive immunity, which lead to immune complex-mediated complement activation, are defining characteristics of RA.325,326 Antibodies or soluble decoy receptors are used to combat pro-inflammatory cytokines such GM-CSF, TNF-, and IL-6.325,326 Recent research suggests that MSC-EXOs decrease the immune system’s response to RA.327,328 Injection of co-gene DBA/1 fibroblasts secreting GAL-1 slowed the progression of arthritis by preventing proliferation and lowering the proportion of mature T and B cell subsets.329 MSC-EXOs have been shown to have anti-inflammatory effects on T and B lymphocytes in the collagen-induced arthritis (CIA) mouse model.330 The induction of TGF-β and IL-10 by vesicles derived from MSCs are less effective compared to MSCs alone.331 Chen et al reported that MSC-EXOs carrying miRNA-150-5p, target the matrix metalloproteinase 14 (MMP14) and subsequently, decrease migration and invasion of fibroblast-like synoviocytes (FLS) and downregulated the process of angiogenesis responsible proteins like MMP14 and VEGF in HUVECs.332 The serum and synovial fluid levels of the autoreactive infiltrating chemokines CCL2 and CXCL12 were considerably reduced after intraarticular injection of hUCMSC-derived exosomes.333 Pigs receiving intra-articular injections of BM-MSC-EVs saw a decrease in the number of synovial lymphocytes and a down-regulation of TNF-α production, which served to reduce inflammation.334 Altogether, MSC-EVs exhibited potential beneficial effects for the treatment of RA. Exosome-derived lncRNA affected the migration of activated macrophages and significantly decreased the levels of MMP-2 and MMP-13.335 MiR-17 suppresses regulatory T cells by targeting TGFBR II in RA. Exosome-derived lncRNA altered the movement of activated macrophages and drastically reduced the levels of MMP-2 and MMP-13. miR-155 and miR-146-a are found in exosomes generated from DCs have a significant role in regulating immunological response and inflammatory response in RA.336–339 Exosomal ncRNAs collectively contribute to the onset and progression of RA through controlling immunological and inflammatory cells.
Type 1 Diabetes Mellitus (Insulin-Dependent Diabetes Mellitus)
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease influenced by genetic, immune, and environmental factors. Insulin resistance and low insulin levels are caused by the autoimmune loss of pancreatic cells that produce insulin.340 The cause of type 1 diabetes and its contributing variables are the loss of immunological tolerance in autoreactive B cells, CD4+T cells, and CD8+.341 Ezquer et al demonstrated that anti-diabetic effects of MSC by normalized Th1/Th2 response in diabetic group compared to control and its restore immune response balance in pancreas by modulation of endocrine activity.342 MSC-EXOs improved diabetic rat’s cognitive deficits and restored damaged astrocytes and neurons.Co-administration of MSC-EXOs and islet transplantation increased regulatory T cell activities, T cell populations, and upregulation of IL-4, IL-10, and TGF- cytokines while downregulating IL-17 and IFNγ- cytokines and suppressing PBMC proliferation. By suppressing the growth of Th1 cells, it improves graft survival in a syngeneic mouse model of T1DM and delays the onset of autoimmune diabetes.343,344 Exosomes from BM-MSCs alleviated cognitive impairment in diabetic rats by mending damaged neurons and astrocytes.345 When ADMSC-EXOs are administered, anti-inflammatory factors (such IL-10) are expressed more frequently, the Treg population grows, and the immune system is suppressed, resulting in autoimmune damage.346 According to a study, MSC-EVs reduced islet inflammation, which increased plasma insulin levels and prevented mice from developing type 1 diabetes.347
Uveitis
Uveitis is an autoimmune condition that severely impairs vision.348 Corticosteroids were employed to overcome the vision impairment. However, prolonged steroid use results in glaucoma. Consequently, a fresh approach to treatment is required. For instance, Bai et al showed that MSC-EXOs from the human umbilical cord lessened the severity of EAU by preventing inflammatory leukocyte infiltration into the eyes.349 Exosomes containing CD73 interact with CD39 on activated immune cells to inhibit proliferation through an increase in adenosine synthesis. Using EAU mice, an in vivo investigation showed the exosome-treated EAU mice showed eyes that resembled normal mouse retinas, free of structural damage and inflammatory infiltration. As a result, the retinal photoreceptors and other organs in the untreated mice suffered serious damage.347,349 According to a different study, mice treated with MSC-EXOs demonstrated significantly lower CD3+ T cell infiltration into the retina and lower levels of macrophage migration to the retina than EAU mice treated with PBS.349 MSC-EVs improved EAU by directly inhibiting the development of Th1 and Th17 cells rather than inducing Tregs to inhibit T cell proliferation.347
Systemic Lupus Erythematosus (SLE)
SLE is a systemic, persistent autoimmune illness that is impacted by a number of variables. Multi-organ damage results from SLE’s increased inflammation and complicated autoimmune diseases.350–352 Profound T and B lymphocyte activation, elevated levels of pro-inflammatory cytokines, and immune complex material sedimentation are the causes of SLE.353 Immune complex triggers cause nephritis, which is brought on by an influx of sizable inflammatory cells.354 The administration of hBM-MSCs increased survival rates by lowering autoantibodies and reduced glomerulonephritis by preventing the growth of Tfh in animals.354 Additionally, allogeneic bone marrow mesenchymal stem cell transplantation (MSCT) may be able to treat various organ dysfunctions.355 The MSC-EXOs’ cargoes have a crucial role in the immunological and inflammatory control of SLE.356,357 Proinflammatory cytokines including TNF-α, IL-1, IL-6, and other inflammatory mediators can be induced by exosomes generated from SLE patients.357
Inflammatory Bowel Disease (IBD)
IBD is a chronic, nonspecific, relapsing inflammatory gastrointestinal disease.MSC- EXOs components have potential targets for the diagnosis and treatment of IBD. For instance, an in vivo study using mouse model of IBD revealed that hUC-MSC-EXOs treatment decreased the infiltration of macrophages in colon tissue and inhibited the expression of IL-7.358 MSC-EXOs carrying miR-146a, effectively down-regulated TNF receptor-associated factor 6 (TRAF6) and IL-1 receptor associated kinase 1 (IRAK1) expression, inhibited pro-inflammatory cytokine and enhanced the expression of IL-10.359 hUC-MSC-EXOs protects intestinal barrier by down regulation of pro-inflammatory cytokines in colon tissue, and up-regulated the levels of anti-inflammatory cytokines.360 Mice study revealed that OEMSC-EXOs significantly enhanced the colitis by modulating the immune response of Th-cells.361 AD-MSC-EXOs significantly recovered the percentage of Treg in the spleen and improved inflammation in DSS induced acute colitis.362 hBM-MSC-EXOs protects intestinal barrier integrity by down regulation of inflammatory responses, polarized M2b macrophages.363 Dextran sulphate sodium induced mouse model study suggests that inflammatory responses are significantly controlled by inducing the production of M2 macrophages.302,364 Mice treated with MSC-EVs promote repair and regeneration of damaged epithelial cells by production of higher levels of immunosuppressive factors (IL-10 and TGF-β) and low level of IL-1β, NO, and IL-18 by depressing NF-κB and iN-OS-driven signaling in 2,4,6-trinitrobenzene sulfonic acid induced colitis.359,364,365 According to the results of all these findings, MSC-EXOs can act as a significant regulator and novel therapeutic agent by reducing inflammatory responses. They can also successfully block immune responses and promote the survival and regeneration of wounded cells. Although MSC-EXOs play a key role in IBD, further research is still required to pinpoint the molecular basis of MSC-EXOs participation in autoimmune disease.
Role of MSC-EXOs on COVID-19
Patients who had immune-mediated inflammatory diseases were impacted by COVID-19 and were thought to be at a high risk of contracting SARS-CoV-2 and developing severe COVID-19. An overwhelming host immunological response, or “cytokine storm”, caused by COVID-19 is characterised by an overproduction of growth factors, chemokines, and pro-inflammatory cytokines like TNF and IL-6.366,367 Targeting pro-inflammatory cytokines are the best tools and are therapeutic targets in the treatment of patients affected by immune-mediated inflammatory diseases and also involved in re-generation.368,369 Hence, to find out effective therapies for the treatment of patients with severe stages of the disease like COVID-19 is inevitable MSC-EXOs can promote the survival of alveolar macrophages and change their phenotype from pro-inflammatory (M1) into the anti-inflammatory (M2) polarization, which has been confirmed by at least two studies.370,371 MSC-EXOs could improve some complications of COVID-19 such as cytokine storm, acute respiratory distress syndrome (ARDS) and acute lung injury (ALI). The stem cell secretome (SCS) showed multifarious activities against ant-fibrotic, anti-inflammatory, immunomodulatory, and angiogenic biological activities.372–374 MSC exosomes have the capacity to control inflammation through cytokines and immunological response through immune cells.375,376 According to a study on mice, MSC-EXOs can treat liver damage by reducing the formation of collagen (type I/III) and hepatocytes’ shift from epithelial to mesenchymal state. MSC-EXOs significantly modulate immunological responses. MSC-EXOs also include microRNAs (miRNAs), which control key biological processes such cell division, death, and host immune responses.377 MSC-EXOs are regarded as a suitable vector for the administration of tailored antiviral medications for the treatment of COVID-19 because of their distinct properties.378 Exosomes produced by MSCs are paracrine effectors that are comparable to the parental cell and have the capacity to sustain healing capabilities, making MSC-EXOs an appealing replacement for MSCs in the treatment of a variety of disorders.379 Bone marrow derived exosomes, a complex mixture of signalling nanovesicles produced by BM-MSC-EXOs, have the ability to reverse the inhibition of host antiviral defences and downregulate the cytokine storm that characterise COVID-19.380 A variety of chemokines, growth factors, mRNA, and miRNA found in BM-MSC-EXOs have anti-inflammatory, regenerative, and immunomodulatory properties. The paracrine and endocrine mediators known as exosomes provide BM-MSCs their ability to repair, making them a good candidate for treating COVID-19.381 Intravenous administration of bone marrow-derived exosomes reduced alveolar inflammation, enhanced edema clearance, restoration of leaky epithelial membranes, and other sequelae of cytokine storm.382,383 BM-MSC-EXOs were used in the first known clinical research to treat severe COVID-19.384 In patients hospitalised with severe COVID-19, it was found that a single intravenous dosage of bone marrow-derived exosomes corrected hypoxia, immunological reconstitution, and downregulation of cytokine storm without causing any negative consequences. In addition to SARS-CoV-2 ARDS and COVID-19, exosomes from BM-MSCs may be used in a variety of inflammatory disease states, including traditional ARDS, chronic obstructive pulmonary disease, sepsis, autoimmune illness, and cancer.385–388 In numerous preclinical models, MSC-EXOs demonstrated immunomodulatory and antiinfection properties that had therapeutic potential. Patients with moderate to severe ARDS brought on by COVID-19 may benefit from MSC-EXOs because they can improve oxygenation and reduce cytokine storm.389 Due to their special abilities to modulate the immune system and reduce inflammation; MSC-EXOs are an intriguing treatment approach for the ongoing COVID-19 epidemic. Most likely, MSC-EXOs can stimulate endogenous repair of wounded lungs as well as prevent the cytokine storm brought on by excessive immune responses. Immune deficiency, inflammation, ARDS, and other lung illnesses may all be treated using MSC-EXOs.390,391 MSC-EXOs can therefore be utilised to treat pneumonia caused by COVID-19. To combat COVID-19, MSC-EXOs can be used as a nanoplatform for therapies and drug delivery.
The Potential of MSC-EXOs in the Treatment of COVID-19
The primary pathogenesis of COVID-19 is overactivation of the immune system. MSCs are known to induce anti-inflammatory macrophages, regulatory T cells and dendritic cells. At present, the treatment for COVID-19 is mostly symptomatic and supportive, though anti-inflammatory and antiviral treatment has been employed.392 MSC-exosomes are able to transfer cargoes to target cells and tissues, resulting in changes to gene expression and in the behavior of target cells. MSC-derived exosomes as cell-free therapeutics due to the unique properties of MSC-EXOs including high stability, low immunogenicity, easy storage, and ability to cross the blood-brain barrier compared to their counter parts.393 MSC-secreted exosomes are shown to both regulate immunity through interacting with immune cells and inhibit the inflammatory response through cytokines.375,394 Animal study revealed that MSC-derived exosomes potentially change the phenotype of alveolar macrophages (M1) toward an anti-inflammatory state (M2) with enhanced phagocytic activities. Macrophages treated with MSC-derived exosomes were able to reduce the alveolar inflammation in mice bearing the endotoxin-induced lung injuries.395 MSC-EXOs acted as a TLR signaling mediator and enhanced the transfer of regulatory miR-451 to the macrophages, thereby ameliorating the TNF-α secretion and suppressing the excess inflammatory responses to the lung injury.396 Exosomes derived from human amnion epithelial cells could suppress the activated neutrophils and T cells proliferation and decrease the percentage of interstitial macrophage in the lungs.397 MSC-secreted exosomes may be employed in the treatment of immune deficiency, inflammation, ARDS, and other lung diseases.390,391 Therefore, MSC-secreted exosomes play significant role for the treatment of COVID-19. A study observed that miR-21-5p delivery by the MSC-exosomes protected lung epithelial cells against oxidative stress-induced cell death.398 In vivo study suggested that administration of MSC-exosomes increased the proliferation of lung epithelial cells. These exosomes contain growth factor FGF2, which has the regenerative capacity and is important in lung development.399 Varderidou-Minasian et al reported that the quick entry into the blood, circulation, retention and clearance of MSC-EXOs is significantly potential compared to MSCs.400 For example, the detection of MSCs in the lung parenchymal cells found that after 24 hrs of treatment, conversely MSC-Exos found that within 1 hrtreatment and remained there for up to 7 days compared to their counterparts. Intravenous injection of MSC-exosomes improved the conditions of COVID-19 patients via clinical symptoms, oxygenation, serum markers of acute inflammation, neutrophil and lymphocyte counts without any side effects.384 The immunomodulatory effects of MSCexosomes inhibit IL-1, IL-6, NK cells, effector and cytotoxic T cells through antiinflammatory cargo, such as IDO, HLA-G, PD-L1, galectin-1, IL-10, TGF-β1 and HGF.401,402 MSC-exosomes contains miRNAs that play a role in COVID-19, including miRNA-145, miRNA-126, miRNA199a, miRNA-221, miRNA-27 and Let-7f.403,404 APCs produce less Ag/MHC molecules on their surface as a result of miRNAs delivered by MSC exosomes, which results in less effector T cell activation. The ability of macrophages, NK cells, T cells, and B cells to prevent infection is also mediated by miRNAs transported by MSC-exosomes.405 Clinical study confirmed that exosomes derived from the amniotic fluid are highly effective in treating the respiratory failure of COVID-19 patients.406 In silico analysis revealed that MSC-EVs miRNA potential to modulate COVID-19 induced increased cytokines, cell death and coagulation disturbance caused by COVID-19.407 EVs bearing hACE2 explicitly block the SARS-CoV-2 entry and thereby inhibit viral replication. MSC-EXOs bearing miR-7-5p, miR-24-3p, miR-145-5p and miR223-3p directly inhibit S protein expression and SARS-CoV-2 replication.408 EVs from amniotic fluid-derived improved respiratory failure in a COVID-19 long hauler.409 Wharton’s jelly MSC-derived EVs showed significantly reduced the expression of nuclear NF-jB p65 and suppression of the cytokine storm in COVID-19 patients suffering from diabetes mellitus or renal disease.410 All these studies concluded that MSC-EXOs potentially involved for the treatment COVID-19.
Challenges and Strategies of Use of MSC-EXOs
The important challenges for the application exosomes derived from stem cells is to identify the molecules involved in paracrine action of stem cells, which facilitate to open new therapeutics avenues using exosomes including the production, processing and manufacturing aspects, particularly isolation, purification and characterization.411 Although, variety of isolation techniques/methods are available such as ultracentrifugation, size, immunoaffinity captured, precipitation and microfluidics, still to prepare clinical grade exosomes are critical factor. Another important factor is, there is no uniform method to separate exosomes, and also each and every method has its own advantages and disadvantages.412 Therefore, MSC-EXOs separation methods must be uniform and standardized. For instance, the contents of cargoes are different among various separating methods.412 For example, ultracentrifugation is well known and common method, which produces significant purity but the yield is low.412 Therefore, it is required to explore a more efficient method for purification and yield and near future developments. The most important obstacle is the yield of exosomes, which can be improved using combination of currently available methods such as 3D microcarrier-based suspension culture promoted by certain bio-active materials. To increase the yield, engineering of full artificial exosomes is necessary which can be relatively controllable composition and clear therapeutic pathway.
The next challenge is source of EVs/exosomes. Physiological conditions and behavior of cells are important factor to provide consistent exosome quality and yield. Next, the important challenge is isolation and storage of exosomes, which are critical, adequate and appropriate technologies to produce quality exosomes for the safety of both donor and recipients. Although exosomes have great potential to treat various types of diseases, still several challenges are remained elusive. The important task is, the source and contents of the exosomes derived from various culture conditions must be determined using standardized protocol and also the conditions of storage, preservation, half-life of freshly isolated and cryopreserved exosomes and also transportation of exosomes needs to be solved.103 Further challenges are warranted to understand and precise determination of exact components involved to treat neurological, particularly epileptic encephalopathy, Parkinson’s, cancer, angiogenesis and autoimmune and inflammatory diseases. Further, several challenges are required to probe the therapeutic effect of MSC-EXOs to maintain the long-term effect, and know the facts of negative effect, which are very important criteria for the correct use of exosomes to treat various diseases. Furthermore, although MSC-EXOs are superior to MSCs, to increase the efficacy, and purification and the scale-up technology needs to be improved before it can be used in clinical practice.
Conclusion and Future Perspectives
The exosomes released from stem cells have been utilized for numerous therapeutic purposes and to develop novel regenerative approaches. The EVs from stem cells exert protective effects in the treatment of various diseases by regulating various pathological conditions. MSC-EXOs offer a more possibility of cell-free therapy than parental MSCs due to the regenerative and anti-inflammatory effect. The unique properties MSC-EXOs such as immunomodulatory effects allow treating different inflammatory and autoimmune diseases. Finding from several studies indicated that exosomes secreted by MSCs play significant role in cancer progression. Conversely, MSC-EXOs can also suppress tumors through regulating immune responses and intercellular signaling. MSC-EXOs used as drug delivery systems are considered to deliver targeted tumor therapy. MSC-EXOs shows potential effects on suppression of several autoreactive cells in autoimmune diseases. Due to their ability to modulate DAn survival, MSC-secretome considered as a promising therapeutic tool for several neuro-degenerative diseases.
The future perspectives need to be concentrated on various aspects including stability, long-term preservation, purification processes and the precise molecular contents are essential; it would help produce ready-to-use batches of exosomes, which could be easily transported. Culture conditions such as cell density, passage number, hypoxia, extracellular matrix, and mechanical stress can influence the production. Hence, the derived exosomes must be characterized by various analytical techniques including size distribution, TEM, SEM, Nanotrack, proteomics, and miRNA profiling, which are essential parameters for therapeutic purposes for various diseases. However, the exact mechanisms of the effects of MSC-EXOs on cancer need to be explored and tested and the utility of exosomes as a delivery vehicle. Hence, additional studies of MSC-EXOs are required to determine their roles in the pathogenesis of cancer and to provide a new tool for cancer diagnosis and therapeutics. More studies are required to illuminate unclear aspects of cell-free therapy using MSC-EXOs. Thus, understanding the complexity of MSC-EXOs, and how its miRNA content interacts with cells involved in the molecular, cellular and functional mechanisms is of great importance in all kinds of diseases. More studies are necessary to explore the molecular mechanisms of those exosomes harboring lncRNAs in the pathogenesis, potential pathways, and development of multi-target-based strategies for RA. Since exosomes are derived from various types of stem cells and may provide different response in target cells, thus detailed investigations are necessary to identify the optimal cell types, functional status, and molecular contents and engineering of exosomes are essential to provide platforms for durable retention and prolonged release of cargos in the injured myocardium. Standards and guidelines should be established for isolation, purification, and identification of surface biomarkers from the samples of diseases patients for the use of the clinical setting. Another important issue needs to resolve is to reduce the lot-to-lot variation regarding primary naive MSCs. Collectively, findings from various studies imply that MSC-derived exosomes possess promising therapeutic capacity and these can be exploited as exosome-based therapy for the treatment of a variety of diseases as drug delivery vehicle and biomarkers (Figure 5). However, many efforts are required towards determining standards methods to enhance effective, efficacy and safety therapy, which will speed up clinical trials and patients speed recovery by implementation of MSC-EXOs as therapeutic agents. Finally, determination of optimal dose and safety measurements are inevitable parameters for the development of an effective exosome-based nanoplatform to expand the therapeutic potential.
Acknowledgments
We thank Dr. Ramakrishnan Muthuswamy, Associate Professor, Nanjing Forestry University, China for helping image generation using BioRender software.
Funding
This study was supported by Development of a new precise cytosine base editor (JBGS (2021) 025), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the 111 Project D18007, and Yangzhou city and Yangzhou University corporation (YZ2021161/YZ2022187).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi:10.1084/jem.183.3.1161
2. Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871(2):455–468. doi:10.1016/j.bbcan.2019.04.004
3. De la Torre Gomez C, Goreham RV, Bech Serra JJ, Nann T, Kussmann M. “Exosomics”-a review of biophysics, biology and biochemistry of exosomes with a focus on human breast milk. Front Genet. 2018;9:92. doi:10.3389/fgene.2018.00092
4. Grigor’eva AE, Dyrkheeva NS, Bryzgunova OE, Tamkovich SN, Chelobanov BP, Ryabchikova EI. Contamination of exosome preparations, isolated from biological fluids. Biomed Khim. 2017;63(1):91–96. doi:10.18097/PBMC2017630191
5. Torregrosa Paredes P, Esser J, Admyre C, et al. Bronchoalveolar lavage fluid exosomes contribute to cytokine and leukotriene production in allergic asthma. Allergy. 2012;67(7):911–919. doi:10.1111/j.1398-9995.2012.02835
6. Gatti JL, Métayer S, Belghazi M, Dacheux F, Dacheux JL. Identification, proteomic profiling, and origin of ram epididymal fluid exosome-like vesicles. Biol Reprod. 2005;72(6):1452–1465. doi:10.1095/biolreprod.104.036426
7. Asea A, Jean-Pierre C, Kaur P, et al. Heat shock protein-containing exosomes in mid-trimester amniotic fluids. J Reprod Immunol. 2008;79(1):12–17. doi:10.1016/j.jri.2008.06.001
8. Masyuk AI, Huang BQ, Ward CJ, et al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol. 2010;299(4):G990–G999. doi:10.1152/ajpgi.00093.2010
9. Battaglia R, Palini S, Vento ME, et al. Identification of extracellular vesicles and characterization of miRNA expression profiles in human blastocoel fluid. Sci Rep. 2019;9(1):84. doi:10.1038/s41598-018-36452-7
10. Val S, Jeong S, Poley M, et al. Purification and characterization of microRNAs within middle ear fluid exosomes: implication in otitis media pathophysiology. Pediatr Res. 2017;81(6):911–918. doi:10.1038/pr.2017.25
11. Zhu LP, Tian T, Wang JY, et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8(22):6163–6177. doi:10.7150/thno.28021
12. Conde-Vancells J, Rodriguez-Suarez E, Embade N, et al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res. 2008;7(12):5157–5166. doi:10.1021/pr8004887
13. Subra C, Grand D, Laulagnier K, et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J Lipid Res. 2010;51(8):2105–2120. doi:10.1194/jlr.M003657
14. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–9420. doi:10.1016/S0021-9258(18)48095-7
15. Martins VR, Dias MS, Hainaut P. Tumor-cell-derived microvesicles as carriers of molecular information in cancer. Curr Opin Oncol. 2013;25(1):66–75. doi:10.1097/CCO.0b013e32835b7c81
16. Katsman D, Stackpole EJ, Domin DR, Farber DB. Embryonic stem cell-derived microvesicles induce gene expression changes in Müller cells of the retina. PLoS One. 2012;7(11):e50417. doi:10.1371/journal.pone.0050417
17. Vestad B, Llorente A, Neurauter A, et al. Size and concentration analyses of extracellular vesicles by nanoparticle tracking analysis: a variation study. J Extracell Vesicles. 2017;6(1):1344087. doi:10.1080/20013078.2017.1344087
18. Jung JH, Fu X, Yang PC. Exosomes generated from iPSC-derivatives: new direction for stem cell therapy in human heart diseases. Circ Res. 2017;120(2):407–417. doi:10.1161/CIRCRESAHA.116.309307
19. Kishore R, Khan M. More than tiny sacks: stem cell exosomes as cell-free modality for cardiac repair. Circ Res. 2016;118(2):330–343. doi:10.1161/CIRCRESAHA.115.307654
20. Wiklander OPB, Brennan MÁ, Lötvall J, Breakefield XO, El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11(492):eaav8521. doi:10.1126/scitranslmed.aav8521
21. Zhu L, Kalimuthu S, Gangadaran P, et al. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics. 2017;7(10):2732–27451. doi:10.7150/thno.18752
22. Han C, Sun X, Liu L, et al. Exosomes and their therapeutic potentials of stem cells. Stem Cells Int. 2016;2016:7653489. doi:10.1155/2016/7653489
23. Fuster-Matanzo A, Gessler F, Leonardi T, Iraci N, Pluchino S. Acellular approaches for regenerative medicine: on the verge of clinical trials with extracellular membrane vesicles? Stem Cell Res Ther. 2015;6:227. doi:10.1186/s13287-015-0232-9
24. Riazifar M, Pone EJ, Lötvall J, Zhao W. Stem cell extracellular vesicles: extended messages of regeneration. Annu Rev Pharmacol Toxicol. 2017;57:125–154. doi:10.1146/annurev-pharmtox-061616-030146
25. Lamichhane TN, Sokic S, Schardt JS, Raiker RS, Lin JW, Jay SM. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2015;21(1):45–54. doi:10.1089/ten.TEB.2014.0300
26. Perets N, Betzer O, Shapira R, et al. Golden exosomes selectively target brain pathologies in neurodegenerative and neurodevelopmental disorders. Nano Lett. 2019;19(6):3422–3431. doi:10.1021/acs.nanolett.8b04148
27. György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439–464. doi:10.1146/annurev-pharmtox-010814-124630
28. Betzer O, Perets N, Angel A, et al. In vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano. 2017;11(11):10883–10893. doi:10.1021/acsnano.7b04495
29. Liu JT, Lamprecht MP, Duncan SA. Using human induced pluripotent stem cell-derived hepatocyte-like cells for drug discovery. J Vis Exp. 2018;135:57194. doi:10.3791/57194
30. Liu C, Xu X, Li B, et al. Single-exosome-counting immunoassays for cancer diagnostics. Nano Lett. 2018;18(7):4226–4232. doi:10.1021/acs.nanolett.8b01184
31. Borrelli DA, Yankson K, Shukla N, Vilanilam G, Ticer T, Wolfram J. Extracellular vesicle therapeutics for liver disease. J Control Release. 2018;273:86–98. doi:10.1016/j.jconrel.2018.01.022
32. Lv LL, Feng Y, Tang TT, Liu BC. New insight into the role of extracellular vesicles in kidney disease. J Cell Mol Med. 2019;23(2):731–739. doi:10.1111/jcmm.14101
33. Rufino-Ramos D, Albuquerque PR, Carmona V, Perfeito R, Nobre RJ, Pereira de Almeida L. Extracellular vesicles: novel promising delivery systems for therapy of brain diseases. J Control Release. 2017;262:247–258. doi:10.1016/j.jconrel.2017.07.001
34. Bei Y, Xu T, Lv D, et al. Exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury. Basic Res Cardiol. 2017;112(4):38. doi:10.1007/s00395-017-0628-z
35. Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1(1):98–110.
36. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30(6):836–848. doi:10.1016/j.ccell.2016.10.009
37. Huang XY, Huang ZL, Huang J, et al. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J Exp Clin Cancer Res. 2020;39(1):20. doi:10.1186/s13046-020-1529-9
38. Sun Z, Wang L, Dong L, Wang X. Emerging role of exosome signalling in maintaining cancer stem cell dynamic equilibrium. J Cell Mol Med. 2018;22(8):3719–3728. doi:10.1111/jcmm.13676
39. Seo N, Akiyoshi K, Shiku H. Exosome-mediated regulation of tumor immunology. Cancer Sci. 2018;109(10):2998–3004. doi:10.1111/cas.13735
40. Al-Sowayan BS, Al-Shareeda AT, Alrfaei BM. Cancer stem cell-exosomes, unexposed player in tumorigenicity. Front Pharmacol. 2020;11:384. doi:10.3389/fphar.2020.00384
41. Shen Z, Huang W, Liu J, Tian J, Wang S, Rui K. Effects of mesenchymal stem cell-derived exosomes on autoimmune diseases. Front Immunol. 2021;12:749192. doi:10.3389/fimmu.2021.749192
42. Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392–402. doi:10.1016/j.stem.2013.09.006
43. Bazzoni R, Takam Kamga P, Tanasi I, Krampera M. Extracellular vesicle-dependent communication between mesenchymal stromal cells and immune effector cells. Front Cell Dev Biol. 2020;8:596079. doi:10.3389/fcell.2020.596079
44. Chiasserini D, van Weering JR, Piersma SR, et al. Proteomic analysis of cerebrospinal fluid extracellular vesicles: a comprehensive dataset. J Proteomics. 2014;106:191–204. doi:10.1016/j.jprot.2014.04.028
45. García-Romero N, Carrión-Navarro J, Esteban-Rubio S, et al. DNA sequences within glioma-derived extracellular vesicles can cross the intact blood-brain barrier and be detected in peripheral blood of patients. Oncotarget. 2017;8(1):1416–1428. doi:10.18632/oncotarget.13635
46. Graham EM, Burd I, Everett AD, Northington FJ. Blood biomarkers for evaluation of perinatal encephalopathy. Front Pharmacol. 2016;7:196. doi:10.3389/fphar.2016.00196
47. Huang S, Ge X, Yu J, et al. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 2018;32(1):512–528. doi:10.1096/fj.201700673R
48. Xian P, Hei Y, Wang R, et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019;9(20):5956–5975. doi:10.7150/thno.33872
49. Van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64(3):676–705. doi:10.1124/pr.112.005983
50. Baek G, Choi H, Kim Y, Lee HC, Choi C. Mesenchymal stem cell-derived extracellular vesicles as therapeutics and as a drug delivery platform. Stem Cells Transl Med. 2019;8(9):880–886. doi:10.1002/sctm.18-0226
51. Massa M, Croce S, Campanelli R, et al. Clinical applications of mesenchymal stem/stromal cell derived extracellular vesicles: therapeutic potential of an acellular product. Diagnostics. 2020;10(12):999. doi:10.3390/diagnostics10120999
52. Moghadasi S, Elveny M, Rahman HS, et al. A paradigm shift in cell-free approach: the emerging role of MSCs-derived exosomes in regenerative medicine. J Transl Med. 2021;19(1):302. doi:10.1186/s12967-021-02980-6
53. Bamba C, Singh SP, Choudhury S. Can mesenchymal stem cell therapy be the interim management of COVID‐19? Drug Discov Ther. 2020;14(3):
54. Hu NW, Corbett GT, Moore S, et al. Extracellular forms of Aβ and Tau from iPSC models of alzheimer’s disease disrupt synaptic plasticity. Cell Rep. 2018;23(7):1932–1938. doi:10.1016/j.celrep.2018.04.040
55. Shahsavari A, Weeratunga P, Ovchinnikov DA, Whitworth DJ. Pluripotency and immunomodulatory signatures of canine induced pluripotent stem cell-derived mesenchymal stromal cells are similar to harvested mesenchymal stromal cells. Sci Rep. 2021;11(1):3486. doi:10.1038/s41598-021-82856-3
56. Barile L, Moccetti T, Marbán E, Vassalli G. Roles of exosomes in cardioprotection. Eur Heart J. 2017;38(18):1372–1379. doi:10.1093/eurheartj/ehw304
57. Taheri B, Soleimani M, Fekri Aval S, Esmaeili E, Bazi Z, Zarghami N. Induced pluripotent stem cell-derived extracellular vesicles: a novel approach for cell-free regenerative medicine. J Cell Physiol. 2019;234(6):8455–8464. doi:10.1002/jcp.27775
58. Kishore R, Garikipati VNS, Gumpert A. Tiny shuttles for information transfer: exosomes in cardiac health and disease. J Cardiovasc Transl Res. 2016;9(3):169–175. doi:10.1007/s12265-016-9682-4
59. Gao X, Ran N, Dong X, et al. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci Transl Med. 2018;10(444):eaat0195. doi:10.1126/scitranslmed.aat0195
60. Lin Y, Anderson JD, Rahnama LMA, Gu SV, Knowlton AA. Exosomes in disease and regeneration: biological functions, diagnostics, and beneficial effects. Am J Physiol Heart Circ Physiol. 2020;319(6):H1162–H1180. doi:10.1152/ajpheart.00075.2020
61. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi:10.1126/science.aau6977
62. Shi Y, Shi H, Nomi A, Lei-Lei Z, Zhang B, Qian H. Mesenchymal stem cell-derived extracellular vesicles: a new impetus of promoting angiogenesis in tissue regeneration. Cytotherapy. 2019;21(5):497–508. doi:10.1016/j.jcyt.2018.11.012
63. Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464(7290):864–869. doi:10.1038/nature08849
64. Jadli AS, Ballasy N, Edalat P, Patel VB. Inside(sight) of tiny communicator: exosome biogenesis, secretion, and uptake. Mol Cell Biochem. 2020;467(1–2):77–94. doi:10.1007/s11010-020-03703-z
65. Hsu C, Morohashi Y, Yoshimura S, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189(2):223–232. doi:10.1083/jcb.200911018
66. Maheshwari S, Singh AK, Arya RK, Pandey D, Singh A, Datta D. Exosomes: emerging players of intercellular communication in tumor microenvironment. Discoveries. 2014;2(3):e26 doi:10.15190/d.2014.18
67. Turturici G, Tinnirello R, Sconzo G, Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am J Physiol Cell Physiol. 2014;306(7):C621–C633. doi:10.1152/ajpcell.00228.2013
68. Segura E, Guérin C, Hogg N, Amigorena S, Théry C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J Immunol. 2007;179(3):1489–1496. doi:10.4049/jimmunol.179.3.1489
69. O’Loughlin AJ, Woffindale CA, Wood MJ. Exosomes and the emerging field of exosome-based gene therapy. Curr Gene Ther. 2012;12(4):262–274. doi:10.2174/156652312802083594
70. Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:10. doi:10.3402/jev.v3.24641
71. Rana S, Yue S, Stadel D, Zöller M. Toward tailored exosomes: the exosomaltetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. 2012;44(9):1574–1584. doi:10.1016/j.biocel.2012.06.018
72. Parolini I, Federici C, Raggi C, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284(49):34211–34222. doi:10.1074/jbc.M109.041152
73. Nakamura Y, Miyaki S, Ishitobi H, et al. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589(11):1257–1265. doi:10.1016/j.febslet.2015.03.031
74. Koga Y, Yasunaga M, Moriya Y, et al. Exosome can prevent RNase from degrading microRNA in feces. J Gastrointest Oncol. 2011;2(4):215–222. doi:10.3978/j.issn.2078-6891.2011.015
75. Andreu Z, Yáñez-Mó M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. doi:10.3389/fimmu.2014.00442
76. Jeske R, Bejoy J, Marzano M, Li Y. Human pluripotent stem cell-derived extracellular vesicles: characteristics and applications. Tissue Eng Part B Rev. 2020;26(2):129–144. doi:10.1089/ten.TEB.2019.0252
77. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi:10.1146/annurev-cellbio-101512-122326
78. Ismail N, Wang Y, Dakhlallah D, et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013;121(6):984–995. doi:10.1182/blood-2011-08-374793
79. Lee JK, Park SR, Jung BK, et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One. 2013;8(12):e84256. doi:10.1371/journal.pone.0084256
80. Nakamura Y, Kita S, Tanaka Y, et al. Adiponectin stimulates exosome release to enhance mesenchymal stem-cell-driven therapy of heart failure in mice. Mol Ther. 2020;28(10):2203–2219. doi:10.1016/j.ymthe.2020.06.026
81. Zhang Z, Xu R, Yang Y, et al. Micro/nano-textured hierarchical titanium topography promotes exosome biogenesis and secretion to improve osseointegration. J Nanobiotechnology. 2021;19(1):78. doi:10.1186/s12951-021-00826-3
82. Marzano M, Bou-Dargham MJ, Cone AS, et al. Biogenesis of extracellular vesicles produced from human-stem-cell-derived cortical spheroids exposed to iron oxides. ACS Biomater Sci Eng. 2021;7(3):1111–1122. doi:10.1021/acsbiomaterials.0c01286
83. Peng X, Li X, Yang S, et al. LINC00511 drives invasive behavior in hepatocellular carcinoma by regulating exosome secretion and invadopodia formation. J Exp Clin Cancer Res. 2021;40(1):183. doi:10.1186/s13046-021-01990-y
84. Ramos TL, Snchez-Abarca LI, Muntin S, et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun Sign. 2016;14:1–14. doi:10.1186/s12964-015-0124-8
85. Haraszti RA, Didiot MC, Sapp E, et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles. 2016;5:32570. doi:10.3402/jev.v5.32570
86. Charoenviriyakul C, Takahashi Y, Morishita M, Matsumoto A, Nishikawa M, Takakura Y. Cell type-specific and common characteristics of exosomes derived from mouse cell lines: yield, physicochemical properties, and pharmacokinetics. Eur J Pharm Sci. 2017;96:316–322. doi:10.1016/j.ejps.2016.10.009
87. Álvarez-viejo M. Mesenchymal stem cells from different sources and their derived exosomes: a pre-clinical perspective. World J Stem Cells. 2020;12(2):100–109. doi:10.4252/wjsc.v12.i2.100
88. Gurunathan S, Kang MH, Kim JH. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int J Nanomedicine. 2021;16:1281–1312. doi:10.2147/IJN.S291956
89. Ti D, Hao H, Tong C, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308. doi:10.1186/s12967-015-0642-6
90. Wang J, Bonacquisti EE, Brown AD, Nguyen J. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells. 2020;9(3):660. doi:10.3390/cells9030660
91. Kita S, Shimomura I. Stimulation of exosome biogenesis by adiponectin, a circulating factor secreted from adipocytes. J Biochem. 2021;169(2):173–179. doi:10.1093/jb/mvaa105
92. Ruan XF, Ju CW, Shen Y, et al. SuxiaoJiuxin pill promotes exosome secretion from mouse cardiac mesenchymal stem cells in vitro. Acta Pharmacol Sin. 2018;39(4):569–578. doi:10.1038/aps.2018.19
93. Levéen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8(16):1875–1887. doi:10.1101/gad.8.16.1875
94. Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8(16):1888–1896. doi:10.1101/gad.8.16.1888
95. Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126(14):3047–3055. doi:10.1242/dev.126.14.3047
96. Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol. 1994;125(4):917–928. doi:10.1083/jcb.125.4.917
97. Dhar K, Dhar G, Majumder M, et al. Tumor cell-derived PDGF-B potentiates mouse mesenchymal stem cells-pericytes transition and recruitment through an interaction with NRP-1. Mol Cancer. 2010;9:209. doi:10.1186/1476-4598-9-209
98. Ball SG, Shuttleworth CA, Kielty CM. Platelet-derived growth factor receptors regulate mesenchymal stem cell fate: implications for neovascularization. Expert Opin Biol Ther. 2010;10(1):57–71. doi:10.1517/14712590903379510
99. Wang M, Feng R, Zhang JM, et al. Dysregulated megakaryocyte distribution associated with nestin+ mesenchymal stem cells in immune thrombocytopenia. Blood Adv. 2019;3(9):1416–1428. doi:10.1182/bloodadvances.2018026690
100. Chen Y, Pu Q, Ma Y, et al. Aging reprograms the hematopoietic-vascular niche to impede regeneration and promote fibrosis. Cell Metab. 2021;33(2):395–410.e4. doi:10.1016/j.cmet.2020.11.019
101. Bagheri HS, Mousavi M, Rezabakhsh A, et al. Low-level laser irradiation at a high power intensity increased human endothelial cell exosome secretion via Wnt signaling. Lasers Med Sci. 2018;33(5):1131–1145. doi:10.1007/s10103-018-2495-8
102. Liu D, Gu G, Gan L, et al. Identification of a CTRP9 C-Terminal polypeptide capable of enhancing bone-derived mesenchymal stem cell cardioprotection through promoting angiogenic exosome production. Redox Biol. 2021;41:101929. doi:10.1016/j.redox.2021.101929
103. Lopatina T, Bruno S, Tetta C, Kalinina N, Porta M, Camussi G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal. 2014;12:26. doi:10.1186/1478-811X-12-26
104. Wen C, Lin L, Zou R, Lin F, Liu Y. Mesenchymal stem cell-derived exosome mediated long non-coding RNA KLF3-AS1 represses autophagy and apoptosis of chondrocytes in osteoarthritis. Cell Cycle. 2022;21(3):289–303. doi:10.1080/15384101.2021.2019411
105. Yu Z, Wen Y, Jiang N, et al. TNF-α stimulation enhances the neuroprotective effects of gingival MSCs derived exosomes in retinal ischemia-reperfusion injury via the MEG3/miR-21a-5p axis. Biomaterials. 2022;284:121484. doi:10.1016/j.biomaterials.2022.121484
106. Zhu H, Lan L, Zhang Y, et al. Epidermal growth factor stimulates exosomal microRNA-21 derived from mesenchymal stem cells to ameliorate aGVHD by modulating regulatory T cells. FASEB J. 2020;34(6):7372–7386. doi:10.1096/fj.201900847RRRR
107. Liu W, Li L, Rong Y, et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196–212. doi:10.1016/j.actbio.2019.12.020
108. Geßner A, Koch B, Klann K, et al. Characterization of extracellular vesicles from preconditioned human adipose-derived stromal/stem cells. Int J Mol Sci. 2021;22(6):2873. doi:10.3390/ijms22062873
109. Liu H, Li PW, Yang WQ, et al. Identification of non-invasive biomarkers for chronic atrophic gastritis from serum exosomal microRNAs. BMC Cancer. 2019;19(1):129. doi:10.1186/s12885-019-5328-7
110. Haraszti RA, Miller R, Dubuke ML, et al. Serum deprivation of mesenchymal stem cells improves exosome activity and alters lipid and protein composition. iScience. 2019;16:230–241. doi:10.1016/j.isci.2019.05.029
111. Guo S, Debbi L, Zohar B, et al. Stimulating extracellular vesicles production from engineered tissues by mechanical forces. Nano Lett. 2021;21(6):2497–2504. doi:10.1021/acs.nanolett.0c04834
112. Yan L, Wu X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol Toxicol. 2020;36(2):165–178. doi:10.1007/s10565-019-09504-5
113. Ambattu LA, Ramesan S, Dekiwadia C, Hanssen E, Li H, Yeo LY. High frequency acoustic cell stimulation promotes exosome generation regulated by a calcium-dependent mechanism. Commun Biol. 2020;3(1):553. doi:10.1038/s42003-020-01277-6
114. Ahn SH, Ryu SW, Choi H, You S, Park J, Choi C. Manufacturing therapeutic exosomes: from bench to industry. Mol Cells. 2022;45(5):284–290. doi:10.14348/molcells.2022.2033
115. Colao IL, Corteling R, Bracewell D, Wall I. Manufacturing exosomes: a promising therapeutic platform. Trends Mol Med. 2018;24(3):242–256. doi:10.1016/j.molmed.2018.01.006
116. Patel GK, Khan MA, Zubair H, et al. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci Rep. 2019;9(1):5335. doi:10.1038/s41598-019-41800-2
117. Du W, Zhang K, Zhang S, et al. Enhanced proangiogenic potential of mesenchymal stem cell-derived exosomes stimulated by a nitric oxide releasing polymer. Biomaterials. 2017;133:70–81. doi:10.1016/j.biomaterials.2017.04.030
118. Liu L, Liu Y, Feng C, et al. Lithium-containing biomaterials stimulate bone marrow stromal cell-derived exosomal miR-130a secretion to promote angiogenesis. Biomaterials. 2019;192:523–536. doi:10.1016/j.biomaterials.2018.11.007
119. Park DJ, Yun WS, Kim WC, et al. Improvement of stem cell-derived exosome release efficiency by surface-modified nanoparticles. J Nanobiotechnology. 2020;18(1):178. doi:10.1186/s12951-020-00739-7
120. Wu Z, He D, Li H. Bioglass enhances the production of exosomes and improves their capability of promoting vascularization. Bioact Mater. 2020;6(3):823–835. doi:10.1016/j.bioactmat.2020.09.011
121. Pegtel DM, Peferoen L, Amor S. Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos Trans R Soc Lond B Biol Sci. 2014;369(1652):20130516. doi:10.1098/rstb.2013.0516
122. Xin H, Li Y, Chopp M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front Cell Neurosci. 2014;8:377. doi:10.3389/fncel.2014.00377
123. Burnley-Hall N, Willis G, Davis J, Rees DA, James PE. Nitrite-derived nitric oxide reduces hypoxia-inducible factor 1α-mediated extracellular vesicle production by endothelial cells. Nitric Oxide. 2017;63:1–12. doi:10.1016/j.niox.2016.12.005
124. Wang K, Ye L, Lu H, et al. TNF-α promotes extracellular vesicle release in mouse astrocytes through glutaminase. J Neuroinflammation. 2017;14(1):87. doi:10.1186/s12974-017-0853-2
125. Gurunathan S, Kang MH, Jeyaraj M, Kim JH. Platinum nanoparticles enhance exosome release in human lung epithelial adenocarcinoma cancer cells (A549): oxidative stress and the ceramide pathway are key players. Int J Nanomedicine. 2021;16:515–538. doi:10.2147/IJN.S291138
126. Andjus P, Kosanović M, Milićević K, et al. Extracellular vesicles as innovative tool for diagnosis, regeneration and protection against neurological damage. Int J Mol Sci. 2020;21(18):6859. doi:10.3390/ijms21186859
127. Zhu Y, Wang Y, Zhao B, et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8(1):64. doi:10.1186/s13287-017-0510-9
128. Pusic AD, Kraig RP. Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia. 2014;62(2):284–299. doi:10.1002/glia.22606
129. Yuyama K, Sun H, Sakai S, et al. Decreased amyloid-β pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J Biol Chem. 2014;289(35):24488–24498. doi:10.1074/jbc.M114.577213
130. Yuyama K, Sun H, Usuki S, et al. A potential function for neuronal exosomes: sequestering intracerebral amyloid-β peptide. FEBS Lett. 2015;589(1):84–88. doi:10.1016/j.febslet.2014.11.027
131. Haney MJ, Klyachko NL, Zhao Y, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. doi:10.1016/j.jconrel.2015.03.033
132. Jarmalavičiūtė A, Tunaitis V, Pivoraitė U, Venalis A, Pivoriūnas A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy. 2015;17(7):932–939. doi:10.1016/j.jcyt.2014.07.013
133. Elia CA, Tamborini M, Rasile M, et al. Intracerebral injection of extracellular vesicles from mesenchymal stem cells exerts reduced Aβ plaque burden in early stages of a preclinical model of alzheimer’s disease. Cells. 2019;8(9):1059. doi:10.3390/cells8091059
134. Doeppner TR, Herz J, Görgens A, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4(10):1131–1143. doi:10.5966/sctm.2015-0078
135. Xin H, Li Y, Liu Z, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31(12):2737–2746. doi:10.1002/stem.1409
136. Xin H, Katakowski M, Wang F, et al. MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke. 2017;48(3):747–753. doi:10.1161/STROKEAHA.116.015204
137. Drommelschmidt K, Serdar M, Bendix I, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav Immun. 2017;60:220–232. doi:10.1016/j.bbi.2016.11.011
138. Ophelders DR, Wolfs TG, Jellema RK, et al. Mesenchymal stromal cell-derived extracellular vesicles protect the fetal brain after hypoxia-ischemia. Stem Cells Transl Med. 2016;5(6):754–763. doi:10.5966/sctm.2015-0197
139. Wang J, De Veirman K, Faict S, et al. Multiple myeloma exosomes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. J Pathol. 2016;239(2):162–173. doi:10.1002/path.4712
140. Ni H, Yang S, Siaw-Debrah F, et al. Exosomes derived from bone mesenchymal stem cells ameliorate early inflammatory responses following traumatic brain injury. Front Neurosci. 2019;13:14. doi:10.3389/fnins.2019.00014
141. Harting MT, Srivastava AK, Zhaorigetu S, et al. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells. 2018;36(1):79–90. doi:10.1002/stem.2730
142. Cui GH, Wu J, Mou FF, et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018;32(2):654–668. doi:10.1096/fj.201700600R
143. Cui C, Ye X, Chopp M, et al. miR-145 regulates diabetes-bone marrow stromal cell-induced neurorestorative effects in diabetes stroke rats. Stem Cells Transl Med. 2016;5(12):1656–1667. doi:10.5966/sctm.2015-0349
144. Xu G, Ao R, Zhi Z, Jia J, Yu B. miR-21 and miR-19b delivered by hMSC-derived EVs regulate the apoptosis and differentiation of neurons in patients with spinal cord injury. J Cell Physiol. 2019;234(7):10205–10217. doi:10.1002/jcp.27690
145. Zhao Y, Gan Y, Xu G, Hua K, Liu D. Exosomes from MSCs overexpressing microRNA-223-3p attenuate cerebral ischemia through inhibiting microglial M1 polarization mediated inflammation. Life Sci. 2020;260:118403. doi:10.1016/j.lfs.2020.118403
146. Xu H, Zhao G, Zhang Y, et al. Mesenchymal stem cell-derived exosomal microRNA-133b suppresses glioma progression via Wnt/β-catenin signaling pathway by targeting EZH2. Stem Cell Res Ther. 2019;10(1):381. doi:10.1186/s13287-019-1446-z
147. Lee M, Ban JJ, Yang S, Im W, Kim M. The exosome of adipose-derived stem cells reduces β-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer’s disease. Brain Res. 2018;1691:87–93. doi:10.1016/j.brainres.2018.03.034
148. Sun G, Li G, Li D, et al. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C Mater Biol Appl. 2018;89:194–204. doi:10.1016/j.msec.2018.04.006
149. Li Z, Li Q, Tong K, et al. BMSC-derived exosomes promote tendon-bone healing after anterior cruciate ligament reconstruction by regulating M1/M2 macrophage polarization in rats. Stem Cell Res Ther. 2022;13(1):295. doi:10.1186/s13287-022-02975-0
150. Liu B, Zheng W, Dai L, Fu S, Shi E. Bone marrow mesenchymal stem cell derived exosomal miR-455-5p protects against spinal cord ischemia reperfusion injury. Tissue Cell. 2022;74:101678. doi:10.1016/j.tice.2021.101678
151. Luo H, Ye G, Liu Y, et al. miR-150-3p enhances neuroprotective effects of neural stem cell exosomes after hypoxic-ischemic brain injury by targeting CASP2. Neurosci Lett. 2022;779:136635. doi:10.1016/j.neulet.2022.136635
152. Su W, Li Z, Jia Y, et al. microRNA-21a-5p/PDCD4 axis regulates mesenchymal stem cell-induced neuroprotection in acute glaucoma. J Mol Cell Biol. 2017;9(4):289–301. doi:10.1093/jmcb/mjx022
153. Min W, Wu Y, Fang Y, et al. Bone marrow mesenchymal stem cells-derived exosomal microRNA-124-3p attenuates hypoxic-ischemic brain damage through depressing tumor necrosis factor receptor associated factor 6 in newborn rats. Bioengineered. 2022;13(2):3194–3206. doi:10.1080/21655979.2021.2016094
154. Li T, Gu J, Yang O, Wang J, Wang Y, Kong J. Bone marrow mesenchymal stem cell-derived exosomal miRNA-29c decreases cardiac ischemia/reperfusion injury through inhibition of excessive autophagy via the PTEN/Akt/mTOR signaling pathway. Circ J. 2020;84(8):1304–1311. doi:10.1253/circj.CJ-19-1060
155. Li H, Liao Y, Gao L, et al. Coronary serum exosomes derived from patients with myocardial ischemia regulate angiogenesis through the miR-939-mediated nitric oxide signaling pathway. Theranostics. 2018;8(8):2079–2093. doi:10.7150/thno.21895
156. Zhao H, Li Y, Chen L, et al. HucMSCs-Derived miR-206-knockdown exosomes contribute to neuroprotection in subarachnoid hemorrhage induced early brain injury by targeting BDNF. Neuroscience. 2019;417:11–23. doi:10.1016/j.neuroscience.2019.07.051
157. Yang B, Duan W, Wei L, et al. Bone marrow mesenchymal stem cell-derived hepatocyte-like cell exosomes reduce hepatic ischemia/reperfusion injury by enhancing autophagy. Stem Cells Dev. 2020;29(6):372–379. doi:10.1089/scd.2019.0194
158. Gu J, Jin ZS, Wang CM, Yan XF, Mao YQ, Chen S. Bone marrow mesenchymal stem cell-derived exosomes improves spinal cord function after injury in rats by activating autophagy. Drug Des Devel Ther. 2020;14:1621–1631. doi:10.2147/DDDT.S237502
159. Cheng C, Chen X, Wang Y, et al. MSCs-derived exosomes attenuate ischemia-reperfusion brain injury and inhibit microglia apoptosis might via exosomal miR-26a-5p mediated suppression of CDK6. Mol Med. 2021;27(1):67. doi:10.1186/s10020-021-00324-0
160. Jiang X, Lew KS, Chen Q, Richards AM, Wang P. Human mesenchymal stem cell-derived exosomes reduce ischemia/reperfusion injury by the inhibitions of apoptosis and autophagy. Curr Pharm Des. 2018;24(44):5334–5341. doi:10.2174/1381612825666190119130441
161. Fu S, Fu S, Ma X, Yang X, Ling J. miR-875-5p regulates IR and inflammation via targeting TXNRD1 in gestational diabetes rats. Mol Med Rep. 2021;23(5):303. doi:10.3892/mmr.2021.11942
162. Yang T, Cai C, Peng A, Liu J, Wang Q. Exosomes derived from cochlear spiral ganglion progenitor cells prevent cochlea damage from ischemia-reperfusion injury via inhibiting the inflammatory process. Cell Tissue Res. 2021;386(2):239–247. doi:10.1007/s00441-021-03468-x
163. Xin W, Qiang S, Jianing D, et al. Human bone marrow mesenchymal stem cell-derived exosomes attenuate blood-spinal cord barrier disruption via the TIMP2/MMP pathway after acute spinal cord injury. Mol Neurobiol. 2021;58(12):6490–6504. doi:10.1007/s12035-021-02565-w
164. Ke Y, Fan X, Hao R, et al. Human embryonic stem cell-derived extracellular vesicles alleviate retinal degeneration by upregulating Oct4 to promote retinal Müller cell retrodifferentiation via HSP90. Stem Cell Res Ther. 2021;12(1):21. doi:10.1186/s13287-020-02034-6
165. Mu J, Li L, Wu J, et al. Hypoxia-stimulated mesenchymal stem cell-derived exosomes loaded by adhesive hydrogel for effective angiogenic treatment of spinal cord injury. Biomater Sci. 2022;10(7):1803–1811. doi:10.1039/d1bm01722e
166. Shu J, Jiang L, Wang M, et al. Human bone marrow mesenchymal stem cells-derived exosomes protect against nerve injury via regulating immune microenvironment in neonatal hypoxic-ischemic brain damage model. Immunobiology. 2022;227(3):152178. doi:10.1016/j.imbio.2022.152178
167. Zhou H, Zhou J, Teng H, Yang H, Qiu J, Li X. MiR-145 enriched exosomes derived from bone marrow-derived mesenchymal stem cells protects against cerebral ischemia-reperfusion injury through downregulation of FOXO1. Biochem Biophys Res Commun. 2022;632:92–99. doi:10.1016/j.bbrc.2022.09.089
168. Cui L, Luo W, Jiang W, et al. Human umbilical cord mesenchymal stem cell-derived exosomes promote neurological function recovery in rat after traumatic brain injury by inhibiting the activation of microglia and astrocyte. Regen Ther. 2022;21:282–287. doi:10.1016/j.reth.2022.07.005
169. Moshé SL, Perucca E, Ryvlin P, Tomson T. Epilepsy: new advances. Lancet. 2015;385(9971):884–898. doi:10.1016/S0140-6736(14)60456-6
170. Guerrini R, Marini C, Barba C. Generalized epilepsies. Handb Clin Neurol. 2019;161:3–15. doi:10.1016/B978-0-444-64142-7.00038-2
171. Nolte-’t Hoen E, Cremer T, Gallo RC, Margolis LB. Extracellular vesicles and viruses: are they close relatives? Proc Natl Acad Sci USA. 2016;113(33):9155–9161. doi:10.1073/pnas.1605146113
172. Laso-García F, Ramos-Cejudo J, Carrillo-Salinas FJ, et al. Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLoS One. 2018;13(9):e0202590. doi:10.1371/journal.pone.0202590
173. Wang H, Sui H, Zheng Y, et al. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3beta pathway. Nanoscale. 2019;11(15):7481–7496. doi:10.1039/c9nr01255a
174. Zhou Y, Wen L-L, Li Y-F, et al. Exosomes derived from bone marrow mesenchymal stem cells protect the injured spinal cord by inhibiting pericyte pyroptosis. Neural Regen Res. 2022;17(1):194–202. doi:10.4103/1673-5374.314323
175. Zhang Y, Chopp M, Meng Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122(4):856–867. doi:10.3171/2014.11.JNS14770
176. Sisa C, Kholia S, Naylor J, et al. Mesenchymal stromal cell derived extracellular vesicles reduce hypoxia-ischaemia induced perinatal brain injury. Front Physiol. 2019;10:282. doi:10.3389/fphys.2019.00282
177. Pitkänen A, Löscher W, Vezzani A, et al. Advances in the development of biomarkers for epilepsy. Lancet Neurol. 2016;15(8):843–856. doi:10.1016/S1474-4422(16)00112-5
178. Reynolds JL, Mahajan SD. Transmigration of tetraspanin 2 (Tspan2) siRNA via microglia derived exosomes across the blood brain barrier modifies the production of immune mediators by microglia cells. J Neuroimmune Pharmacol. 2020;15(3):554–563. doi:10.1007/s11481-019-09895-6
179. Upadhya D, Hattiangady B, Castro OW, et al. Human induced pluripotent stem cell-derived MGE cell grafting after status epilepticus attenuates chronic epilepsy and comorbidities via synaptic integration. Proc Natl Acad Sci USA. 2019;116(1):287–296. doi:10.1073/pnas.1814185115
180. Xiang L, Ren Y, Cai H, Zhao W, Song Y. MicroRNA-132 aggravates epileptiform discharges via suppression of BDNF/TrkB signaling in cultured hippocampal neurons. Brain Res. 2015;1622:484–495. doi:10.1016/j.brainres.2015.06.046
181. Korotkov A, Broekaart DWM, Banchaewa L, et al. microRNA-132 is overexpressed in glia in temporal lobe epilepsy and reduces the expression of pro-epileptogenic factors in human cultured astrocytes. Glia. 2020;68(1):60–75. doi:10.1002/glia.23700
182. Cantone M, Küspert M, Reiprich S, et al. A gene regulatory architecture that controls region-independent dynamics of oligodendrocyte differentiation. Glia. 2019;67(5):825–843. doi:10.1002/glia.23569
183. Wang H, Moyano AL, Ma Z, et al. miR-219 cooperates with miR-338 in myelination and promotes myelin repair in the CNS. Dev Cell. 2017;40(6):566–582.e5. doi:10.1016/j.devcel.2017.03.001
184. Milbreta U, Lin J, Pinese C, et al. Scaffold-mediated sustained, non-viral delivery of miR-219/miR-338 promotes CNS remyelination. Mol Ther. 2019;27(2):411–423. doi:10.1016/j.ymthe.2018.11.016
185. Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7(1):31–40. doi:10.1038/nrneurol.2010.178
186. Yuen AWC, Keezer MR, Sander JW. Epilepsy is a neurological and a systemic disorder. Epilepsy Behav. 2018;78:57–61. doi:10.1016/j.yebeh.2017.10.010
187. Shi LM, Chen RJ, Zhang H, Jiang CM, Gong J. Cerebrospinal fluid neuron specific enolase, interleukin-1β and erythropoietin concentrations in children after seizures. Childs Nerv Syst. 2017;33(5):805–811. doi:10.1007/s00381-017-3359-4
188. Dey A, Kang X, Qiu J, Du Y, Jiang J. Anti-inflammatory small molecules to treat seizures and epilepsy: from bench to bedside. Trends Pharmacol Sci. 2016;37(6):463–484. doi:10.1016/j.tips.2016.03.001
189. Vieira ÉL, de Oliveira GN, Lessa JM, et al. Interleukin-1β plasma levels are associated with depression in temporal lobe epilepsy. Epilepsy Behav. 2015;53:131–134. doi:10.1016/j.yebeh.2015.09.035
190. Li M, Li C, Yu H, et al. Lentivirus-mediated interleukin-1β (IL-1β) knock-down in the hippocampus alleviates lipopolysaccharide (LPS)-induced memory deficits and anxiety- and depression-like behaviors in mice. J Neuroinflammation. 2017;14(1):190. doi:10.1186/s12974-017-0964-9
191. Ma M, Li B, Zhang M, et al. Therapeutic effects of mesenchymal stem cell-derived exosomes on retinal detachment. Exp Eye Res. 2020;191:107899. doi:10.1016/j.exer.2019.107899
192. Che F, Du H, Zhang W, Cheng Z, Tong Y. MicroRNA-132 modifies angiogenesis in patients with ischemic cerebrovascular disease by suppressing the NF-κB and VEGF pathway. Mol Med Rep. 2018;17(2):2724–2730. doi:10.3892/mmr.2017.8138
193. Barr TL, Latour LL, Lee KY, et al. Blood-brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke. 2010;41(3):e123–e128. doi:10.1161/STROKEAHA.109.570515
194. Zhao Z, Zlokovic BV. Remote control of BBB: a tale of exosomes and microRNA. Cell Res. 2017;27(7):849–850. doi:10.1038/cr.2017.71
195. Rüber T, David B, Lüchters G, et al. Evidence for peri-ictal blood-brain barrier dysfunction in patients with epilepsy. Brain. 2018;141(10):2952–2965. doi:10.1093/brain/awy242
196. Rempe RG, Hartz AMS, Soldner ELB, et al. Matrix metalloproteinase-mediated blood-brain barrier dysfunction in epilepsy. J Neurosci. 2018;38(18):4301–4315. doi:10.1523/JNEUROSCI.2751-17.2018
197. Zuo X, Lu J, Manaenko A, et al. MicroRNA-132 attenuates cerebral injury by protecting blood-brain-barrier in MCAO mice. Exp Neurol. 2019;316:12–19. doi:10.1016/j.expneurol.2019.03.017
198. Long Q, Upadhya D, Hattiangady B, et al. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci USA. 2017;114(17):E3536–E3545. doi:10.1073/pnas.1703920114
199. Shetty AK, Turner DA. Aging impairs axonal sprouting response of dentate granule cells following target loss and partial deafferentation. J Comp Neurol. 1999;414(2):238–254. doi:10.1002/(sici)1096-9861(19991115)414:2<238::aid-cne7>3.0.co;2-a
200. Shetty AK, Zaman V, Hattiangady B. Repair of the injured adult hippocampus through graft-mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy. J Neurosci. 2005;25(37):8391–8401. doi:10.1523/JNEUROSCI.1538-05.2005
201. Hattiangady B, Rao MS, Zaman V, Shetty AK. Incorporation of embryonic CA3 cell grafts into the adult hippocampus at 4-months after injury: effects of combined neurotrophic supplementation and caspase inhibition. Neuroscience. 2006;139(4):1369–1383. doi:10.1016/j.neuroscience.2006.01.058
202. Hester MS, Hosford BE, Santos VR, et al. Impact of rapamycin on status epilepticus induced hippocampal pathology and weight gain. Exp Neurol. 2016;280:1–12. doi:10.1016/j.expneurol.2016.03.015
203. Wang X, Sha L, Sun N, Shen Y, Xu Q. Deletion of mTOR in reactive astrocytes suppresses chronic seizures in a mouse model of temporal lobe epilepsy. Mol Neurobiol. 2017;54(1):175–187. doi:10.1007/s12035-015-9590-7
204. Goetzl EJ, Abner EL, Jicha GA, Kapogiannis D, Schwartz JB. Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer’s disease. FASEB J. 2018;32(2):888–893. doi:10.1096/fj.201700731R
205. Shetty AV, Matrana MR, Atkinson BJ, Flaherty AL, Jonasch E, Tannir NM. Outcomes of patients with metastatic renal cell carcinoma and end-stage renal disease receiving dialysis and targeted therapies: a single institution experience. Clin Genitourin Cancer. 2014;12(5):348–353. doi:10.1016/j.clgc.2014.01.004
206. Upadhya D, Shetty AK. Extracellular vesicles as therapeutics for brain injury and disease. Curr Pharm Des. 2019;25(33):3500–3505. doi:10.2174/1381612825666191014164950
207. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. doi:10.1016/S0140-6736(14)61393-3
208. De Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–535. doi:10.1016/S1474-4422(06)70471-9
209. Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and preventionx. Lancet Neurol. 2016;15(12):1257–1272. doi:10.1016/S1474-4422(16)30230-7
210. Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol. 2013;9(1):13–24. doi:10.1038/nrneurol.2012.242
211. Khoo TK, Yarnall AJ, Duncan GW, et al. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology. 2013;80(3):276–281. doi:10.1212/WNL.0b013e31827deb74
212. Alvarez-Erviti L, Couch Y, Richardson J, Cooper JM, Wood MJ. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci Res. 2011;69(4):337–342. doi:10.1016/j.neures.2010.12.020
213. Stefanis L, Emmanouilidou E, Pantazopoulou M, Kirik D, Vekrellis K, Tofaris GK. How is alpha-synuclein cleared from the cell? J Neurochem. 2019;150(5):577–590. doi:10.1111/jnc.14704
214. Ishii T, Warabi E, Mann GE. Circadian control of BDNF-mediated Nrf2 activation in astrocytes protects dopaminergic neurons from ferroptosis. Free Radic Biol Med. 2019;133:169–178. doi:10.1016/j.freeradbiomed.2018.09.002
215. Shakespear N, Ogura M, Yamaki J, Homma Y. Astrocyte-Derived Exosomal microRNA miR-200a-3p prevents MPP+-induced apoptotic cell death through down-regulation of MKK4. Neurochem Res. 2020;45(5):1020–1033. doi:10.1007/s11064-020-02977-5
216. Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–1972. doi:10.1038/jcbfm.2012.126
217. Zhuang X, Xiang X, Grizzle W, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19(10):1769–1779. doi:10.1038/mt.2011.164
218. Lai CP, Breakefield XO. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front Physiol. 2012;3:228. doi:10.3389/fphys.2012.00228
219. Marote A, Teixeira FG, Mendes-Pinheiro B, Salgado AJ. MSCs-derived exosomes: cell-secreted nanovesicles with regenerative potential. Front Pharmacol. 2016;7:231. doi:10.3389/fphar.2016.00231
220. Teixeira FG, Carvalho MM, Sousa N, Salgado AJ. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci. 2013;70(20):3871–3882. doi:10.1007/s00018-013-1290-8
221. Teixeira MG, Rodrigues LC. Teixeira and Rodrigues Respond. Am J Public Health. 2016;106(8):e9. doi:10.2105/AJPH.2016.303249
222. Qu M, Lin Q, Huang L, et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J Control Release. 2018;287:156–166. doi:10.1016/j.jconrel.2018.08.035
223. Vilaça-Faria H, Salgado AJ, Teixeira FG. Mesenchymal stem cells-derived exosomes: a new possible therapeutic strategy for parkinson’s disease? Cells. 2019;8(2):118. doi:10.3390/cells8020118
224. Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6(1):127. doi:10.1186/s13287-015-0116-z
225. Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. doi:10.1186/s12967-015-0417-0
226. de Rivero Vaccari JP, Dietrich WD, Keane RW. Therapeutics targeting the inflammasome after central nervous system injury. Transl Res. 2016;167(1):35–45. doi:10.1016/j.trsl.2015.05.003
227. Han D, Wu C, Xiong Q, Zhou L, Tian Y. Anti-inflammatory mechanism of bone marrow mesenchymal stem cell transplantation in rat model of spinal cord injury. Cell Biochem Biophys. 2015;71(3):1341–1347. doi:10.1007/s12013-014-0354-1
228. Ohmichi T, Kasai T, Shinomoto M, et al. Quantification of blood caffeine levels in patients with parkinson’s disease and multiple system atrophy by caffeine ELISA. Front Neurol. 2020;11:580127. doi:10.3389/fneur.2020.580127
229. Malliaras K, Terrovitis J. Cardiomyocyte proliferation vs progenitor cells in myocardial regeneration: the debate continues. Glob Cardiol Sci Pract. 2013;2013(3):303–315. doi:10.5339/gcsp.2013.37
230. Srivastava D, Ivey KN. Potential of stem-cell-based therapies for heart disease. Nature. 2006;441(7097):1097–1099. doi:10.1038/nature04961
231. Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med. 2014;92(4):387–397. doi:10.1007/s00109-013-1110-5
232. Lee C, Mitsialis SA, Aslam M, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126(22):2601–2611. doi:10.1161/CIRCULATIONAHA.112.114173
233. Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem. 2015;37(6):2415–2424. doi:10.1159/000438594
234. Rezaie J, Rahbarghazi R, Pezeshki M, et al. Cardioprotective role of extracellular vesicles: a highlight on exosome beneficial effects in cardiovascular diseases. J Cell Physiol. 2019;234(12):21732–21745. doi:10.1002/jcp.28894
235. Li B, Lu Y, Wang H, et al. miR-221/222 enhance the tumorigenicity of human breast cancer stem cells via modulation of PTEN/Akt pathway. Biomed Pharmacother. 2016;79:93–101. doi:10.1016/j.biopha.2016.01.045
236. Yu B, Kim HW, Gong M, et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol. 2015;182:349–360. doi:10.1016/j.ijcard.2014.12.043
237. Baharlooi H, Salehi Z, MinbashiMoeini M, Rezaei N, Azimi M. Immunomodulatory potential of human mesenchymal stem cells and their exosomes on multiple sclerosis. Adv Pharm Bull. 2022;12(2):389–397. doi:10.34172/apb.2022.038
238. Yu X, Deng Q, Bode AM, Dong Z, Cao Y. The role of necroptosis, an alternative form of cell death, in cancer therapy. Expert Rev Anticancer Ther. 2013;13(7):883–893. doi:10.1586/14737140.2013.811180
239. Ju YT, Kwag SJ, Park HJ, et al. Decreased expression of heat shock protein. 20 in colorectal cancer and its implication in tumorigenesis. J Cell Biochem. 2015;116(2):277–286. doi:10.1002/jcb.24966
240. Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10(3):301–312. doi:10.1016/j.scr.2013.01.002
241. Wang N, Chen C, Yang D, et al. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim Biophys Acta Mol Basis Dis. 2017;1863(8):2085–2092. doi:10.1016/j.bbadis.2017.02.023
242. Wang K, Jiang Z, Webster KA, et al. EnhancedCardioprotection by human endometrium mesenchymal stem cells driven by exosomal MicroRNA-21. Stem Cells Transl Med. 2017;6(1):209–222. doi:10.5966/sctm.2015-0386
243. Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113(6):810–834. doi:10.1161/CIRCRESAHA.113.300219
244. Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. doi:10.3389/fphys.2012.00359
245. Komaki M, Numata Y, Morioka C, et al. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res Ther. 2017;8(1):219. doi:10.1186/s13287-017-0660-9
246. Ma J, Zhao Y, Sun L, et al. Exosomes derived from akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl Med. 2017;6(1):51–59. doi:10.5966/sctm.2016-0038
247. Zhang JK, Zhang Z, Guo ZA, et al. The BMSC-derived exosomal lncRNA Mir9-3hg suppresses cardiomyocyte ferroptosis in ischemia-reperfusion mice via the Pum2/PRDX6 axis. Nutr Metab Cardiovasc Dis. 2022;32(2):515–527. doi:10.1016/j.numecd.2021.10.017
248. Gong M, Wang M, Xu J, et al. Nano-sized extracellular vesicles secreted from GATA-4 modified mesenchymal stem cells promote angiogenesis by delivering Let-7 miRNAs. Cells. 2022;11(9):1573. doi:10.3390/cells11091573
249. Roccaro AM, Sacco A, Maiso P, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123(4):1542–1555. doi:10.1172/JCI66517
250. Ning H, Chen H, Deng J, et al. Exosomes secreted by FNDC5-BMMSCs protect myocardial infarction by anti-inflammation and macrophage polarization via NF-κB signaling pathway and Nrf2/HO-1 axis. Stem Cell Res Ther. 2021;12(1):519. doi:10.1186/s13287-021-02591-4
251. Ni J, Liu Y, Kang L, et al. Human trophoblast-derived exosomes attenuate doxorubicin-induced cardiac injury by regulating miR-200b and downstream Zeb1. J Nanobiotechnology. 2020;18(1):171. doi:10.1186/s12951-020-00733-z
252. Sun L, Zhu W, Zhao P, et al. Long noncoding RNA UCA1 from hypoxia-conditioned hMSC-derived exosomes: a novel molecular target for cardioprotection through miR-873-5p/XIAP axis. Cell Death Dis. 2020;11(8):696. doi:10.1038/s41419-020-02783-5
253. Zheng H, Liang X, Han Q, et al. Hemin enhances the cardioprotective effects of mesenchymal stem cell-derived exosomes against infarction via amelioration of cardiomyocyte senescence. J Nanobiotechnology. 2021;19(1):332. doi:10.1186/s12951-021-01077-y
254. Xu L, Fan Y, Wu L, et al. Exosomes from bone marrow mesenchymal stem cells with overexpressed Nrf2 inhibit cardiac fibrosis in rats with atrial fibrillation. Cardiovasc Ther. 2022;2022:2687807. doi:10.1155/2022/2687807
255. Fu DL, Jiang H, Li CY, Gao T, Liu MR, Li HW. MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction. Eur Rev Med Pharmacol Sci. 2020;24(19):10107–10117. doi:10.26355/eurrev_202010_23230
256. Pan J, Alimujiang M, Chen Q, Shi H, Luo X. Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction-induced myocardial damage via downregulation of early growth response factor 1. J Cell Biochem. 2019;120(3):4433–4443. doi:10.1002/jcb.27731
257. Santoso MR, Ikeda G, Tada Y, et al. Exosomes from induced pluripotent stem cell-derived cardiomyocytes promote autophagy for myocardial repair. J Am Heart Assoc. 2020;9(6):e014345. doi:10.1161/JAHA.119.014345
258. Deng M, Yuan H, Liu S, Hu Z, Xiao H. Exosome-transmitted LINC00461 promotes multiple myeloma cell proliferation and suppresses apoptosis by modulating microRNA/BCL-2 expression. Cytotherapy. 2019;21(1):96–106. doi:10.1016/j.jcyt.2018.10.006
259. Zhang G, Wan Z, Liu Z, Liu D, Zhao Z, Leng Y. Exosomes derived from BMSCs ameliorate intestinal ischemia-reperfusion injury by regulating miR-144-3p-mediated oxidative stress. Dig Dis Sci. 2022;67(11):5090–5106. doi:10.1007/s10620-022-07546-0
260. Mao S, Zhao J, Zhang ZJ, Zhao Q. MiR-183-5p overexpression in bone mesenchymal stem cell-derived exosomes protects against myocardial ischemia/reperfusion injury by targeting FOXO1. Immunobiology. 2022;227(3):152204. doi:10.1016/j.imbio.2022.152204
261. Luo F, Guo W, Liu W. Exosomes derived from bone marrow mesenchymal stem cells inhibit human aortic vascular smooth muscle cells calcification via the miR-15a/15b/16/NFATc3/OCN axis. Biochem Biophys Res Commun. 2022;635:65–76. doi:10.1016/j.bbrc.2022.09.076
262. Volarevic V, Markovic BS, Gazdic M, et al. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15(1):36–45. doi:10.7150/ijms.21666
263. Choudhery MS, Harris DT. Stem cell therapy for COVID-19: possibilities and challenges. Cell Biol Int. 2020;44(11):2182–2191. doi:10.1002/cbin.11440
264. Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–222. doi:10.1016/j.scr.2009.12.003
265. Hu GW, Li Q, Niu X, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. 2015;6(1):10. doi:10.1186/scrt546
266. Wang L, Yin P, Wang J, et al. Delivery of mesenchymal stem cells-derived extracellular vesicles with enriched miR-185 inhibits progression of OPMD. ArtifCellsNanomedBiotechnol. 2019;47(1):2481–2491. doi:10.1080/21691401.2019.1623232
267. Blázquez R, Sánchez-Margallo FM, Álvarez V, et al. Murine embryos exposed to human endometrial MSCs-derived extracellular vesicles exhibit higher VEGF/PDGF AA release, increased blastomere count and hatching rates. PLoS One. 2018;13(4):e0196080. doi:10.1371/journal.pone.0196080
268. Gong M, Yu B, Wang J, et al. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget. 2017;8(28):45200–45212. doi:10.18632/oncotarget.16778
269. Ye M, Ni Q, Qi H, et al. Exosomes derived from human induced pluripotent stem cells-endothelial cells promotes postnatal angiogenesis in mice bearing ischemic limbs. Int J Biol Sci. 2019;15(1):158–168. doi:10.7150/ijbs.28392
270. Heo JS, Kim S. Human adipose mesenchymal stem cells modulate inflammation and angiogenesis through exosomes. Sci Rep. 2022;12(1):2776. doi:10.1038/s41598-022-06824-1
271. Li G, Chen Y, Han Y, Ma T, Han Y. Human antigen R promotes angiogenesis of endothelial cells cultured with adipose stem cells derived exosomes via overexpression of vascular endothelial growth factor in vitro. Adipocyte. 2021;10(1):475–482. doi:10.1080/21623945.2021.1982577
272. Zhang D, Li D, Shen L, et al. Exosomes derived from Piwil2-induced cancer stem cells transform fibroblasts into cancer-associated fibroblasts. Oncol Rep. 2020;43(4):1125–1132. doi:10.3892/or.2020.7496
273. Liu P, Zhang Q, Mi J, et al. Exosomes derived from stem cells of human deciduous exfoliated teeth inhibit angiogenesis in vivo and in vitro via the transfer of miR-100-5p and miR-1246. Stem Cell Res Ther. 2022;13(1):89. doi:10.1186/s13287-022-02764-9
274. Treps L, Perret R, Edmond S, Ricard D, Gavard J. Glioblastoma stem-like cells secrete the pro-angiogenic VEGF-A factor in extracellular vesicles. J Extracell Vesicles. 2017;6(1):1359479. doi:10.1080/20013078.2017.1359479
275. Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. doi:10.1016/j.stem.2018.05.004
276. Gu H, Ji R, Zhang X, et al. Exosomes derived from human mesenchymal stem cells promote gastric cancer cell growth and migration via the activation of the Akt pathway. Mol Med Rep. 2016;14(4):3452–3458. doi:10.3892/mmr.2016.5625
277. Wang J, Hendrix A, Hernot S, et al. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood. 2014;124(4):555–566. doi:10.1182/blood-2014-03-562439
278. Wang M, Zhao C, Shi H, et al. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br J Cancer. 2014;110(5):1199–1210. doi:10.1038/bjc.2014.14
279. Lin R, Wang S, Zhao RC. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013;383(1–2):13–20. doi:10.1007/s11010-013-1746-z
280. Cao X, Han ZB, Zhao H, Liu Q. Transplantation of mesenchymal stem cells recruits trophic macrophages to induce pancreatic beta cell regeneration in diabetic mice. Int J Biochem Cell Biol. 2014;53:372–379. doi:10.1016/j.biocel.2014.06.003
281. Yu M, Liu W, Li J, et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020;11(1):350. doi:10.1186/s13287-020-01824-2
282. Liang Y, Zhang D, Li L, et al. Exosomal microRNA-144 from bone marrow-derived mesenchymal stem cells inhibits the progression of non-small cell lung cancer by targeting CCNE1 and CCNE2. Stem Cell Res Ther. 2020;11(1):87. doi:10.1186/s13287-020-1580-7
283. Qi J, Zhang R, Wang Y. Exosomal miR-21-5p derived from bone marrow mesenchymal stem cells promote osteosarcoma cell proliferation and invasion by targeting PIK3R1. J Cell Mol Med. 2021;25(23):11016–11030. doi:10.1111/jcmm.17024
284. Zhao W, Qin P, Zhang D, et al. Long non-coding RNA PVT1 encapsulated in bone marrow mesenchymal stem cell-derived exosomes promotes osteosarcoma growth and metastasis by stabilizing ERG and sponging miR-183-5p. Aging. 2019;11(21):9581–9596. doi:10.18632/aging.102406
285. Qin F, Tang H, Zhang Y, Zhang Z, Huang P, Zhu J. Bone marrow-derived mesenchymal stem cell-derived exosomal microRNA-208a promotes osteosarcoma cell proliferation, migration, and invasion. J Cell Physiol. 2020;235(5):4734–4745. doi:10.1002/jcp.29351
286. He Z, Li W, Zheng T, Liu D, Zhao S. Human umbilical cord mesenchymal stem cells-derived exosomes deliver microRNA-375 to downregulate ENAH and thus retard esophageal squamous cell carcinoma progression. J Exp Clin Cancer Res. 2020;39(1):140. doi:10.1186/s13046-020-01631-w
287. Bolandi Z, Mokhberian N, Eftekhary M, et al. Adipose derived mesenchymal stem cell exosomes loaded with miR-10a promote the differentiation of Th17 and Treg from naive CD4+ T cell. Life Sci. 2020;259:118218. doi:10.1016/j.lfs.2020.118218
288. Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi:10.1038/367645a0
289. Zhao S, Mi Y, Guan B, et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. 2020;13(1):156. doi:10.1186/s13045-020-00991-2
290. Sun Z, Wang L, Zhou Y, et al. Glioblastoma stem cell-derived exosomes enhance stemness and tumorigenicity of glioma cells by transferring notch1 protein. Cell Mol Neurobiol. 2020;40(5):767–784. doi:10.1007/s10571-019-00771-8
291. Li W, Han Y, Zhao Z, et al. Oral mucosal mesenchymal stem cell-derived exosomes: a potential therapeutic target in oral premalignant lesions. Int J Oncol. 2019;54(5):1567–1578. doi:10.3892/ijo.2019.4756
292. Wang L, Yang G, Zhao D, et al. CD103-positive CSC exosome promotes EMT of clear cell renal cell carcinoma: role of remote MiR-19b-3p. Mol Cancer. 2019;18(1):86. doi:10.1186/s12943-019-0997-z
293. Grange C, Tapparo M, Collino F, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71(15):5346–5356. doi:10.1158/0008-5472.CAN-11-0241
294. Wang ZF, Liao F, Wu H, Dai J. Glioma stem cells-derived exosomal miR-26a promotes angiogenesis of microvessel endothelial cells in glioma. J Exp Clin Cancer Res. 2019;38(1):201. doi:10.1186/s13046-019-1181-4
295. Wang JH, Liu XL, Sun JM, Yang JH, Xu DH, Yan SS. Role of mesenchymal stem cell derived extracellular vesicles in autoimmunity: a systematic review. World J Stem Cells. 2020;12(8):879–896. doi:10.4252/wjsc.v12.i8.879
296. Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi:10.1038/ni.3002
297. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–3506. doi:10.1182/blood-2007-02-069716
298. Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917. doi:10.1155/2015/394917
299. de Castro LL, Lopes-Pacheco M, Weiss DJ, Cruz FF, Rocco PRM. Current understanding of the immunosuppressive properties of mesenchymal stromal cells. J Mol Med. 2019;97(5):605–618. doi:10.1007/s00109-019-01776-y
300. Shokri MR, Bozorgmehr M, Ghanavatinejad A, et al. Human menstrual blood-derived stromal/stem cells modulate functional features of natural killer cells. Sci Rep. 2019;9(1):10007. doi:10.1038/s41598-019-46316-3
301. Cosenza S, Ruiz M, Maumus M, Jorgensen C, Noël D. Pathogenic or therapeutic extracellular vesicles in rheumatic diseases: role of mesenchymal stem cell-derived vesicles. Int J Mol Sci. 2017;18(4):889. doi:10.3390/ijms18040889
302. Cao L, Xu H, Wang G, Liu M, Tian D, Yuan Z. Extracellular vesicles derived from bone marrow mesenchymal stem cells attenuate dextran sodium sulfate-induced ulcerative colitis by promoting M2 macrophage polarization. Int Immunopharmacol. 2019;72:264–274. doi:10.1016/j.intimp.2019.04.020
303. Song Y, Dou H, Li X, et al. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cells. 2017;35(5):1208–1221. doi:10.1002/stem.2564
304. Zhao H, Shang Q, Pan Z, et al. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018;67(2):235–247. doi:10.2337/db17-0356
305. Rui K, Hong Y, Zhu Q, et al. Olfactory ecto-mesenchymal stem cell-derived exosomes ameliorate murine Sjögren’s syndrome by modulating the function of myeloid-derived suppressor cells. Cell Mol Immunol. 2021;18(2):440–451. doi:10.1038/s41423-020-00587-3
306. Fan Y, Herr F, Vernochet A, Mennesson B, Oberlin E, Durrbach A. Human fetal liver mesenchymal stem cell-derived exosomes impair natural killer cell function. Stem Cells Dev. 2019;28(1):44–55. doi:10.1089/scd.2018.0015
307. Budoni M, Fierabracci A, Luciano R, Petrini S, Di Ciommo V, Muraca M. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant. 2013;22:369–379. doi:10.3727/096368911X582769
308. Adamo A, Brandi J, Caligola S, et al. Extracellular vesicles mediate mesenchymal stromal cell-dependent regulation of B cell PI3K-AKT signaling pathway and actin cytoskeleton. Front Immunol. 2019;10:446. doi:10.3389/fimmu.2019.00446
309. Tavasolian F, Hosseini AZ, Soudi S, Naderi M. miRNA-146a improves immunomodulatory effects of MSC-derived exosomes in rheumatoid arthritis. Curr Gene Ther. 2020;20(4):297–312. doi:10.2174/1566523220666200916120708
310. Dendrou CA, Fugger L. Immunomodulation in multiple sclerosis: promises and pitfalls. Curr Opin Immunol. 2017;49:37–43. doi:10.1016/j.coi.2017.08.013
311. Martin R. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis and their application for new therapeutic strategies. J Neural Transm Suppl. 1997;49:53–67. doi:10.1007/978-3-7091-6844-8_6
312. Giovannoni G, Soelberg Sorensen P, Cook S, et al. MRI outcomes with cladribine tablets for multiple sclerosis in the CLARITY study. J Neurol. 2013;260(4):1136–1146. doi:10.1007/s00415-012-6775-0
313. Nakhaei-Nejad M, Barilla D, Lee CH, Blevins G, Giuliani F. Characterization of lymphopenia in patients with MS treated with dimethyl fumarate and fingolimod [published correction appears in Neurol NeuroimmunolNeuroinflamm. Neurol Neuroimmunol Neuroinflamm. 2017;5(2):e432. doi:10.1212/NXI.0000000000000432
314. Wang W, Zhou H, Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur J Med Chem. 2018;158:502–516. doi:10.1016/j.ejmech.2018.09.027
315. Herrero C, Pérez-Simón JA. Immunomodulatory effect of mesenchymal stem cells. Braz J Med Biol Res. 2010;43(5):425–430. doi:10.1590/s0100-879x2010007500033
316. Nasri F, Mohtasebi MS, Hashemi E, Zarrabi M, Gholijani N, Sarvestani EK. Therapeutic efficacy of mesenchymal stem cells and mesenchymal stem cells-derived neural progenitors in experimental autoimmune encephalomyelitis. Int J Stem Cells. 2018;11(1):68–77. doi:10.15283/ijsc17052
317. Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Dalir-Naghadeh B. Phenotypic modulation of auto-reactive cells by insertion of tolerogenic molecules via MSC-derived exosomes. Vet Res Forum. 2012;3(4):257–261.
318. Kaisey M, Lashgari G, Fert-Bober J, Ontaneda D, Solomon AJ, Sicotte NL. An update on diagnostic laboratory biomarkers for multiple sclerosis. Curr Neurol Neurosci Rep. 2022;22(10):675–688. doi:10.1007/s11910-022-01227-1
319. Li L, Wang R, Jia Y, Rong R, Xu M, Zhu T. Exosomes derived from mesenchymal stem cells ameliorate renal ischemic-reperfusion injury through inhibiting inflammation and cell apoptosis. Front Med. 2019;6:269. doi:10.3389/fmed.2019.00269
320. Hosseini Shamili F, Alibolandi M, Rafatpanah H, et al. Immunomodulatory properties of MSC-derived exosomes armed with high affinity aptamer toward mylein as a platform for reducing multiple sclerosis clinical score. J Control Release. 2019;299:149–164. doi:10.1016/j.jconrel.2019.02.032
321. Li Z, Ye H, Cai X, et al. Bone marrow-mesenchymal stem cells modulate microglial activation in the peri-infarct area in rats during the acute phase of stroke. Brain Res Bull. 2019;153:324–333. doi:10.1016/j.brainresbull.2019.10.001
322. Pusic AD, Pusic KM, Kraig RP. What are exosomes and how can they be used in multiple sclerosis therapy? Expert Rev Neurother. 2014;14(4):353–355. doi:10.1586/14737175.2014.890893
323. MacGregor AJ, Snieder H, Rigby AS, et al. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 2000;43(1):30–37. doi:10.1002/1529-0131(200001)43:1<30::AID-ANR5>3.0.CO;2-B
324. Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358(9285):903–911. doi:10.1016/S0140-6736(01)06075-5
325. Malmström V, Catrina AI, Klareskog L. The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nat Rev Immunol. 2017;17(1):60–75. doi:10.1038/nri.2016.124
326. McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389(10086):2328–2337. doi:10.1016/S0140-6736(17)31472-1
327. Withrow J, Murphy C, Liu Y, Hunter M, Fulzele S, Hamrick MW. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther. 2016;18(1):286. doi:10.1186/s13075-016-1178-8
328. Wang S, Zhu R, Li H, Li J, Han Q, Zhao RC. Mesenchymal stem cells and immune disorders: from basic science to clinical transition. Front Med. 2019;13(2):138–151. doi:10.1007/s11684-018-0627-y
329. Rabinovich GA, Daly G, Dreja H, et al. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J Exp Med. 1999;190(3):385–398. doi:10.1084/jem.190.3.385
330. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7(1):16214. doi:10.1038/s41598-017-15376-8
331. Conforti A, Scarsella M, Starc N, et al. Microvescicles derived from mesenchymal stromal cells are not as effective as their cellular counterpart in the ability to modulate immune responses in vitro. Stem Cells Dev. 2014;23(21):2591–2599. doi:10.1089/scd.2014.0091e
332. Chen Z, Wang H, Xia Y, Yan F, Lu Y. Therapeutic potential of mesenchymal cell-derived miRNA-150-5p-expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J Immunol. 2018;201(8):2472–2482. doi:10.4049/jimmunol.1800304
333. Levraut M, Martis N, Viau P, Suarez F, Queyrel V. Refractory sarcoidosis-like systemic granulomatosis responding to ruxolitinib. Ann Rheum Dis. 2019;78:1606. doi:10.1136/annrheumdis-2019-215387
334. Casado JG, Blázquez R, Vela FJ, Álvarez V, Tarazona R, Sánchez-Margallo FM. Mesenchymal stem cell-derived exosomes: immunomodulatory evaluation in an antigen-induced synovitis porcine model. Front Vet Sci. 2017;4:39. doi:10.3389/fvets.2017.00039
335. Song J, Kim D, Han J, Kim Y, Lee M, Jin EJ. PBMC and exosome-derivedHotair is a critical regulator and potent marker for rheumatoid arthritis. Clin Exp Med. 2015;15(1):121–126. doi:10.1007/s10238-013-0271-4
336. Wang L, Wang C, Jia X, Yu J. Circulating exosomal miR-17 inhibits the induction of regulatory T cells via suppressing TGFBR II expression in rheumatoid arthritis. Cell Physiol Biochem. 2018;50(5):1754–1763. doi:10.1159/000494793
337. Nakasa T, Miyaki S, Okubo A, et al. Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum. 2008;58(5):1284–1292. doi:10.1002/art.23429
338. Kurowska-Stolarska M, Alivernini S, Ballantine LE, et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci USA. 2011;108(27):11193–11198. doi:10.1073/pnas.1019536108
339. Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103(33):12481–12486. doi:10.1073/pnas.0605298103
340. Acharjee S, Ghosh B, Al-Dhubiab BE, Nair AB. Understanding type 1 diabetes: etiology and models. Can J Diabetes. 2013;37(4):269–276. doi:10.1016/j.jcjd.2013.05.001
341. Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–1300. doi:10.1038/nature08933
342. Ezquer ME, Ezquer FE, Arango-Rodríguez ML, Conget PA. MSC transplantation: a promising therapeutic strategy to manage the onset and progression of diabetic nephropathy. Biol Res. 2012;45(3):289–296. doi:10.4067/S0716-97602012000300010
343. Wen D, Peng Y, Liu D, Weizmann Y, Mahato RI. Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. J Control Release. 2016;238:166–175. doi:10.1016/j.jconrel.2016.07.044
344. Kota DJ, Wiggins LL, Yoon N, Lee RH. TSG-6 produced by hMSCs delays the onset of autoimmune diabetes by suppressing Th1 development and enhancing tolerogenicity. Diabetes. 2013;62(6):2048–2058. doi:10.2337/db12-0931
345. Nakano M, Nagaishi K, Konari N, et al. Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci Rep. 2016;6:24805. doi:10.1038/srep24805
346. Nojehdehi S, Soudi S, Hesampour A, Rasouli S, Soleimani M, Hashemi SM. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J Cell Biochem. 2018;119(11):9433–9443. doi:10.1002/jcb.27260
347. Shigemoto-Kuroda T, Oh JY, Kim DK, et al. MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and uveoretinitis. Stem Cell Rep. 2017;8(5):1214–1225. doi:10.1016/j.stemcr.2017.04.008
348. Prete M, Dammacco R, Fatone MC, Racanelli V. Autoimmune uveitis: clinical, pathogenetic, and therapeutic features. Clin Exp Med. 2016;16(2):125–136. doi:10.1007/s10238-015-0345-6
349. Bai L, Shao H, Wang H, et al. Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci Rep. 2017;7(1):4323. doi:10.1038/s41598-017-04559-y
350. Al-Shobaili HA, Rasheed Z. Immunological studies of oxidized superoxide dismutase in patients with systemic lupus erythematosus. Correlation with disease induction and progression. Saudi Med J. 2012;33(11):1177–1184.
351. Colasanti T, Maselli A, Conti F, et al. Autoantibodies to estrogen receptor α interfere with T lymphocyte homeostasis and are associated with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2012;64(3):778–787. doi:10.1002/art.33400
352. Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–2121. doi:10.1056/NEJMra1100359
353. Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358(9):929–939. doi:10.1056/NEJMra071297N
354. Jang E, Jeong M, Kim S, et al. Infusion of human bone marrow-derived mesenchymal stem cells alleviates autoimmune nephritis in a lupus model by suppressing follicular helper T-cell development. Cell Transplant. 2016;25(1):1–15. doi:10.3727/096368915X688173
355. Sun L, Akiyama K, Zhang H, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27(6):1421–1432. doi:10.1002/stem.68
356. Perez-Hernandez J, Redon J, Cortes R. Extracellular vesicles as therapeutic agents in systemic lupus erythematosus. Int J Mol Sci. 2017;18(4):717. doi:10.3390/ijms18040717
357. Lee JY, Park JK, Lee EY, Lee EB, Song YW. Circulating exosomes from patients with systemic lupus erythematosus induce a proinflammatory immune response. Arthritis Res Ther. 2016;18(1):264. doi:10.1186/s13075-016-1159-y
358. Mao F, Wu Y, Tang X, et al. Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. Biomed Res Int. 2017;2017:5356760. doi:10.1155/2017/5356760
359. Wu H, Fan H, Shou Z, et al. Extracellular vesicles containing miR-146a attenuate experimental colitis by targeting TRAF6 and IRAK1. Int Immunopharmacol. 2019;68:204–212. doi:10.1016/j.intimp.2018.12.043
360. Yang S, Liang X, Song J, et al. A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res Ther. 2021;12(1):315. doi:10.1186/s13287-021-02404-8
361. Tian J, Zhu Q, Zhang Y, et al. OlfactoryEcto-mesenchymal stem cell-derived exosomes ameliorate experimental colitis via modulating Th1/Th17 and treg cell responses. Front Immunol. 2020;11:598322. doi:10.3389/fimmu.2020.598322
362. Heidari N, Abbasi-Kenarsari H, Namaki S, et al. Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J Cell Physiol. 2021;236(8):5906–5920. doi:10.1002/jcp.30275
363. Liu H, Liang Z, Wang F, et al. Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight. 2019;4(24):e131273. doi:10.1172/jci.insight.131273
364. Eiro N, Fraile M, González-Jubete A, González LO, Vizoso FJ. Mesenchymal (stem) stromal cells based as new therapeutic alternative in inflammatory bowel disease: basic mechanisms, experimental and clinical evidence, and challenges. Int J Mol Sci. 2022;23(16):8905. doi:10.3390/ijms23168905
365. Yang J, Liu XX, Fan H, et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One. 2015;10(10):e0140551. doi:10.1371/journal.pone.0140551
366. Huang Y, Yang C, Xu XF, Xu W, Liu SW. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41(9):1141–1149. doi:10.1038/s41401-020-0485-4
367. Najm A, Alunno A, Mariette X, et al. Pathophysiology of acute respiratory syndrome coronavirus 2 infection: a systematic literature review to inform EULAR points to consider. RMD Open. 2021;7(1):e001549. doi:10.1136/rmdopen-2020-001549
368. Schett G, Sticherling M, Neurath MF. COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat Rev Immunol. 2020;20(5):271–272. doi:10.1038/s41577-020-0312-7
369. Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10(1):5. doi:10.1186/s13287-019-1165-5
370. He X, Dong Z, Cao Y, et al. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019;2019:7132708. doi:10.1155/2019/7132708
371. Park J, Kim S, Lim H, et al. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax. 2019;74(1):43–50. doi:10.1136/thoraxjnl-2018-211576
372. Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev Rep. 2020;16(3):427–433. doi:10.1007/s12015-020-09973-w
373. Hu S, Park J, Liu A, et al. Mesenchymal stem cell microvesicles restore protein permeability across primary cultures of injured human lung microvascular endothelial cells. Stem Cells Transl Med. 2018;7(8):
374. Zheng ZX. Stem cell therapy: a promising treatment for COVID-19. World J Clin Cases. 2021;9(36):11148–11155. doi:10.12998/wjcc.v9.i36.11148
375. Kojima M, Gimenes-Junior JA, Chan TW, et al. Exosomes in postshock mesenteric lymph are key mediators of acute lung injury triggering the macrophage activation via Toll-like receptor 4. FASEB J. 2018;32(1):97–110. doi:10.1096/fj.201700488R
376. Yang R, Liao Y, Wang L, et al. Exosomes derived from M2b macrophages attenuate DSS-induced colitis. Front Immunol. 2019;10:2346. doi:10.3389/fimmu.2019.02346
377. Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35(4):851–858. doi:10.1002/stem.2575
378. Khalaj K, Figueira RL, Antounians L, Lauriti G, Zani A. Systematic review of extracellular vesicle-based treatments for lung injury: are EVs a potential therapy for COVID-19? J Extracell Vesicles. 2020;9(1):1795365. doi:10.1080/20013078.2020.1795365
379. Joo HS, Suh JH, Lee HJ, Bang ES, Lee JM. Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int J Mol Sci. 2020;21(3):727. doi:10.3390/ijms21030727
380. Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208. doi:10.1007/s00018-017-2595-9
381. Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15(3):4142–4157. doi:10.3390/ijms15034142
382. Wang M, Yuan Q, Xie L. Mesenchymal stem cell-based immunomodulation: properties and clinical application. Stem Cells Int. 2018;2018:3057624. doi:10.1155/2018/3057624
383. Lee JH, Park J, Lee JW. Therapeutic use of mesenchymal stem cell-derived extracellular vesicles in acute lung injury. Transfusion. 2019;59(S1):876–883. doi:10.1111/trf.14838
384. Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29(12):747–754. doi:10.1089/scd.2020.0080
385. Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a Phase 1 clinical trial. Lancet Respir Med. 2015;3(1):24–32. doi:10.1016/S2213-2600(14)70291-7
386. Peng F, Tu L, Yang Y, et al. Management and treatment of COVID-19: the Chinese experience. Can J Cardiol. 2020;36(6):915–930. doi:10.1016/j.cjca.2020.04.010
387. Hicok K, Vangsness T, Dordevic M. Exosome origins: why the cell source matters. Stem Cells Regen Med. 2020;4:1–4. doi:10.33425/2639-9512.1040
388. Shao M, Xu Q, Wu Z, et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res Ther. 2020;11(1):37. doi:10.1186/s13287-020-1550-0
389. Moreira A, Naqvi R, Hall K, et al. Mesenchymal stromal cell conditioned media for lung disease: a systematic review and meta-analysis of preclinical studies. Respir Res. 2019;20(1):239. doi:10.1186/s12931-019-1212-x
390. Fujita Y, Kadota T, Araya J, Ochiya T, Kuwano K. Clinical application of mesenchymal stem cell-derived extracellular vesicle-based therapeutics for inflammatory lung diseases. J Clin Med. 2018;7(10):355. doi:10.3390/jcm7100355
391. Abraham A, Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl Med. 2020;9(1):28–38. doi:10.1002/sctm.19-0205
392. Wu YC, Chen CS, Chan YJ. The outbreak of COVID-19: an overview. J Chin Med Assoc. 2020;83(3):217. doi:10.1097/JCMA.0000000000000270
393. Yin K, Wang S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomarker Res. 2019;7(1):8. doi:10.1186/s40364-019-0159-x
394. Yang C, Lim W, Park J, Park S, You S, Song G. Anti-inflammatory effects of mesenchymal stem cell-derived exosomal microRNA-146a-5p and microRNA-548e-5p on human trophoblast cells. Mol Hum Reprod. 2019;25(11):755–771. doi:10.1093/molehr/gaz054
395. Morrison TJ, Jackson MV, Cunningham EK, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275–1286. doi:10.1164/rccm.201701-0170OC
396. Phinney DG, Di Giuseppe M, Njah J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. doi:10.1038/ncomms9472
397. Tan JL, Lau SN, Leaw B, et al. Amnion epithelial cell-derived exosomes restrict lung injury and enhance endogenous lung repair. Stem Cells Transl Med. 2018;7(2):180–196. doi:10.1002/sctm.17-0185
398. Zhang W, Xu L, Chen M, et al.. Effect of overexpression of microRNA-21-5p on early apoptosis of type II alveolar epithelial cells in rats with hyperoxic acute lung injury. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31(8):978–982. Chinese. doi:10.3760/cma.j.issn.2095-4352.2019.08.013
399. Kim YS, Kim JY, Cho R, et al. Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Exp Mol Med. 2017;49(1):e284. doi:10.1038/emm.2016.127
400. Varderidou-Minasian S, Lorenowicz MJ. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics. 2020;10(3):5979–5997. doi:10.7150/thno.40122
401. Al-Khawaga S, Abdelalim EM. Potential application of mesenchymal stem cells and their exosomes in lung injury: an emerging therapeutic option for COVID-19 patients. Stem Cell Res Ther. 2020;11(1):437. doi:10.1186/s13287-020-01963-6
402. Allan D, Tieu A, Lalu M, Burger D. Mesenchymal stromal cell derived extracellular vesicles for regenerative therapy and immune modulation: progress and challenges toward clinical application. Stem Cells Transl Med. 2020;9(1):39–46. doi:10.1002/sctm.19-0114
403. Basiri A, Mansouri F, Azari A, et al. Stem cell therapy potency in personalizing severe COVID-19 treatment. Stem Cell Rev Rep. 2021;17(1):193–213. doi:10.1007/s12015-020-10110-w
404. Wang J, Huang R, Xu Q, et al. Mesenchymal stem cell-derived extracellular vesicles alleviate acute lung injury via transfer of miR-27a-3p. Crit Care Med. 2020;48(7):e599–e610. doi:10.1097/CCM.0000000000004315
405. Lucas C, Wong P, Klein J, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(777821):463–469. doi:10.1038/s41586-020-2588-y
406. Mitrani MI, Bellio MA, Sagel A, et al. Case report: administration of amniotic fluid-derived nanoparticles in three severely ill COVID-19 patients. Front Med. 2021;8:242. doi:10.3389/fmed.2021.583842
407. Schultz IC, Bertoni APS, Wink MR. Mesenchymal stem cell derived extracellular vesicles carrying miRNA as a potential multi target therapy to COVID-19: an in silico analysis. Stem Cell Rev Rep. 2021;17:341–356. doi:10.1007/s12015-021-10122-0
408. Wang Y, Zhu X, Jiang XM, et al. Decreased inhibition of exosomal miRNAs on SARS-CoV-2 replication underlies poor outcomes in elderly people and diabetic patients. Signal Transduct Target Ther. 2021;6(1):300. doi:10.1038/s41392-021-00716-y
409. Mitrani MI, Bellio MA, Meglin A, et al. Treatment of a COVID-19 long hauler with an amniotic fluid-derived extracellular vesicle biologic. Respir Med Case Rep. 2021;34:101502. doi:10.1016/j.rmcr.2021.101502
410. Khanh VC, Fukushige M, Chang YH, et al. Wharton’s jelly mesenchymal stem cell-derived extracellular vesicles reduce SARS-CoV2-induced inflammatory cytokines under high glucose and uremic toxin conditions. Stem Cells Dev. 2021;30(15):758–772. doi:10.1089/scd.2021.0065
411. Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38(6):754–763. doi:10.1038/aps.2017.12
412. Li J, Tan M, Xiang Q, Zhou Z, Yan H. Thrombin-activated platelet-derived exosomes regulate endothelial cell expression of ICAM-1 via microRNA-223 during the thrombosis-inflammation response. Thromb Res. 2017;154:96–105. doi:10.1016/j.thromres.2017.04.016
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.