Back to Journals » Drug Design, Development and Therapy » Volume 8
Bioactive protein fraction DLBS1033 containing lumbrokinase isolated from Lumbricus rubellus: ex vivo, in vivo, and pharmaceutic studies
Authors Tjandrawinata R , Trisina J, Rahayu P, Prasetya L, Hanafiah A, Rachmawati H
Received 11 April 2014
Accepted for publication 23 May 2014
Published 25 September 2014 Volume 2014:8 Pages 1585—1593
DOI https://doi.org/10.2147/DDDT.S66007
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Raymond R Tjandrawinata,1 Jessica Trisina,1 Puji Rahayu,1 Lorentius Agung Prasetya,1 Aang Hanafiah,2 Heni Rachmawati3
1Dexa Laboratories of Biomolecular Sciences, Dexa Medica, Cikarang, Indonesia; 2National Nuclear Energy Agency, Bandung, Indonesia; 3School of Pharmacy, Bandung Institute of Technology, Bandung, Indonesia
Abstract: DLBS1033 is a bioactive protein fraction isolated from Lumbricus rubellus that tends to be unstable when exposed to the gastrointestinal environment. Accordingly, appropriate pharmaceutical development is needed to maximize absorption of the protein fraction in the gastrointestinal tract. In vitro, ex vivo, and in vivo stability assays were performed to study the stability of the bioactive protein fraction in gastric conditions. The bioactive protein fraction DLBS1033 was found to be unstable at low pH and in gastric fluid. The “enteric coating” formulation showed no leakage in gastric fluid–like medium and possessed a good release profile in simulated intestinal medium. DLBS1033 was absorbed through the small intestine in an intact protein form, confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) analysis. This result confirmed that an enteric coating formula using methacrylic acid copolymer could protect DLBS1033 from the acidic condition of the stomach by preventing the release of DLBS1033 in the stomach, while promoting its release when reaching the intestine. From the blood concentration–versus-time curve, 99mTc-DLBS1033 showed a circulation half-life of 70 minutes. This relatively long biological half-life supports its function as a thrombolytic protein. Thus, an enteric delivery system is considered the best approach for DLBS1033 as an oral thrombolytic agent.
Keywords: bioactive protein fraction, enteric coated tablet, pharmacodynamic
Introduction
Bioactive protein fraction DLBS1033, which is derived from earthworms or Lumbricus rubellus, has been found to be potential as an agent for prevention and treatment of thrombosis-related disease.1 The active constituent of DLBS1033, among many proteins, is a group of serine proteases known as lumbrokinase, which has already been investigated and shown to be well absorbed through intestinal epithelium.2 Many studies on lumbrokinase have been conducted, and collectively, these proteins serve in thrombolytic and antithrombolytic activities. Our laboratory has been focusing on the study of natural products such as DLBS1033,1,3 and especially on their pharmacological activities.1,4,5 As in the case of chemical agents, the long-term use of therapeutic agents from natural sources requires a convenient route to maximize patient compliance, with oral administration being the most preferred route. A lingering problem experienced in the oral solid formulation of drugs that contain protein as an active ingredient is the instability of the protein in the harsh condition of the gastrointestinal (GI) tract. Protein tends to be unstable in the stomach, due to the low gastric fluid pH and gastric protease activity.6 One of the common formulation strategies to overcome this problem is to prevent the drug from having direct contact with the environment of the stomach with use of an “enteric coating”. Enteric coatings have been shown to facilitate the delivery of therapeutic agent into the intestinal region without disturbance by the harsh gastric condition.7,8 The primary use of enteric coating in this investigation was to maintain the activity of proteins, such as lumbrokinase, that are unstable when exposed to gastric fluid. The most commonly used pH-sensitive enteric polymers for this application include cellulose acetate phthalate, cellulose acetate trimellitate, hydroxypropyl methylcellulose phthalate, and methacrylic acid copolymers.9–11
The present study investigated the characteristic and stability of DLBS1033 in the GI tract and examined the release profile when coated with a methacrylic acid copolymer formulation. DLBS1033 extract was also examined to confirm its permeability through the Wistar rat intestine. Blood clearance analysis of DLBS1033 labeled with the radioisotope 99mTc was undertaken in an animal model to evaluate the in vivo half-life.
Materials and methods
Materials
DLBS1033 was prepared at Dexa Laboratories of Biomolecular Sciences (Cikarang, Indonesia) as described previously.1 Bovine serum albumin (BSA) fraction V, human plasminogen, and thrombin for biochemistry were purchased from Merck KGaA (Darmstadt, Germany). Human fibrinogen fraction I-S and fetal calf serum were obtained from Sigma-Aldrich Corp (St Louis, MO, USA). Low-molecular-weight marker protein and Sephacryl® S-300HR were purchased from GE Healthcare (Little Chalfont, UK). Na99mTcO4 was obtained from National Nuclear Energy Agency (Tangerang, Indonesia). Other reagents used were of analytical grade.
Animals
Six male Rattus norvegicus strain Wistar rats (D’Wistar, West Java, Indonesia) weighing around 200–250 g were used for the experiment. The rats were housed under standard condition (18°C–25°C, relative humidity <70%, under a 12/12 hours light/dark cycle). All protocols using animals were evaluated and approved (approval number DOC-DLBS-BIOL-VVR-APF-011/Intestinal Absorption Study of DLBS1033) by the Animal Care and Use Committee of Dexa Laboratories of Biomolecular Sciences (DLBS-ACUC), according to the requirements and standards set out by the Association for Assessment and Accreditation of Laboratory Animal Care.
The influence of pH on the in vitro solubility and stability of DLBS1033
Mixtures of 61.25 mg/mL DLBS1033 were prepared in three different pH solutions: purified water pH ±6.0; 20 mM sodium phosphate-buffered saline (PBS) pH 7.4; and 20 mM sodium acetate buffer pH 2.8. Each mixture was mixed by vortex for 15 minutes and centrifuged at 3,000 rpm for 10 minutes. The supernatant was collected and used for zymography, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), protease activity, and protein content assay.
Ex vivo and in vivo gastric stability of DLBS1033
Rats were fasted for 2 days prior to the experiment, with free access to drinking water. Initially, rats were anesthetized with ketamine 75 mg/kg by intramuscular (IM) injection, followed with acepromazine 2.5 mg/kg IM. Purified water 2 mL per oral (PO) was administered to all rats. After 1 hour, surgery was performed to collect gastric fluid.
For the ex vivo gastric stability test, DLBS1033 powder was made into a mixture, at a concentration of 61.25 mg/mL, with the gastric fluid obtained from the rat and incubated at 37°C for 1 hour. The mixture was then centrifuged at 3,000 rpm for 10 minutes, and the supernatant was used for zymography, SDS-PAGE, protease activity assay, and protein content assay.
For the in vivo gastric stability study, DLBS1033 powder was made into a mixture, at a concentration of 61.25 mg/mL, with purified water and homogenized for 15 minutes using vortex. The mixture was then given PO to each rat 1 hour before surgery. Surgery was performed to collect some gastric fluid. The gastric fluid was then centrifuged at 3,000 rpm for 10 minutes. The supernatant was taken and used for zymography, SDS-PAGE, protease activity assay, and protein content assay.
Assays of DLBS1033
Characterization of DLBS1033 was conducted as previously described by Trisina et al.1 SDS PAGE was run using a 12% polyacrylamide gel and stained using Coomassie Brilliant Blue R-250. The molecular weight was determined using low-molecular-weight marker standards. For the zymography analysis, fibrinogen (0.3%–1%) was incorporated into 12% native polyacrylamide gel electrophoresis (PAGE).
Intestinal permeability assay of DLBS1033
The rats were fasted for 12 hours before the experiment, with normal access to drinking water. Prior to surgery, the rats were anesthetized. The small intestine was washed with 10 mL PBS (1×) and 7 mL of DLBS1033 solution, respectively. Then, the small intestine (duodenum, jejunum, and ileum) was filled with DLBS1033 solution and tied with a string. The small intestine was isolated and placed into a chamber that contained 50 mL of saline. A sample of 600 μL solution was taken out of the chamber at different time points, to be analyzed using protease assay, zymography, and fibrin plate assay. The removed solution was then replaced with fresh saline.
Radiolabeling and purification of 99mTc-DLBS1033
Direct labeling on DLBS1033 was done using Na99mTcO4. First, a mixture of 50 μg SnCl2 · 2H2O and 500 μL of DLBS1033 were prepared in PBS (pH 6–7). Then, Na99mTcO4, with radioactivity of ±3–5 mCi, was added into the mixture and mixed by vortex for 1 minute. The radioactivity of the mixture was measured with a Deluxe Isotope Calibrator II (Victoreen Inc, Modling, Austria). The mixture was then incubated for 15 minutes at 37°C. The Direct labelling resulted in 99mTcO4-DLBS1033. However, there were 99mTcO4- that are not attached to the DLBS1033. Therefore, the purity of 99mTcO4-DLBS1033 from the excess 99mTcO4− was determined using paper chromatography, with Whatman® cellulose chromatography paper 3mm Chr sheets (Sigma-Aldrich, St Louis, USA) as the stationary phase and acetone-free water as the mobile phase. After the chromatography process was done, the Whatman 3MM paper was dried and cut in 1 cm lengths. The pieces of paper were measured for radioactivity, using an ORTEC 4890 preamplifier sca (Ortec, Milan, Italy) to determine the retardation factor (Rf) and purity.
The 99MTc-DLBS1033 was purified using Sephacryl S-300HR, eluted with PBS pH 7.4, and collected into fractions. Fractions that contained 99MTc-DLBS1033 were collected and used for the blood clearance study.
In vivo blood clearance study of 99mTc-DLBS1033
The rats were given 0.01 mCi/mL of 99mTc-DLBS1033 intravenously (IV). Blood sampling was performed at ten time points (5, 10, 15, 20, 30, 45, 60, 90, 120, and 180 minutes) following the injection. Each blood sample was weighed, and its radioactivity was measured with the single-channel analyzer apparatus. Blood clearance analysis was done following the two-compartment model and calculated using Multifit Software (University of Groningen, Groningen, Netherlands). The resulting data was expressed as plasma concentration (cps/g) as a function of time.
Formulation and physical characteristics
Enteric-coated tablet formulation of DLBS1033
The enteric coated tablet formulation of DLBS1033 for this study was prepared at Dexa Laboratories of Biomolecular Sciences. The core tablet of DLBS1033 was obtained by direct compression of a mixture composed of DLBS1033, croscarmellose sodium, talc, and magnesium stearate. Methacrylic acid copolymer was used as the enteric coating agent. The coating suspension was composed of methacrylic acid copolymers 23.06% weight/weight (w/w), Eurolake Ponceau 4R 0.76% (w/w), talc 6.20% (w/w), titanium dioxide 0.67% (w/w), propylene glycol 2.34% (w/w), and purified water 67.1% (w/w).
Disintegration and dissolution study of DLBS1033 enteric coated formulation
A disintegration test was performed to examine the tablet’s integrity in a simulation of gastric fluid. The tablet disintegration test of the DLBS1033 enteric coated tablet (Dexa Medica, Tangerang, Indonesia) was performed, as described in USP XXXI, 31st edition of United States Pharmacopeia using a disintegration tester type ZT 222 (Erweka GmbH, Heusenstamm, Germany).12 Six enteric coated tablets were placed into different tubes containing 0.1 N of hydrochloric acid fuming 37% (Merck Millipore, Billerica, USA), without a disk, for 2 hours, after which the tablets were placed in PBS pH 6.8, with a disk, in each tube. The disintegration media used for this test was maintained at 37°C±2°C. The time required for disintegration of the enteric coated tablet was measured and recorded.
The DLBS1033 enteric coated tablets dissolution profile was evaluated, using a DT-700 dissolution apparatus, type 2 (paddle) (Erweka, Heusenstamn, Germany) and using two different media: HCl 0.1 N, which represented the human stomach condition, for 120 minutes and subsequently, PBS pH 6.8, which represented the condition in human intestine, for 40 minutes; both analyses were done at 37°C±0.5°C.
Results
Solubility and stability of DLBS1033 in different pH
This assay was performed to determine the solubility and stability of DLBS1033 in different pH conditions. Table 1 shows the protease activity and protein content of DLBS1033 in different pH media. The protease activity and protein content of DLBS1033 in sodium acetate buffer were lower than that of DLBS1033 in purified water and PBS. Lower protease activity was also apparent from the weaker band in the zymogram result of in vitro sample in sodium acetate buffer compared with that in purified water and PBS (Figure 1).
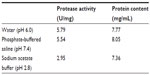  | Table 1 Protease activity and protein content of DLBS1033 in different media |
Ex vivo and in vivo gastric stability of DLBS1033
Table 2 shows that the isolated gastric fluid only contained a very small amount of protein with no proteolytic enzyme activity. The mixture of DLBS1033 sample and gastric fluid showed similar protease activity and protein content to the in vitro result of the DLBS1033 sample in sodium acetate buffer.
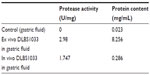  | Table 2 DLBS1033 solubility in gastric fluid |
The zymography result of the in vivo samples (Figure 2) showed extensive degradation of the DLBS1033 protein fraction compared with that found in the in vitro and ex vivo samples. This result can be inferred from the small amount of protein and weak protease activity compared with that of the ex vivo condition.
Intestinal permeability assay of DLBS1033
This assay was performed to investigate transport of DLBS1033 across the intestinal membrane. The results showed that the active proteolytic components of DLBS1033 were transported through the rat intestine in intact form. The zymogram result (Figure 3) showed that the proteolytic enzyme activity appeared after 20 minutes of incubation and continued until the 150th minute.
  | Figure 3 Zymogram of permeated DLBS1033 at different time points. |
The fibrin plate result showed fibrinolytic activity of DLBS1033 (Figure 4). The pattern on the fibrin plate showed a similar pattern to that of the zymogram ie, DLBS1033 was detected in the medium outside the intestine after 20 minutes of incubation and gradually increased until the 150th minute. This is shown with the increment of lysed fibrin volume, due to the enhancement of fibrinolytic activity, in the samples (Figure 5).
  | Figure 5 Fibrin plate result of DLBS1033 after intestinal permeability assay. |
Radiolabelling and purification of 99mTc-DLBS1033
The result of labelling Na-99mTcO4 to DLBS1033 was 99mTc-DLBS1033. Two possible impurities were 99mTcO2 and 99mTcO4−. The 99mTcO2 fractions represented the free reduced Tc form, whereas 99mTcO4− represented Tc that did not react with DLBS1033. The purity of 99mTc-DLBS1033 was determined using the paper chromatography method. Figure 6 shows that paper chromatography was used to separate 99mTcO4− from 99mTc-DLBS1033. This is shown from the different Rf values for 99mTc-DLBS1033 and 99mTcO4−. While 99mTcO2 was inseparable from 99mTc-DLBS1033, the percentage of purity of the 99mTc-DLBS1033 and 99mTcO2 combination was 99.93%±0.015%. Further purification, using Sephacryl column chromatography, was done to separate 99mTc-DLBS1033 and 99mTcO2. Pure 99mTc-DLBS1033 was obtained, with a 70.56% yield.
  | Figure 6 Paper chromatography profile of 99mTc-DLBS1033. |
Determination of circulation half-life of 99mTc-DLBS1033
Figure 7 shows the percentage of radioactivity remaining in the blood as a function of time. As indicated in Figure 7, the radioactivity of the samples reached the baseline after 180 minutes.
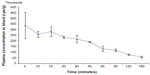  | Figure 7 Blood clearance of 99mTc-DLBS1033 in rats (n=3). |
The in vivo clearance study showed that after 1 hour, 55% of injected radioactivity remained in the circulation. The biological half-life of the 99mTc-DLBS1033 was found to be 70 minutes, or 1.16 hours.
Disintegration and dissolution study of DLBS1033 enteric coated formulation
Disintegration studies and analysis of the dissolution profile of the enteric coated tablet formulation of DLBS1033 were performed in acidic as well as neutral environments. Based on the disintegration as well as dissolution tests in acidic medium (pH 2.8), all tablets showed no sign of disintegration or leakage. However, in the PBS medium at pH 7.4, the tablets disintegrated completely before 30 minutes. These expected results were depicted in Figure 8, while the release percentage in PBS pH 6.8 increased gradually from 0 to 40 minutes (Figure 9). The t80 value, or the time where the released percentage had reached 80%, was achieved between 20 to 30 minutes, while the t90 (where the released percentage reached 90%) was found between 30 to 40 minutes. The cumulative percentage released at the end of the study (40 minutes) was 93.72%, as shown in Figure 9.
  | Figure 8 Drug-release profile of DLBS1033 enteric coated tablet in acidic medium (pH 2.8). |
  | Figure 9 Drug-release profile of DLBS1033 enteric coated tablet in phosphate buffer medium (PBS) (pH 7). |
Discussion
It is generally believed that digestive enzyme activity plays a critical role in the inactivation of enzymes/proteins in the GI tract.13 DLBS1033 was designed to be given by oral administration; therefore its stability in gastric fluid in the presence of highly active digestive enzymes needed to be analyzed. This paper shows the results of in vitro, ex vivo, and in vivo studies of DLBS1033 in simulated GI conditions, undertaken to assess the physicochemical characteristic and stability of DLBS1033 for oral administration.
In the in vitro study, DLBS1033 was mixed into solutions of different pH to assess its solubility and stability with respect to pH. The sample extracted with sodium acetate buffer showed fewer proteolytic bands on the zymograph gel; further, the intensity of the existing bands was not as strong as that seen with samples extracted with purified water and PBS (Figure 1). The zymogram and SDS PAGE results further confirmed the low solubility and instability of DLBS1033 in acidic solution. These results confirm our previous report by Trisina et al, describing that DLBS1033 was stable in neutral to alkaline pH conditions.1
The ex vivo data was in line with the in vitro result (Figures 1 and 2) – both studies showed similar protease activity and protein content of DLBS1033 in gastric fluid mixture. It was also discovered that the isolated gastric fluid contained a very small amount of protein (0.023 mg/mL) with no proteolytic activity. This might have been caused by reduced secretion of gastric protease due to the fasting conditions of the rats prior to the experiment. This result supports the in vitro data regarding the protein instability in low pH, though it did not provide sufficient explanation of the effects of digestive enzymes on DLBS1033.
An in vivo gastric experiment was performed to further analyze the stability of DLBS1033 in the stomach since in vivo study is best for describing the real interaction between DLBS1033 and gastric fluid components, such as gastric acid and gastric protease. The in vivo data showed that DLBS1033 was degraded in the presence of gastric protease (Table 2).
Poor bioavailability through the GI tract has always been a major challenge for oral delivery of therapeutic proteins.14 This poor bioavailability is generally due to presystemic enzymatic degradation, poor membrane penetration of the GI mucosa, hepatic metabolism, and the unique physicochemical characteristics of protein drugs.15,16 However, previous studies have suggested that lumbrokinase could be absorbed in its intact form through the intestinal epithelium and mostly absorbed, by passive diffusion, through the paracellular route.2,17,18 Various methods have been developed to detect the absorption of lumbrokinase from the intestine, using antibody detection,2 fluorescence markers,18,19 western blotting, and fibrin zymography.17 In this study, absorption of the active components of DLBS1033 to the intestines was assayed using isolated intestines, and the activity was detected with fibrinogen zymography and fibrin plate assay. The study of intestinal absorption was done to ensure that the absorbed DLBS1033 active components were in their intact and active form. The fibrinogen zymogram result revealed components of DLBS1033 with fibrinogenolytic activity that could be preserved after absorption. The fibrin plate result also confirmed that the presence of fibrinogenolytic activity in the fibrin plate assay was due to the DLBS1033 sample that was absorbed into the intestine.
99mTc-labeled DLBS1033 (99mTc-DLBS1033) was given through the IV route to the tested laboratory animals. Among the available methods for studying blood clearance of drugs in animals, radiolabeling technique using 99mTc has been one of the preferred options due to its several advantages, such as favorable radiation characteristics, relatively low cost, ready availability, and also easy preparation of stable conjugates.
In vivo clearance in rats revealed that there was quite a rapid wash out of the 99mTc-DLBS1033 from the blood circulation (Figure 7). The half-life of 99mTc itself reached 6 hours so the decreasing radioactivity count from the blood was caused more by the clearance of 99mTc-DLBS1033 and not by radioactivity decay.
The short in vivo half-life of currently available thrombolytic agents, such as streptokinase (30 minutes) and tissue plasminogen activator (tPA) (5 minutes) often limit their efficacies as an efficient blood clot dissolving agent.20 Thus, with longer biological half-life (70 minutes), DLBS1033 could be more effective for thrombolytic therapy.
According to Martignoni et al,21 generally, small animals tend to eliminate drugs or foreign substances more rapidly than humans. This is due to their higher relative amount of hepatic enzymes compared with humans. Therefore, the biological half-life of DLBS1033 in human is most likely to be longer than 70 minutes. An allometric equation by Sarver et al was used to estimate the biological half-life in humans, based on the biological half-life data in rats.22 Based on this equation, the biological half-life of DLBS1033 in humans is estimated to be around 4.9 hours.
Although the active components of DLBS1033 can be absorbed through the intestine, exposure of DLBS1033 to the gastric environment can cause significant degradation and thus reduce the protein bioavailability. To ensure that DLBS1033 can be delivered safely through the stomach without any degradation and reach the intestine in its intact and active form, DLBS1033 must be formulated properly. One way to protect DLBS1033 from the gastric environment and modify its release of the drug substance is with use of an enteric coating. This was done by applying methacrylic acid copolymers on DLBS1033 as an enteric coating layer. The coating layer is deemed to be a delayed-release dosage format that plays a particular role in optimizing the oral delivery of DLBS1033 so that the drug can be efficiently absorbed in the designated compartment.
An aqueous dispersion of methacrylic acid copolymers is commonly used as a coating agent in the pharmaceutical industry. Because it possesses a free carboxylic acid group on its polymer backbone, methacrylic acid exhibits a differential pH solubility profile. It is almost insoluble in aqueous medium at low pH, but in the condition where the pH increases to a specific pH, it experiences a sharp, well-defined increase in solubility. Methacrylic acid copolymers are soluble in intestinal fluid, from pH 5.5, therefore the active ingredient will be released in the upper part of the small intestine after the drug has passed through the stomach. Use of propylene glycol as a plasticizer is essential in improving the flexibility of the polymer and reducing the brittleness caused by the polymers.33 In this role, the coating agent acts as a barrier for the drug against the high acidity of gastric juice and maintains the drug until it is absorbed in the small intestine. Therefore, the therapeutic agent can stay in its optimal condition to confer its biological activity in promoting blood flow.
Drug absorption from its solid dosage form after oral administration depends on the release of drug substance from the formula, the dissolution or solubility of the drug under physiological conditions, and the permeability across the GI tract. In vitro tests, including disintegration and dissolution tests, were performed to assess the physical attributes of the enteric coating formulation in protecting DLBS1033 protein from the harsh conditions in the stomach. DLBS1033 coated tablets were subjected to disintegration tests, using an acidic medium to represent gastric conditions, followed by PBS pH 6.8, which represented intestine conditions. Based on the disintegration test in acidic medium for 2 hours, all tablets showed no sign of disintegration or leakage (Figure 2). However, in the PBS medium, the tablets disintegrated completely before 30 minutes (Figure 3). This result showed the superior physical protection of methacrylic acid copolymers as an enteric coating agent against gastric fluid. The dissolution profile of the DLBS1033 enteric coated tablet showed excellent physical resistance to acidic medium, with a release percentage of 0% in 2 hours (Figure 2). Altering the dissolution medium to simulate small intestine condition (PBS pH 6.8) led to rapid release of the DLBS1033 protein component. The rapid release of protein will support the absorption of the active component in the designated compartment. Aside from protecting the drug from gastric conditions and promoting optimal drug absorption in the designated compartment, the coating process is also applied to DLBS1033 for several other reasons, such as protection of the drug from the environment (moisture, air, light), to improve stability, for taste masking, to minimize patient/operator contact with drug substance, skin allergies to improve product identity and appearance, to improve ease of swallowing, to lessen mechanical resistance, and to reduce abrasion and attrition during handling.24
Aside from delivery factors, one of the toughest hindrances of the available thrombolytic therapies is the short half-life and rapid clearance of the respective therapeutic proteins.25,26 It is known that therapeutic proteins are removed from circulation via numerous pathways: degradation by proteolysis, Fcγ receptor-mediated clearance, target-mediated clearance, nonspecific endocytosis, and the formation of immune complexes followed by complement- or Fc receptor-mediated clearance mechanisms.27 Previous study has shown that one of the important inhibitors of proteolytic-based therapeutic proteins, including lumbrokinase, is α2-macroglobulin. High concentrations of α2-macroglobulin in the circulation can inhibit almost all four classes of proteins – cysteine proteases, serine proteases, aspartyl proteases, and metalloproteases.28
Aside from the physical properties of DLBS1033 in different pHs, its appropriate formulation, as well as its disintegration and dissolution profile; the half-life, and clearance of therapeutic proteins in the blood circulation are also considered to be important factors in determining the success of thrombolytic agents. This study evaluated the half-life and blood clearance profile of DLBS1033, using an animal model. The study utilized radioactive labeling to observe the blood clearance of DLBS1033 and to evaluate the half-life period of DLBS1033.
Conclusion
In the gastric compartment, DLBS1033 was degraded through acidic and enzymatic cleavage. The intestinal environment seems to be the most appropriate location to release and to absorb this active protein. Therefore, an enteric coating formulation using a methacrylic acid copolymer is a good approach, not only to prevent premature release of DLBS1033 after oral administration, but also, to promote the release of the protein for intestinal absorption. In addition, a relatively long biological half-life of this active protein after oral administration and intestinal absorption further supports the formulation of DLBS1033 in an enteric coated dosage form.
Acknowledgments
The authors thank Audrey Clarissa, Venni Carolina, and Hanna Christabel Rouli for careful reading of the manuscript.
Disclosure
The authors report no conflict of interest in this work.
References
Trisina J, Sunardi F, Suhartono MT, Tjandrawinata RR. DLBS1033, a protein extract from Lumbricus rubellus, possesses antithrombotic and thrombolytic activities. J Biomed Biotechnol. 2011;2011:519652. | |
Fan Q, Wu C, Li L, et al. Some features of intestinal absorption of intact fibrinolytic enzyme III-1 from Lumbricus rubellus. Biochim Biophys Acta. 2001;1526(3):286–292. | |
Sukandar EY, Anggadireja K, Sigit JI, Adnyana IK, Tjandrawinata RR. Toxicity studies of a bioactive protein with antithrombotic-thrombolytic activity, DLBS1033. Drug Chem Toxicol. 2014;37(1):8–16. | |
Tjandrawinata RR, Arifin PF, Tandrasasmita OM, Rahmi D, Aripin A. DLBS1425, a Phaleria macrocarpa (Scheff.) Boerl. extract confers anti proliferative and proapoptosis effects via eicosanoid pathway. J Exp Ther Oncol. 2010;8(3):187–201. | |
Tjandrawinata RR, Suastika K, Nofiarny D. DLBS3233 extract, a novel insulin sensitizer with negligible risk of hypoglycemia: A phase-I study. Int J Diabetes and Metab. 2012;21:13–20. | |
Shaji J, Patole V. Protein and peptide drug delivery: oral approaches. Indian J Pharm Sci. 2008;70(3):269–277. | |
Huyghebaert N, Vermeire A, Neirynck S, Steidler L, Remaut E, Remon JP. Development of an enteric-coated formulation containing freeze-dried, viable recombinant Lactococcus lactis for the ileal mucosal delivery of human interleukin-10. Eur J Pharm Biopharm. 2005;60(3):349–359. | |
Shastri PN, Ubale RV, D’Souza MJ. Implementation of mixture design for formulation of albumin containing enteric-coated spray-dried microparticles. Drug Dev Ind Pharm. 2013;39(2):164–175. | |
Hashmat D, Shoaib MH, Mehmood ZA, Bushra R, Yousuf RI, Lakhani F. Development of enteric coated flurbiprofen tablets using Opadry/Acryl-Eze system – a technical note. AAPS PharmSciTech. 2008;9(1):116–121. | |
Porter SC. Coating of pharmaceutical dosage forms. In: Troy DB, editor. Remington: The Science and Practice of Pharmacy. 21st ed. Baltimore, MD: Lippincott Williams & Wilkins; 2006:929–938. | |
Guo HX, Heinämäki J, Yliruusi J. Diffusion of a freely water-soluble drug in aqueous enteric-coated pellets. AAPS PharmSciTech. 2002;3(2):E16. | |
The United States Pharmacoppia, 31st ed. United States Pharmacopoeia convention, Inc., Rockville (2008). | |
Prusty AK, Sahu SK. Biodegradable nanoparticles – a novel approach for oral administration of biological products. International Journal of Pharmaceutical Sciences and Nanotechnology. 2009;2(2):503–508. | |
Mustata G, Dinh SM. Approaches to oral drug delivery for challenging molecules. Crit Rev Ther Drug Carrier Syst. 2006;23(2):111–135. | |
Mahato RI, Narang AS, Thoma L, Miller DD. Emerging trends in oral delivery of peptide and protein drugs. Crit Rev Ther Drug Carrier Syst. 2003;20(2–3):153–214. | |
Hamman JH, Enslin GM, Kotzé AF. Oral delivery of peptide drugs: barriers and developments. BioDrugs. 2005;19(3):165–177. | |
Yan XM, Kim CH, Lee CK, Shin JS, Cho IH, Sohn UD. Intestinal absorption of fibrinolytic and proteolytic lumbrokinase extracted from earthworm, Eisenia andrei. Korean J Physiol Pharmacol. 2010;14(2):71–75. | |
Yu Q, Li P, Yang Q. Improving the absorption of earthworm fibrinolytic enzymes with mucosal enhancers. Pharm Biol. 2010;48(7):816–821. | |
Cheng MB, Wang JC, Li YH, et al. Characterization of water-in-oil microemulsion for oral delivery of earthworm fibrinolytic enzyme. J Control Release. 2008;129(1):41–48. | |
Wu XC, Ye R, Duan Y, Wong SL. Engineering of plasmin-resistant forms of streptokinase and their production in Bacillus subtilis: streptokinase with longer functional half-life. Appl Environ Microbiol. 1998;64(3):824–829. | |
Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2(6):875–894. | |
Sarver JG, White D, Erhardt P, Bachmann K. Estimating xenobiotic half-lives in humans from rat data: influence of log P. Environ Health Perspect. 1997;105(11):1204–1209. | |
Snejdrova E, Dittrich M. Pharmaceutical applications of plasticized polymers. In: Luqman M, editor. Recent Advances in Plasticizers. Rijeka: InTechopen.com; 2012:69–82. Available from: http://www.intechopen.com/books/recent-advances-in-plasticizers/pharmaceutical-applications-of-plasticized-polymers. Accessed June 23, 2014. | |
Lee BJ. Pharmaceutical preformulation: physicochemical properties of excipients and powders and tablet characterization, In: Cox Gad S, editor. Pharmaceutical Manufacturing Handbook: Production and Processes. Hoboken, NJ: John Wiley & Sons, Inc.; 2007:879–931. | |
Chandler WL, Alessi MC, Aillaud MF, Henderson P, Vague P, Juhan-Vague I. Clearance of tissue plasminogen activator (TPA) and TPA/plasminogen activator inhibitor type 1 (PAI-1) complex: relationship to elevated TPA antigen in patients with high PAI-1 activity levels. Circulation. 1997;96(3):761–768. | |
Köhler M, Sen S, Miyashita C, et al. Half-life of single-chain urokinase-type plasminogen activator (scu-PA) and two-chain urokinase-type plasminogen activator (tcu-PA) in patients with acute myocardial infarction. Thromb Res. 1991;62(1–2):75–81. | |
Vugmeyster Y, Xu X, Theil FP, Khawli LA, Leach MW. Pharmacokinetics and toxicology of therapeutic proteins: Advances and challenges. World J Biol Chem. 2012;3(4):73–92. | |
Wu C, Li L, Zhao J, Fan Q, Tian WX, He RQ. Effect of alpha2M on earthworm fibrinolytic enzyme III-1 from Lumbricus rubellus. Int J Biol Macromol. 2002;31(1–3):71–77. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



