Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Biliverdin Protects Against Cerebral Ischemia/Reperfusion Injury by Regulating the miR-27a-3p/Rgs1 Axis
Authors Li J, Peng L, Bai W, Peng P, Chen W, Yang W, Shao J
Received 6 January 2021
Accepted for publication 2 April 2021
Published 22 April 2021 Volume 2021:17 Pages 1165—1181
DOI https://doi.org/10.2147/NDT.S300773
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jun Chen
Junjie Li,* Lijia Peng,* Wenya Bai, Peihua Peng, Wendong Chen, Wei Yang, Jianlin Shao
Department of Anesthesiology, First Affiliated Hospital, Kunming Medical University, Kunming City, 650032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianlin Shao
Department of Anesthesiology, First Affiliated Hospital of Kunming Medical University, 295 Xichang Road, Wuhua, Kunming, Yunnan, 650000, People’s Republic of China
Tel +86-871-6532488-2755
Email [email protected]
Background: We have previously demonstrated that biliverdin has neuroprotective effects that ameliorate cerebral ischemia/reperfusion (I/R) injury in rats. However, the underlying mechanism is unknown. This study aimed at elucidating on the modulatory role of miR-27a-3p on Rgs1 as a mechanism by which biliverdin affects cerebral I/R injury.
Methods: Middle cerebral artery occlusion/reperfusion (MCAO/R) was used to establish I/R rat models while oxygen glucose deprivation/reoxygenation (OGD/R) was used to induce hippocampal neurons to establish I/R models in vitro. Infarct volume was assessed by TTC staining. Apoptotic analyses of ischemic cortical neurons and cells were performed by TUNEL staining and flow cytometry, respectively. Cell viability was assessed by the CCK-8 assay while the target of miR-27a-3p was determined by double luciferase reporter assay. Relative expression levels of miR-27a-3p and Rgs1 (in vivo and in vitro) as well as markers of inflammation and apoptosis (in vitro) were detected by RT-qPCR. Then, Elisa and western blot were used to assess protein expression levels of inflammatory and apoptotic markers in vitro.
Results: Biliverdin suppressed inflammation and apoptosis in hippocampal neurons upon OGD/R, and reduced cerebral infarction volume as well as apoptosis in the MCAO/R rat model. Furthermore, biliverdin upregulated miR-27a-3p and downregulated hippocampal neuron Rgs1 after OGD/R as well as in rat brain tissues after cerebral I/R. Bioinformatic analysis revealed an miR-27a-3p docking site in the 3ʹ-UTR region of Rgs1. Luciferase reporter assays showed that Rgs1 is an miR-27a-3p target. Moreover, miR-27a-3p upregulation inhibited OGD/R-triggered inflammation and suppressed neuronal apoptosis. Rgs1 knockdown suppressed OGD/R-triggered inflammation and decreased neuronal apoptosis while miR-27a-3p downregulation reversed the protective effect of Rgs1 knockdown. Moreover, miR-27a-3p overexpression and Rgs1 silencing suppressed NF-κB (p65) expression.
Conclusion: Biliverdin protects against cerebral I/R injury by regulating the miR-27a-3p/Rgs1 axis, thereby inhibiting inflammation and apoptosis.
Keywords: cerebral ischemia/reperfusion, biliverdin, miR-27a-3p, Rgs1, inflammation, apoptosis
Introduction
Globally, stroke, a common cerebrovascular disease, is the 2nd main cause of mortalities and the main cause of disabilities.1 Among them, ischemic stroke accounts for the highest proportion of all strokes, and in China, the proportion was as high as 87%.2 Blood flow reperfusion promotes injury or dysfunction in some ischemic brain tissues, resulting in cerebral ischemia/reperfusion (I/R) injury.3 Due to its complexity, there are no effective preventive and therapeutic options for cerebral I/R injuries. During reperfusion, intracellular calcium overload, reactive oxygen accumulation, neurotoxic effects of excitatory amino acids, neuroinflammation, and apoptosis combine to cause serious or even irreversible damage to ischemic brain tissues.4 Inflammation and apoptosis are the main causes of nerve cell damage after I/R.5 A series of intracellular signaling molecules are activated by I/R, which activates related signaling pathways to promote inflammation and apoptosis. For instance, nuclear factor-mediated signaling constitutes the most frequent inflammatory cascade. Entry of phosphorylated NF-κB/p-p65 into the nucleus promotes the generation of TNF-α, IL-6, and IL-1β, which elevates ischemic brain tissue inflammation.6 Therefore, inhibition of reperfusion injury-associated neuroinflammation and apoptosis after ischemic stroke is an attractive strategy for stroke treatment.
In vitro and in vivo studies have shown that Biliverdin, a catalytic product of heme oxygenase-1 has strong anti-inflammatory and anti-apoptotic effects. It inhibits LPS-induced inflammation and relieves acute lung injury in rats.7 In addition, it can also alleviate septica-induced intestinal diseases by regulating inflammatory cascades and significantly reducing gene expression levels of inflammatory cytokines.8 Moreover, exogenous biliverdin suppresses the number of inflammatory cells and inflammatory protein (MMP-2 and MMP-9) expression, thereby inhibiting inflammatory responses and reducing apoptosis in corneal cells.9 We have previously shown that biliverdin inhibits inflammatory cytokine expression in ischemic brain tissues, thereby reducing cerebral infarction volume in rats after cerebral I/R injury.10 However, the anti-inflammatory and anti-apoptotic mechanisms of biliverdin have not yet been elucidated.
MiRNA comprises a class of endogenous non-coding small RNA molecules comprising 21–25 nucleotides. MiRNA is capable of inhibiting protein expression by specifically binding one or more 3 ʹ-UTR mRNA sequences, thereby regulating gene expression post-transcriptionally.11,12 MiRNAs influence the pathogenesis of various diseases, including nervous system disorders, and are, therefore, considered potential therapeutic targets in the nervous system.13 Cerebral ischemia/reperfusion injury alters the expression profile of many miRNAs that may be involved in the injury or recovery processes.14,15 MiR-27a-3p, located on chromosome 19, is thought to be oncogenic in various malignancies, including colorectal, stomach, and esophageal cancers.16 However, studies have evaluated the anti-inflammatory, anti-apoptotic and proliferative effects of miR-27a-3p in non-neoplastic diseases. MiR-27a-3p has been found to be poorly expressed in cartilage tissues of arthritic rats, and its upregulation significantly suppresses the expression of pro-inflammatory factors, TNF-α and IL-6.17 MiR-27a-3p upregulation has been shown to inhibit LPS-triggered acute lung injury in mice by suppressing apoptosis through TLR4/MyD88/NF-κB signaling regulation.18 Additionally, miR-27a-3p represses inflammatory responses by targeting TLR4, thereby suppressing the generation of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) in hyperglycemic retinal pigment epithelial cells (RPE).19 MiR-27a-3p upregulation was shown to target PLK2 to inhibit knee arthritis associated chondrocyte apoptosis in rats.20 However, its role in cerebral I/R injury along with the underlying mechanisms have not been elucidated. Previously, we reported that miR-27a-3p is significantly downregulated in the ischemic cortex of rats with cerebral I/R injuries and that biliverdin treatment significantly upregulates its expression. Bioinformatic analysis revealed that Regulating G protein signaling 1 (Rgs1) may be an miR-27a-3p target.21 Rgs1 is postulated to be a negative modulator of G-protein-coupled receptor signaling.22 Rgs1 regulates macrophage accumulation in atherosclerotic lesions and plays a catalytic role in leukocyte transport and vascular inflammation.23 In addition, Rgs1 silencing was shown to suppress inflammatory responses by inactivating TLR4 signaling and reducing the quantities of pro-inflammatory factors like TNF-α, IL-1β, and IL-17 in rheumatoid arthritis rat models.24 However, the role of Rgs1 in ischemic stroke has not been established.
In this study, we evaluated the effects of biliverdin in hippocampal neurons administered with oxygen-glucose deprivation/reoxygenation (OGD/R) in vitro as well as in a rat model of middle cerebral artery occlusion/reperfusion (MCAO/R). The in vivo and in vitro effects of biliverdin on miR-27a-3p and Rgs1 were also evaluated. This study offers novel insights into the mechanisms by which biliverdin improves cerebral I/R injury.
Materials and Methods
Experimental Animals and Groups
Adult male Sprague-Dawley rats, weighing 200–240 g, were acquired from the department of experimental animals, Kunming Medical University (Kunming, China). They were housed in standard cages with a 12-hour light/dark cycle, at 21–25°C, 45–50% humidity, with ad libitum access to water, as well as food. Ethical approval for animal use in this study was provided by the animal ethical committee of Kunming Medical University (NO. KMMU2020005). Animal procedures adhered to the NIH guidelines for Laboratory Animal Care and Safety. We randomly assigned the rats into the sham group (neck incision only, no cerebral I/R and treatment, n=15), the MCAO/R group (given cerebral I/R and saline treatment, n=15) and the MCAO/R+BV group (given cerebral I/R and biliverdin treatment, n=15).
Middle Cerebral Artery Occlusion/Reperfusion (MCAO/R) Model
The MCAO/R model was established as previously described.10 Briefly, rats were intraperitoneally administered with 10% chloral hydrate (100 mg/kg; Kemiou Chemical Reagent Co. Ltd., Tianjin, China) for anesthetization. The right common carotid artery (CCA), external carotid artery (ECA) along with the internal carotid artery (ICA) were exposed and carefully separated. The CCA and ECA were ligated after which we inserted a nylon thread wrapped with polylysine (0.24 mm diameter) (Cinontech Co. Ltd., Beijin, China) into the ICA from the ECA stump. The sham group rats were subjected to the same procedure, but without insertion of the nylon thread. Two h after tMCAO, the nylon thread was withdrawn to restore cerebral blood flow. Upon being awakened, rats were evaluated using the Zea-Longa scoring system,25 where 4 points = failure to spontaneously walk or loss of consciousness, 3 = paw tilted inwards when walking, 2 = paw rotated inwards when walking, 1 = side front paw could not fully stretch, 0 = no signs of neurologic impairment. We excluded rats with scores of 0 or 4 points from the study.
2, 3, 5-Triphenyl-Tetrazolium Chloride (TTC) Staining
TTC staining was used to assess infarct volume in the ischemic brain (n=5 for each group). Briefly, brain tissues were collected 24 h after reperfusion and sliced into 5 coronal sections (2 mm thick) that were inoculated in 2% TTC (Jiancheng Technology Co. Ltd., Nanjing, China) and incubated for 20 min at 37°C. Then, the sections were rinsed in PBS 1X and fixed in 4% PFA at room temperature for 24 h. After acquiring the color images of the segments, Image J version 1.43 was used to assess infarct area on each section and to calculate percentage cerebral infarct volume as follows: (contralateral hemisphere volume-volume of non-ischemic ipsilateral hemisphere)/the contralateral hemisphere, as previously reported.26
TUNEL Staining
Apoptotic analysis of ischemic cortical neurons was done by TUNEL staining. After 24 h of reperfusion, brain tissues were fixed (n=5 for each group) in 4% PFA for 48 h and embedded in paraffin. Then, they were cut into several coronal sections by a secant and TUNEL staining (Servicebio Technology Co. Ltd., Wuhan, China) performed according to the manufacturer’s instructions. Sections were then stained with DAPI. TUNEL (red) and DAPI positive (blue) cells were examined by fluorescence microscopy (Observer.A1, ZEISS, Germany). Five 400 X different visual fields were randomly selected to count the TUNEL positive cells and the total number of cells in each visual field; then, the positive rate of apoptotic cells per field was calculated.
Cell Culture
HEK-293T cells (KCB 200744YJ, Kunming, China) were acquired from Kunming Institute of Zoology, and cultured in DMEM enriched with 10% FBS (Sigma-Aldrich Inc., MO, USA). Primary hippocampal neurons from neonatal SD rats were cultured as previously described.27 Briefly, the hippocampus was resected, and hippocampal neurons isolated in trypsin-EDTA (0.25%), after which they were cultured in neurobasal media (21,103–049, GIBCO, Thermo Fisher Scientific Inc., MA, USA) supplemented with GlutaMAX and 2% B-27 plus glucose (4.5 g/L) for 7 days. They were cultured for 14 more days in media supplemented with 15 mM glucose, 5% equine serum (Sigma-Aldrich), and 5% FBS (Sigma-Aldrich). Cells were grown in a humidified incubator at 37°C, 5% CO2.
OGD/R Model of Hippocampal Neurons
An in vitro cerebral I/R injury model was created using the OGD/R method as previously described.28 Normal culture medium was replaced with sugar-free DMEM, after which hippocampal neurons were cultured at 37°C under low oxygen levels (1% O2 + 94% N2 + 5% CO2) in a tri-gas incubator (CCL-170T-8; Esco Micro Pte. Ltd., Singapore). After 2 h of culture, the culture medium was replaced with normal culture media and cultured at normal oxygen levels (95% air + 5% CO2) for 0–12 h. Cell viability was evaluated at 3, 6, 9, and 12 h after reoxygenation.
Biliverdin (BV) Treatment
BV hydrochloride (Merck & Co., USA) was dissolved in 0.2 M NaOH and pH adjusted to 7.4 using HCl. Study animals were intraperitoneally injected with 35 mg/kg BV (in 2 mL) 15 min prior to reperfusion, and at 4 h, 12 h and 20 h after reperfusion. Cells were treated with BV at 0.3, 1, 2, 3, 5, and 10 μg/mL. Cell viability after OGD/R was then assessed, and the optimal concentration selected for cell treatment during reoxygenation.
Cell Transfection
MiR-27a-3p-mimic (miR-mimic, sense sequence: 5ʹ-UUCACAGUGGCUAAGUUCCGC-3ʹ, antisense sequence:5ʹ-GCGGAACUUAGCCACUGUGAA-3ʹ), miR-27a-3p-mimic negative control (miR-mimic-NC, sense sequence: 5ʹ-UUUGUACUACACAAAAGUACUG-3ʹ, antisense sequence: 5ʹ-CAGUACUUUUGUGUAGUACAAA-3ʹ), miR-27a-3p-inhibitor (miR-inhibitor, sequence: 5ʹ-GCGGAACUUAGCCACUGUGAA-3ʹ), miR-27a-3p-inhibitor negative control (miR-inhibitor-NC, sequence: 5ʹ-CAGUACUUUUGUGUAGUACAAA-3ʹ) (Ribobio Biotechnology Co. Ltd., Guangzhou, China) and anti-Rgs1 short hairpin RNA (shRgs1, sequence: 001-GCATATCTAAGATCTATGATC, 002-GGGATGAAATCGTCCAAGTCC, 003-GGCTTGTGAAGACTATAAGAA, 004-GTCGAATTCAGTGAAGAGAAC) (GeneCopoeia Inc., Guangzhou, China) were synthesized. Then, they were transfected into cells using Polybrene lentivirus (Genechem Co., Ltd, Shanghai, China) according to manufacturer’s instructions 72 h before OGD/R.
Cell Viability Assessment
CCK-8 assessment was used to evaluate cell viability. To this end, 10×103 cells/well were plated onto 96-well plates and 10 μL CCK-8 solution (CK04-500T, Dojindo, Japan) added. Afterwards, they were incubated at 37°C for 1 h and absorbance read at 450 nm using a microplate reader. Cell viability was determined as follows: cell viability = (OD450 value of normal cells - OD450 value of experimental group)/OD450 value of normal cells × 100%.
Annexin V-FITC/PI Staining and Flow Cytometry
The Annexin V-FITC apoptosis kit (556,547, BD, USA) was used to assess apoptosis. Approximately 1×105 cells were collected and stained with Annexin V-FITC (5 μL) and PI (10 μL) for 15 min in the dark. Then, 400 μL of 1X binding buffer was introduced and apoptosis evaluated by flow cytometry (Millipore Inc., MA, USA).
Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was isolated from brain tissues (n=5 for each group, 6 h after reperfusion) and cultured neurons using the TRIzol™ reagent (15,566–026, Thermo Fisher Scientific Inc., MA, USA) and stored at −80°C until use. All-in-one ™ miRNA First-strand cDNA synthesis kit 2.0 (Genecopoeia Inc., Guangzhou, China) as well as SureScriptTM First-strand cDNA synthesis kit (Genecopoeia Inc., Guangzhou, China) were used to synthesize cDNA according to manufacturer instructions. MiR-27a-3p expression was evaluated using All-in-One™ miRNA qPCR Kit (Genecopoeia Inc., Guangzhou, China) with U6 as the reference gene. RT-qPCR was performed using the SYBR Green reaction mix on a PIKOREAL96-qPCR instrument (Thermo Fisher Scientific, Inc., MASS, USA) using the following conditions: 95°C, 10 min for one cycles, 95°C, 10 s for 40 cycles, 60°C for 20 s, and 72°C for 15 s. Primer sequences are shown in Table 1 (Qingke Biotech, Beijing, China). The 2−ΔΔCt approach was used to compute relative expression levels with GAPDH as the reference gene.
 |
Table 1 Sequences of Primers for RT-qPCR in Present Study |
ELISA and Western Blot Analysis
Concentrations of pro-inflammatory cytokines (IL-1β, TNF-α, IL-6) in cell supernatants were evaluated using a commercial ELISA kit (Neobioscience Co. Ltd., Shenzhen, China) according to manufacturer instructions.
Brain tissues from infarcted rat cortex (same samples analyzed by RT-qPCR, 6 h after reperfusion) were lysed using lysate (Beyotime Institute of Biotechnology, Shanghai, China) and cleared by centrifugation at 4°C, 12,000 rpm for 15 mins. Cell lysing was performed on ice in a RIPA buffer that had been freshly enriched with protease suppressors. The BCA protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China) was used to assay protein concentrations according to manufacturer instructions. Protein samples (25 μg) were resolved by 12% SDS-PAGE (SDS-PAGE protein loading buffer (5X), P0015L, Beyotime Institute of Biotechnology, Shanghai, China) and transfer-embedded onto 0.2 μm PVDF membranes (EMD Millipore, Billerica, MA, USA). Blocking using 5% milk in TBST was performed at room temperature for 40 min and incubated with anti-Rgs1 (1:1000, ab117077, Abcam, Cambridge, UK), anti-Cleaved-caspase-3 (1:500, ab2302, Abcam), anti-Bax (1:2000, EPR18283, Abcam), anti-Bcl-2 (1:1000, ab194583, Abcam), anti-NF-κB/p-p65 (1:500, ab76302, Abcam), and anti-β-actin (1:5000, ab6276, Abcam) primary antibodies in 5% BSA-TBST at 4°C overnight. They were washed thrice using TBST for 6 min each and incubated with a HRP-labelled secondary antibody at room temperature for 40 min. Then, membranes were rinsed three times using TBST for 15 min each and signal developed using enhanced chemiluminescence (Beyotime Institute of Biotechnology, Shanghai, China). Mean gray values were assessed using Image J 1.43 while protein band intensity was normalized to β-actin.
Double Luciferase Reporter Assay
TargetScan version 7.2 (http://www.targetscan.org/vert_72/) was used to predict putative binding sites between miR-27a-3p and Rgs1. Next, double luciferase enzyme reporter assays were used to evaluate interactions between miR-27a-3p and Rgs1. The Rgs1 3 ʹ-UTR bearing the miR-27a-3p binding sequence was cloned into pEZX-MT05 plasmid (Genecopoeia Inc., Guangzhou, China) to generate a WT-Rgs1 vector. The Rgs1 3 ʹ-UTR bearing mutant miR-27a-3p binding sequence was used to generate the MUT-Rgs1 vector. WT-Rgs1 and MUT-Rgs1 vectors were transferred into HEK-293T cells. Cells were transfected with miR-mimic and miR-mimic-NC as negative controls. Firefly and Renilla luciferase enzyme activities were assayed 48 h later using a dual luciferase reporting system (BK-L96C; Zhongsheng Beikong Biological Technology Co., Ltd, Beijing, China). Relative luciferase activity was determined using firefly luciferase as the standard.
Statistical Analyses
Statistical analyses were performed using SPSS 21.0. Data are presented as mean±SD. Comparisons between 2 groups were done using Student’s t-test while for multiple group comparisons, one-way ANOVA followed by Tukey’s post hoc test was used. p <0.05 was set as the threshold for statistical significance.
Results
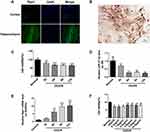
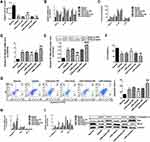
Identification of Hippocampal Neurons and Cell Viability After OGD/R
Immunofluorescence analysis of normal rat brain tissues revealed that Rgs1 expression was elevated in the hippocampus and relatively suppressed in the cortex. Therefore, we used the primary hippocampal neurons for subsequent in vitro experiments (Figure 1A). Cultured cells were identified as hippocampal neurons by histochemical staining of the NeuN marker (Figure 1B). Next, we established the hippocampus neuron OGD/R model of cerebral I/R injury in vitro. Cell vitality testing revealed that relative to the normal group, cell vitality was remarkably low after OGD treatment for 2 h and reoxygenation after the 9th, and 3rd, 6th, and 12th h. Cell vitality was lowest after the 12th h, but was remarkably different between the 6th and 9th h (Figure 1C).
MiR-27a-3p and Rgs1 Expression in Hippocampal Neurons Upon OGD/R Injury
RT-qPCR analyses revealed that after OGD/R, miR-27a-3p expression in the hippocampal neurons was significantly suppressed at various timepoints relative to the normal group, with the lowest expression at the 12th h (Figure 1D). However, Rgs1 expression gradually increased and was highest at the 12th h (Figure 1E). These findings imply that miR-27a-3p and Rgs1 are involved in OGD/R injury. Therefore, the 12 h timepoint was selected for subsequent experiments.
Therapeutic Concentrations of Biliverdin in Cell Experiments
To assess its effects on cell viability, cells were with various concentrations of biliverdin and assessed by the CCK-8 assay. CCK-8 analysis revealed that in the other groups, viability was significantly low relative to the normal group. Compared to the OGD/R group, biliverdin (0.3 and 1 μg/mL) did not exhibit significant effects on viability. However, the 2, 3, 5, and 10 μg/mL treated groups had remarkably higher viabilities relative to the OGD/R group but there were no remarkable differences between treated groups (Figure 1F). Therefore, 2 μg/mL was selected as the optimal biliverdin concentration in the subsequent analyses.
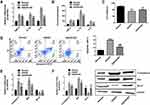
Effects of Biliverdin on Inflammation, Cell Viability, and Apoptosis of Hippocampal Cells Following OGD/R Injury
To elucidate on the protective effects of biliverdin on OGD/R-damaged hippocampal neurons, the effects of biliverdin on inflammatory markers, cell viability, and apoptosis were evaluated. Relative to the normal group, mRNA and protein expression levels (in supernatant) of pro-inflammatory cytokines (IL-6, TNF-α, and IL-1β) were significantly elevated in OGD/R hippocampal neurons. However, biliverdin treatment remarkably suppressed mRNA and protein expression levels of these inflammatory factors (Figure 2A and B). Cell viability tests revealed that relative to the normal group, OGD/R significantly reduced hippocampal cell viability, while biliverdin treatment remarkably enhanced cell viability following OGD/R injury (Figure 2C). Besides, flow cytometry showed that biliverdin remarkably suppressed OGD/R-induced cell apoptosis (Figure 2D). Relative to the normal control, OGD/R significantly elevated caspase-3 and Bax expression at the mRNA and protein levels while suppressing Bcl-2 expression. However, biliverdin remarkably suppressed caspase-3 as well as Bax expression and promoted Bcl-2 expression (Figure 2E and F). These findings show that biliverdin protects against OGD/R injury by suppressing apoptosis and inflammation.
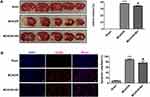
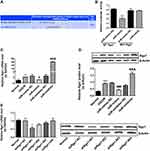
Effects of Biliverdin on Cerebral Infarction Volume and Apoptosis in MCAO/R Rats
The effects of biliverdin on cerebral infarction and cortical neuronal apoptosis after cerebral I/R injury were evaluated using a rat model of MCAO/R. TTC staining showed that relative to the normal control group, cerebral infarction volume was remarkably higher in the MCAO/R group and that biliverdin remarkably reduced cerebral infarction volume relative to the MCAO/R group (Figure 3A). TUNEL staining revealed that relative to the normal group, apoptotic cell numbers were remarkably higher in the MCAO/R group while biliverdin remarkably reduced apoptotic cell numbers relative to the MCAO/R group (Figure 3B).
Effects of Biliverdin on the Expression of miR-27a-3p and Rgs1 in OGD/R-Induced Hippocampal Neurons and MCAO/R Rat Cortex and Hippocampal Tissues
To establish the molecular basis of the effects of biliverdin, we evaluated miR-27a-3p as well as Rgs1 expression levels in the hippocampal neurons and in rat ischemic cortex and hippocampus. In the OGD/R group, miR-27a-3p expression was remarkably suppressed in contrast to the normal group, while Rgs1 mRNA and protein expression levels were remarkably elevated relative to the normal group. However, in contrast to the OGD/R group, biliverdin treatment remarkably elevated miR-27a-3p and suppressed Rgs1 mRNA as well as protein expressions (Figure 4A, D and G). In the cortex and hippocampus, MCAO/R remarkably suppressed miR-27a-3p expression and upregulated Rgs1 mRNA and protein expression levels. Relative to the MCAO/R group, biliverdin was shown to remarkably enhance miR-27a-3p expression while suppressing Rgs1 mRNA and protein expression levels (Figure 4B, C, E, F, H and I). These findings suggest that biliverdin exerts its effects through miR-27a-3p and Rgs1, and that the two have a negative regulatory relationship.
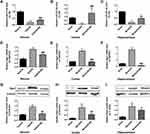
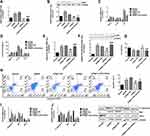
Effects of miR-27a-3p Mimic or Inhibitor on Inflammation, Cell Viability, and Apoptosis in Hippocampal Neurons After OGD/R Injury
To investigate the effects of miR-27a-3p in hippocampal OGD/R-injured neurons, an miR-27a-3p mimic or inhibitor were transfected into cells using a lentivirus, and an OGD/R model was constructed to assess inflammation, cell viability, and apoptosis. MiR-27a-3p levels in the OGD/R group were remarkably low when compared to the normal control group while the miR-27a-3p level was remarkably elevated in the miR-mimic group when compared to the miR-mimic-NC group. However, in contrast to the miR-inhibitor-NC group, expression levels of miR-27a-3p in the miR-inhibitor group were remarkably decreased (Figure 5A).
Pro-inflammatory cytokine expression assessment showed that TNF-α, IL-1β, and IL-6 mRNA and protein expression levels were markedly elevated in the OGD/R group when compared to the normal group. However, compared to the miR-mimic-NC group, TNF-α, IL-1β and IL-6 mRNA and protein (in supernatant) expression levels were remarkably low in the miR-mimic group. However, in contrast with the miR-inhibitor-NC group, TNF-α, IL-1β and IL-6 mRNA as well as protein (supernatant) expression levels were remarkably higher in the miR-inhibitor group (Figure 5B and C). Viabilities of the OGD/R group neurons were found to be remarkably lower than in the normal group. Relative to the miR-mimic-NC group, viabilities of the miR-mimic neurons were significantly higher. However, relative to miR-inhibitor-NC, viabilities of the miR-inhibitor group neurons were remarkably low (Figure 5F)
Flow cytometry revealed a significantly higher apoptosis rate of OGD/R neurons relative to the normal group. In contrast to the miR-mimic-NC group, apoptosis was remarkably suppressed in the miR-mimic group while compared to miR-inhibitor-NC, apoptosis was remarkably higher in the miR-inhibitor group neurons (Figure 5G). Relative to normal controls, mRNA and protein expressions of apoptotic factors (caspase-3 and Bax) were remarkably upregulated in the OGD/R group, while Bcl-2 was remarkably downregulated. Compared to the miR-mimic-NC group, mRNA and protein expressions of caspase-3 and Bax were significantly low in the miR-mimic group, while the expression levels of Bcl-2 mRNA were remarkably upregulated. However, relative to miR-inhibitor-NC, caspase-3 and Bax mRNA as well as protein expressions were remarkably upregulated in the miR-inhibitor while Bcl-2 was remarkably downregulated (Figure 5H and I). These findings imply that miR-27a-3p upregulation protects against hippocampal OGD/R-induced neuronal damage by suppressing inflammation and apoptosis.
Impacts of miR-27a-3p Mimic or Inhibitor on NF-κB/P65 Expression Levels in the Hippocampal Neurons After OGD/R Injury
To assess the effects of miR-27a-3p on inflammatory signaling pathways, we evaluated the expression levels of NF-κB/p65, a key modulator of inflammation. In contrast to the normal group, NF-κB/p65 mRNA and p-p65 protein quantities were markedly upregulated in the OGD/R group, while in contrast to the miR-mimic-NC group, NF-κB/p65 transcripts and p-p65 protein quantities were remarkably downregulated in the miR-mimic group. However, NF-κB/p65 mRNA and p-p65 protein expression levels were significantly upregulated in the miR-inhibitor group than in the miR-inhibitor-NC group (Figure 5D and E).
MiR-27a-3p Directly Targets and Inhibits Rgs1 Expression
To establish if the protective effects of miR-27a-3p on OGD/R-injured hippocampal neurons is mediated by Rgs1, we evaluated the relationship between miR-27a-3p and Rgs1. Bioinformatics analysis revealed that Rgs1 is a downstream miR-27a-3p target, and that the 3 ʹ-UTR region of Rgs1 bears an miR-27a-3p docking site (Figure 6A). Dual luciferase assessment of whether miR-27a-3p directly targets Rgs1 revealed remarkably low luciferase activity in the WT-Rgs1+miR-mimic group relative to the WT-Rgs1+miR-mimic-NC group. However, luciferase activity in the MUT-Rgs1+miR-mimic group did not remarkably differ from that of the MUT-Rgs1+miR-mimic-NC group (Figure 6B). These findings indicate that miR-27a-3p directly targets and regulates Rgs1. Additionally, Rgs1 transcripts and protein expression levels in the OGD/R-injured neurons were remarkably higher relative to the normal group. In contrast with the miR-mimic-NC group, Rgs1 expression levels were remarkably low in the miR-mimic group. However, in contrast with the miR-inhibitor-NC group, Rgs1 expression levels were remarkably upregulated in the miR-inhibitor group (Figure 6C and D), indicating that miR-27a-3p directly regulates Rgs1.
Effects of shRgs1 on Hippocampal Neuronal Viability, Inflammation, and Apoptosis After OGD/R
Out of the 4 anti-Rgs1 shRNA we constructed, shRgs1-001 was shown to potently silence Rgs1 expression (Figure 6E). Next, we transfected hippocampal neurons with shRgs1 or shRgs+miR-27a-3p inhibitor and established an OGD/R model to further evaluate the role of Rgs1. RT-qPCR and WB analysis revealed that Rgs1 mRNA and protein expression levels were elevated in the OGD/R group in contrast to the normal group. However, their expressions were remarkably suppressed in the shRgs1 group relative to the shRgs1-NC group. Moreover, relative to the shRgs1 group, Rgs1 was remarkably upregulated in the shRgs1+miR-inhibitor group (Figure 7A and B). Analysis of pro-inflammatory markers revealed that relative to the normal group, IL-1β, TNF-α, IL-6 transcripts and protein (in supernatant) expression levels were remarkably elevated in the OGD/R group, while their expressions in the shRgs1 group were remarkably low than in the shRgs1-NC group. However, relative to the shRgs1 group, TNF-α, IL-1β, and IL-6 mRNA and protein (in supernatant) expression levels in the shRgs1+miR-inhibitor group were remarkably elevated (Figure 7C and D). Neuronal vitality in the OGD/R group was remarkably low relative to the normal group and remarkably elevated in the shRgs1 group when compared to the shRgs1-NC group. However, relative to the shRgs1 group, cell vitality was remarkably low in the shRgs1+miR-inhibitor group (Figure 7G). Apoptosis analysis revealed higher apoptosis rates in the OGD/R group than in the normal group. Relative to the shRgs1-NC group, the apoptosis rate was remarkably low in the shRgs1 group. However, relative to the shRgs1 group, apoptosis was remarkably higher in the shRgs1+miR-inhibitor group (Figure 7H). Compared to normal controls, RT-qPCR and WB analysis revealed that caspase-3 and Bax expressions were remarkably upregulated in the OGD/R group while Bcl-2 was remarkably downregulated. Relative to the shRgs1-NC group, caspase-3 and Bax mRNA and protein expressions were remarkably suppressed in the shRgs1 group while bcl-2 expression was upregulated. Compared to the shRgs1 group, caspase-3 and Bax mRNA along with protein expression levels in the shRgs1+miR-inhibitor group were markedly upregulated, while Bcl-2 mRNA expressions were remarkably reduced. However, Bcl-2 protein expression levels did not change remarkably (Figure 7I and J). These results suggest that silencing Rgs1 suppresses OGD/R-induced neuronal apoptosis and inflammatory responses, while miR-27a-3p inhibition elevates Rgs1 expression and reverses the protective effects of shRgs1.
Effects of shRgs1 on NF-κB/P65 Expression in Hippocampal Neurons After OGD/R Injury
To assess the effects of Rgs1 on inflammatory signaling pathways, we evaluated NF-κB/p65 expression levels. Relative to the normal control, NF-κB/p65 transcripts and p-p65 protein expressions were remarkably upregulated in the OGD/R group; however, they were remarkably suppressed in the shRgs1 group in contrast with the shRgs1-NC group. Nevertheless, NF-κB/p65 transcripts and p-p65 protein expression levels were remarkably elevated in the shRgs1+miR-inhibitor group relative to the shRgs1 group (Figure 7E and F). These findings suggest that shRgs1 suppresses NF-κB/p65 expression, thereby, inhibiting inflammatory responses.
Discussion
Cerebral I/R injury often causes irreversible neurological impairment in patients. Inflammation and apoptosis contribute to the pathogenesis of ischemic brain and secondary reperfusion injury. Anti-inflammatory and anti-apoptotic therapies may be effective against cerebral I/R injury.5 Biliverdin, a product of heme oxygenase-1, has anti-inflammatory, anti-apoptotic and anti-oxidative effects in various diseases.29,30 However, the molecular basis of its action is unclear. We have previously shown that biliverdin remarkably enhances miR-27a-3p expression and suppresses mRNA Rgs1 expression. Bioinformatic analysis identified Rgs1 as a possible miR-27a-3p target.21 However, the interaction between the two and how they regulate cerebral I/R injury have not been confirmed.
Several in vivo and in vitro models have shown that biliverdin confer anti-inflammatory as well as anti-apoptotic effects. It can inhibit lipopolysaccharide (LPS)-induced C5aR, decreased TNF-α and IL-6 expression via the mTOR pathway in Macrophages, thereby preventing inflammatory response.31 In addition, Lv et al32 showed that biliverdin protected against cisplatin-induced apoptosis of renal tubular epithelial cells. Other scholars have reported that biliverdin prevent I/R-related destructive events. Tian et al33 showed that biliverdin can significantly reduce the levels of peroxides, pro-inflammatory cytokines and apoptosis index in isolated lung tissue after I/R, thereby protecting lung tissue from I/R injury. Moreover, administration of biliverdin significantly suppressed IRI-induced liver dysfunction in swine.34 However, the role and mechanism of biliverdin in ischemic stroke has not been reported. In this study, we found that biliverdin remarkably suppressed generation of pro-inflammatory cytokines and inhibited apoptosis in OGD/R-injured neurons. Additionally, biliverdin reduced cerebral infarction volume and decreased apoptosis in ischemic cortex after MCAO/R in rats. These data demonstrate the anti-inflammatory and anti-apoptotic effects of biliverdin in cerebral I/R models.
Furthermore, the results demonstrated that miR-27a-3p levels were downregulated whereas Rgs1 levels were increased in hippocampal neurons (in vitro) of OGD/R injury and in MCAO/R-activated rat cortex and hippocampus (in vivo). However, biliverdin treatment ameliorated these effects. This was consistent with results from gene chip analysis in our previous report, suggesting that miR-27a-3p and Rgs1 may mediate the effects of biliverdin. MiR-27a-3p has been reported to have anti-inflammatory and anti-apoptotic effects in many diseases.17,35,36 Recently, Shang et al37 showed that miR-27a-3p overexpression suppressed LPS-triggered inflammatory responses and apoptosis in alveolar epithelial cells through targeting FOXO3 and repressing NAPDH/ROS activation. In addition, Liao et al38 showed that miR-27a-3p directly targeted FosB to inhibit apoptosis and inflammation responses in immunoglobulin a nephropathy. To further test the role of miR-27a-3p in cerebral I/R injury, miR-27a-3p mimic or inhibitor and corresponding negative controls were transfected into OGD/R-injured neurons using lentivirus. Results from this analysis confirmed that inflammation and apoptosis were aggravated following OGD/R-triggered injury. However, overexpression of miR-27a-3p remarkably suppressed inflammation and apoptosis, which is consistent with the effects of biliverdin. This suggests that upregulation of miR-27a-3p also exerts anti-inflammatory and anti-apoptotic effects in I/R model and may mediate the protective effects of biliverdin. Zhang et al39 showed that overexpression of miR-27a-3p regulated TLR4 and inhibited NF-κB signaling, thereby suppressing TNF-α and IL-6 expression and repressing inflammatory response upon spinal cord injury. Moreover, another study showed that sevoflurane protected against LPS-triggered acute lung injury by increasing miR-27a-3p expression and suppressing inflammation via inhibition of TLR4/MyD88/NF-κB signaling.40 Together, these studies demonstrate that miR-27a-3p overexpression suppresses inflammation by repressing TLR4/NF-κB signaling. The TLR4/NF-κB cascade plays a role in the induction of inflammation. Overexpression of p-p65, the active form of NF-κB, increases cytokine production and promotes inflammation in neurological disorders.41,42 We established that miR-27a-3p-mimic suppressed NF-κB expression, whereas miR-27a-3p-inhibitor enhanced its expression in OGD/R-injured neurons. This indicates that the anti-inflammatory effects of miR-27a-3p may be achieved by repression of NF-κB signaling in cerebral I/R injury (in vitro). Here, we show, for the first time, that miR-27a-3p upregulation prevents apoptosis and inflammation during cerebral I/R injury and mediates the protective effects of biliverdin. Our data also indicate that miR-27a-3p inhibits inflammation by suppressing the NF-κB cascade.
In this study, the dual luciferase reporter gene assay showed that the relative fluorescence intensity of the recombinant plasmid containing Rgs1 mRNA 3 ʹ-UTR was significantly reduced after treatment with miR-27a-3p mimic, indicating that miR-27a-3p inhibited the expression of Rgs1. Since miRNA can bind to the 3 ʹ-UTR of mRNA and inhibit the translation of target mRNA, we speculate that the anti-inflammatory and anti-apoptotic effects of miR-27a-3p might be mediated by downregulation of Rgs1.
Rgs1 is a negative modulator of G-protein-coupled receptor signaling and is reported to be a risk factor associated with poor prognosis in various diseases.43,44 Rgs1 is also expressed in both monocytes and tissue macrophages and modulates immune and inflammatory responses.22 Studies of atherosclerosis-prone mice have implicated Rgs1 in macrophage-mediated vascular inflammation associated with atherosclerotic plaques.45 Here, downregulation of Rgs1 remarkably suppressed inflammatory response and neuronal apoptosis in OGD/R-injured neurons, which is congruent with the effects of biliverdin and miR-27a-3p overexpression. Collectively, these data show that suppression of Rgs1 expression may mediate the anti-inflammatory and anti-apoptotic effects of biliverdin and miR-27a-3p overexpression. Furthermore, NF-κB expression was remarkably downregulated by Rgs1 silencing, implying that Rgs1 downregulation prevents inflammation by suppressing NF-κB signaling. A recent study found that downregulation of Rgs1 suppressed TLR signaling, thereby reducing serum concentration of pro-inflammatory cytokines and suppressing inflammation in rats with rheumatoid arthritis, which is consistent with our results in OGD/R-triggered hippocampal neuronal injury.24 Of note, inhibiting miR-27a-3p expression following Rgs1 silencing reverted the protective properties of Rgs1 silencing, further indicating that Rgs1 is a direct target of miR-27a-3p. A single miRNA may target multiple mRNAs and one mRNA may be targeted by multiple miRNAs,12 our data imply that the anti-inflammatory as well as anti-apoptotic properties of miR-27a-3p are at least in part mediated through targeting Rgs1. Our data show for the first time that downregulation Rgs1 prevents inflammation and apoptosis in a model of cerebral I/R injury in vitro, and that this effect is directly modulated by miR-27a-3p.
Although a lot of research has been done in recent years, the pathological mechanism of cerebral ischemia-reperfusion injury is still largely unknown. In the current study, we found that biliverdin can protect against cerebral I/R injury by regulating the miR-27a-3p/Rgs1 axis, suggesting that biliverdin may be a potential treatment of ischemic stroke. However, the effectiveness and mechanism of biliverdin need to be further studied.
Conclusion
To the best of our knowledge, this is the first report to show that biliverdin upregulates miR-27a-3p to target Rgs1, thereby inhibiting inflammation by suppressing NF-κB signaling, and reducing apoptosis in hippocampal neurons in vitro. It also confers neuroprotective effects against cerebral I/R injury by reducing brain infarct volume and apoptosis of neurocytes in vivo (Figure 8). These findings expand our understanding of the anti-inflammatory and anti-apoptotic mechanisms of biliverdin in cerebral I/R injury.
Ethical Statement
Animal use of this study adhered to National Institutes of Health guidelines for Laboratory Animal Care and Safety. Ethical approval for animal use was granted by the Animal Ethics Committee of Kunming Medical University (NO. KMMU2020005).
Funding
This work was subsidized by the National Natural Science Foundation of China (Grant No.81760248; 81960250), and the Key Applied and Basic Research Program in Yunnan Province (Grant No. 2018FA042), and the Yunling Industry Technology Leading Talent Training program of Yunnan Province (Grant No. YLXL20170054).
Disclosure
All authors have claimed no conflict of interest in this research.
References
1. Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38(02):208–211. doi:10.1055/s-0038-1649503
2. Wang Y, Li Z, Zhao X, et al. Effect of a multifaceted quality improvement intervention on hospital personnel adherence to performance measures in patients with acute ischemic stroke in China: a randomized clinical trial. JAMA. 2018;320(3):245–254. doi:10.1001/jama.2018.8802
3. Hong P, Li F, Gu R, et al. Inhibition of NLRP3 inflammasome ameliorates cerebral ischemia-reperfusion injury in diabetic mice. Neural Plast. 2018;2018:9163521. doi:10.1155/2018/9163521
4. Feng S, Aa N, Geng J, et al. Pharmacokinetic and metabolomic analyses of the neuroprotective effects of salvianolic acid A in a rat ischemic stroke model. Acta Pharmacologica Sinica. 2017;38(11):1435–1444. doi:10.1038/aps.2017.114
5. Chai Z, Gong J, Zheng P, et al. Inhibition of miR-19a-3p decreases cerebral ischemia/reperfusion injury by targeting IGFBP3 in vivo and in vitro. Biol Res. 2020;53(1):17. doi:10.1186/s40659-020-00280-9
6. Kim T, Shin J, Chung K, et al. Anti-inflammatory mechanisms of koreanaside A, a lignan isolated from the flower of forsythia koreana, against LPS-induced macrophage activation and DSS-induced colitis mice: the crucial role of AP-1, NF-κB, and JAK/STAT signaling. Cells. 2019;8(10):1163. doi:10.3390/cells8101163
7. Sarady-Andrews JK, Liu F, Gallo D, et al. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2005;289(6):L1131–7. doi:10.1152/ajplung.00458.2004
8. Overhaus M, Moore BA, Barbato JE, et al. Biliverdin protects against polymicrobial sepsis by modulating inflammatory mediators. Am J Physiol Gastrointest Liver Physiol. 2006;290(4):G695–G703. doi:10.1152/ajpgi.00152.2005
9. Bellner L, Wolstein J, Patil KA, et al. Biliverdin rescues the HO-2 null mouse phenotype of unresolved chronic inflammation following corneal epithelial injury. Invest Ophthalmol Vis Sci. 2011;52(6):3246–3253. doi:10.1167/iovs.10-6219
10. Li J, Zou Z, Liu J, et al. Biliverdin administration ameliorates cerebral ischemia reperfusion injury in rats and is associated with proinflammatory factor downregulation. Exp Ther Med. 2017;14(1):671–679. doi:10.3892/etm.2017.4549
11. Su Q, Lv X, Ye Z, et al. The mechanism of miR-142-3p in coronary microembolization-induced myocardiac injury via regulating target gene IRAK-1. Cell Death Dis. 2019;10(2):61. doi:10.1038/s41419-019-1341-7
12. Liu W, Miao Y, Zhang L, et al. MiR-211 protects cerebral ischemia/reperfusion injury by inhibiting cell apoptosis. Bioengineered. 2020;11(1):189–200. doi:10.1080/21655979.2020.1729322
13. Korotkov A, Mills JD, Gorter JA, et al. Systematic review and meta-analysis of differentially expressed miRNAs in experimental and human temporal lobe epilepsy. Sci Rep. 2017;7(1):11592. doi:10.1038/s41598-017-11510-8
14. Cairns MJ, Kocerha J. Advances in non-coding RNA profiling for neurological diseases. Front Genet. 2013;4:5. doi:10.3389/fgene.2013.00005
15. Liu FJ, Lim KY, Kaur P, et al. microRNAs involved in regulating spontaneous recovery in embolic stroke model. PLoS One. 2013;8(6):e66393. doi:10.1371/journal.pone.0066393
16. Ben W, Zhang G, Huang Y, et al. MiR-27a-3p regulated the aggressive phenotypes of cervical cancer by targeting FBXW7. Cancer Manag Res. 2020;12:2925–2935. doi:10.2147/CMAR.S234897
17. Zhang F, Wang Z, Zhang H, et al. MiR-27a alleviates osteoarthritis in rabbits via inhibiting inflammation. Eur Rev Med Pharmacol Sci. 2019;23(3 Suppl):89–95. doi:10.26355/eurrev_201908_18634
18. Ju M, Liu B, He H, et al. MicroRNA-27a alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis through modulating TLR4/MyD88/NF-κB pathway. Cell Cycle. 2018;17(16):2001–2018. doi:10.1080/15384101.2018.1509635
19. Tang X, Dai Y, Wang X, et al. MicroRNA-27a protects retinal pigment epithelial cells under high glucose conditions by targeting TLR4. Exp Ther Med. 2018;16(1):452–458. doi:10.3892/etm.2018.6150
20. Liu W, Zha Z, Wang H. Upregulation of microRNA-27a inhibits synovial angiogenesis and chondrocyte apoptosis in knee osteoarthritis rats through the inhibition of PLK2. J Cell Physiol. 2019;234(12):22972–22984. doi:10.1002/jcp.28858
21. Zou Z, Liu J, Chang C, et al. Biliverdin administration regulates the microRNA-mRNA expressional network associated with neuroprotection in cerebral ischemia reperfusion injury in rats. Int J Mol Med. 2019;43(3):1356–1372. doi:10.3892/ijmm.2019.4064
22. Xie Z, Chan EC, Druey KM. R4 Regulator of G Protein Signaling (RGS) proteins in inflammation and immunity. AAPS J. 2016;18(2):294–304. doi:10.1208/s12248-015-9847-0
23. Patel J, McNeill E, Douglas G, et al. RGS1 regulates myeloid cell accumulation in atherosclerosis and aortic aneurysm rupture through altered chemokine signalling. Nat Commun. 2015;6(1):6614. doi:10.1038/ncomms7614
24. Hu X, Tang J, Zeng G, et al. RGS1 silencing inhibits the inflammatory response and angiogenesis in rheumatoid arthritis rats through the inactivation of toll-like receptor signaling pathway. J Cell Physiol. 2019;234(11):20432–20442. doi:10.1002/jcp.28645
25. Meng Y, Ding Z, Wang H, et al. Effect of microRNA-155 on angiogenesis after cerebral infarction of rats through AT1R/VEGFR2 pathway. Asian Pac J Trop Med. 2015;8(10):829–835. doi:10.1016/j.apjtm.2015.09.009
26. Hu Q, Chen C, Yan J, et al. Therapeutic application of gene silencing MMP-9 in a middle cerebral artery occlusion-induced focal ischemia rat model. Exp Neurol. 2009;216(1):35–46. doi:10.1016/j.expneurol.2008.11.007
27. Liu A, Zhang W, Wang S, et al. HMGB-1/RAGE signaling inhibition by dioscin attenuates hippocampal neuron damage induced by oxygen-glucose deprivation/reperfusion. Exp Ther Med. 2020;20(6):231. doi:10.3892/etm.2020.9361
28. Huang W, Liu X, Cao J, et al. miR-134 regulates ischemia/reperfusion injury-induced neuronal cell death by regulating CREB signaling. J Mol Neurosci. 2015;55(4):821–829. doi:10.1007/s12031-014-0434-0
29. Shiels RG, Hewage W, Pennell EN, et al. Biliverdin and bilirubin sulfonate inhibit monosodium urate induced sterile inflammation in the rat. Eur J Pharm Sci. 2020;155:105546. doi:10.1016/j.ejps.2020.105546
30. Baylor JL, Butler MW. Immune challenge-induced oxidative damage may be mitigated by biliverdin. J Exp Biol. 2019;222(6):jeb200055. doi:10.1242/jeb.200055
31. Bisht K, Wegiel B, Tampe J, et al. Biliverdin modulates the expression of C5aR in response to endotoxin in part via mTOR signaling. Biochem Biophys Res Commun. 2014;449(1):94–99. doi:10.1016/j.bbrc.2014.04.150
32. Lv Q, Yao Y, Wang W, et al. Biliverdin protects against cisplatin-induced apoptosis of renal tubular epithelial cells. J Huazhong Univ Sci Technolog Med Sci. 2016;36(1):48–52. doi:10.1007/s11596-016-1540-8
33. Tian W, Weng P, Sheng Q, et al. Biliverdin protects the isolated rat lungs from ischemia-reperfusion injury via antioxidative, anti-inflammatory and anti-apoptotic effects. Chin Med J. 2017;130(7):859–865. doi:10.4103/0366-6999.202735
34. Andria B, Bracco A, Attanasio C, et al. Biliverdin protects against liver ischemia reperfusion injury in swine. PLoS One. 2013;8(7):e69972. doi:10.1371/journal.pone.0069972
35. Cai C, Min S, Yan B, et al. MiR-27a promotes the autophagy and apoptosis of IL-1β treated-articular chondrocytes in osteoarthritis through PI3K/AKT/mTOR signaling. Aging. 2019;11(16):6371–6384. doi:10.18632/aging.102194
36. Qiu W, Xu M, Zhu X, et al. MicroRNA-27a alleviates IL-1β-induced inflammatory response and articular cartilage degradation via TLR4/NF-κB signaling pathway in articular chondrocytes. Int Immunopharmacol. 2019;76:105839. doi:10.1016/j.intimp.2019.105839
37. Shang J, Wang L, Tan L, et al. MiR-27a-3p overexpression mitigates inflammation and apoptosis of lipopolysaccharides-induced alveolar epithelial cells by targeting FOXO3 and suppressing the activation of NAPDH/ROS. Biochem Biophys Res Commun. 2020;533(4):723–731. doi:10.1016/j.bbrc.2020.07.126
38. Liao Y, Wang Z, Wang L, et al. MicroRNA-27a-3p directly targets FosB to regulate cell proliferation, apoptosis, and inflammation responses in immunoglobulin a nephropathy. Biochem Biophys Res Commun. 2020;529(4):1124–1130. doi:10.1016/j.bbrc.2020.06.115
39. Zhang P, Li L, Zhang D, et al. Over-expressed miR-27a-3p inhibits inflammatory response to spinal cord injury by decreasing TLR4. Eur Rev Med Pharmacol Sci. 2018;22(17):5416–5423. doi:10.26355/eurrev_201809_15800
40. Wang Y, Zhang X, Tian J, et al. Sevoflurane alleviates LPS-induced acute lung injury via the microRNA-27a-3p/TLR4/MyD88/NF-κB signaling pathway. Int J Mol Med. 2019;44(2):479–490. doi:10.3892/ijmm.2019.4217
41. Zusso M, Lunardi V, Franceschini D, et al. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J Neuroinflammation. 2019;16(1):148. doi:10.1186/s12974-019-1538-9
42. Li D, Wang Y, Jin X, et al. NK cell-derived exosomes carry miR-207 and alleviate depression-like symptoms in mice. J Neuroinflammation. 2020;17(1):126. doi:10.1186/s12974-020-01787-4
43. Roh J, Shin S, Lee A, et al. RGS1 expression is associated with poor prognosis in multiple myeloma. J Clin Pathol. 2017;70(3):202–207. doi:10.1136/jclinpath-2016-203713
44. Carreras J, Kikuti YY, Beà S, et al. Clinicopathological characteristics and genomic profile of primary sinonasal tract diffuse large B cell lymphoma (DLBCL) reveals gain at 1q31 and RGS1 encoding protein; high RGS1 immunohistochemical expression associates with poor overall survival in DLBCL not otherwise specified (NOS). Histopathology. 2017;70(4):595–621. doi:10.1111/his.13106
45. Wan W, Murphy PM. Regulation of atherogenesis by chemokines and chemokine receptors. Arch Immunol Ther Exp (Warsz). 2013;61(1):1–14. doi:10.1007/s00005-012-0202-1
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.