Back to Journals » Journal of Pain Research » Volume 13
Bilateral Erector Spinae Plane Blocks for Open Posterior Lumbar Surgery
Authors Zhang TJ , Zhang JJ, Qu ZY, Zhang HY, Qiu Y, Hua Z
Received 4 February 2020
Accepted for publication 6 March 2020
Published 5 April 2020 Volume 2020:13 Pages 709—717
DOI https://doi.org/10.2147/JPR.S248171
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Teng-Jiao Zhang,1,2 Jing-Jing Zhang,1,2 Zong-Yang Qu,1,2 Hong-Ye Zhang,1,2 Yong Qiu,1,2 Zhen Hua1,2
1Department of Anesthesiology, Beijing Hospital, National Center of Gerontology, Beijing, People’s Republic of China; 2Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China
Correspondence: Zhen Hua
Department of Anesthesiology, Beijing Hospital, National Center of Gerontology, No. 1 Dahua Road, Beijing 100730, People’s Republic of China
Tel/Fax +86 10 8513 6409
Email [email protected]
Purpose: Erector spinae plane block (ESPB) is a newly reported interfascial plane block in pain management, and it can block the nerves exactly in line with the area of the posterior lumbar surgery. The objective of this research was to determine the effectiveness of pre-operative ESPB in enhancing recovery of posterior lumbar surgery.
Patients and Methods: A total of 60 patients undergoing open posterior lumbar decompression surgery under general anesthesia were randomized into two groups. T12 group was performed pre-operatively bilateral ESPB with ropivacaine at the T12 level, but control group did not receive the block. The primary outcome was the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) score at 10 minutes after extubation. Secondary outcomes included intraoperative sufentanil consumption, postoperative morphine consumption, first time to ambulation after surgery and hospital length of stay after surgery. All participants were followed up to hospital discharge.
Results: The mean (SD) MOAA/S scores at 10 minutes after extubation were 4.2 (95% CI, 4.0 to 4.4), and 3.4 (95% CI, 3.2 to 3.6) in the T12 and control groups (P < 0.001), respectively. Intraoperative sufentanil consumption (P =0.007) and postoperative morphine consumption (P =0.003) were lower in the T12 group than in the control group. Although first time to ambulation after surgery was sooner in the T12 group than in the control group (P =0.003), hospital length of stay was similar (P=0.054).
Conclusion: Pre-operative bilateral ESPB at T12 can enhance recovery after posterior lumbar surgery and reduce perioperative opioid consumption.
Keywords: posterior lumbar surgery, erector spinae plane block, enhanced recovery after surgery, regional anesthesia
Introduction
Posterior lumbar surgery is one of the most common operations performed in the world to address back and leg pain.1 Such patients frequently present with chronic pre-operative pain, but they also experience surgery-related new onset acute pain in the early postoperative period. The pain is severe enough that patients usually require significant amounts of intravenous opioids in the first 48–72 hours, as well as possibly experiencing increased complications and delayed recovery.2 Pain is one of the main obstacles to achieving enhanced recovery after surgery (ERAS), the responsibility thus falls to the anesthesiologist to modify management to increase both the quality and outcomes of peri-operative care. Multimodal analgesia is critical both to ERAS and to achieve the target of Dreaming (drinking, eating and mobilizing).3
It has been hypothesized that a reduction in the surgical stress responses will lead to a reduced incidence of postoperative organ dysfunction and thereby to an improved outcome. Moreover, in addition to the consequences of pain in the immediate postoperative period, acute pain may trigger long-term neuronal changes that result in the development of chronic pain.2 In addition to their analgesic effects, regional anesthesia reduces the surgical stress response and cardiac, pulmonary, thromboembolic, and renal complications.4 There have been many advances in optimizing management of perioperative analgesia to facilitate enhanced recovery, with multimodal analgesia regimens now the standard practice in ERAS protocols. Regional anesthesia, as an important component of multimodal analgesic regimens, would seem to be one of ideal choices for improving perioperative stress and pain management. However, in lumbar spinal surgery, it has been primarily confined to neuraxial techniques, namely, epidural analgesia and intrathecal opioids.5 Neuraxial techniques have not been widely adopted in spinal surgery because of their side effects and limitations. Local anesthetic wound infiltration is commonly performed but its benefit tends to be short-lived.5,6 Ultrasound-guided erector spinae plane block (ESPB) is a recently described technique for providing thoracic analgesia.7 The injection of local anesthetics into the erector spinae plane (ESP) provides analgesia for several dermatomes, due to its cranial-caudal spread. The proposed mechanism of action of the ESPB is via blockade of the dorsal and ventral rami of the spinal nerves.7,8 Anatomy and imaging studies suggest that the local anesthetic injected into the ESP spreads cranially and caudally as the plane maintains continuity along the spine. Part of the appeal of the ESPB could be that it is easy to implement and gains indirect access to the paravertebral space, and provides analgesia with lower risk for pneumothorax compared with neuraxial block and paravertebral block.8 In addition, the block has a reduced risk of direct spinal cord injury, epidural hematoma, and central infection compared with neuraxial and paravertebral block.
Anatomically, the dorsal ramus of the thoracolumbar spinal nerves passes by the posterior surface of the transverse process of the corresponding lower vertebra and is distributed to the back.9 Generally, the dorsal ramus may innervate two to three segments caudal to its initial nerve root. Injection of 20–25 mL into the ESP produces clinical and radiographic evidence of spread, extending at least three vertebral levels cranially and caudally from the site of injection.7,8 This enhanced coverage allows for effective truncal analgesia in surgeries where incisions are widely spaced, or where the surgical field or wound dressings prevent injection at a level congruent with the surgical site. Based on the proposed mechanism of action and recent reports,10 we hypothesized that if performed at the T12 transverse process level, ESPB may provide effective truncal analgesia for posterior lumbar surgery. To determine the effectiveness of pre-operative ESPB in enhancing recovery of posterior lumbar surgery, we conducted a randomized controlled study comparing T12 ESPB group to control group.
Patients and Methods
Participants
A diagram of the overall study design is shown in Figure 1. This prospective randomized controlled study was conducted in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee of Beijing Hospital (approval no. 2018BJYYEC-011-01) and written informed consent was obtained from all subjects participating in the trial. The trial was registered prior to patient enrollment at chictr.org.cn (no. ChiCTR1800015002, Principal investigator: Zong-Yang Qu, Date of registration: 2018-02-27). From April 2018 to May 2019, ASA physical status I–III patients (prolapsed lumbar intervertebral disk, lumbar stenosis) aged 18 to 80 years old who underwent open posterior lumbar decompression surgery in Beijing Hospital were recruited to participate in the study. All patients provided written informed consent to participate in the study. Exclusion criteria: hepatic or renal insufficiency, preoperative cognitive dysfunction or communication disorder, allergy to amide-type local anesthetics, back puncture site infection, neuromuscular disease, emergency surgery, chronic opioids use, and participation in another clinical study.
 |
Figure 1 The Consolidated Standards of Reporting Trials (CONSORT) flow diagram of the study. ESPB, erector spinae plane block. |
Enrollment was performed by a physician investigator. Allocation of T12 ESPB group or control group was performed by the investigator physician with treatment divulged by sequentially numbered sealed envelopes before injection. T12 group received bilateral ultrasound-guided single-injection ESPB at the T12 level with ropivacaine, control group just received an ultrasound scan but did not receive block. The patient, anesthesiologist responsible for the operation, and evaluating physician (ie, outcome adjudicator) were blinded to the assignment.
Treatment
The patient was taken to the anesthesia preparation room 1 hour before the operation. Bilateral ultrasound-guided ESPBs were performed pre-operatively for patients in T12 ESPB group. Each patient was placed in the left lateral position under routine monitoring. A low-frequency convex array ultrasound transducer (Konica Minolta, Tokyo, Japan) covered in a sterile sleeve was then placed in a transverse orientation at this level to identify the tip of the T12 transverse process. The T12 transverse process was identified by the twelfth rib. The tip of the transverse process was centered on the ultrasound screen, and the probe was then rotated into a longitudinal orientation to produce a parasagittal view. Following local infiltration of anesthesia into the superficial tissues, an echogenic 21-gauge block needle (Pajunk, Geisingen, Germany) was inserted out-of-plane to the ultrasound beam in a lateral-to-medial direction until contact was made with the T12 transverse process. A total of 25 mL ropivacaine 0.3% was then injected into the ESP (Figure 2A). The procedure was repeated on the contralateral side. Forty minutes after the ESPB, the cutaneous sensory block was assessed using a pinprick test over the back in every patient (Figure 2B). Surgical technique and postoperative care followed standard local clinical practice.
Anesthesia was induced with propofol 1–2 mg kg−1, sufentanil 0.2–0.3 ug kg−1 and cisatracurium 0.2–0.3 mg kg−1. Anesthesia was maintained with sevoflurane in a 50:50 air: oxygen mixture and nitrous oxide in a 1:1. When necessary, sufentanil and cisatracurium were administered intra-operatively. Warming of every patient was kept with a heater during the surgery. Postoperatively, the cisatracurium was antagonized using neostigmine and atropine. All patients were transferred from the operating room to the post-anesthesia care unit (PACU) and stayed for at least 30 minutes. Following surgery, all groups were treated with a patient-controlled intravenous analgesia (PCIA) with morphine (0.5 mg mL−1) for 48 hours postoperative analgesia. The run parameter was set to 0.5 mL h−1 basal infusion, 2 mL bolus with 8-minute lockout interval.
Data Collection and Outcomes
The baseline data recorded included pain scores over the past week at rest and movement on a 0 to 10 numerical rating scale (NRS, 10 cm written pain scale showing all integers: 0 was no pain, and 10 was the worst pain imaginable), as well as baseline demographic and clinical variables. The intraoperative data recorded included sufentanil dosage, blood pressure, heart rate, neural coordination assessment after extubation (be able to quickly move lower limbs as directed in normal tone), PaCO2 20 minutes after extubation, duration of anesthesia, and duration of anesthesia recovery (residence time at operating room and PACU after extubation). Any adverse events were recorded, such as local anesthetic toxicity, allergy, block failure, acute postoperative pain, nausea, vomiting, agitation, and delirium. The first follow-up was 24 hours post-surgery, at which time NRS pain scores at rest and movement, first time of morphine bolus, morphine dosage and effective bolus times were assessed. The same clinical variables were recorded 48 hours post-surgery. In the following days, the first time to ambulation after surgery, hospital length of stay post-surgery, and any complications were recorded.
The primary outcome measure was the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S; Supplementary Table 1) score,11 which was assessed at 10 minutes after extubation. MOAA/S scores at 20 and 30 minutes after extubation were also recorded. The secondary outcome measures included sufentanil dosage, morphine dosage 24 and 48 hours post-surgery, first time to ambulation after surgery and hospital length of stay post-surgery. The outcome adjudicator was blinded to the assignment.
Statistical Analysis
A power analysis to determine sample size was performed before initiation of the study based on the estimated differences among the two groups for the primary outcome measure from a pilot study. The assumptions included a mean (SD) MOAA/S score at 10 minutes after extubation of 4.5 (1.0) in the T12 group and 3.5 (1.0) in the control group. Using an alpha level of 0.05 (type I error) in the two groups, we determined that 24 patients per group would have 90% power to detect a mean difference of 1.0 in MOAA/S score. To account for an anticipated 20% dropout rate, 30 participants were needed in each group.
For quantitative variables, data are expressed as the mean (SD) or median (Q1-Q3), and normality was tested with Shapiro–Wilk tests. When obeying a normal distribution, data were analyzed using Student’s t-tests or repeated measures two-way ANOVA, with a Bonferroni post hoc test, as indicated in the main text or figure captions as appropriate. When not obeying a normal distribution, data were analyzed using Mann–Whitney U-tests. For categorical variables, data are expressed as number (proportion) and were analyzed using chi-square tests or Fisher exact tests based on expected cell counts, except ordered multi-categorical variables using Mann–Whitney U-tests, as indicated in the table or figure captions as appropriate. Generally, a P value less than an alpha of 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics 21.0 (IBM, Armonk, NY, USA).
Results
Among the 76 patients enrolled in the study, 60 patients completed the study protocol. Table 1 shows the baseline demographic and clinical data by group assignment. There were no statistically significant differences in any variables at baseline among the two groups.
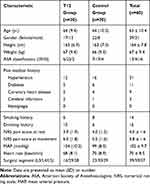 |
Table 1 Baseline Demographic and Clinical Characteristics of Study Participants |
The primary outcome of the MOAA/S score at 10 minutes after extubation was significantly different in the two groups (Figure 3A): 4.2 ± 0.50 (95% CI, 4.0 to 4.4) in the T12 group, and 3.4 ± 0.56 (95% CI, 3.2 to 3.6) in the control group, P <0.001; with similar differences 20 minutes after extubation (P <0.001). There were no significant differences between the two groups 30 minutes after extubation (P = 0.103).
Intraoperative data are shown in Table 2. The intraoperative dosages of sufentanil were lower in the T12 group than in the control group (P = 0.007). For neural coordination assessment after extubation, only 10 (33.3%) control group patients were able to quickly move their lower limbs as directed in normal tone compared to 27 (90%) patients in the T12 group (P <0.001). The duration of anesthesia recovery (min) was shorter in the T12 group than in the control group (P <0.001). There were no significant differences among the two groups in terms of duration of anesthesia or PaCO2 20 minutes after extubation.
 |
Table 2 Intraoperative Outcomes |
Follow-up outcomes following surgery are shown in Table 3. The first time of morphine bolus was later in the T12 group than in the control group (P <0.001). The postoperative cumulative morphine consumption (mg) at 24 hours (Figure 3B) was 9.1 ± 2.1 (95% CI, 8.6 to 9.9) in the T12 group, and 21.8 ± 3.4 (95% CI, 20.5 to 23.1) in the control group (P =0.003), with similar differences in effective bolus times (P =0.008). The postoperative cumulative morphine consumption and effective bolus times at 48 hours had similar differences to those at 24 hours. The first time to ambulation after surgery was sooner in the T12 group than in the control group (P =0.003). There were no significant differences among the two groups in terms of NRS pain scores at rest or movement at 24 and 48 hours, as well as in the hospital length of stay post-surgery.
 |
Table 3 Follow-Up Outcomes |
There were no serious adverse events in any patients, including local anesthetic toxicity (post-block), allergy, and block failure. One patient experienced agitation after extubation (self-relieving after 10 minutes) in the T12 group. Two patients removed PCIA due to vomiting within 24 hours after surgery in the T12 groups. One patient removed PCIA due to vomiting within 24 hours after surgery, and another patient experienced supraventricular tachycardia in the control group. There were no differences in the incidence of complications (P =0.99).
Discussion
The main findings in the study are that pre-operative bilateral ESPB at T12 enhanced recovery after posterior lumbar surgery, reduced perioperative opioids consumption, enhanced anesthesia emergence, and shortened the first time to ambulation after surgery.
The paraspinal muscles, bony tissues and posterior skin are innervated by the dorsal rami of the spinal nerves.9 In ESPB, local anesthetic is injected deep into the erector spinae muscle and acts on the dorsal rami of spinal nerves at multiple levels by spreading within the musculofascial plane. The T12 ESPB produced analgesia in the surgical area and relieved the tension of paraspinal muscles, thereby enhancing recovery of posterior lumbar surgery by reducing the consumption of perioperative anesthetic. The MOAA/S score was selected as the primary outcome measure to evaluate the effect of ESPB on the anesthesia emergence. All patients, regardless of whether they were in the intervention or control group, received satisfactory surgeries with smooth hemodynamic parameters and adequate postoperative analgesia without significant differences.
Clinical studies have shown anterior-posterior truncal analgesia with ESPB for surgeries involving thoracic and abdominal regions.7,12 Cadaveric studies have confirmed successful staining of both ventral and dorsal rami of multiple spinal nerves located above and below the injection site when dye has been injected into the deep plane of the erector spinae muscle.8,13 Anatomy and imaging studies indicate that the likely mechanism of action is diffusion of local anesthetic anteriorly through the connective tissues spanning the adjacent transverse processes and into the vicinity of the spinal nerve roots.7,8,13 However, a recent study found that there was no spread of dye anteriorly to the paravertebral space, and the ventral rami were not blocked.14 Local anesthetic blocking the dorsal rami of the spinal nerves may be the main mechanism of ESPB. Consistent with this finding, we also found that there were rarely discernible cutaneous sensory blocks on the pinprick test in the anterior trunk of the ESPB patients (Figure 2B). The lack of an observed motor block, which may hinder postoperative neurologic testing and limb activity, is an additional potential advantage of ESPB. The lack of correlation between the degree of analgesia and motor block achieved may be explained by the limited amount of local anesthetic that actually reaches the lumbar ventral rami and nerve roots, the delayed onset (40 minutes post-block), or the low concentration of local anesthetic.
Posterior lumbar surgery is one of the most painful surgical procedures, with median pain scores (using the 0–10 NRS) on the first postoperative day ranging from 5 (spinal decompression) to 7 (spinal fusion).1 Patients are often kept in the hospital, primarily to manage their pain, and interventions targeted at reducing opioid requirements and optimizing analgesia have been shown to result in a shorter length of stay.3,5 Opioids have traditionally been the mainstay of analgesia therapy, but they may not always adequately control pain, and at high doses, they are associated with significant adverse effects and the risk of dependence.3 Additionally, as traditional regional anesthesia techniques of epidural anesthesia, paravertebral blocks include risks of inadvertent motor blockade, deep vein thrombosis, hypotension, urinary retention, pneumothorax, vascular puncture, and epidural hematoma.6 ESPB provides effective truncal analgesia with only a simple injection, and avoids the risks associated with opioids and with more invasive traditional regional anesthesia techniques.10,15,16
This capacity for extensive cranial-caudal spread is a unique advantage of the ESPB, allowing it to be performed at a distance from the surgical field, thus minimizing the risk of microbial contamination. This is in contrast to another recently described regional analgesic technique for spine surgery, the thoracolumbar interfacial plane block, which requires injection at a vertebral level congruent with the surgical site.17,18 In addition, the thoracolumbar interfacial plane block is considered to be incomplete analgesia due to the fact that it only partially blocks the posterior rami of the thoracolumbar spinal nerves.19 The retrolaminar block that has been reported to offer analgesia for lumbar surgery has the same deficiencies as the thoracolumbar interfacial plane block.20 In addition, the retrolaminar block may be opposed by an orthopedist owing to the need for a drug injection around the midline of the spine. Limitations of the ESPB include the need to perform bilateral injections for incisions that cross the midline and the limited duration of analgesia with single injections. However, the ESPB also lends itself well to catheter insertion for intermittent boluses or continuous infusions of local anesthetics. The ESPB is a technique that can be performed quickly and simply by all surgeons, it is reliable and consistent, it is opioid sparing, and it has minimal complications.15 Finally, we would like to note that as with all plane blocks, there is likely to be interindividual variability in the physical spread of local anesthetic and the consequent intensity and extent of analgesia.
There are several limitations to our study. First, our study was designed for bilateral single injections rather than catheter insertion for intermittent boluses or continuous infusions of local anesthetics. It is possible that some patients might not have derived long-term benefits, thereby underestimating the clinical effectiveness of ESPB. Second, to ensure patient safety, our study design precluded blinding of the block physicians who were not responsible for outcome evaluation. Third, no sham injection was performed in the control group, and the placebo effect of injection could not, therefore, be assessed. This may have introduced bias and amplified differences. Finally, patients who had a history of chronic opioids use were excluded, because chronic opioids use may create a tolerance to the sedative effects of opioids. This may affect the generality of recruited participants.
Conclusion
In summary, we have demonstrated the potential of bilateral ESPB as an effective regional anesthetic technique when performed at the level of the T12 transverse process, enhancing recovery after posterior lumbar surgery by reducing perioperative opioids consumption.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author.
Ethics and Consent Statement
Ethics approval was obtained from the Ethics Committee of Beijing Hospital. Written informed consent was obtained from all subjects prior to the study.
Acknowledgments
This research was funded by the Ministry of Human Resources and Social Security of the People’s Republic of China (BJ-2017-039).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118(4):934–944. doi:10.1097/ALN.0b013e31828866b3
2. Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a cochrane systematic review and meta-analysis †. Br J Anaesth. 2013;111(5):711–720. doi:10.1093/bja/aet213
3. Levy N, Mills P, Mythen M. Is the pursuit of DREAMing (drinking, eating and mobilising) the ultimate goal of anaesthesia? Anaesthesia. 2016;71(9):1008–1012. doi:10.1111/anae.2016.71.issue-9
4. Tan M, Law LS, Gan TJ. Optimizing pain management to facilitate enhanced recovery after surgery pathways. Can J Anaesth. 2015;62(2):203–218. doi:10.1007/s12630-014-0275-x
5. Puvanesarajah V, Liauw JA, Lo SF, Lina IA, Witham TF, Gottschalk A. Analgesic therapy for major spine surgery. Neurosurg Rev. 2015;38(3):407–418. (). doi:10.1007/s10143-015-0605-7
6. Gottschalk A, Freitag M, Tank S, et al. Quality of postoperative pain using an intraoperatively placed epidural catheter after major lumbar spinal surgery. Anesthesiology. 2004;101(1):175–180. doi:10.1097/00000542-200407000-00027
7. Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41(5):621–627. doi:10.1097/AAP.0000000000000451
8. Adhikary SD, Bernard S, Lopez H, Chin KJ. Erector spinae plane block versus retrolaminar block: a magnetic resonance imaging and anatomical study. Reg Anesth Pain Med. 2018;43(7):756–762. doi:10.1097/AAP.0000000000000798
9. Saito T, Steinke H, Miyaki T, et al. Analysis of the posterior ramus of the lumbar spinal nerve: the structure of the posterior ramus of the spinal nerve. Anesthesiology. 2013;118(1):88–94. doi:10.1097/ALN.0b013e318272f40a
10. Melvin JP, Schrot RJ, Chu GM, Chin KJ. Low thoracic erector spinae plane block for perioperative analgesia in lumbosacral spine surgery: a case series. Can J Anaesth. 2018;65(9):1057–1065. doi:10.1007/s12630-018-1145-8
11. Pambianco DJ, Vargo JJ, Pruitt RE, Hardi R, Martin JF. Computer-assisted personalized sedation for upper endoscopy and colonoscopy: a comparative, multicenter randomized study. Gastrointest Endosc. 2011;73(4):765–772. doi:10.1016/j.gie.2010.10.031
12. Chin KJ, Adhikary S, Sarwani N, Forero M. The analgesic efficacy of pre-operative bilateral erector spinae plane (ESP) blocks in patients having ventral hernia repair. Anaesthesia. 2017;72(4):452–460. doi:10.1111/anae.13814
13. Yang HM, Choi YJ, Kwon HJ. O J, Cho TH, Kim SH. Comparison of injectate spread and nerve involvement between retrolaminar and erector spinae plane blocks in the thoracic region: a cadaveric study. Anaesthesia. 2018;73(10):1244–1250. doi:10.1111/anae.14408
14. Ivanusic J, Konishi Y, Barrington MJ. A cadaveric study investigating the mechanism of action of erector spinae blockade. Reg Anesth Pain Med. 2018;43(6):567–571. doi:10.1097/AAP.0000000000000789
15. El-Boghdadly K, Pawa A. The erector spinae plane block: plane and simple. Anaesthesia. 2017;72(4):434–438. doi:10.1111/anae.13830
16. Tsui BCH, Fonseca A, Munshey F, McFadyen G, Caruso TJ. The erector spinae plane (ESP) block: a pooled review of 242 cases. J Clin Anesth. 2019;53:29–34. doi:10.1016/j.jclinane.2018.09.036
17. Hand WR, Taylor JM, Harvey NR, et al. Thoracolumbar interfascial plane (TLIP) block: a pilot study in volunteers. Can J Anaesth. 2015;62(11):1196–1200. doi:10.1007/s12630-015-0431-y
18. Li C, Jia J, Qin Z, Tang Z. The use of ultrasound-guided modified thoracolumbar interfascial plane (TLIP) block for multi-level lumbar spinal surgery. J Clin Anesth. 2018;46:49–51. doi:10.1016/j.jclinane.2018.01.018
19. Wang AZ, Fan K. Ultrasound-guided posterior ramus of spinal nerve block for anesthesia and analgesia in lumbar spinal surgery. J Clin Anesth. 2019;52:48–49. doi:10.1016/j.jclinane.2018.08.032
20. Ueshima H, Hara E, Otake H. Lumbar vertebra surgery performed with a bilateral retrolaminar block. J Clin Anesth. 2017;37:114. doi:10.1016/j.jclinane.2016.12.021
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


