Back to Journals » Journal of Inflammation Research » Volume 16
Bifidobacterium longum Administration Diminishes Parasitemia and Inflammation During Plasmodium berghei Infection in Mice
Authors Fitri LE , Sardjono TW, Winaris N , Pawestri AR , Endharti AT , Norahmawati E, Handayani D, Kurniawan SN , Azizah S, Alifia LI, Asiyah R , Ayuningtyas TR
Received 20 December 2022
Accepted for publication 17 February 2023
Published 27 March 2023 Volume 2023:16 Pages 1393—1404
DOI https://doi.org/10.2147/JIR.S400782
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Loeki Enggar Fitri,1,2 Teguh Wahju Sardjono,1,2 Nuning Winaris,1,2 Aulia Rahmi Pawestri,1,2 Agustina Tri Endharti,1 Eviana Norahmawati,3 Dian Handayani,4 Shahdevi Nandar Kurniawan,5 Syafiatul Azizah,6 Lustyafa Inassani Alifia,6,7 Rokhmatul Asiyah,6,8 Tita Rachma Ayuningtyas6
1Department of Parasitology, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia; 2AIDS, Toxoplasma, Opportunistic Disease, and Malaria (ATOM) Research Group, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia; 3Department of Anatomical Pathology, Dr. Saiful Anwar Hospital/Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia; 4Department of Nutrition, Faculty of Health Sciences, Universitas Brawijaya, Malang, Indonesia; 5Department of Neurology, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia; 6Master Program in Biomedical Sciences, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia; 7Department of Parasitology, Faculty of Medicine, University of Muhammadiyah Malang, Malang, Indonesia; 8State University of Malang, Malang, Indonesia
Correspondence: Teguh Wahju Sardjono, Department of Parasitology, Faculty of Medicine Universitas Brawijaya, Jl. Veteran, Malang, 65145, Indonesia, Tel +62 341 569117, Fax +62 341 564755, Email [email protected]
Purpose: During Plasmodium berghei (P. berghei) infection, infected erythrocytes are sequestered in gut tissues through microvascular circulation, leading to dysbiosis. This study aimed to investigate the effect of Lactobacillus casei (L. casei) and Bifidobacterium longum (B. longum) administration on the parasitemia level, gut microbiota composition, expression of cluster of differentiation 103 (CD103) in intestinal dendritic and T regulatory cells (T reg), plasma interferon gamma (IFN-γ) and tumor necrosis factor (TNF-α) levels in P. berghei infected mice.
Methods: P. berghei was inoculated intraperitoneally. Infected mice were randomly divided into 5 groups and treated with either L. casei, B. longum, or the combination of both for 5 days before up to 6 days post-infection (p.i). The control group was treated with phosphate-buffered saline (PBS), while uninfected mice were used as negative control. Levels of CD103 and forkhead box P3 (FoxP3) expression were measured by direct immunofluorescense, while plasma IFN-γ and TNF-α level were determined using enzyme-linked immunosorbent assay (ELISA).
Results: All treated groups showed an increase in parasitemia from day 2 to day 6 p.i, which was significant at day 2 p.i (p = 0.001), with the group receiving B. longum displaying the lowest degree of parasitemia. Significant reduction in plasma IFN-γ and TNF-α levels was observed in the group receiving B. longum (p = 0.022 and p = 0.026, respectively). The CD103 and FoxP3 expression was highest in the group receiving B. longum (p = 0.01 and p = 0.02, respectively).
Conclusion: B. longum showed the best protective effect against Plasmodium infection by reducing the degree of parasitemia and modulating the gut immunity. This provides a basis for further research involving probiotic supplementation in immunity modulation of infectious diseases.
Keywords: Bifidobacterium longum, inflammation, Plasmodium, sequestration
Introduction
During the blood stage of the Plasmodium spp. life cycle, the infected erythrocytes are not only present in the peripheral blood, but also sequestered in various tissues and elicit an immune cascade associated with dysbiosis. In human, sequestration of infected erythrocytes was observed in various organs, including in the gastrointestinal tract, comprising the colon, jejunum, ileum, and stomach.1 In vivo studies of C57 black 6 (C57BL/6) mice also showed sequestration of infected erythrocytes in sections of the cecum.2 Specific immune cell populations were noted in the lamina propria of the small and large intestine of C57BL/6 mice infected with Plasmodium yoelii (P. yoelii) within 14–21 days post-infection (p.i.), where macrophages and CD8 T cells peaked on day 14, while monocytes and neutrophils peaked on day 21 p.i. This influx of monocytes and neutrophils indicated an inflammatory lamina propria at the peak of P. yoelii infection.3 In addition, anatomical changes were present in the gastrointestinal tract of the malaria model mice, including significant shortening of the intestines from the duodenum to the colon. Microscopically, there were shortening of the villi, increasing depth of crypts, thickening of the mucin layer, and microscopic bleeding.4 These conditions might result from dysbiosis in Plasmodium spp. infection, which refers to the changes in the composition and function of microbiota. This, in turn, affects the decrease or rise of the number, metabolites, or enzymatic activity of both beneficial and pathogenic bacteria.5,6 Several studies have reported dysbiosis during malaria infection, such as an increase of Proteobacteria and Verrucomicrobia, and decrease of Lactobacillus spp. in P. berghei-infected mice.4 Moreover, a reduction in Firmicutes and increase of Bacteroidetes abundance were also described on the day tenth day post P. yoelii infection in vivo.2 A study by Toukam et al revealed that P. berghei ANKA (PbA)-infected mice receiving gavage of L. sakei probiotic isolated from traditional fermented milk for 7 and 14 days showed a decrease in the degree of parasitemia at 72 hours post-infection compared to non-treated group.7 Another study from Mahajan et al, that observed the effect of L. casei probiotic as adjuvant therapy with chloroquine in C57BL/6 mice infected with P. berghei, showed that the combination of L. casei and chloroquine resulted in a suppression of the degree of parasitemia.8
Dysbiosis due to Plasmodium infection could affect intestinal immune response, abnormal cytokine production, and the severity of malaria. This condition aids in polarization of macrophages and dendritic cells (DCs), inducing T helper 1 (Th 1) and Th17 cells to produce proinflammatory cytokines, such as IL-6, IL-12, TNF-α, interleukin (IL)-1β, and IFN-γ.9 Plasmodium infection-related dysbiosis also results in CD103+ intestinal DCs to lose their ability to mature, capture, present antigens to T cells, and migrate towards T cells to initiate an adaptive immune response. On the other hand, inflammatory DCs will release pro-inflammatory cytokines IL-12/IL-27 and produce IFN-γ, where IFN-γ could directly inhibit the formation of FoxP3 T reg cells.10,11 It is intriguing to further elucidate the intestinal immune response during Plasmodium infection after probiotic administration. Therefore, this study investigated the effect of L. casei and B. longum probiotic supplementation on the degree of parasitemia and gut immune responses in P. berghei-infected mice.
Materials and Methods
Bacterial Culture of L. casei and B. longum
Isolates of L. casei and B. longum were obtained from the Food and Nutritional Culture Collection (FNCC), Gajah Mada University (Yogyakarta, Indonesia). The bacteria were grown in 11 mL of De Man, Rogosa, and Sharpe agar (MRS broth and incubated at 37°C for 24 hours). After harvesting, their optical density (OD) was measured using spectrophotometry at a wavelength of 600 nm. Then, 1 mL of the bacteria was vortexed and centrifuged at 4000 rpm for 15 minutes. The pellets were resuspended in PBS to a certain volume. This procedure was performed for 11 days during the probiotic intervention. Gram staining was carried out every day to determine the morphology and ensure the purity of the bacteria.
Mice and Grouping
Animal experiment conducted in this study has been approved by the Ethics Committee of the Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia (ethical approval number 168/EC/KEPK/06/2021). Six-week-old male C57BL/6 mice weighing 22 to 30 grams were purchased from PT. Biomedical Technology Indonesia (Bogor, Indonesia). All mice were acclimatized to the living environment 2 weeks prior to the experiment. Two mice were infected with P. berghei to produce donor mice. They were fed standard diet and water ad libitum and housed at a temperature of 21°C under a 12-hour light–dark cycle. After the acclimatization period, 28 male mice were randomly allocated into five groups. All groups except the negative control group (Neg) was infected with 5×106 cells of P. berghei intraperitoneally. The treatment group each received either L. casei at 109 colony forming unit/100 µL (CFU/100 µL) (group I), B. longum 109 at CFU/100 µL (group II), or a combination of L. casei and B. longum each at 5×108 CFU/100 µL (group III). The negative and positive control groups received PBS instead of the probiotics. The probiotics were administered orally from 5 days before until day 6 post P. berghei inoculation. The weight of each mouse was measured daily. The survival rate was determined every day post-infection (Appendix 1).
Preparation and Inoculation of P. berghei
Plasmodium berghei ANKA strain were obtained from the Laboratory of Parasitology, Universitas Brawijaya (Malang, Indonesia). Pellets of P. berghei-infected erythrocytes were thawed and centrifuged at 2000 rpm for 5 minutes. The pellets were then washed twice using the Roswell Park Memorial Institute (RPMI) medium and diluted as needed for intraperitoneal inoculation at 5×106 parasite cells per 0.2 mL of diluted erythrocytes per mice. The parasitemia was confirmed using Giemsa-stained thin blood smear according the World Health Organization (WHO) protocol.12 The degrees of parasitemia were counted under light microscope with a 1000x magnification and the infection rate was calculated per 1000 erythrocytes.
Tissue Collection
All mice were sacrificed at day 6 p.i and the intestinal organs were collected. Ileum and colon were collected and fixed using 10% neutral buffered formalin (NBF). After the fixation process, a dehydration process was carried out to remove the water content in the organs by gradually placing the samples into 70%, 80%, and 90% ethanol for 5 minutes each, followed by absolute ethanol for 2×10 minutes, and finally xylol for 2×10 minutes. Then, the samples were embedded and blocked in a liquid paraffin block and cut using a microtome with a slide thickness of 2–10 µm. The length of colon tissues was measured. The proximal 3 cm sections were collected and stored in 10% NBF for 24 hours. The tissues were then processed using a Tissue Processor Thermo Scientific STP 120 for 16 hours. The blocks were cut into 3–5 μm thickness, fixed onto slides and stained with hematoxylin and eosin (H&E).
Measurement of Plasma TNF-α and IFN-γ Levels
The blood was collected from the heart into ethylenediaminetetraacetic (EDTA) tubes and centrifuged for 15 minutes at 3000 rpm to retrieve the plasma. TNF-α nd IFN-γ level was measured using a commercial ELISA Kit (Elabscience, IFN-γ cat. number E-EL-M004896T, TNF-α cat. number E-EL-M004996T, Texas, US) according to manufacturer’s instruction. Briefly, 100 μL of undiluted samples was added to the anti-mouse IFN-γ or TNF-α precoated ELISA plates and incubated for 90 minutes at 37°C. After the liquid removal, the biotinylated detecting antibody was added immediately and incubated for 1 hour at 37°C. Following 3 times washing, the HRP-conjugated working solution was added and incubated for 30 min at 37°C. Then, the substrate was added and incubated in the dark for about 15 min at 37°C. Finally, the stopping solution was added and the optical density (OD) was measured immediately using a microplate reader at 450 nm.
Determination of CD103 and FoxP3 Expression
The CD103 and FoxP3 expression were measured using direct immunofluorescence. Slides were heated at a temperature of 60°C and rehydrated by sequentially placing the samples on xylol (2 × 10 minutes), absolute ethanol (2 × 10 minutes), 90% ethanol (1 × 5 minutes), 80% ethanol (1 × 5 minutes), 70% ethanol (1 × 5 minutes), and sterile distilled water (3 × 5 minutes). Next, the antigen retrieval process is carried out by immersing the slides in a chamber containing citrate buffer, pH 6.0, and placed in a water bath at 95°C for 20 minutes. Then, the slides were washed 3 times with PBS for 5 minutes each. Permeabilization was performed by adding PBS Triton-X 100 0.1% for 5 minutes. The slides were then blocked with 3% bovine serum albumin (BSA) for 30 minutes. The slides were then incubated overnight at 4°C with the primary antibody, PE-conjugated CD103 PE (Santa Cruz Biotechnology, Inc., cat. number sc-53085, Heidelberg, Germany) or fluorescein isothiocyanate (FITC) conjugated FoxP3 (Santa Cruz Biotechnology, Inc., cat. number sc-53876, Heidelberg, Germany). The next day, after washing, the slides were stained with 4’,6’-diamidino-2-phenylindole (DAPI) at 1:1000 dilution for 5 minutes and washed with PBS. They were then mounted with mounting medium and cover slips were put in place. The expression was observed using Olympus IX7 fluorescence microscope and Cellsense Standard (Olympus, Japan). Analysis was performed using the Image J software.
Histopathological Observation of the Colon
Microscopic observation of colonic tissue was conducted using Olympus BX 51 (Olympus, Japan). Observation of each sample used five fields of view under 100x magnification to assess the level of damage, followed by 400x magnification to observe inflammatory cells characterized by the presence of neutrophils. The histological score was determined based on the severity of inflammation and the level of architectural damage. The scoring system refered to is published in Erben et al.13
Data Analysis
The data of the degree of parasitemia, weight changes, survival rates, plasma TNF-α and IFN-γ levels, CD103 and FoxP3 expression, and colon histopathology were shown as mean ± SD and analyzed by the Statistical Product and Service Solutions (SPSS) v.25 for Windows program. The normality of the data distribution was tested using the Shapiro Wilk normality test. The difference among groups were tested using one-way ANOVA or Kruskal Wallis with a confidence level of 0.05. Tukey and Games-Howell multiple comparison test was carried out to determine the significant differences between each treatment group in this study. The survival rate was analyzed using Log-rank (Mantel-Cox) test.
Results
Body Weight and Survival Rate
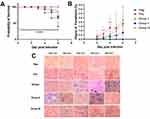
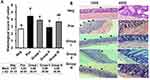
The body weight of mice in each group was measured daily. After P. berghei infection, the body weight of each group fluctuated (Appendix 2). Nevertheless, there were no significant differences of body weight among groups. In groups I and II, the percentage of mortality began to increase at day 4 p.i, while in the positive control group it started to rise on day 5. At day 6 p.i., the survival rate of the positive control group was 72%, group I and group II, where the mice exhibited the highest level of parasitemia, the survival rate was 67.2%, while group III showed 100% survival rate. Oddly, the negative control showed the lowest survival rate among the groups of 39.52%, as some mice died due to unforeseen circumstances, which was presumed to be cardiac stress (Figure 1A). The survival rate at day 6 p.i. showed significant difference among groups (p = 0.04).
The Degree of Parasitemia in P. berghei Infected Mice
All treatment groups showed an increase in the degree of parasitemia from day 2 to day 6 post-infection. At day 2 p.i., parasitemia was significantly different among groups (p = 0.001). When compared to the positive control, Post-hoc Tukey multi-comparison test showed significant differences toward group I and group III (p = 0.12), group I (p = 0.002), and group II (p = 0.08). At day 3, significant difference of parasitemia was also observed among groups (p = 0.001), while multi comparison test showed significant difference between the positive control group with group I (p = 0.025) and group II (p = 0.016). Day 4 p.i. showed significant difference among treatment groups (p = 0.001), while multi-comparison test showed difference between the positive control group with group I (p = 0.062) and group II (p = 0.007). Day 5 and day 6 p.i. also showed significant differences among groups (p = 0.007; p = 0.008). Group II receiving B. longum showed the lowest degree of parasitemia at day 6 p.i. compared to other groups (Figure 1B).
In the positive control group, the ring form of P. berghei appeared from day 2 p.i., showed double chromatin dots at day 5, and multiple infected erythrocytes at day 6 (Figure 1C). In the treatment group, the ring form appeared at day 4 p.i. and began to rise at day 6.
CD103 and FoxP3 Expression
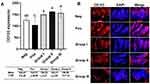
Intestinal dendritic cell CD103 expression from ileal samples was identified using PE-conjugated anti-CD103 antibody (red) (Figure 2). The results showed an increase in the CD103 expression in group I, II, and III, where the expression of CD103 in group III was lower compared to group II. One-way ANOVA showed significant differences among the treatment groups (p = 0.01). Post-hoc Tukey multi-comparison test showed significant difference between the positive control and all treatment groups (p = 0.012; p = 0.03; p = 0.02, respectively).
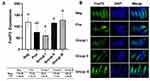
FoxP3 expression in the ileum was identified using FITC-labeled anti-FoxP3 antibody (green) (Figure 3). FoxP3 expression in group I was lower compared to positive control, while group II and III showed an increase in FoxP3 expression compared to group I and positive control. One-way ANOVA showed significant difference among the treatment groups (p = 0.002). Post-hoc Tukey’s multi-comparison test showed significant differences between the negative control group and group I (p-0.021), group III and positive control (p = 0.024), group I and group II (p = 0.031), and group I and group III (p = 0.004) (Figure 3).
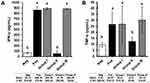
Systemic Levels of Pro-Inflammatory Cytokines TNF-α and IFN-γ
Consistently, the systemic IFN-γ and TNF-α levels (Figure 4) measured from group II receiving B. longum were significantly lower compared to positive control and other treatment groups (p = 0.022 and p = 0.026, respectively). The levels of these pro-inflammatory cytokines were similar to those of the negative control group.
Histopathological Parameters of the Colon
Histopathological assessment (Figure 5) of the positive control group showed erosion and ulceration in several parts, as well widespread inflammation at the mucosal, submucosal, and transmural layers. In group II, the extent of damage was less compared to the positive control. Focal erosion and inflammation were found on the mucosa, which was similar to the negative control group. The histological score of the negative control and group II were significantly lower compared to positive control (p = 0.035 and p = 0.05 respectively) and other treatment groups. There were no significant differences (p = 0.341) in colon lengths among groups (Appendix 3).
Discussion
This study demonstrates the effects of probiotics on the gut immunity during P. berghei infection. During infection, there were no significant differences observed in body weight among the treatment and control groups. This result correlated with a previous study that reported no significant changes in weight in mice infected with the blood stage of P. chabaudi AS parasites.14 In this study, the average weight began to decline at day 4 post-infection, which was correlated with other studies using P. berghei-infected mice receiving heat-killed L. sakei HS-1 treatment.15
All groups showed significant differences in survival rates, suggesting that probiotic administration affected survival rates in Plasmodium infection. This effect by probiotics was in contrast to a previous study performed by Chen et al that showed vitamin A treatments did not increase the survival rate in P. berghei infected mice.16
Compared to the untreated positive control group, the groups receiving L. casei (group I) and B. longum (group II) showed lower parasitemia levels. This finding was supported by a previous study that showed lower parasite burden in P. yoelii-infected mice treated with probiotics Lactobacillus and Bifidobacterium.17 Another study revealed that PbA-infected mice receiving oral administration of L. sakei presented a gradual and significant dose- and treatment duration-dependent reduction of the level of parasitemia compared to the untreated group.7 B. longum is thought to act against acid stress by altering the structure and permeability of the cell membrane to prevent hydrogen ions from entering the cell. BBMN68 strain of B. longum was also able to tolerate bile salt stress by maintaining the efflux and hydrolysis of bile salts. Therefore, this probiotic can reach the intestine, colonize, and interact with immune cells.18
In this study, the group receiving L. casei showed higher parasitemia compared to the untreated group. Similar findings were reported in a previous study where the level of parasitemia was higher in mice treated with heat-killed L. sakei HS-1 in PbA-infected C57BL/6 mice.15 However, another study performed by Mahajan in 2021 investigating the effect of probiotic L. casei as adjuvant therapy in P. berghei infected C57BL/6 mice showed contrasting results, where L. casei and chloroquine combination resulted in complete suppression of parasitemia level.8 A study investigating probiotics as pretreatment showed that P. chabaudi infected mice had lower parasitemia compared to control group. In this study, oral administration of L. casei was likely to form a successful colonization and bacterial adhesion in the intestinal tract, which then affected the immune cells.19 Another study performed by Taniguchi et al showed that PbA infection in C57CL/B6 and BALB/c evoked different pathologies and dysbiosis. C57BL/6 mice infected with PbA developed experimental cerebral malaria (ECM) and died within 2 weeks, despite the low level of parasitemia. Meanwhile, the BALB/c mice infected with PbA died more than 3 weeks after infection with high parasitemia degree and the absence of ECM.4
The interaction between probiotics and host immune cells in the intestinal ecosystem is important to regulate the gut immunity.20 In this study, we measured intestinal DCs CD103 expression from ileal samples. The CD103 expression in the untreated group was significantly lower compared to the treatment groups. This finding might be associated with the pathology of malaria infection, where the DCs lose their ability in parasite clearance after phagocyting infected erythrocytes or free merozoites.16 Another study showed similar results, where the levels of CD86, CD83, and HLA-DR decreased significantly in Indonesian patients infected with P. falciparum and P. vivax, indicating the decrease of DC maturation. Low amount of these markers was associated with a rise of spontaneous apoptosis and the declining DC ability to capture, mature, and present antigens to the effector T cells.10
In the groups treated with L. casei and B. longum, the expression of CD103 was higher compared to the untreated group. The maturation of DCs is impaired during malaria infection, which causes a loss in antigen presenting cell (APC) function. This immature DCs also lose the ability to interact with T cells and initiate adaptive immune response.16 The findings support the result in our study, where significantly higher level of FoxP3 expression was observed in the group treated with B. longum, as well as the group receiving a combination of L. casei and B. longum. The sequestration of infected erythrocytes in the intestinal microvasculature will cause inflammation which will trigger a local intestinal immune response. In the intestinal immune response, APCs play an important role in maintaining the balance between activation of the immune response and tolerance in the intestine. In this case, intestinal DCs play a role in maintaining gut homeostasis. Mucosal DCs originating from intestinal tissue are distributed in Peyer’s patches, mesenteric lymph nodes (MLN), and lamina propria (LP). Under normal and healthy LP conditions, the identified intestinal DCs are immature cells, which are characterized by the low expression of co-stimulators and cytokines. In line with this study, the study of Strauch et al in colitis-induced mice reported a decrease in the expression of CD103 DCs in the LP and MLN of the colon, as well as an increase in mature DCs expressing the CD80 co-stimulator molecule, resulting in dysregulation of the intestinal mucosa.11
This study found that the groups receiving L. casei or B. longum both had high expression of CD103. Previous studies examining vitamin A pretreatment in Plasmodium mice showed that vitamin A supplementation pretreatment was able to improve DCs that were impaired and mature in DCs (MHC II mDCs) during infection with PbA. Impaired maturation of DCs during malaria infection also results in loss of APC function. These immature DCs also lose the ability to migrate towards T cells to initiate an adaptive immune response.16 It is known that mucosal DC CD103 has an important role in promoting the expression of the gut homing receptor CCR9 on T cells, as well as the formation of FoxP3 Tregs the formation of FoxP3 Tregs in the gut with transforming growth factor beta (TGF-β) and retinoic acid as cofactors.11,21
T reg cells are a subpopulation of T lymphocytes with an immunoregulatory function, in which they are able to inhibit the activation and proliferation of autoreactive T cells, secrete cytokines such as IL-10 and TGF-β, downregulate T helper cells, and maintain intestinal homeostasis and immune tolerance.22 The formation of effector T cells is very important for immunity against blood-stage Plasmodium. However, Plasmodium parasites have the ability to evade T-cell immunity, through antigenic diversity mechanisms, clonal antigenic variations, and mechanisms of impairment of DC cell maturation by infected erythrocytes.23 In observing the intestinal expression of FoxP3 Treg, the positive control group that was only infected with P. berghei had lower FoxP3 expression compared to the P2 group that was treated with B. longum and the P3 group that was given L. casei + B. longum. In line with this research, a study by Cheng et al which examined the effect of pretreatment of vitamin A supplements in Plasmodium-infected mice showed that during malaria infection, vitamin A promotes the differentiation of regulatory T cells which can be induced by FoxP3+ by inducing DCs to express CD103.16
Furthermore, the result in this study showed a significant reduction of IFN-γ and TNF-α plasma levels in the group treated with B. longum. Other study suggested beneficial effect of B. longum. Oral administration of B. longum in Salmonellosis model was significantly reduced IFN-γ levels and protecting the intestinal epithelium histologically.24 In colitis model mice, there was also a significant decrease in IFN-γ level after administration of B. longum.4
In an in vivo study of malaria in mice, an increase in IFN-γ was found in mice infected with malaria compared to the control group.25 The increase of IFN-γ could be beneficial for host because IFN-γ induces epithelial permeability for movement of epithelial bridging neutrophils that destroys bacteria, parasites, and other pathogens in the intestinal lumen. On the other hand, during malaria infection, excess IFN-γ may trigger dysbiosis in the gastrointestinal tract followed by changes in the immune system. In this study, systemic IFN-γ was increased in the positive control group. Excessive pro-inflammatory cytokine, IFN-γ may suppress T follicular helper (Tfh) cell differentiation CXC chemokine receptor 3 (CXCR3) which acts as a B cell activator. Expansion of atypical Tbet+ B cells is also mediated by IFN-γ signaling inhibitors on the B cell receptors (BCR) and reduces effector function. As a result, there is a decrease in the antibodies produced by effector B cells or plasma cells to fight malaria infection.6
TNF-α production in the early phase of malaria is related to absorption of the parasite burden, but overproduction in the late phase is associated with severity. The dual role of TNF-α indicates that the regulation and timing of pro-inflammatory cytokine production is essential for controlling infection.26
In this study, there were histological damages such as ulceration, erosion, and inflammation in the Plasmodium infected group. There was also an increasing histological score in this group. Administration of probiotics has proven to significantly reduce histological damage, especially in the B. longum group. In this study, there were no significant differences in colon length among the groups. Our result contradicts other study, in which they showed significant changes in the gastrointestinal tract of the malaria mice model, including shortening of the intestine from the duodenum to the colon, shortening of the villi, increased depth of crypts, thickening of the mucin layer, and microscopic bleeding.4
In malaria infection, increased IFN-γ may impact the anatomy and histology of the colon. Increased IFN-γ induce damage the intestinal epithelium by inhibiting proliferation, increasing apoptosis, and increasing the permeability of intestinal epithelial cells. The effects of IFN-γ are according to the duration of exposure. IFN-γ in excessive condition will block β-catenin, then reduce proliferation. The influence of interferon-γ will also activate transcription through JAK/STAT signals to cause excessive apoptosis. IFN-γ induce the expression of intercellular adhesion molecule-1 (ICAM-1) at the apical membrane of T84 cells and increased the number of adherent neutrophils. Neutrophil and ICAM-1 binding modulate myosin light chain kinase (MCLK) phosphorylation so that the tight junction will break down and ruin the epithelial of intestine.27 In addition, the gastrointestinal inflammation that occurs during malaria infection induces extensive tissue fibrosis and a stiff colon, which is unable to perform peristaltic movement or absorb fluids, thus leading to diarrhea. Under conditions of fibrosis expansion, it can affect the muscle layer and myenteric plexus. The accumulation of damage that occurs is marked macroscopically in the form of shortening of the gastrointestinal tract.4,28 There was a significant shortening of the small intestines, cecum, and colon in C57CL/B6 mice infected with PbA. A histological assessment in C57CL/B6 mice infected with PbA showed shortening of villi in the small intestines, detachment of intestinal epithelia, destruction of villi and microscopic bleeding. Meanwhile, there were no histological changes in whole intestines in BALB/c mice infected with PbA, despite the shortening of the colon. Therefore, the most severe effect of PbA infection is presented in C57CL/B6 mice.4
The mechanism of L. casei and B. longum in decreasing the parasitemia remains unclear. However, the immunomodulatory properties of each probiotic bacterium, such as enzymes, antimicrobial peptides and short chain fatty acids (SCFAs), might play a role against malaria infections. A study from Gupta et al reported that antimicrobial properties from L. plantarum LR/14 have anti-Plasmodial activity in chloroquine-sensitive P. falciparum. This study investigated anti-microbial peptides (AMPs) produced from L. plantarum LR-14 and showed a decrease in cell viability in both tested strains of P. falciparum. This result suggested that differences in the membrane composition, such as interaction of the positively charged peptides from the bacteria, may affect the negatively charged surface molecules of the parasites. This condition results in an increase in erythrocyte membrane fluidity, alteration of the host cell’s lipid, fatty acid, protein composition, phospholipid distribution, and increased membrane permeability. These modifications lead to the construction of erythrocyte membrane channels known as the new permeability pathways (NPPs), thus allowing the selective entry of low molecular weight molecules to the infected erythrocytes.29
On the other hand, Bifidobacterium is also known to have protective effect against Plasmodium infection through the immunomodulatory action of surface associated molecules. The immunomodulatory protein of B. longum, the extracellular serine protease inhibitor (serpin), has the ability to bind and irreversibly inactivate proteases. The targets of serpin secreted by B. longum are pro-inflammatory proteases and human neutrophil.30 Bifidobacteria are also known to produce SCFAs such as acetate and lactate, which are the main end-products of the Bifidobacteria catabolism. The acetate produced by Bifidobacteria is used as substrates for other microbes, mainly for the butyrate and propionate producers. Butyrate and propionate are known to have anti-inflammatory effects in the gut, and promotes and regulates the pool of colonic T reg cells. Butyrate acts by inhibiting histone deacetylase (HDAC) activity in DC and T cells, which leads to differentiation of Treg cells and increase the expression of Foxp3, the only transcription factor for Treg cells. These mechanisms are suggested to be mediated by free fatty acid receptor 3 (FFAR3) and GPR109A, the butyrate receptors in epithelial and immune cells.30,31
The colonization of these probiotics in the intestine needs to be further investigated to reveal the mechanism of immune modulation during Plasmodium infection. Other studies exploring the therapeutic effects of these probiotics also need to be conducted, for instance by comparing the Plasmodium-infected probiotic-treated group with untreated groups, thus determining the therapeutic effect and the percentage of parasitemia growth inhibition by probiotic administration. This study has several technical limitations. In our experimental design in which there was no positive control group that was given standard antimalarial drugs. The T reg population, which is much higher in the colon, needs to be measured in future studies, as well as the local expression of cytokines. Upcoming analysis related to the effect of these probiotic on gut microbiota composition and their metabolites, such as SCFAs, needs to be conducted. Additionally, the marker of intestinal DCs maturation (such as CD80, CD86, MHCII) in malaria model, as well as local cytokines production, needs to be measured in the future studies.
Conclusion
In summary, the intestinal immune system accounts for a large component of the tissue immunity due to its large surface area and constant exposure to microbiota. B. longum administration in P. berghei-infected model showed beneficial effect in suppressing the degree of parasitemia and modulate gut immunity by increasing expression of CD103, FoxP3 and reduced TNF-α and IFN-γ plasma levels.
Abbreviations
µL, microliter; ANOVA, Analysis of Variance; APC, Antigen Presenting Cells; BCR, B Cell Receptors; NBF, Neutral Buffered Formalin; C57BL/6, C57 Black 6; CCR9, C-C Chemokine Type Receptor 9; CD, Cluster of differentiation; CFU, Colony Forming Unit; CXCR3, CXC Motif; Chemokine Receptor 3; DC, Dendritic Cells; ELISA, Enzyme-linked immunosorbent assay; FITC, Fluorescein isothiocyanate; FoxP3, Forkhead box P3; H&E, Hematoxylin and Eosin; ICAM-1, intercellular adhesion molecule-1; IFN, Interferon; IL, Interleukin; JAK/STAT, Janus; kinase/signal transducers and activators of transcription; LP, Lamina Propria; MCLK, myosin light chain kinase; MHC, major histocompatibility complex; mL, milliliter; MLB, Mesenteric Lymph Node; OD, Optical Density; P.i, Post infection; PBS, Phosphate Buffer Saline; PE, Phycoerythrin; RPM, Revolutions Per Minute; SPSS, Statistical Package for the Social Sciences; Th, T helper; TNF; Tumor Necrosis Factor; Treg, T regulator; WHO, World Health Organization.
Acknowledgments
This study was funded by the research grant of the Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia (grant number 3125/UN10.F08/PN/2021) and AIDS, Toxoplasma, Opportunistic Disease, and Malaria (ATOM) Research Group, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Milner DA, Lee JJ, Frantzreb C, et al. Quantitative assessment of multiorgan sequestration of parasites in fatal pediatric cerebral malaria. J Infect Dis. 2015;212(8):1317–1321. doi:10.1093/infdis/jiv205
2. Mooney JP, Lokken KL, Byndloss MX, et al. Inflammation-associated alterations to the intestinal microbiota reduce colonization resistance against non-typhoidal Salmonella during concurrent malaria parasite infection. Sci Rep. 2015;5:1–11. doi:10.1038/srep14603
3. Denny JE, Powers JB, Castro HF, et al. Differential sensitivity to plasmodium yoelii infection in C57BL/6 mice impacts gut-liver axis homeostasis. Sci Rep. 2019;9(1):1–15. doi:10.1038/s41598-019-40266-6
4. Taniguchi T, Miyauchi E, Nakamura S, et al. Plasmodium berghei ANKA causes intestinal malaria associated with dysbiosis. Sci Rep. 2015;5:1–13. doi:10.1038/srep15699
5. Hosseini Jazani N, Shahabi S. Gut microbiota, dysbiosis and immune system: a brief review. J Res Appl Basic Med Sci. 2019;5(2):77–81.
6. Lee MSJ, Coban C. Unforeseen pathologies caused by malaria. Int Immunol. 2018;30(3):121–129. doi:10.1093/intimm/dxx076
7. Toukam LL, Tatsinkou Fossi B, Taiwe GS, et al. In vivo antimalarial activity of a probiotic Bacterium lactobacillus sakei isolated from traditionally fermented milk in BALB/c mice infected with Plasmodium berghei ANKA. J Ethnopharmacol. 2021;280:114448. doi:10.1016/j.jep.2021.114448
8. Mahajan E, Sinha S, Bhatia A, et al. Evaluation of the effect of probiotic as add-on therapy with conventional therapy and alone in malaria induced mice. BMC Res Notes. 2021;14(1):1–5. doi:10.1186/s13104-021-05661-1
9. Mukherjee S. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier Connect, the company‘s public news and information; 2020.
10. Amorim KNS, Chagas DC, Sulczewski FB, Boscardin SB. Dendritic cells and their multiple roles during malaria infection. J Immunol Res. 2016;2016:1.
11. Strauch UG, Grunwald N, Obermeier F, et al. Loss of CD103+ intestinal dendritic cells during colonic inflammation. World J Gastroenterol. 2010;16(1):21–29. doi:10.3748/wjg.v16.i1.21
12. World Health Organization. Giemsa Staining of Malaria Blood Films. Malaria Microscopy Standard Operating Procedure—MM-SOP-07A. Geneva, Switzerland: World Health Organization; 2016:1–6.
13. Erben U, Loddenkemper C, Doerfel K, et al. Original article A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol. 2014;2014:4557–4576.
14. Mooney JP, DonVito SM, Lim R, et al. Intestinal inflammation and increased intestinal permeability in Plasmodium chabaudi AS infected mice. Wellcome Open Res. 2022;7:134. doi:10.12688/wellcomeopenres.17781.2
15. Shimada M, Hayakawa HE, Matsuoka H. Heat-killed Lactobacillus sakei HS-1 mitigates small intestinal pathophysiology on Plasmodium berghei ANKA infected C57BL/6 mice. Jichi Med Univ J. 2021;43:13–19.
16. Chen G, Du Y-T, Liu J-H, et al. Modulation of anti-malaria immunity by vitamin A in C57BL/6J mice infected with heterogenic plasmodium. Int Immunopharmacol. 2019;76:105882. doi:10.1016/j.intimp.2019.105882
17. Villarino NF, LeCleir GR, Denny JE, et al. Composition of the gut microbiota modulates the severity of malaria. Proc Natl Acad Sci USA. 2016;113(8):2235–2240. doi:10.1073/pnas.1504887113
18. Zhang C, Yu Z, Zhao J, et al. Colonization and probiotic function of Bifidobacterium longum. J Funct Foods. 2019;53(1800):157–165. doi:10.1016/j.jff.2018.12.022
19. Martínez-Gómez F, Ixta-Rodríguez O, Aguilar-Figueroa B, et al. Lactobacillus casei ssp. rhamnosus aumenta la protección no específica contra Plasmodium chabaudi AS en ratones. Salud Publica Mex. 2006;48(6):498–503. doi:10.1590/S0036-36342006000600008
20. Wang S, Ye Q, Zeng X, Qiao S. Functions of macrophages in the maintenance of intestinal homeostasis. J Immunol Res. 2019;2019:1.
21. Coombes JL, Siddiqui KRR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β -and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757–1764. doi:10.1084/jem.20070590
22. Zhou A, Yuan Y, Yang M, et al. Crosstalk between the gut microbiota and epithelial cells under physiological and infectious conditions. Front Cell Infect Microbiol. 2022;12:1–11.
23. Hisaeda H, Maekawa Y, Iwakawa D, et al. Escape of malaria parasites from host immunity requires CD4 +CD25+ regulatory T cells. Nat Med. 2004;10(1):29–30. doi:10.1038/nm975
24. Silva AM, Barbosa FHF, Duarte R, et al. Effect of Bifidobacterium longum ingestion on experimental salmonellosis in mice. J Appl Microbiol. 2004;97(1):29–37. doi:10.1111/j.1365-2672.2004.02265.x
25. Murr NJ, Olender TB, Smith MR, et al. Plasmodium chabaudi infection alters intestinal morphology and mucosal innate immunity in moderately malnourished mice. Nutrients. 2021;13(3):1–16. doi:10.3390/nu13030913
26. Leão L, Puty B, Dolabela MF, et al. Association of cerebral malaria and TNF-α levels: a systematic review. BMC Infect Dis. 2020;20:1.
27. Andrews C, McLean MH, Durum SK. Cytokine tuning of intestinal epithelial function. Front Immunol. 2018;9:1. doi:10.3389/fimmu.2018.01270
28. Xu X, Lin S, Yang Y, et al. Histological and ultrastructural changes of the colon in dextran sodium sulfate‑induced mouse colitis. Exp Ther Med. 2020;2020:1.
29. Gupta R, Rajendran V, Ghosh PC, et al. Assessment of anti-plasmodial activity of non-hemolytic, non-immunogenic, non-toxic antimicrobial peptides (AMPs LR14) produced by lactobacillus plantarum LR/14. Drugs RD. 2014;14(2):95–103. doi:10.1007/s40268-014-0043-y
30. Ruiz L, Delgado S, Ruas-Madiedo P, et al. Bifidobacteria and their molecular communication with the immune system. Front Microbiol. 2017;8:2345. doi:10.3389/fmicb.2017.02345
31. Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi:10.1038/nature12726
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.