Back to Journals » Drug Design, Development and Therapy » Volume 17
Bibliometric Study of Adaptogens in Dermatology: Pharmacophylogeny, Phytochemistry, and Pharmacological Mechanisms
Authors Liu XX, Chen CY, Li L, Guo MM, He YF, Meng H, Dong YM, Xiao PG, Yi F
Received 23 November 2022
Accepted for publication 24 January 2023
Published 6 February 2023 Volume 2023:17 Pages 341—361
DOI https://doi.org/10.2147/DDDT.S395256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Xiao-Xing Liu,1– 3 Chun-Yu Chen,1– 3 Li Li,1– 3 Miao-Miao Guo,1– 3 Yi-Fan He,1– 3 Hong Meng,1– 3 Yin-Mao Dong,1– 3 Pei-Gen Xiao,4 Fan Yi1– 3
1Beijing Key Laboratory of Plant Resources Research and Development, College of Chemistry and Materials Engineering, Beijing Technology and Business University, Beijing, People’s Republic of China; 2Key Laboratory of Cosmetic, China National Light Industry, Beijing Technology and Business University, Beijing, People’s Republic of China; 3Institute of Cosmetic Regulatory Science, Beijing Technology and Business University, Beijing, People’s Republic of China; 4Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Fan Yi, Email [email protected]
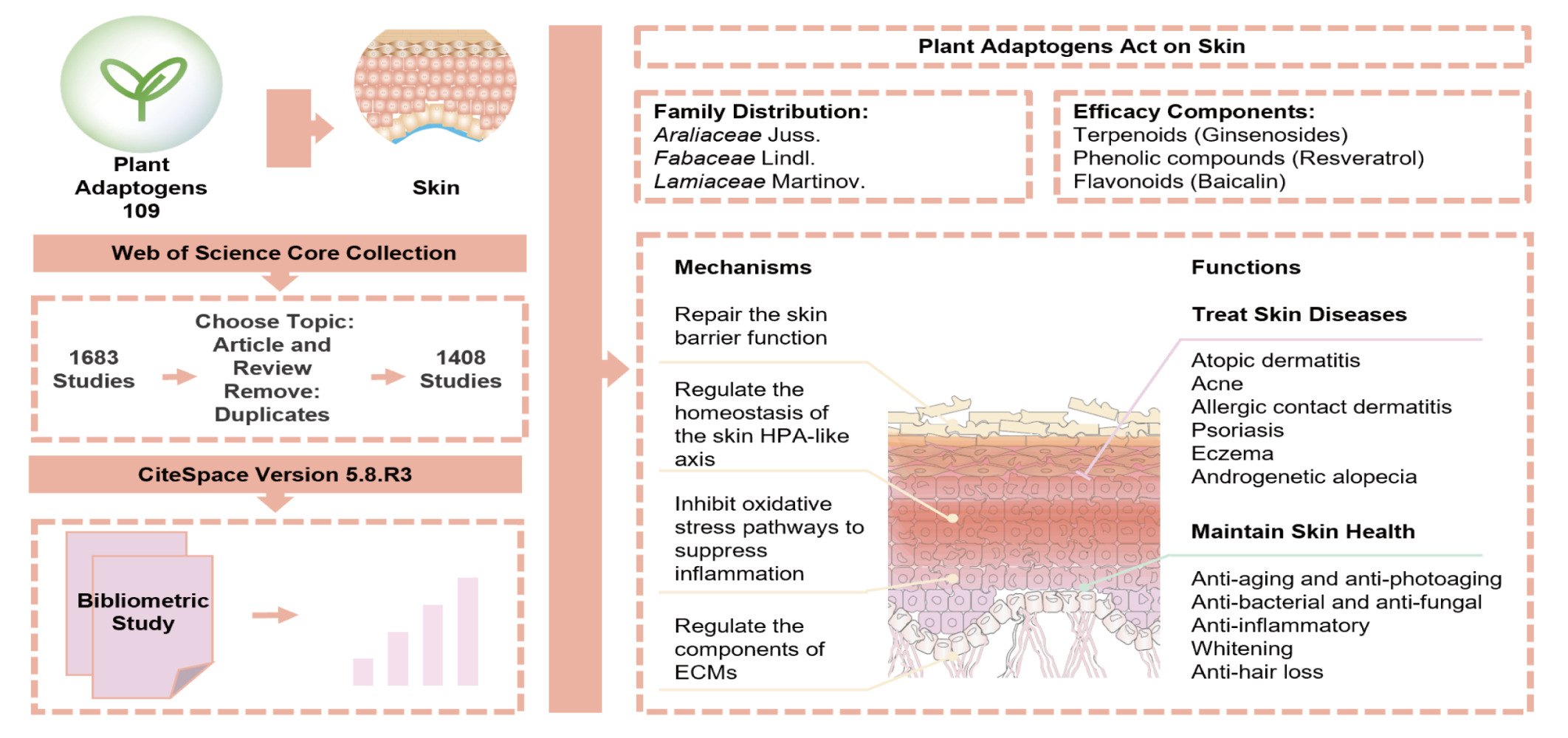
Background: Adaptogens are a class of medicinal plants that can nonspecifically enhance human resistance. Most of the plant adaptogens have relevant applications in dermatology, but there are still few studies related to their particular action and co-operative mechanisms in topical skin application.
Methods: Plant adaptogens related articles and reviews that published between 1999 and 2022 were obtained from the Web of Science Core Collection database. Various bibliographic elements were collected, including the annual number of publications, countries/regions, and keywords. CiteSpace, a scientometric software, was used to conduct bibliometric analyses. Also, the patsnap global patent database was used to analyze the patent situation of plant adaptogens in the field of cosmetics up to 2021.
Results: We found that the effects of plant adaptogens on skin diseases mainly involve atopic dermatitis, acne, allergic contact dermatitis, psoriasis, eczema, and androgenetic alopecia, etc. And the effects on skin health mainly involve anti-aging and anti-photoaging, anti-bacterial and anti-fungal, anti-inflammatory, whitening, and anti-hair loss, etc. Also, based on the results of patent analysis, it is found that the effects of plant adaptogens on skin mainly focus on aging retardation. The dermatological effects of plant adaptogens are mainly from Fabaceae Lindl., Araliaceae Juss. and Lamiaceae Martinov., and their mainly efficacy phytochemical components are terpenoids, phenolic compounds and flavonoids.
Conclusion: The plant adaptogens can repair the skin barrier and maintain skin homeostasis by regulating the skin HPA-like axis, influencing the oxidative stress pathway to inhibit inflammation, and regulating the extracellular matrix (ECM) components to maintain a dynamic equilibrium, ultimately achieving the treatment of skin diseases and the maintenance of a healthy state.
Keywords: plant adaptogens, dermatology, skin HPA-like axis, ECMs, skin homeostasis, pharmacophylogeny
Graphical Abstract:
Introduction
The Definition and Evolution of Adaptogen
The definition of “adaptogen” was first introduced in the mid-twentieth century by the Soviet toxicologist Lazarev1 and is defined as medicinal substances that enhance human resistance non-specifically.2,3 This definition emphasizes that the mode of action of adaptogens is non-specific, ie, they do not act on only one pathway and one target, but act in a multi-pathway and multi-target network in humans. The concept of ‘adaptogen’ evolved dynamically over the next decades, and in 1969 Brekhman and Dardymov4 proposed that: (A) the adaptogen should be nontoxic and cause minimal impairment of the physiological functions of the organism; (B) the action underlying the effects of the adaptogen should be nonspecific, ie, it should enhance resistance to the adverse effects of physical, chemical and biological factors. (C) the adaptogen should have a stimulant effect without side effects, such as the insomnia produced by stimulants. This definition is based on the original definition and further emphasizes that the adaptogen should be safe and free of toxic side effects while being effective. In 1998, the US Food and Drug Administration (FDA) provided an official definition of an adaptogen: an adaptogen is a metabolic regulator that improves the ability of an organism to adapt to its environment and avoid external damage.5 It indicates that after half a century of development, the new concept of adaptogens has been officially recognized. Finally, in 2018, Panossian et al summarized “adaptogen” as a natural compound or plant extract that can improve the adaptability and survival of an organism under stress.6 Compared to the previous definitions, this one is more explicit about the properties and medicinal results of adaptogens. Plant adaptogens are part of the classes of adaptogens. They have the advantages of diversity, high value and safety, and are valuable for exploitation.
Basic Information on Plant Adaptogens
Family Distribution Characteristics
The range of plant adaptogens covered in this study is mainly based on 109 plant adaptogens summarized in the literatures.7,8 Their detailed information is provided in Supplementary Table 1. The results of the family distribution of the plant adaptogens are shown in Table 1 (TOP 8, frequency > 3), from which it can be seen that the plant adaptogens are more frequently distributed in Araliaceae family, Fabaceae family and Lamiaceae family. The Araliaceae family contains many classic plant adaptogens, such as Panax ginseng C.A.Mey. and Eleutherococcus senticosus (Rupr. and Maxim.) Maxim.; the Fabaceae family is the third largest family of seed plants and has an extremely wide distribution. And the most studied genus is Rhodiola, which includes Rhodiola crenulata (Hook.f. and Thomson) H. Ohba, Rhodiola heterodonta (Hook. f. and Thomson) Boriss., Rhodiola rosea L., and Rhodiola imbricata Edgew.
 |
Table 1 Top 8 Families of Plant Adaptogens |
Characteristics of Phytochemical Structure
Panossian A7,8 summarized the plants reported to have anti-stress (adaptive) activity and classified them according to the phytochemical structure of their main active ingredients, with the final results shown in Table 2.
 |
Table 2 Classification of the Phytochemical Structure of the Main Active Ingredients of the Plant Adaptogens |
Most of the plant adaptogens reported in the literature above are from Araliaceae family. The active ingredients are represented by triterpene saponins, especially the tetracyclic backbone, which is structurally similar to cortisol. The cortisol component is one of the downstream products of the central HPA axis. In contrast, lignans and their glycosides, phenylpropane derivatives and phenylethyl derivatives are aromatic compounds structurally similar to catecholamines.
Research Issues of Plant Adaptogens in the Field of Skin
Modern pharmacological studies have shown that plant adaptogens can act in multiple pathways and targets in the human body, affecting the hypothalamic-pituitary-adrenal axis (HPA axis) and the neuro-endocrine-immune system to nonspecifically enhance the resistance of the body under various external stress conditions.9 It indicates that the common mechanism of the endogenous action of plant adaptogens has been intensively studied. However, there is a wide variety of plant adaptogens and no studies have yet pointed out the characteristics of their family frequency distribution.
Some plant adaptogens have historically been used externally on the skin to treat and improve skin-related issues. Plant adaptogens have potential roles in the skin field, but there are still some problems: (i) It is not clear which skin diseases and skin health issues plant adaptogens can act on. (ii) It is not clear the phytochemical structure commonality of the efficacious substances of plant adaptogens acting on the skin. (iii) It is not clear the commonality of the mechanism of action of plant adaptogens acting on skin diseases and skin cosmetic problems. These are the scientific problems that need to be solved for the development and application of plant adaptogens to the skin field. (iv) It has been pointed out that a similar central HPA regulatory system exists in the skin that maintains skin homeostasis.10,11 Giving us a hint that plant adaptogens are used internally for the regulation of body homeostasis by the HPA axis, could there be a similar mechanism for external application to the skin?
In this paper, based on bibliometric analysis, patent analysis, and literature survey analysis of the mechanisms of action of plant adaptogens in the skin field, we summarized the efficacy of plant adaptogens for topical use on the skin and the commonality of family distribution, phytochemistry and pharmacological mechanisms of action of plant adaptogens for external application to the skin.
Methods
Data Source and Collection
Data for the bibliometric analysis were taken from the Web of Science core collection and searched from 1999, to 2022. The 109 plant adaptogens listed in Supplementary Table 1, including ginseng, were paired with Skin disease, Dermatosis, Dermatology, and Cosmetic, respectively, for a full-text search, yielding a total of 1683 publications. Conditional screening and deduplication operations were performed on these studies, and 1408 articles were ultimately obtained (up to April 2022). The process of literature data screening and collection is shown in Figure 1. And data for the patent analysis was taken from the global patents disclosed in the patsnap global patent database. The number of “adaptogen” patent applications in the field of cosmetics was 1364, after the searched, manual read and removed duplicates, ultimately obtained 655 patents (up to Sept. 2021).
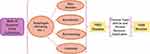 |
Figure 1 Literature collection and screening process. |
Data Analysis and Visualization
CiteSpace version 5.8. R3 software was used to perform bibliometric analysis on the acquired literature. Besides, we used Microsoft Excel 2021 to conduct data analyses and visualization, such as annual publication counts, country/region analysis, and keywords co-occurrence analysis.
Results
Trends in Annual Issuance
Based on the statistical analysis of the overall research on the application of plant adaptogens in the field of dermatology and the changes in the annual number of publications, the development and research status of this field could be effectively evaluated, and the trends in the future development of this research field could be further predicted.
The 1408 papers collected from 1999 to 2022 were counted by year, and the results are shown in Figure 2. Overall, it seems that the annual number of publications in the field of plant adaptogens related to skin shows an increasing trend over time. In 2018, Panossian6 proposed a new definition of adaptogen, and in the same year, our research team9 published an article to sort out the relevant comparative studies of plant adaptogens and ginseng from various countries, which together contributed to the research fervor of plant adaptogens. The number of research results on plant adaptogens in the field of skin exceeded 100 for the first time in 2018 and has maintained a steady growth trend since then, indicating that more and more relevant studies will be conducted in this field in the future.
 |
Figure 2 Trends in the number of articles issued per year. |
Country/Region Analysis
After ranking the countries in the world by the number of publications in the field of plant adaptogens and skin-related conditions from 1999 to 2022, the top five countries in terms of number of publications and their ranking results were collected in Table 3. The analysis shows that the country with the most research in this field is India. India, China, and South Korea all have their own traditional medicine practices, and India has more research on “Indian ginseng” (Withania somnifera (L.) Dunal). In China and South Korea, the focus is on the development and application of Panax ginseng C.A.Mey. and Eleutherococcus senticosus (Rupr. and Maxim.) Maxim. Plant adaptogens have also been studied in the United States and Europe, the head markets of nutraceuticals, because of their excellent health effects.
 |
Table 3 Top 5 Countries in Terms of Number of Articles Published |
Keywords Co-Occurrence Analysis
Research hotspots can reflect the focus and trends of research, and keyword analysis is a common means to analyse research hotspots. The results of keyword co-occurrence analysis can reflect the current research hotspots of plant adaptogens in skin-related fields. The keywords related to active ingredients and those related to skin diseases and those related to skin health were classified and combined in the keyword cooccurrence results; for example, acne and Propionibacterium acnes were classified as acne, and the final results are shown in Table 4 and Table 5, and Figure 3.
 |
Table 4 Keyword Co-Occurrence Results (Active Ingredients) |
 |
Table 5 Keyword Co-Occurrence Results (Pharmacological Effects) |
 |
Figure 3 Keyword co-occurrence results (pharmacological effects). |
The key words related to the study of active ingredients of plant adaptogens were sorted, and the results are shown in Table 4; the active ingredients were found to be phenols, volatile oils, flavonoids, terpenoids, fatty acids, vitamins, polysaccharides and alkaloids in order. Grapes, curcumin and ginseng have been studied more in dermatology. Grapes are common cash crops, and the main active ingredients include phenols, flavonoids, fatty acids and vitamins; curcumin is widely used in the food industry as a flavouring and pigment in China and abroad and is a phenolic compounds; ginseng is a component of the most representative plant adaptogens, and the main active ingredients are terpenoids and volatile oils. Volatile oils generally comprise various components, and most of them contain terpenoids in addition to aliphatic and aromatic compounds.
The key words related to the pharmacological effects of the plant adaptogens were sorted, and the results are shown in Table 5 and Figure 3. The skin disease-related aspects were atopic dermatitis, acne, allergic contact dermatitis, psoriasis, androgenetic alopecia, and eczema; the skin health-related aspects were anti-aging and anti-photoaging, anti-bacterial and anti-fungal, anti-inflammatory, whitening, and anti-hair loss.
The most frequent keyword related to the pharmacological effects was “antioxidant activity”, and the frequency of keywords in the top 20, including “antioxidant”, “oxidative stress” and “antioxidant activity”, together indicate that research on the use of plant adaptogens in the field of dermatology is more focused on antioxidants. It has been noted that oxidative stress caused by the accumulation of reactive oxygen species exacerbates skin aging and inflammation and plays an important role in hair loss12–14 and that NF-κB in epithelial cells is important for maintaining the skin barrier.15 In recent years, the incidences of oral diseases associated with oxidative stress have also increased.16
Based on the results of the bibliometric analysis of plant adaptogens in skin-related fields from 1999 to 2022, we understand that there is an overall upwards trend in the number of annual publications in this field and therefore predict that more relevant studies will be conducted in the future. In terms of the geographic distribution of research, most of the research in this field is distributed in India, China and South Korea, and all three countries have their own traditional medicine practices. In terms of keyword co-occurrence analysis results (active ingredients), there are more studies on phenols, volatile oils, flavonoids, and terpenoids; in terms of keyword co-occurrence analysis results (pharmacological effects), studies on plant adaptogens related to skin diseases include studies on atopic dermatitis, acne, allergic contact dermatitis, psoriasis, eczema, and androgenetic alopecia; studies on plant adaptogens related to skin health include studies on their anti-aging and anti-photoaging, anti-bacterial and anti-fungal, anti-inflammatory, whitening and anti-hair loss effects.
By using bibliometric analysis, we identified the status of the development of plant adaptogens in skin-related fields, including the research trends, the geographical research areas, the more researched active ingredient classifications and the researched pharmacological efficacy. Further, we will explore the mechanistic information on the role of plant adaptogens in skin-related diseases from both skin disease-related and skin health-related aspects.
Mechanism of Action of Plant Adaptogens Associated with Skin Diseases
Based on the results of the above bibliometric analysis, plant adaptogens were used for skin disease applications related to atopic dermatitis, acne, allergic contact dermatitis, psoriasis, eczema, and androgenetic alopecia, and further literature research was conducted to obtain the mechanism of action underlying the effects of plant adaptogens related to these skin diseases. The results are detailed in Supplementary Table 2.
Atopic Dermatitis
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by recurrent episodes, pleomorphic lesions, dry skin, and intense pruritus.17 Atopic dermatitis has complex pathophysiological mechanisms, including those involving genetic factors, the impaired skin barrier, changes in immune response and the disruption of skin microbial balance.18
Dermatologists often prescribe glucocorticosteroid treatment, but long-term use of glucocorticosteroids can cause many side effects.19,20 Patients and their families often demand herbal treatments with similar efficacy and fewer side effects. This concern makes the use of adaptogens promising as they are effective while being safe and free of toxic side effects.
The total flavonoids of sea buckthorn (Elaeagnus rhamnoides (L.) A. Nelson. (Syn. Hippophae rhamnoides L.)), Curcumin from turmeric (Curcuma longa) and thymoquinone from Nigella sativa L., etc., can inhibit the immune inflammatory response of the skin in patients with atopic dermatitis.21–23 The ginsenoside Rg5 from Panax ginseng C.A. Mey. can reduce the level of skin inflammation and oxidative stress in patients.24 The hypericin from Hypericum perforatum L. and neferine from Nelumbo nucifera Gaertn. can repair the skin barrier and improve the symptoms of skin damage.25,26
Acne
Acne is a chronic inflammatory skin disease that occurs mainly in areas of the skin with a high density of sebaceous glands, such as the face, back and chest, and mainly develops during early and late adolescence.27 The pathogenesis of acne mainly involves increased sebum production, excessive proliferation of keratinocytes, skin inflammation and over proliferation of Propionibacterium acnes.28 Psychological stress can lead to increased levels of serum CRH, CRH receptors, and cortisol. CRH (which can be released from central or peripheral nerve fibers or produced by skin cells themselves) acts as the most upstream trigger of the HPA axis to initiate the stress response and induce the HPA axis response. This is why CRH is called a stress response hormone. Stimulation of sebocytes by stress responders (eg CRH) increases the secretion of sebum and pro-inflammatory mediators, which can cause or exacerbate acne symptoms.10 It has been found that human sebocytes can autocrine CRH and its receptors, induce sebum synthesis, exhibit independent HPA axis-like functions, and participate in pathological processes such as acne and androgenetic alopecia.29
The main treatment options for acne include topical or systemic treatments, such as antibiotics, and physical therapy, such as cryotherapy.30 However, the problem of the increasing drug resistance of Propionibacterium acnes has emerged, and new treatments are needed.
Curcumin from turmeric (Curcuma longa),31–33 Hypericin from Hypericum perforatum L. and salidroside from Rhodiola crenulata (Hook.f. and Thomson) H. Ohba can reduce the level of oxidative damage and inflammatory response in acne lesions by inhibiting the activity of Propionibacterium acnes.34,35 Resveratrol in Vitis vinifera L. can reduce the area of skin lesions.36 The thymoquinone in Nigella sativa L. can reduce the symptoms of skin lesions.37
Allergic Contact Dermatitis
Allergic contact dermatitis is a delayed hypersensitivity reaction characterized by erythema and scaling in the acute phase and mossy lesions and hyperkeratosis in the chronic phase.38 Treatment principles should be based on avoiding contact, and local application of corticosteroids and other drugs can improve the condition, but the long-term use of these drugs is not appropriate.39
Delta(9)-tetrahydrocannabinol (THC) in Cannabis sativa L. can reduce the severity of allergic contact dermatitis by reducing the production of pro-inflammatory mediators.40
Psoriasis
Psoriasis is a chronic inflammatory disease with immune-mediated polygenic regulation that is clinically characterized by erythema, scaling, pruritus and pain, is difficult to treat and is prone to relapse.41 The aetiology of psoriasis is complex, and its development may involve T-cell differentiation, inflammatory cell infiltration, and proliferation.42 Abnormal expression of CRH/CRHR1 in the skin disrupts local homeostasis and leads to abnormal differentiation and proliferation of keratin-forming cells, which can induce or exacerbate the onset and progression of psoriasis43 Local upregulation of CRH in the skin can activate mast cells, impair skin immune function, and exacerbate skin inflammation such as psoriasis11 Topical application of corticosteroids along with treatment can lead to adverse effects, such as dermatome atrophy and capillary dilation, which limits the widespread use of corticosteroids.44
The madecassoside and asiatic acid in Centella asiatica (L.) Urb. can, reduce mast cell infiltration and reduce levels of skin inflammation by inhibiting the secretion of inflammatory factors such as IL-23.45,46 The tanshinone IIA in Salvia miltiorrhiza Bunge can inhibit keratinocyte proliferation, and induce apoptosis via the caspase pathway.47 Nimbolide in Azadirachta indica A. Juss. can significantly improve the size and severity of psoriasis lesions by inhibiting the expression of inflammatory and proliferative mediators.48
Eczema
Eczema is a chronic recurrent skin disease characterized by pruritus and skin barrier dysfunction.49 Clinical treatment includes chemical and physical, local and systemic treatment methods. Patients with mild to moderate disease are usually treated with basic therapy and local glucocorticoid therapy, while patients with moderate to severe disease are usually treated with systemic therapy, such as ultraviolet light therapy.50
Thymoquinone from Nigella sativa L. can reduce the production of ROS and proinflammatory regulators by inhibiting the Akt/NFκB pathway.51 Ammonium glycyrrhizate in Glycyrrhiza glabra L. can reduce the traumatic inflammatory response and the severity of eczema patients.52
Androgenetic Alopecia
Androgenetic alopecia is a common form of progressive hair loss due to androgenic factors. The pathogenesis of the condition may be based on age, genetic factors and androgens, with histopathological findings showing a decreased anagen/resting phase ratio.53 Pharmacological treatment for androgenetic alopecia includes topical minoxidil, anti-androgen drugs and 5-alpha reductase inhibitors.54 Since hair loss requires long-term treatment, it becomes especially important to minimize the side effects associated with medication.
Ginsenoside Ro in Panax ginseng C.A. Mey.,55 Baicalin in Scutellaria baicalensis Georgi,56 and Proanthocyanidins in Vitis vinifera L. can regulate the balance of androgen levels, promote hair follicle cell proliferation and hair growth, and reduce the symptoms of hair loss.57
Furthermore, an experimental study demonstrated that CRH and ACTH increase local cortisol secretion in the skin of human hair follicle keratin-forming cells and that hydrocortisone downregulates CRH receptor expression in human hair follicle keratin-forming cells, similar to the classical central HPA system.58 This result was able to demonstrate that human hair follicles have a well-established HPA axis-like function, suggesting that hair follicles are independent peripheral autoregulatory HPA axis organizations. Stimulating factors such as stress activate stress mediators in human scalp hair follicles, triggering human hair follicle-like HPA axis activity, inducing stress responses in the hair follicle, impairing normal human hair growth and exacerbating androgenic symptoms.59
It is well documented that a functionally similar peripheral HPA system exists in the skin and plays an important role in stress-induced skin problems.60,61 The aforementioned skin disorders are closely related to stress. Corticotropin releasing hormone (CRH), the most upstream substance of the stress-induced HPA axis, has been shown to be widely present in epidermal and follicular keratin-forming cells, melanocytes, fibroblasts, sebocytes, and mast cells of the skin.61–64 HPA axis-like hormones such as CRH have also been shown to be aberrantly expressed in immune inflammation-related skin diseases such as acne and psoriasis.11,65
A total of 35 plant adaptogens were studied in the above dermatophytes, accounting for 32.11% of the plant adaptogens in Supplementary Table 1, involving 27 families. The highest frequencies were Lamiaceae family (Ocimum tenuiflorum L. (Syn. Ocimum sanctum L.), Salvia miltiorrhiza Bunge, Scutellaria baicalensis Georgi) and Fabaceae family (Caesalpinia bonduc (L.) Roxb., Glycyrrhiza glabra L., Trigonella foenum‐graecum L.), have 3 plants each. The Zingiberaceae family, Araliaceae family, Rubiaceae family, and Myrtaceae family have 2 respectively, and the remaining families have 1. The most common active ingredient compound categories were, in order, curcumin and other phenolic compounds (16), ginsenosides and other terpenoids (16), and baicalein and other flavonoids (13).
Mechanism of Action of Plant Adaptogens Associated with Skin Health
Patent Analysis
Many botanicals are being used in cosmetics, even if most of them are not currently considered plant adaptogens. The use and marketability of the concept of “adaptogen” in cosmetics is gradually increasing which started with classical plant adaptogens, such as ginseng. This practice is more common now than before the concept of “adaptogen” was introduced. The use and marketing of the concept of “adaptogen” in the cosmetic field is gradually increasing. Based on the current patent situation in this field, we summarized and analysed the trends in the development of plant adaptogens in cosmetics.
Application Trends
The results are shown in Figure 4. The number of patent applications is relatively small, and the annual number of patent applications fluctuates, which indicates that there is less research in this field and that the technology is just beginning to be developed.
 |
Figure 4 Trends in the frequency of “adaptogen” patent applications in the field of cosmetics. |
Application Geography
Based on the information in Figure 5, from the viewpoint of the nations and countries in which the patent applications were filed, the United States and South Korea have more studies and wider technology layout, while China has many studies but only domestic layouts. In terms of patents, few studies on plant adaptogens technology have been conducted, and there is much room for further studies.
 |
Figure 5 Market countries and source countries of patent technology related to adaptogens in cosmetics. |
Frequency of the Use of Adaptive Plants
Figure 6 shows the results of an analysis of the frequency of the use of plant adaptogens in cosmetic patents. It can be seen that the classic “plant adaptogens”, such as Panax ginseng C.A. Mey., Rhodiola rosea L., and Eleutherococcus senticosus (Rupr. and Maxim.)Maxim. are the most studied in cosmetics. The main effects of ginseng include delaying aging, improving cellular resistance to oxidative stress and immunity, enhancing scalp cell immunity, improving scalp antioxidant and anti-aging properties, activating scalp hair follicle cells, improving scalp blood circulation, increasing skin blood flow and helping to prevent hair loss.
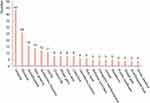 |
Figure 6 Top 20 frequency of appearance of plant adaptogens in cosmetic field patents. |
The main effects of Rhodiola rosea L. are to increase endurance, improve anxiety, rejuvenate the skin, delay aging, protect the skin from negative environmental factors, scavenge free radicals and antioxidants, promote the recovery of the physiological properties of the skin, stimulate tissue metabolism and promote anti-inflammatory effects, thus restoring and stimulating hair growth, strengthening the heart and calming and regulating the body’s metabolism. The main effects of Eleutherococcus senticosus (Rupr. and Maxim.)Maxim. involve increasing the body’s defensive power, with tonic and antioxidant effects.
Efficacy Claims of Adaptive Plants
From the efficacy claims of plant adaptogens in the cosmetic field as presented in Figure 7, the most important effect of plant adaptogens added to cosmetics is antiaging. Based on the information of cosmetic dosage forms obtained from patents related to “adaptogen” in the field of cosmetics, creams and milk appear most frequently, followed by masks, hair products, oral products, etc., which correspond to the efficacy of anti-hair loss, hair growth promotion, and oral disease prevention. The efficacy claims of reducing skin inflammation, enhancing skin protection, and preventing or treating repair of damaged skin all specify the role of plant adaptogens used in cosmetics that also promote anti-inflammatory repair of the skin barrier. It can be seen that in addition to common facial use, the development of hair and oral products is also a priority for the application of plant adaptogens.
 |
Figure 7 Efficacy claims of adaptive plants in cosmetic field patents. |
In general, the number of patents related to plant adaptogens in the field of cosmetics is relatively small. The United States and South Korea have not only large number of patent applications but also a wide layout. China has applied for many patents but only domestically. Ginseng and other classic plant adaptogens appear more frequently in cosmetic patent applications, which shows that the recognition of plant adaptogens in the cosmetic field is increasing, while there is still much room for application development. The most claimed effects of plant adaptogens in the cosmetic field are anti-aging, followed by anti-hair loss, anti-inflammatory, skin barrier repair, etc.
Plant Adaptogens Related to Skin Health
Based on the results of the above bibliometric analysis, it was determined that plant adaptogens are associated with anti-aging and anti-photoaging, antibacterial and anti-fungal, anti-inflammatory, whitening, and anti-hair loss effects in health skin applications, and further literature research was conducted to obtain the mechanisms of action of plant adaptogens related to these health skin applications. The results are detailed in Supplementary Table 3.
Skin inflammation can lead to impaired skin barrier function, which in turn can lead to a range of skin health problems and, in severe cases, can even lead to skin diseases, such as atopic dermatitis and psoriasis. Under normal circumstances, the amount of hair loss and new growth dynamically changes, and the overall number of hairs is maintained. Stress and endocrine disorders can lead to hair loss, affecting hair growth, circulation and density.66 Pathological hair loss includes androgenetic alopecia and baldness. Anti-inflammatory and anti-hair loss-related contents have been briefly described above in the article, so we will not go into further detail.
Anti-Aging and Anti-Photoaging
Skin aging is a complex process that generally includes normal skin aging processes and photoaging. Normal skin aging is a genetically determined aging phenomenon that occurs with the passage of time. The skin shows atrophy, loss of elasticity, slowing of metabolic activity, and loss of collagen.67 The mechanisms underlying skin aging include the production of reactive oxygen species, mitochondrial DNA mutations, telomere shortening, and changes in hormone levels.68
Skin photoaging is an aging phenomenon that occurs when the skin is exposed to ultraviolet light for a long period of time, resulting in cumulative damage to the skin. The skin manifests with wrinkles and roughness, dryness and dullness, sagging skin, and dilated capillaries.67 The key hallmarks of photoaging are increased levels of reactive oxygen species and DNA damage, as well as increased expression levels of inflammatory factors.69
The Centella asiatica glycosides of Centella asiatica (L.) Urb.,70 Salvianolic acid B from Salvia miltiorrhiza Bunge can downregulate MMP-1 expression and upregulate the expression of ECMs, such as type I collagen, type III collagen and elastin.71 Changes in the expression of MMPs components lead to increased degradation of protein components in ECMs, which eventually present in the skin as skin thinning, decreased elasticity, and increased wrinkles, promoting the skin aging phenotype.
Curcumin from turmeric (Curcuma longa) can prevent skin photoaging by inhibiting UV-induced gelatinase activation.72 Organosulfur compounds from Allium sativum L. can inhibit free radical damage to DNA and prevent skin photoaging.73,74 Both rhodiol glycosides and rhodiogenin from Rhodiola macrophylla Rhodiola (Hook. f. and Thomson) H. Ohba were found to be effective in ameliorating UV-induced apoptosis and regulating inflammatory expression levels in aging cells.75
Anti-Bacterial and Anti-Fungal Activities
The skin defends organisms against the external environment and can protect organisms from infection by foreign germs. However, when some bacteria or fungi overgrow on the skin surface, they can disrupt the microecological balance of the skin surface flora, leading to cosmetic results. For example, excessive infection of the skin by Propionibacterium acnes can lead to acne, and excessive infection of the scalp by Malassezia can lead to dandruff.
Cryptotanshinone in Salvia miltiorrhiza Bunge,76 Baicalin from Scutellaria baicalensis Georgi can inhibit the biofilm activity of bacterial, such as Staphylococcus epidermidis and Pseudomonas aeruginosa, to exert antibacterial activity.77 The ethanolic extract of the roots of Asparagus racemosus Willd. can inhibit Malassezia activity and promote scalp health.78 Ethanolic extracts of Terminalia chebula Retz. can prevent periodontal disease by inhibiting the growth of plaque bacteria.79
Whitening
Human skin colour is mainly affected by the amount of melanin produced by melanocytes in the epidermis. Melanin production involves many enzymatic catalysis and chemical reactions, and tyrosinase is the key enzyme involved in the melanogenesis process. Inhibition of tyrosinase activity can achieve effective skin whitening. Skin whitening can also be achieved by inhibiting the migration of produced melanin and by promoting melanin metabolism.
Gedunin from Neem Azadirachta indica A. Juss., ginsenosides from Ginseng,80,81 and Centella asiatica glycosides from Centella asiatica (L.) Urb. can reduce melanin synthesis by inhibiting the expression and activity of tyrosinase TYR.82 Gomisin N in Schisandra chinensis (Turcz.) Baill.,83 Baicalin from Scutellaria baicalensis Georgi,84 and Zerumbone in Zingiber officinale Roscoe can inhibit melanin gene expression, melanin generation and transport, ultimately achieve the effect of whitening skin.85
The topical application of plant adaptogens to the skin can serve to maintain skin homeostasis and protect the skin barrier. Both patent and bibliometric analyses show that the effects of plant adaptogens on skin health are mainly related to the delay of skin aging. The stability of the protein composition of ECMs is essential for the delay of skin aging.
A total of 80 plant adaptogens were studied in the above dermatophytes, accounting for 73.39% of the plant adaptogens in Supplementary Table 1, involving 49 families. The highest frequency were Fabaceae family (Albizia julibrissin Durazz., Butea monosperma (Lam.) Taub. Caesalpinia bonduc (L.) Roxb., Cicer arietinum L., Glycyrrhiza glabra L., Melilotus officinalis (L.) Pall., Mucuna pruriens (L.) DC., Pueraria tuberosa (Roxb. ex Willd.) DC., Trigonella foenum‐graecum L., Sutherlandia frutescens (L.) R.Br.), Araliaceae family (Aralia elata (Miq) Seem., Eleutherococcus senticosus (Rupr. and Maxim.)Maxim., Eleutherococcus sessiliflorus (Rupr. and Maxim.) S.Y. Hu (syn Acanthopanax sessiliflorus (Rupr. and Maxim.) Seem.), Oplopanax elatus (Nakai) Nakai (Syn.Echinopanax elatum Nakai), Panax ginseng C.A.Mey., Panax notoginseng (Burk.) FH Chen), Lamiaceae family (Ajuga turkestanica (Regel) Briq., Ocimum tenuiflorum L. (Syn.Ocimum sanctum L.), Prunella vulgaris L., Salvia miltiorrhiza Bunge, Scutellaria baicalensis Georgi) and Cucurbitaceae family (Bryonia alba L., Lagenaria siceraria (Molina) Standl., Momordica charantia L.), have 10,6,5,3 respectively. Followed by the Crassulaceae family, Zingiberaceae family, Meliaceae family, Solanaceae family, Rutaceae family, Apiaceae family, Rubiaceae family, Myrtaceae family, Menispermaceae family, Apocynaceae family, and Malvaceae family, have 2 respectively, and the remaining families have 1 respectively. The highest active ingredient compound categories were, in order, ginsenosides and other terpenoids (23), curcumin and other phenolic compounds (15), and baicalin and other flavonoids (11).
Discussion
Prediction of Plant Adaptogens to Maintain Skin Homeostasis
The skin is one of the largest organs in mammals and plays an important role in innate immunity. Skin research should not focus solely on the detailed cellular composition, immune factors or protein structure. More systematic consideration should be given to the structural composition of the skin and the overall relevance of the various layers. It not only acts as a physical barrier to protect the host from mechanical damage, UV exposure, and environmental toxins but also serves as an immune barrier against various pathogens.86 Skin immune homeostasis refers to the process of defending against external stimuli such as stress, environmental influences, and microorganisms while removing apoptotic cells and promoting tissue recovery from wounds, inflammation, and disease.87 Innate immune mechanisms coordinate skin immune homeostasis, which relies on a complex network of distinct cell types, the involvement of cytokines, and tight regulation of complement system activation.
Unlike the overall body homeostasis, the skin homeostasis system consists of various inflammatory factors, ECMs, and cellular tissue structure morphology, forming a complete and independent system.88 A regulatory mechanism similar to the central HPA axis exists in the skin.11 The cutaneous HPA axis can respond to endogenous and exogenous stressors to maintain local homeostasis. The HPA axis is one of the neuroendocrine networks used by the body to cope with psychological stress (PS).89 Glucocorticoids (GCs) are key effector molecules of the HPA axis and are essential for skin homeostasis. The cutaneous HPA-like axis acts in an auto/paracrine manner to modify skin stress responses. Keratinocytes can produce not only hormones such as CRH, ACTH and cortisol but also neurotransmitters (such as epinephrine, norepinephrine, dopamine, histamine, acetylcholine, etc.), neurotrophic factors (such as nerve growth factor and brain-derived neurotrophic factor), and neuropeptides that also respond to stress, such as substance P. In addition to synthesizing neurotransmitters and neurohormones, keratinocytes express their respective receptors. Thus, the epidermis continuously senses the environment and responds to various stressors (humidity, temperature, skin surface pH, skin microbiota, injury and PS) to maintain epidermal homeostasis and modulate skin barrier function.10 Among various stressors, PS plays a key role in the skin barrier and affects the immune response by promoting the secretion of stress-related neuropeptide/cytokines and altering HPA axis-related hormones.86
Bibliometrics show that plant adaptogens directly improve the regulation of oxidative stress and inflammatory factors. Ginseng and other representative plant adaptogens have antioxidant effects on the skin.90 Their antioxidant effects can directly scavenge free radicals, inhibit the generation of reactive oxygen species, and indirectly activate the antioxidant enzyme defence system. This result also confirms the results of bibliometric analysis: more plant adaptogens are related to scavenging free radicals and antioxidation. Antioxidation is a type of antiaging, which also corresponds to the results of patent analysis in the field of cosmetics: antiaging is the most important effect of plant adaptogens applied to cosmetics.91 In addition to the main antiaging effects, representative plant adaptogens such as ginseng also have anti-inflammatory effects, which are mainly reflected in the inhibition of the NF-κB signalling pathway and the reduction in the expression levels of inflammatory factors such as TNF-α. This mechanism corresponds to the bibliometric keyword co-occurrence results shown in Figure 3: NF-κB is one of the most frequently occurring keywords in the literature studies on the effects of plant adaptogens on the skin. Figure 7 Results of efficacy claims in patents filed by plant adaptogens in the field of cosmetics show that plant adaptogens also have anti-inflammatory and skin barrier repair effects in cosmetics.
A comprehensive literature analysis further speculates that the common mechanism may be a direct action on CRH receptors involved in the skin’s HPA-like axis or a direct action on skin cells to activate antioxidant defense systems and inhibit oxidative stress-induced cellular damage, while promoting the production and homeostatic balance of ECMs protein constituents. The stabilization of ECMs constituents plays a key role in tissue homeostasis and repair.92 While the ECMs has long been thought to have primarily passive functions by providing physical stability to tissues, detailed characterization of its physical structure and biochemical properties has revealed an unprecedentedly broad range of functions. It is now clear that the dynamic balance of the components of ECMs maintains a balance of tissue compartmentalization and dynamic interactions between embedded resident and recruited inflammatory cells in response to pathological changes in the skin caused by various external stimuli.The protein components of ECMs mainly include core maternal proteins (collagen, glycoproteins, proteoglycans) and maternal-associated proteins (ECM regulatory factors, ECM accessory proteins, secretory factors).93 The ECMs is an evolutionarily conserved, highly flexible and dynamic scaffold that is constantly changing through controlled remodelling and reorganization processes. The different layers of the skin, the epidermis and dermis, are separated by the basement membrane (BM), a complex mixture of ECMs proteins.94 ECMs surround and interact closely with dermal cells such as fibroblasts, immune cells, and vascular cells, providing physical support for these cells. It also allows cells to sense the changing conditions in their local environment, facilitating communication between cells and promoting cellular adaptation to this changing microenvironment.95 Nonetheless, changes in ECMs composition can directly contribute to or induce pathological conditions, which is a hallmark of the aging process.94 The ECMs is further involved in transmitting important signals to recruit inflammatory cells in response to danger signals. At present, many studies have also proven that plant adaptogens can act to stimulate the production of ECMs, thereby alleviating the occurrence of inflammation.96
Therefore, in summary, the skin has a complete homeostatic system in which related inflammatory factors, the HPA-like axis and the ECMs can regulate each other so that the skin maintains a dynamic and stable state (Figure 8A). Once the skin receives external stimulation, skin homeostasis is destroyed, which leads to a series of inflammatory reactions, resulting in abnormal internal and external structures and functions of cells, which in turn accelerate the occurrence of aging and diseases (Figure 8B). Plant adaptogens can suppress inflammation by acting directly on the skin HPA axis or directly by inhibiting oxidative stress pathways. There are also many studies showing that plant adaptogens can modulate the expression profile of ECMs. Plant adaptogens can repair barrier function from these three different pathways, maintain skin homeostasis, and finally enable the treatment of skin diseases and the maintenance of a healthy state (Figure 8C).
Prospective of Adaptogen Use in Dermatology
Although the number of publications and patent applications related to plant adaptogens and skin is relatively small, classical plant adaptogens such as Panax ginseng C.A.Mey. already have relatively well established research applications in the field of skin health. Plant adaptogens are safe and have no side effects. In the pursuit of healthier skin treatments, more scientific research will focus on the use of plant adaptogens and skin health related areas of research. A diverse and highly valued resource of plant adaptogens will be beneficial for the future development of this field.
Most of the current research on the application of plant adaptogens on the skin and related studies are still limited to expanding the scope of plants with the concept of “adaptogen”, without systematic in-depth studies on their mechanisms of action and efficacy. Therefore, experimental studies are urgently needed to determine the common material basis and biological processes of plant adaptogens on the skin. A thorough assessment of the biological molecular mechanisms by which the topical application of plant adaptogens to the skin affects the health and disease states of skin cells and tissues, enhances the nutritional and immune functions of the skin, thus ensuring skin resistance to the external world and delaying skin aging.
Conclusion
Plant adaptogens can maintain skin homeostasis by affecting the skin HPA-like axis to inhibit skin inflammation and oxidative stress levels, directly affecting the skin oxidative stress system to regulate skin condition, and regulating the balance of ECMs protein components to achieve the protection of normal skin function and healthy condition. Plant adaptogens show great application value in the field of dermatology and can provide valuable references for the selection of plant raw materials in dermatology treatment and skin health industries.
The results of this review will provide a new treatment option and solution for dermatologists and provide a new promising and valuable development direction for the development of cosmetic plant efficacy ingredients, and point out that plant adaptogens are safe and effective, can be applied to the face and head, and have important commercial applications. For the first time, we have sorted out and summarized the current research status of plant adaptogens in skin-related fields, and proposed the phytochemical structure and mechanistic commonalities of the action of plant adaptogens on the skin, which will provide research assistance for subsequent in-depth research on the action of plant adaptogens in skin-related fields.
Abbreviations
ACTH, adreno-cortico-tropic-hormone; AKT, protein kinase B; CRH, corticotropin releasing hormone; ECM, extracellular matrix; ERK, extracellular regulated protein kinases; GCs, glucocorticoids; HPA, hypothalamic–pituitary–adrenal; IFN, interferon; IL, interleukin; MAPK, mitogen-activated protein kinase; MITF, melanocyte inducing transcription factor; MMP, matrix metalloproteinase; NFκB, nuclear factor kappa-B; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species; STAT, signal transducer and activator of transcription; Th, helper T cell; TNF, tumor necrosis factor; TWEL, transepidermal water loss; TYR, tyrosine.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Scientific Research Project of Beijing Educational Committee (KM202010011009) and Beijing Excellent Talent Training Project-Young Individuals (2018000020124G032), all these funds support to Pr. Fan YI.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stix G. Turbocharging the brain. Sci Am. 2009;301(4):46–55. doi:10.1038/scientificamerican1009-46
2. Lazarev NV. Obshchee i spetsificheskoe v deistvii farmakologicheskikh sredstv [General and specific effects of drugs]. Farmakol Toksikol. 1958;21(3):81–86. Turkish.
3. Lazarev NV, Liublina EI, Rozin MA. Patologicheskaia fiziologiia i eksperimental’naia terapiia [States of non-specific increased resistance]. Patol Fiziol Eksp Ter. 1959;3:16–21. Russian.
4. Brekhman II, Dardymov IV. New substances of plant origin which increase nonspecific resistance. Annu Rev Pharmacol. 1969;9(1):419–430. doi:10.1146/annurev.pa.09.040169.002223
5. Winslow LC, Kroll DJ. Herbs as medicines. Arch Intern Med. 1998;158(20):2192–2199. doi:10.1001/archinte.158.20.2192
6. Panossian A, Seo E-J, Efferth T. Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine. 2018;50:257–284. doi:10.1016/j.phymed.2018.09.204
7. Panossian A. Understanding adaptogenic activity: specificity of the pharmacological action of adaptogens and other phytochemicals. Ann N Y Acad Sci. 2017;1401(1):49–64. doi:10.1111/nyas.13399
8. Panossian AG, Efferth T, Shikov AN, et al. Evolution of the adaptogenic concept from traditional use to medical systems: pharmacology of stress- and aging-related diseases. Med Res Rev. 2021;41(1):630–703. doi:10.1002/med.21743
9. Liao LY, He YF, Li L, et al. A preliminary review of studies on adaptogens: comparison of their bioactivity in TCM with that of ginseng-like herbs used worldwide. Chin Med UK. 2018;13:1–12.
10. Saric-Bosanac S, Clark AK, Sivamani RK, Shi VY. The role of hypothalamus-pituitary-adrenal (HPA)-like axis in inflammatory pilosebaceous disorders. Dermatol Online J. 2020;26(2). doi:10.5070/D3262047430
11. O’Kane M, Murphy EP, Kirby B. The role of corticotropin-releasing hormone in immune-mediated cutaneous inflammatory disease. Exp Dermatol. 2006;15(3):143–153. doi:10.1111/j.1600-0625.2006.00382.x
12. Nakai K, Tsuruta D. What are reactive oxygen species, free radicals, and oxidative stress in skin diseases? Int J Mol Sci. 2021;22(19):10799. doi:10.3390/ijms221910799
13. Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K. Oxidative stress in aging human skin. Biomolecules. 2015;5(2):545–589. doi:10.3390/biom5020545
14. Trüeb RM. Oxidative stress and its impact on skin, scalp and hair. Int J Cosmet Sci. 2021;43(Suppl S1). doi:10.1111/ics.12736
15. Pasparakis M. Role of NF-κB in epithelial biology. Immunol Rev. 2012;246(1):346–358. doi:10.1111/j.1600-065X.2012.01109.x
16. Kumar J, Teoh SL, Das S, Mahakknaukrauh P. Oxidative stress in oral diseases: understanding its relation with other systemic diseases. Front Physiol. 2017;8:693. doi:10.3389/fphys.2017.00693
17. Jurakić Tončić R, Marinović B. The role of impaired epidermal barrier function in atopic dermatitis. Acta Dermatovenerol Croat. 2016;24(2):95.
18. Sroka-Tomaszewska J, Trzeciak M. Molecular mechanisms of atopic dermatitis pathogenesis. Int J Mol Sci. 2021;22(8):16. doi:10.3390/ijms22084130
19. Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457–465. doi:10.1517/14740338.2016.1140743
20. Hoffmann J, Gendrisch F, Schempp CM, Wolfle U. New herbal biomedicines for the topical treatment of dermatological disorders. Biomedicines. 2020;8(2):21. doi:10.3390/biomedicines8020027
21. Sharma S, Sethi GS, Naura AS. Curcumin ameliorates ovalbumin-induced atopic dermatitis and blocks the progression of atopic march in mice. Inflammation. 2020;43(1):358–369. doi:10.1007/s10753-019-01126-7
22. Aslam H, Shahzad M, Shabbir A, Irshad S. Immunomodulatory effect of thymoquinone on atopic dermatitis. Mol Immunol. 2018;101:276–283. doi:10.1016/j.molimm.2018.07.013
23. Gu Y, Wang X, Liu F, et al. Total flavonoids of sea buckthorn (Hippophae rhamnoides L.) improve MC903-induced atopic dermatitis-like lesions. J Ethnopharmacol. 2022;292:115195. doi:10.1016/j.jep.2022.115195
24. Ahn S, Siddiqi MH, Aceituno VC, et al. Ginsenoside Rg5: rk1attenuates TNF-α/IFN-γ-induced production of thymus- and activation-regulated chemokine (TARC/CCL17) and LPS-induced NO production via downregulation of NF-κB/p38 MAPK/STAT1 signaling in human keratinocytes and macrophages. In Vitro Cell Dev Biol Anim. 2016;52(3):287–295. doi:10.1007/s11626-015-9983-y
25. Yang -C-C, Hung Y-L, Ko W-C, et al. Effect of neferine on DNCB-induced atopic dermatitis in HaCaT cells and BALB/c mice. Int J Mol Sci. 2021;22:15.
26. Reuter J, Wolfle U, Weckesser S, Schempp C. Which plant for which skin disease? Part 1: atopic dermatitis, psoriasis, acne, condyloma and herpes simplex. J Dtsch Dermatol Ges. 2010;8(10):788–796. doi:10.1111/j.1610-0387.2010.07496.x
27. Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guidelines for the treatment of acne. J Eur Acad Dermatol Venereol. 2012;26(Suppl 1.):1–29.
28. Picardo M, Eichenfield LF, Tan J. Acne and rosacea. Dermatol Ther. 2017;7(Suppl 1):43–52. doi:10.1007/s13555-016-0168-8
29. Zouboulis CC, Seltmann H, Hiroi N, et al. Corticotropin-releasing hormone: an autocrine hormone that promotes lipogenesis in human sebocytes. Proc Natl Acad Sci U S A. 2002;99(10):7148–7153. doi:10.1073/pnas.102180999
30. Fox L, Csongradi C, Aucamp M, du Plessis J, Gerber M. Treatment modalities for acne. Molecules. 2016;21(8):20. doi:10.3390/molecules21081063
31. Hamdy AA, Kassem HA, Awad GEA, et al. In-vitro evaluation of certain Egyptian traditional medicinal plants against Propionibacterium acnes. S Afr J Bot. 2017;109:90–95. doi:10.1016/j.sajb.2016.12.026
32. Zaman S, Akhtar N. Effect of Turmeric (Curcuma longa Zingiberaceae) extract cream on human skin sebum secretion. Trop J Pharm Res. 2013;12(5):665–669.
33. Jain A, Basal E. Inhibition of Propionibacterium acnes-induced mediators of inflammation by Indian herbs. Phytomedicine. 2003;10(1):34–38. doi:10.1078/094471103321648638
34. Barroso RA, Navarro R, Tim CR, et al. Antimicrobial photodynamic therapy against Propionibacterium acnes biofilms using hypericin (Hypericum perforatum) photosensitizer: in vitro study. Lasers Med Sci. 2021;36(6):1235–1240. doi:10.1007/s10103-020-03163-3
35. Coenye T, Brackman G, Rigole P, et al. Eradication of Propionibacterium acnes biofilms by plant extracts and putative identification of icariin, resveratrol and salidroside as active compounds. Phytomedicine. 2012;19(5):409–412. doi:10.1016/j.phymed.2011.10.005
36. Fabbrocini G, Staibano S, De Rosa G, et al. Resveratrol-containing gel for the treatment of acne vulgaris: a single-blind, vehicle-controlled, pilot study. Am J Clin Dermatol. 2011;12(2):133–141. doi:10.2165/11530630-000000000-00000
37. Huang WC, Tsai TH, Huang CJ, et al. Inhibitory effects of wild bitter melon leaf extract on Propionibacterium acnes-induced skin inflammation in mice and cytokine production in vitro. Food Funct. 2015;6(8):2550–2560. doi:10.1039/C5FO00550G
38. Oender M. Allergic contact dermatitis. Turkderm Turk Arch Dermatol Venerol. 2009;43(1):3–9.
39. Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin N Am. 2020;104(1):61–+. doi:10.1016/j.mcna.2019.08.012
40. Gaffal E, Cron M, Glodde N, Tuting T. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68(8):994–1000. doi:10.1111/all.12183
41. Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. 2020;182(4):840–848. doi:10.1111/bjd.18245
42. Chiricozzi A, Romanelli P, Volpe E, Borsellino G, Romanelli M. Scanning the immunopathogenesis of psoriasis. Int J Mol Sci. 2018;19(1):179. doi:10.3390/ijms19010179
43. Zhou C, Yu X, Cai D, Liu C, Li C. Role of corticotropin-releasing hormone and receptor in the pathogenesis of psoriasis. Med Hypotheses. 2009;73(4):513–515. doi:10.1016/j.mehy.2009.02.051
44. Uva L, Miguel D, Pinheiro C, et al. Mechanisms of action of topical corticosteroids in psoriasis. Int J Endocrinol. 2012;2012:16. doi:10.1155/2012/561018
45. OuYang Q, Pan YQ, Luo HQ, Xuan CX, Liu JE, Liu J. MAD ointment ameliorates imiquimod-induced psoriasiform dermatitis by inhibiting the IL-23/IL-17 axis in mice. Int Immunopharmacol. 2016;39:369–376. doi:10.1016/j.intimp.2016.08.013
46. Kukula O, Kirmizikan S, Tiryaki ES, Cicekli MN, Gunaydin C. Asiatic acid exerts an anti-psoriatic effect in the imiquimod-induced psoriasis model in mice. Immunopharmacol Immunotoxicol. 2022;44(3):367–372.
47. Li FL, Xu R, Zeng QC, et al. Tanshinone IIA inhibits growth of keratinocytes through cell cycle arrest and apoptosis: underlying treatment mechanism of psoriasis. Evid Based Complement Altern Med. 2012;2012:14.
48. More NB, Sharma N, Pulivendala G, Bale S, Godugu C. Natural product topical therapy in mitigating imiquimod-induced psoriasis-like skin inflammation-underscoring the anti-psoriatic potential of nimbolide. Indian J Pharmacol. 2021;53(4):278–285. doi:10.4103/ijp.IJP_591_20
49. Sohn A, Frankel A, Patel RV, Goldenberg G. Eczema. Mt Sinai J Med. 2011;78(5):730–739. doi:10.1002/msj.20289
50. Berthold E, Weisshaar E. Treatment of hand eczema. Hautarzt. 2019;70(10):790–796. doi:10.1007/s00105-019-04475-4
51. Kohandel Z, Farkhondeh T, Aschner M, Samarghandian S. Anti-inflammatory effects of thymoquinone and its protective effects against several diseases. Biomed Pharmacother. 2021;138:111492. doi:10.1016/j.biopha.2021.111492
52. Marianecci C, Rinaldi F, Di Marzio L, et al. Ammonium glycyrrhizinate-loaded niosomes as a potential nanotherapeutic system for anti-inflammatory activity in murine models. Int J Nanomedicine. 2014;9:635–651. doi:10.2147/IJN.S55066
53. Kutlubay Z, Baglam S, Engin B, Serdaroglu S. Male androgenetic alopecia. Turkderm Turk Arch Dermatol Venerol. 2014;48:36–39.
54. Piraccini BM, Alessandrini A. Androgenetic alopecia. G Ital Dermatol Venereol. 2014;149(1):15–24.
55. Murata K, Takeshita F, Samukawa K, Tani T, Matsuda H. Effects of ginseng rhizome and ginsenoside ro on testosterone 5 alpha-reductase and hair re-growth in testosterone-treated mice. Phytother Res. 2012;26(1):48–53. doi:10.1002/ptr.3511
56. Kim AR, Kim SN, Jung IK, Kim HH, Park YH, Park WS. The inhibitory effect of Scutellaria baicalensis extract and its active compound, baicalin, on the translocation of the androgen receptor with implications for preventing androgenetic alopecia. Planta Med. 2014;80(2–3):153–158. doi:10.1055/s-0033-1360300
57. Dhariwala MY, Ravikumar P. An overview of herbal alternatives in androgenetic alopecia. J Cosmet Dermatol. 2019;18(4):966–975. doi:10.1111/jocd.12930
58. Ito N, Ito T, Kromminga A, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19(10):1332–1334. doi:10.1096/fj.04-1968fje
59. Fischer TW, Bergmann A, Kruse N, et al. New effects of caffeine on corticotropin-releasing hormone (CRH)-induced stress along the intrafollicular classical hypothalamic-pituitary-adrenal (HPA) axis (CRH-R1/2, IP -R, ACTH, MC-R2) and the neurogenic non-HPA axis (substance P, p75 and TrkA) in ex vivo human male androgenetic scalp hair follicles. Br J Dermatol. 2021;184(1):96–110. doi:10.1111/bjd.19115
60. Rassouli O, Liapakis G, Venihaki M. Role of central and peripheral CRH in skin. Curr Mol Pharmacol. 2018;11(1):72–80. doi:10.2174/1874467209666161026144219
61. Chen Y, Lyga J. Brain-skin connection: stress, inflammation and skin aging. Inflamm Allergy Drug Targets. 2014;13(3):177–190. doi:10.2174/1871528113666140522104422
62. Kono M, Nagata H, Umemura S, Kawana S, Osamura RY. In situ expression of corticotropin-releasing hormone (CRH) and proopiomelanocortin (POMC) genes in human skin. FASEB J. 2001;15(12):2297–2299. doi:10.1096/fj.01-0254fje
63. Slominski A, Wortsman J, Pisarchik A, et al. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15(10):1678–1693. doi:10.1096/fj.00-0850rev
64. Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145(2):941–950.
65. Ganceviciene R, Graziene V, Fimmel S, Zouboulis CC. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160(2):345–352. doi:10.1111/j.1365-2133.2008.08959.x
66. Vinay K, Sawatkar GU, Dogra S. Hair manifestations of endocrine diseases: a brief review. Indian J Dermatol Venereol Leprol. 2018;84(5):528–538. doi:10.4103/ijdvl.IJDVL_671_17
67. Sjerobabski-Masnec I, Situm M. Skin aging. Acta Clin Croat. 2010;49(4):515–518.
68. Tobin DJ. Introduction to skin aging. J Tissue Viability. 2017;26(1):37–46. doi:10.1016/j.jtv.2016.03.002
69. Fitsiou E, Pulido T, Campisi J, Alimirah F, Demaria M. Cellular senescence and the senescence-associated secretory phenotype as drivers of skin photoaging. J Invest Dermatol. 2021;141(4):1119–1126. doi:10.1016/j.jid.2020.09.031
70. Plengmuankhae W, Tantitadapitak C. Low temperature and water dehydration increase the levels of asiaticoside and madecassoside in Centella asiatica (L.) Urban. S Afr J Bot. 2015;97:196–203. doi:10.1016/j.sajb.2015.01.013
71. Meng H, Zhao MM, Yang RY, et al. Salvianolic acid B regulates collagen synthesis: indirect influence on human dermal fibroblasts through the microvascular endothelial cell pathway. J Cosmet Dermatol. 2022;21(7):3007–3015.
72. Muta K, Inomata S, Fukuhara T, et al. Inhibitory effect of the extract of rhizome of &ITCurcuma longa&IT L in gelatinase activity and its effect on human skin. J Biosci Bioeng. 2018;125(3):353–358. doi:10.1016/j.jbiosc.2017.10.001
73. Borek C. Antioxidant health effects of aged garlic extract. J Nutr. 2001;131(3s):1010S–1015S. doi:10.1093/jn/131.3.1010S
74. Imai J, Ide N, Nagae S, Moriguchi T, Matsuura H, Itakura Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. 1994;60(5):417–420. doi:10.1055/s-2006-959522
75. Wang YS, Zhou SS, Shen CY, Jiang JG. Isolation and identification of four antioxidants from Rhodiola crenulata and evaluation of their UV photoprotection capacity in vitro. J Funct Food. 2020;66:11. doi:10.1016/j.jff.2020.103825
76. Zu RL, Yi H, Yi YL, Yong JY, Li Y. Effect of cryptotanshinone on Staphylococcus epidermidis biofilm formation under in vitro conditions. Jundishapur J Microbiol. 2019;12(4):11.
77. Luo J, Dong B, Wang K, et al. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS One. 2017;12(4):e0176883. doi:10.1371/journal.pone.0176883
78. Onlom C, Khanthawong S, Waranuch N, Ingkaninan K. In vitro anti-malassezia activity and potential use in anti-dandruff formulation of Asparagus racemosus. Int J Cosmet Sci. 2014;36(1):74–78. doi:10.1111/ics.12098
79. Lee J, Nho YH, Yun SK, Hwang YS. Use of ethanol extracts of Terminalia chebula to prevent periodontal disease induced by dental plaque bacteria. BMC Complement Altern Med. 2017;17:10. doi:10.1186/s12906-017-1619-1
80. Jeon HJ, Kim K, Kim C, Kim MJ, Kim TO, Lee SE. Molecular mechanisms of anti-melanogenic gedunin derived from neem tree (Azadirachta indica) using b16f10 mouse melanoma cells and early-stage Zebrafish. Plants-Basel. 2021;10(2):11.
81. Kim K. Effect of ginseng and ginsenosides on melanogenesis and their mechanism of action. J Ginseng Res. 2015;39(1):1–6. doi:10.1016/j.jgr.2014.10.006
82. Kwon KJ, Bae S, Kim K, et al. Asiaticoside, a component of Centella asiatica, inhibits melanogenesis in B16F10 mouse melanoma. Mol Med Rep. 2014;10(1):503–507. doi:10.3892/mmr.2014.2159
83. Chae JK, Subedi L, Jeong M, et al. Gomisin N inhibits melanogenesis through regulating the PI3K/Akt and MAPK/ERK signaling pathways in melanocytes. Int J Mol Sci. 2017;18(2):13. doi:10.3390/ijms18020471
84. Kudo M, Kobayashi-Nakamura K, Tsuji-Naito K, Slominski AT. Bifunctional effects of O-methylated flavones from Scutellaria baicalensis Georgi on melanocytes: inhibition of melanin production and intracellular melanosome transport. PLoS One. 2017;12(2):26. doi:10.1371/journal.pone.0171513
85. Oh TI, Jung HJ, Lee YM, et al. Zerumbone, a tropical ginger sesquiterpene of Zingiber officinale Roscoe, attenuates -MSH-induced melanogenesis in B16F10 cells. Int J Mol Sci. 2018;19(10):17. doi:10.3390/ijms19103149
86. Cibrian D, de la Fuente H, Sánchez-Madrid F. Metabolic pathways that control skin homeostasis and inflammation. Trends Mol Med. 2020;26(11):975–986. doi:10.1016/j.molmed.2020.04.004
87. Fernández-Gallego N, Sánchez-Madrid F, Cibrian D. Role of AHR ligands in skin homeostasis and cutaneous inflammation. Cells. 2021;10(11):3176. doi:10.3390/cells10113176
88. Wang T, Li K, Xiao S, Xia Y. A plausible role for Collectins in skin immune homeostasis. Front Immunol. 2021;12:594858. doi:10.3389/fimmu.2021.594858
89. Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265–266:143–149. doi:10.1016/j.mce.2006.12.012
90. Bale S, Venkatesh P, Sunkoju M, Godugu C. An adaptogen: withaferin A ameliorates in vitro and in vivo pulmonary fibrosis by modulating the interplay of fibrotic, matricelluar proteins, and cytokines. Front Pharmacol. 2018;9:248. doi:10.3389/fphar.2018.00248
91. Gu Y, Han J, Jiang C, Zhang Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res Rev. 2020;59:101036. doi:10.1016/j.arr.2020.101036
92. Pfisterer K, Shaw LE, Symmank D, Weninger W. The extracellular matrix in skin inflammation and infection. Front Cell Dev Biol. 2021;9:682414. doi:10.3389/fcell.2021.682414
93. Li M, Li X, Liu B, et al. Time-resolved extracellular matrix atlas of the developing human skin dermis. Front Cell Dev Biol. 2021;9:783456. doi:10.3389/fcell.2021.783456
94. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi:10.1038/nrm3904
95. Bhattacharjee O, Ayyangar U, Kurbet AS, Ashok D, Raghavan S. Unraveling the ECM-immune cell crosstalk in skin diseases. Front Cell Dev Biol. 2019;7:68.
96. Nyström A, Bruckner-Tuderman L. Matrix molecules and skin biology. Semin Cell Dev Biol. 2019;89:136–146. doi:10.1016/j.semcdb.2018.07.025
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.