Back to Journals » Drug Design, Development and Therapy » Volume 18
Bibliometric and Visualization Analysis of Stem Cell Therapy for Erectile Dysfunction
Authors Sun T , Liu Y , Yuan P, Jia Z, Yang J
Received 6 November 2023
Accepted for publication 24 February 2024
Published 8 March 2024 Volume 2024:18 Pages 731—746
DOI https://doi.org/10.2147/DDDT.S448483
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Taotao Sun,1 Yipiao Liu,2 Penghui Yuan,1 Zhankui Jia,1 Jinjian Yang1
1Department of Urology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, 450052, People’s Republic of China; 2Department of Hepatopancreatobiliary Surgery, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, 450052, People’s Republic of China
Correspondence: Taotao Sun; Jinjian Yang, Department of Urology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, 450052, People’s Republic of China, Email [email protected]; [email protected]
Purpose: As a common male disease, erectile dysfunction (ED) seriously affects the physical and mental health of patients. In recent years, studies have continued to point out the great potential of stem cell therapy (SCT) in the treatment of ED. The purpose of this study is to comprehensively analyze the research of SCT for ED and understand the development trends and research frontiers in this field.
Methods: Publications regarding SCT and ED were retrieved and collected from the Web of Science Core Collection. CiteSpace and VOSviewer software were then utilized for bibliometric and visualization analysis.
Results: A total of 524 publications were eventually included in this study. The annual number of publications in this field was increasing year by year. China and the USA were the two most productive countries. Lin GT, Lue TF and Lin CS, and the University of California San Francisco where they worked were the most productive research group and institution, respectively. The journal with the largest number of publications was The Journal of Sexual Medicine, and the following were mostly professional journals of urology and andrology. Diabetes mellitus-induced ED and cavernous nerve injury-related ED were the two most commonly constructed models of ED in studies. Concerning the types of stem cells, mesenchymal stem cells derived from adipose and bone marrow were most frequently used. Moreover, future research would mainly focus on exosomes, tissue engineering technology, extracorporeal shockwave therapy, and clinical translation.
Conclusion: The research of SCT for ED will receive increasing global attention in the future. Our study provided bibliometric and visualization analysis of published literature, helping researchers understand the global landscape and frontiers in this field. More preclinical and clinical studies should be conducted to more deeply explore the underlying mechanisms of treatment and promote clinical translation.
Keywords: erectile dysfunction, stem cells, bibliometric analysis, visualization, VOSviewer, citespace
Introduction
Erectile dysfunction (ED) is considered a condition wherein the penis is insufficiently rigid to achieve satisfactory sexual intercourse. Its prevalence among middle-aged and elderly men over 40 years old is as high as 52% and increases with age.1,2 The number of patients suffering from ED is estimated to reach 322 million in 2025.3 Moreover, its impact on male patients is multifaceted. On the one hand, it affects the quality of sexual life and damages the relationship between couples.4 On the other hand, the penis is the continuation of the blood vessels of the whole body, and ED could serve as a predictor of cardiovascular diseases.5 Timely and effective diagnosis and treatment of ED could assist in enhancing the quality of life in patients and promote physical and mental health.
According to the cause, ED could be divided into three categories, namely the psychogenic, organic, and mixed types.6 Common clinical risk factors include diabetes mellitus (DM), smoking, high blood pressure, radical pelvic surgery, stroke, drugs, aging, and other causes, all of which could promote the occurrence of ED.4,7 Phosphodiesterase type 5 inhibitor (PDE5i) is the most important drug in the current treatment of ED,8 with an effectiveness as high as 83%. However, patients with certain ED subtypes respond poorly to PDE5i, with response rates of 63% in DM-induced ED (DMED) and 48% in cavernous nerve injury (CNI)-related ED (CNI-ED).9 Second and third-line treatments encompass vacuum erection devices, intraurethral suppositories, intracavernous injections, and penile implants.4 However, limitations associated with these strategies, such as the inability to correct the pathological state of the penis and their restricted application due to side effects, cost, and unsatisfactory results,10 which highlight the urgent need for a comprehensive exploration of the pathological mechanism and treatment strategies for ED.
As is well documented, stem cells have the capability of self-renewal and can differentiate into mature cells. According to their differentiation potential, stem cells can be classified as totipotent (eg zygotes), pluripotent (eg embryonic stem cells), multipotent (eg mesenchymal stem cells), oligopotent, and unipotent cells.11,12 The most frequently used and easily obtained stem cells are mesenchymal stem cells, which exert almost no tumorigenic effects and are not subject to ethical restrictions.13 Since Ernst Haeckel proposed the concept of stem cells in 1868, the exploration of stem cells and their application in diseases have been the focus of scientific research and clinical practice.14 Indeed, they hold significant implications in the treatment of various diseases and have considerably promoted the development of regenerative medicine.15 The application of stem cells in ED started later, with no corresponding reports published until the 21st century.16 However, stem cell therapy (SCT) in ED has achieved surprising phased objectives. A number of basic and clinical trials have established that SCT could partially restore erectile function caused by various factors and improve the quality of sexual life.11,17,18 More importantly, stem cells can alleviate not only symptoms but also ED by addressing pathological mechanisms, including differentiating into specific effector cells and secreting numerous growth factors and exosomes, thereby improving the local pathological environment and stimulating tissue repair.16,19,20
While many studies have contributed to the basic research and clinical translation of SCT in ED treatment, researchers are typically unable to quickly grasp current research progress, frontiers, and future development directions in this field based on the complicated literature. Traditional literature reviews are generally associated with a certain degree of subjectivity and one-sidedness, and cannot comprehensively and systematically elucidate the above questions. As a method of document analysis, bibliometrics allows for the aggregation of current research data in a certain field and its quantitative and qualitative analysis.21 CiteSpace and VOSviewer are commonly used analysis software, generating knowledge networks to facilitate researchers in understanding the past, present, and future of this field more conveniently and efficiently.22 Therefore, this study aimed to conduct bibliometric and visualization analysis of research on SCT for ED to provide a theoretical reference for future exploration in this field.
Materials and Methods
Data Sources and Search Strategy
In this study, the Web of Science Core Collection (WoSCC) database was searched for relevant articles. The search formula was set as follows: TS = (“erectile dysfunction” OR “impotence”) AND TS = (“stem cell*” OR “progenitor cell*”). The search was performed on 2023.8.15, and all documents were retrieved and collected within this day to mitigate the risk of time-related bias. The date of publication was limited to 2022 and before, and only publications in English were considered. This search only included original articles and reviews and excluded meeting abstracts, editorial materials, corrections, letters, processing papers, and early access articles. After screening the titles and abstracts, publications not related to the study topic were also excluded. Two researchers searched the database and screened relevant publications. Disagreements were arbitrated by the third researcher until a consensus was reached. Following these criteria, a total of 524 publications were eventually included in this study (Figure 1).
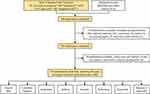 |
Figure 1 Flow diagram of literature selection. |
Complete records and references were exported, including the title, author, journal, country/region, institution, abstract, keywords, references, etc. Given that our study was a bibliometric study and did not involve human or animal participants, the review and approval from the ethics committee were waived.
Data Analysis
In this study, GraphPad Prism 8.0 was used to analyze the publication information of the literature and the source of stem cells. VOSviewer 1.6.16 was utilized to examine the cooperative relationship between institutions and countries. Additionally, Scimago Graphica 1.0.36 was also employed for the country analysis.23,24 Lastly, authors, journals, references, and keywords were explored using CiteSpace 6.2.R4.25,26
Results
The Trend of Publications and Citations
As illustrated in Figure 2, the annual research on SCT for ED generally showed a fluctuating upward trend, indicating that this field has garnered increasing attention from the academic community. The initial study on SCT and ED in WoSCC was published in 2004,27 with the first peak of publication in this field being in 2012. The number of publications after 2017 was basically the same as that in 2012 or even catching up. Documents published from 2007 to 2016 had a relatively high level of citations, with the most cited documents published in 2012. The aforementioned trend in citation levels may be affected by the year of publication; that is, earlier documents were more likely to be cited frequently.
 |
Figure 2 Global trends in the number of articles and cited times. |
Analysis of Countries/Regions and Institutions
In this study, the countries or regions of the authors were ranked by the number of publications related to SCT and ED (Table 1). China (192) and the USA (187) ranked first and second in terms of total number of published articles, respectively, accounting for over 70% of publications. Likewise, the top 2 countries in the H-index were also the USA (45) and China (38). The above results showed that these two countries had enormous advantages in this field and had published many high-quality documents. As for the average citations per item (ACI), the top 3 countries were Canada (64.9), Egypt (59.5), and Belgium (40.5), indicating that these three countries had been involved in research in this field in the early stage. To clearly reflect cooperative relationships, the distribution of countries or regions was re-examined after combining geographical location information (Figure 3A). In general, cooperation between countries globally was close. The USA had the highest intensity of cooperation with other countries, followed by China. The closest cooperation was also between China and the USA. In addition, Europe was currently the research center in this field and had the most frequent cooperation.
 |
Table 1 The Top 10 Most Productive Countries/Regions on the Research of SCT for ED |
Given that authors and institutions require funds to conduct scientific research, the investment of funds is related to the country’s leading level in this field. As summarized in Table 2, the National Natural Science Foundation of China funded the largest number of publications. Eight of the top 10 funds were from China and the USA, which was consistent with the research strengths of these two countries in this field.
 |
Table 2 The Top 10 Funds on the Research of SCT for ED |
The top 10 institutions in terms of number of publications related to SCT and ED are detailed in Table 3. The University of California San Francisco (42) in the USA ranked first, and its ACI (48.1) and H-index (25) were also among the highest. It was followed by several Chinese institutions followed, such as Sun Yat-sen University (26), Shanghai Jiao Tong University (24), and Peking University (21). It is worthwhile mentioning that while Nanjing University did not publish many articles (14), it ranked first in ACI (50.3), signifying that the documents produced by this institution were published earlier or of higher quality. Furthermore, a visual analysis of the institutions was implemented to generate a collaborative network (Figure 3B). The University of California San Francisco remained at the center of the network. Several institutions in China appeared to have initiated their efforts relatively late, indicating that China has made tremendous progress in recent years to achieve its current advantage in this field.
 |
Table 3 The Top 10 Most Productive Institutions on the Research of SCT for ED |
Analysis of Authors
The top 10 most productive authors were ranked according to the number of publications in the field of SCT and ED (Table 4). Lin GT (35), Lue TF (33), and Lin CS (18) were the top 3 authors. These 3 authors were all from the University of California San Francisco, and their ACI and H-index were both at the leading level. Among the 10 authors, 6 were from the USA, 2 were from South Korea, and the remaining two were from Belgium and Italy. No Chinese author ranked among the top 10. This may be ascribed to the fragmented nature of Chinese research and the lack of authors’ influence.
 |
Table 4 The Top 10 Most Productive Authors on the Research of SCT for ED |
The visual network and clustering analysis of authors’ cooperation were performed to reflect collaborations and research directions among authors (Figure 4). Academic groups were formed among American authors, primarily focusing on the research of adipose-derived mesenchymal stem cells (ADSCs). Several authors from China worked closely with academic groups in the USA, such as Liu Jihong and Xin Zhongcheng. Academic groups were also observed among Korean authors, mainly focusing on research on streptozotocin-induced diabetic rats.
Analysis of Journals
Table 5 lists the top 10 journals by number of publications related to SCT and ED. The Journal of Sexual Medicine ranked first in terms of number of publications (62), ACI (38.2), and H-index (28), indicating that this is a popular journal for research in this field. It was followed by the International Journal of Impotence Research (28), Andrology (20), Asian Journal of Andrology (18), and Translational Andrology and Urology (14), which are all professional journals related to urology and andrology. Moreover, the 2022 impact factors (IF) of these journals were all slightly low, ranging from 2.5 to 5.5, which may be limited by the average IF level of professional journals in the field of andrology.
 |
Table 5 The Top 10 Journals Publishing Research on SCT and ED |
The dual-map overlay of journals was plotted to visualize citation relationships between journals (Figure 5).28 The left portion of the map represents citing journals, while the right portion represents cited journals. The two most visible paths on the map (orange and green) represent the most important citation routes in this area. Studies published in Molecular, Biology, Genetics, Health, Nursing, and Medicine-related journals tended to be cited in Molecular, Biology, Immunology, Medicine, Medical, and Clinical-related journals.
Analysis of References
Highly cited references in the field were visualized and are presented in Figure 6A. The publication with the highest citation intensity was published in 2010, titled “Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury”29 This study principally demonstrated the therapeutic effect of ADSCs and cell lysate on erectile function in rats with CNI. Clustering the above highly cited references yielded a total of 6 clusters, namely smooth muscle, ADSCs, ED, coronary artery disease, neurogenic impotence, and endothelial dysfunction (Figure 6B). Most of the highly cited documents were related to research on ADSCs.
Frequent citations of certain documents over a period of time indicated new breakthroughs or hot spots in this field. In other words, the top 20 references with the strongest citation bursts deserve attention (Figure 6C). The strongest one was “Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction”, published by Bivalacqua TJ et al in 2007 (strength = 16.43; from 2008 to 2012).30 In this article, the authors pointed out that genetically modified mesenchymal stem cells could differentiate into endothelial cells (ECs) and smooth muscle cells (SMCs) and ultimately improve erectile function in aged rats. References ranking second and third (strength = 16.36 and 15.97; both from 2017 to 2022) in burst strength were both clinical trials of stem cells used in patients with ED following radical prostatectomy.31,32 The effectiveness and safety of SCT in clinical patients with ED were initially validated. The remaining 4 references whose citation burst period ended in 2022 were 2 clinical trials regarding SCT for the treatment of ED,33,34 1 guideline,35 and 1 review.36 The above results indicated that the clinical application of stem cells seemed to have received more attention in recent years. The remaining references listed in Figure 6 are detailed in the References List.37–48
Analysis of Keywords
Given that keywords represent the critical information in the article, the co-occurrence analysis of keywords was conducted and is presented in Figure 7A. The five keywords with the highest frequency of occurrence were ED (343), rat model (116), radical prostatectomy (83), stem cells (74), and CNI (64), implying that rats were the most commonly used experimental animals in basic research on ED, with CNI being one of the major causes of ED. The results of clustering analysis exposed that high-frequency keywords were summarized into 6 clusters, namely endothelial dysfunction, DM, CNI, endothelial growth factors, ED, and smooth muscle (Figure 7B). Combined with temporal information, a timeline visualization of keywords was performed (Figure 7C). DM and CNI appeared throughout, indicating that these two causes had been the research focus in this area.49,50 Research on ECs and SMCs was ongoing until 2022, signifying that normalization of these two components in the corpus cavernosum was critical to erection.19,20,41
The top 20 keywords with the strongest citation bursts are depicted in Figure 7D. Nitric oxide synthase (NOS) was the keyword with the earliest appearance, highest burst strength, and most extended duration. This enzyme has the ability to catalyze the substrate to generate nitric oxide (NO), which directly initiates and maintains penile erection.51 The citation bursts for intracavernous injection, extracorporeal shockwave therapy, SCT, and delivery continued until 2022, suggesting that these keywords are currently focal points. Intracavernous injection remains the most important approach to introducing stem cells into recipients to exert therapeutic effects. Meanwhile, extracorporeal shockwave therapy not only activates local stem cells/progenitor cells in the penis but also improves the efficacy of SCT when used in conjunction with stem cells.52,53
Analysis of Stem Cell Sources
Considering that stem cells in SCT may originate from different species or tissues, original articles were manually searched for information on stem cell sources. A total of 164 basic experimental articles on exogenous stem cells were included, 10 of which employed stem cells derived from two tissues, so the final count was 174 articles. Regarding the sources of different species, rat-derived stem cells accounted for 62.1% of all stem cells (108), followed by human-derived stem cells accounting for 30.5% (53) (Figure 8A). Concerning the sources of different tissues, adipose (48.3%, 84) ranked first, followed by bone marrow (27.0%, 47) and umbilical cord (8.0%, 14) (Figure 8B). Based on these observations, a comprehensive analysis regarding the sources of stem cells was performed (Figure 8C). Attributed to the fact that rats were the most frequently used experimental animals for exploring ED, rat adipose (66) and rat bone marrow (33) were the top two sources of stem cells. Human adipose (16), human umbilical cord (13), human bone marrow (12), and other sources were lower down the list. In short, whether of rat or human origin, adipose and bone marrow remained the most common tissue sources of stem cells in SCT. In addition, some applications of other sources, such as muscle and urine, were also made.54,55
Discussion
In recent years, research results on SCT have accumulated, and SCT has been found to exert therapeutic effects on various diseases, including diabetes, neurological disorders, cardiovascular disorders, and cancer. In addition, SCT plays a pivotal role in regenerative medicine.12,56 In view of the limitations of traditional treatment methods for ED and the underlying effects of SCT, the latter has progressively become the focus of the ED treatment field and has attracted the attention of an increasing number of researchers. In this study, CiteSpace and VOSviewer were utilized to conduct bibliometric and visualization analysis of research in the field of SCT and ED, aiming to offer valuable insights into the development process, research trends, and research hotspots in this field. Based on the analysis of 524 original studies and reviews, our results showed that the literature involving SCT and ED increased yearly. The above information revealed that SCT is a candidate treatment for ED and is likely to be a key research direction in regenerative medicine for ED in the future.
General Information
China (first) and the USA (second) were the two countries that conducted the highest number of studies on SCT and ED, and their investment in scientific research was also enormous. The strength of China may be slightly inferior, as reflected in its late start, marginally lower ACI and H-index, and the lack of authoritative scholars and high-impact literature. In addition, there were extensive connections and cooperation between countries, institutions, and scholars worldwide, especially between China and the USA. The research group led by Lue TF, Lin GT, and Lin CS from the University of California San Francisco excelled in this field. They have published a large number of studies, many of which were high-impact documents, and have made significant contributions to the development of the field.38,42,43 Publications on the application of SCT in ED tended to be published in specialized journals of urology and andrology, such as The Journal of Sexual Medicine, Andrology, and Asian Journal of Andrology. Moreover, an increasing number of high-quality documents have been published in journals in other fields and comprehensive journals in recent years,49,57,58 indicating that the scientific research community is widely recognizing research in this field.
Mechanism of SCT for ED
Following sexual stimulation, NOS in nerve cells and ECs is activated and generates NO, which diffuses into adjacent SMCs and reduces intracellular Ca2+ concentration, ultimately leading to vasodilation and penile erection.51 The damage to any of the above links may lead to the occurrence of ED. Stem cells possess differentiation potential and could differentiate into nerve cells, ECs, and SMCs after transplantation, thereby replacing damaged tissue and preserving homeostatic function. A large number of bioactive factors are also released in a paracrine manner by implanted stem cells, thereby improving the local pathological environment and accelerating tissue repair.41,59 However, more and more studies have documented that paracrine effects seem to be the primary mechanism of SCT compared with cellular differentiation effects.10,18 Only a small number of stem cells could be detected after transplantation. It is difficult to distinguish whether the restoration of effector cells is due to the differentiation or paracrine activity of stem cells. These are the reasons for the controversy regarding the repair mechanism mentioned above. In addition, the advancement of research on exosomes in recent years uncovered that stem cell-secreted exosomes also play a fundamental role in penile tissue repair.60 Compared with the injection of stem cells, exosomes have many benefits, such as higher stability, no risk of tumorigenesis, and lower immunogenicity.61 Exosomes secreted by stem cells seem to be a promising novel direction for the research of ED.
Improvement of SCT Efficacy
Compared to original stem cells, various methods have been explored to increase the efficacy of SCT for the treatment of ED. Genetically modifying stem cells to manufacture engineered stem cells has been established to enhance cellular function. Stem cells overexpressing a series of growth factors all show beneficial effects on the recovery of erectile function.45,62,63 The expression of certain microRNAs also affects the activity of stem cells; thus, regulating the levels of specific microRNAs in stem cells is another viable strategy.64,65 The enhancement of cellular retention, cellular differentiation, and cellular paracrine effects after transplantation are the mechanisms by which the abovementioned engineered stem cells exert powerful effects.66 The research and application of novel biomaterials have brought new opportunities for the development of SCT in ED. The retention time of stem cells in the corpus cavernosum is extended when combined with materials such as hydrogels, scaffolds, and magnetic nanoparticles.58,67,68 In addition, the combination of stem cells and existing therapies, such as shockwave therapy and certain drugs, could also be effective.52,69 Comprehensive management of the above methods often exerts a powerful therapeutic effect.
Clinical Trials
Although current research on SCT and ED is chiefly centered on animal experiments, there are still some clinical trials exploring the effectiveness and safety of SCT in the treatment of ED.17 Among the 15 retrieved clinical trials (Supplementary Table 1), the types of stem cells used in the studies were diverse, with the majority of patients developing DMED and CNI-ED. All studies showed that patients experienced no severe adverse events or were not at increased risk of severe adverse events during SCT. In these clinical studies, SCT exerted an excellent therapeutic effect on ED. Erectile function was improved in almost all patients except those suffering from incontinence with severe damage to the cavernous nerve. The small study size and short follow-up period are weaknesses of the current studies. High-quality, large-scale, and long-term clinical studies are still needed to further evaluate the feasibility of promoting SCT to clinical patients with ED.
Unresolved Problems
Although the therapeutic effect of SCT on ED has been established, some issues remain unclear. Owing to the limited follow-up time of SCT (2–4 months for animals and up to 12 months for patients), the long-term effects and tumorigenic potential of stem cells after transplantation remain elusive. Intracavernous injection is the predominant route of administration, but several other methods exist, including intravenous injection, injured cavernous nerve cover, and periprostatic implantation. Each administration method has its own advantages and disadvantages. Hence, it is challenging to determine the ideal route of administration. Furthermore, the optimal timing of injection, the number of cells to be injected, the frequency of injection, and the use of autologous or allogeneic cells are yet to be clarified and need to be determined by further research.13
Limitations
Inevitably, there are some limitations in this study. To begin, relevant articles were solely screened in the WoSCC database, the most widely used database globally. Other databases, such as Scopus and Embase, were not included in the search. Next, our study exclusively focused on original studies and reviews. Other types of publications were excluded, which may lead to omissions. Finally, due to the low number of citations in some high-quality documents published in recent years, these documents may be drowned in the vast sea of literature data.
Conclusion
Based on bibliometric and visualization analysis of the research related to SCT and ED, the present study scientifically, comprehensively, and systematically analyzed the research status and frontiers in this field. The research of SCT in ED is booming and receiving increasing attention. The collaboration between different countries, institutions and authors around the world was extensive and drove this field forward together. Our research provided a theoretical reference and guidance for preclinical and clinical research on the use of SCT for the treatment of ED.
Abbreviations
ED, erectile dysfunction; SCT, stem cell therapy; DM, diabetes mellitus; PDE5i, phosphodiesterase type 5 inhibitor; DMED, diabetes mellitus-induced ED; CNI-ED, cavernous nerve injury-related ED; WoSCC, the Web of Science Core Collection; ACI, the average citations per item; ADSCs, adipose-derived mesenchymal stem cells; ECs, endothelial cells; SMCs, smooth muscle cells; NOS, nitric oxide synthase; NO, nitric oxide.
Data Sharing Statement
All data analyzed were included in this paper; further requests can be consulted and data can be obtained from the correspondent author.
Acknowledgments
We would like to thank all authors who participated in the study of SCT and ED, and Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82201775) and the Joint Construction Project between Medical Science and Technology Research Project of Henan Province (No. LHGJ20220343).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Quilter M, Hodges L, von Hurst P, Borman B, Coad J. Male sexual function in New Zealand: a population-based cross-sectional survey of the prevalence of erectile dysfunction in men aged 40–70 years. J Sex Med. 2017;14(7):928–936. doi:10.1016/j.jsxm.2017.05.011
2. Corona G, Lee DM, Forti G, et al. Age-related changes in general and sexual health in middle-aged and older men: results from the European Male Ageing Study (EMAS). J Sex Med. 2010;7(4 Pt 1):1362–1380. doi:10.1111/j.1743-6109.2009.01601.x
3. Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84(1):50–56. doi:10.1046/j.1464-410x.1999.00142.x
4. Yafi FA, Jenkins L, Albersen M, et al. Erectile dysfunction. Nat Rev Dis Primers. 2016;2:16003. doi:10.1038/nrdp.2016.3
5. Gandaglia G, Briganti A, Jackson G, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol. 2014;65(5):968–978. doi:10.1016/j.eururo.2013.08.023
6. Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381(9861):153–165. doi:10.1016/S0140-6736(12)60520-0
7. Allen MS, Walter EE. Erectile dysfunction: an umbrella review of meta-analyses of risk-factors, treatment, and prevalence outcomes. J Sex Med. 2019;16(4):531–541. doi:10.1016/j.jsxm.2019.01.314
8. Sun T, Xu W, Wang J, et al. Saxagliptin alleviates erectile dysfunction through increasing stromal cell-derived factor-1 in diabetes mellitus. Andrology. 2023;11(2):295–306. doi:10.1111/andr.13296
9. Fink HA, Mac Donald R, Rutks IR, Nelson DB, Wilt TJ. Sildenafil for male erectile dysfunction: a systematic review and meta-analysis. Arch Intern Med. 2002;162(12):1349–1360. doi:10.1001/archinte.162.12.1349
10. Wani MM, Rai BP, Webb WR, Madaan S. Is there a role for stem cell therapy in erectile dysfunction secondary to cavernous nerve injury? Network meta-analysis from animal studies and human trials. Ther Adv Urol. 2022;14:17562872221086999. doi:10.1177/17562872221086999
11. Castiglione F, Cakir OO, Satchi M, et al. The current role and implications of stem cell therapy in erectile dysfunction: a transformation from caterpillar to butterfly is required. Eur Urol Focus. 2023;9(1):28–31. doi:10.1016/j.euf.2022.11.009
12. Sarkar A, Saha S, Paul A, Maji A, Roy P, Maity TK. Understanding stem cells and its pivotal role in regenerative medicine. Life Sci. 2021;273:119270. doi:10.1016/j.lfs.2021.119270
13. Campbell JD, Milenkovic U, Usta MF, Albersen M, Bivalacqua TJ. The good, bad, and the ugly of regenerative therapies for erectile dysfunction. Transl Androl Urol. 2020;9(Suppl 2):S252–S261. doi:10.21037/tau.2019.10.06
14. Vasuri F, Fittipaldi S, Pasquinelli G. Arterial calcification: finger-pointing at resident and circulating stem cells. World J Stem Cells. 2014;6(5):540–551. doi:10.4252/wjsc.v6.i5.540
15. Zakrzewski W, Dobrzynski M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10(1):68. doi:10.1186/s13287-019-1165-5
16. Pakpahan C, Ibrahim R, William W, et al. Stem cell therapy and diabetic erectile dysfunction: a critical review. World J Stem Cells. 2021;13(10):1549–1563. doi:10.4252/wjsc.v13.i10.1549
17. Wang B, Gao W, Zheng MY, Lin G, Lue TF. Recent advances in stem cell therapy for erectile dysfunction: a narrative review. Expert Opin Biol Ther. 2023;23(6):565–573. doi:10.1080/14712598.2023.2203811
18. Gur S, Abdel-Mageed AB, Sikka SC, Hellstrom WJG. Advances in stem cell therapy for erectile dysfunction. Expert Opin Biol Ther. 2018;18(11):1137–1150. doi:10.1080/14712598.2018.1534955
19. Yang J, Zhang Y, Zang G, et al. Adipose-derived stem cells improve erectile function partially through the secretion of IGF-1, bFGF, and VEGF in aged rats. Andrology. 2018;6(3):498–509. doi:10.1111/andr.12483
20. Sun T, Xu W, Tu B, et al. Engineered adipose-derived stem cells overexpressing RXFP1 via CRISPR activation ameliorate erectile dysfunction in diabetic rats. Antioxidants. 2023;12(1):171. doi:10.3390/antiox12010171
21. Yang Y, Chen Y, Liu Y, et al. Mesenchymal stem cells and pulmonary fibrosis: a bibliometric and visualization analysis of literature published between 2002 and 2021. Front Pharmacol. 2023;14:1136761. doi:10.3389/fphar.2023.1136761
22. Zhou W, Hu S, Wu Y, et al. A bibliometric analysis of mesenchymal stem cell-derived exosomes in acute lung injury/acute respiratory distress syndrome from 2013 to 2022. Drug Des Devel Ther. 2023;17:2165–2181. doi:10.2147/DDDT.S415659
23. van Eck NJ, Waltman L. Software survey: vOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi:10.1007/s11192-009-0146-3
24. Hassan-Montero Y, De-Moya-Anegón F, Guerrero-Bote VP. SCImago Graphica: a new tool for exploring and visually communicating data. Profes De La Inform. 2022;31:5.
25. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. 2004;101(Suppl 1):5303–5310. doi:10.1073/pnas.0307513100
26. Chen C, Chen Y. Searching for clinical evidence in CiteSpace. AMIA Annu Symp Proc. 2005;2005:121–125.
27. Gonzalez-Cadavid NF, Rajfer J. Therapy of erectile dysfunction: potential future treatments. Endocrine. 2004;23(2–3):167–176. doi:10.1385/ENDO:23:2-3:167
28. Chen C. Leydesdorff LJJotafis, technology. Patterns of connections and movements in dual‐map overlays: a new method of publication portfolio analysis. J Assoc Inform Sci Technol. 2014;65(2):334–351. doi:10.1002/asi.22968
29. Albersen M, Fandel TM, Lin G, et al. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7(10):3331–3340. doi:10.1111/j.1743-6109.2010.01875.x
30. Bivalacqua TJ, Deng W, Kendirci M, et al. Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:3.
31. Haahr MK, Jensen CH, Toyserkani NM, et al. Safety and potential effect of a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: an open-label phase i clinical trial. EBioMedicine. 2016;5:204–210. doi:10.1016/j.ebiom.2016.01.024
32. Yiou R, Hamidou L, Birebent B, et al. Safety of intracavernous bone marrow-mononuclear cells for postradical prostatectomy erectile dysfunction: an open dose-escalation pilot study. Eur Urol. 2016;69(6):988–991. doi:10.1016/j.eururo.2015.09.026
33. Levy JA, Marchand M, Iorio L, Cassini W, Zahalsky MP. Determining the feasibility of managing erectile dysfunction in humans with placental-derived stem cells. J Am Osteopath Assoc. 2016;116(1):e1–5. doi:10.7556/jaoa.2016.007
34. Al Demour S, Jafar H, Adwan S, et al. Safety and potential therapeutic effect of two intracavernous autologous bone marrow derived mesenchymal stem cells injections in diabetic patients with erectile dysfunction: an open label phase I clinical trial. Urol Int. 2018;101(3):358–365. doi:10.1159/000492120
35. Burnett AL, Nehra A, Breau RH, et al. Erectile Dysfunction: AUA Guideline. J Urol. 2018;200(3):633–641. doi:10.1016/j.juro.2018.05.004
36. Matz EL, Terlecki R, Zhang Y, Jackson J, Atala A. Stem cell therapy for erectile dysfunction. Sex Med Rev. 2019;7(2):321–328. doi:10.1016/j.sxmr.2017.12.008
37. Foresta C, Caretta N, Lana A, Cabrelle A, Palu G, Ferlin A. Circulating endothelial progenitor cells in subjects with erectile dysfunction. Int J Impot Res. 2005;17(3):288–290. doi:10.1038/sj.ijir.3901311
38. Garcia MM, Fandel TM, Lin G, et al. Treatment of erectile dysfunction in the obese type 2 diabetic ZDF rat with adipose tissue-derived stem cells. J Sex Med. 2010;7(1 Pt 1):89–98. doi:10.1111/j.1743-6109.2009.01541.x
39. Huang YC, Ning H, Shindel AW, et al. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J Sex Med. 2010;7(4 Pt 1):1391–1400. doi:10.1111/j.1743-6109.2009.01697.x
40. Kendirci M, Trost L, Bakondi B, Whitney MJ, Hellstrom WJ, Spees JL. Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerve injury. J Urol. 2010;184(4):1560–1566. doi:10.1016/j.juro.2010.05.088
41. Qiu X, Lin H, Wang Y, et al. Intracavernous transplantation of bone marrow-derived mesenchymal stem cells restores erectile function of streptozocin-induced diabetic rats. J Sex Med. 2011;8(2):427–436. doi:10.1111/j.1743-6109.2010.02118.x
42. Fandel TM, Albersen M, Lin G, et al. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;61(1):201–210. doi:10.1016/j.eururo.2011.07.061
43. Lin CS, Xin ZC, Wang Z, et al. Stem cell therapy for erectile dysfunction: a critical review. Stem Cells Dev. 2012;21(3):343–351. doi:10.1089/scd.2011.0303
44. Qiu X, Fandel TM, Ferretti L, et al. Both immediate and delayed intracavernous injection of autologous adipose-derived stromal vascular fraction enhances recovery of erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;62(4):720–727. doi:10.1016/j.eururo.2012.02.003
45. Liu G, Sun X, Bian J, et al. Correction of diabetic erectile dysfunction with adipose derived stem cells modified with the vascular endothelial growth factor gene in a rodent diabetic model. PLoS One. 2013;8:8.
46. Alwaal A, Zaid UB, Lin CS, Lue TF. Stem cell treatment of erectile dysfunction. Adv Drug Deliv Rev. 2015;82–83:137–144. doi:10.1016/j.addr.2014.11.012
47. Chen F, Zhang H, Wang Z, et al. Adipose-derived stem cell-derived exosomes ameliorate erectile dysfunction in a rat model of type 2 diabetes. J Sex Med. 2017;14(9):1084–1094. doi:10.1016/j.jsxm.2017.07.005
48. Soebadi MA, Milenkovic U, Weyne E, Castiglione F, Albersen M. Stem cells in male sexual dysfunction: are we getting somewhere? Sex Med Rev. 2017;5(2):222–235. doi:10.1016/j.sxmr.2016.11.002
49. Feng H, Liu Q, Deng Z, et al. Human umbilical cord mesenchymal stem cells ameliorate erectile dysfunction in rats with diabetes mellitus through the attenuation of ferroptosis. Stem Cell Res Ther. 2022;13(1):450. doi:10.1186/s13287-022-03147-w
50. Zhang Z, Nie P, Yang W, Ma X, Chen Z, Wei H. Lipopolysaccharide-preconditioned allogeneic adipose-derived stem cells improve erectile function in a rat model of bilateral cavernous nerve injury. Basic Clin Androl. 2022;32(1):5. doi:10.1186/s12610-022-00156-w
51. Castela A, Costa C. Molecular mechanisms associated with diabetic endothelial-erectile dysfunction. Nat Rev Urol. 2016;13(5):266–274. doi:10.1038/nrurol.2016.23
52. Liu S, Jiang C, Hu J, Chen H, Han B, Xia S. Low-intensity pulsed ultrasound enhanced adipose-derived stem cell-mediated angiogenesis in the treatment of diabetic erectile dysfunction through the Piezo-ERK-VEGF axis. Stem Cells Int. 2022;2022:6202842. doi:10.1155/2022/6202842
53. Qiu X, Lin G, Xin Z, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med. 2013;10(3):738–746. doi:10.1111/jsm.12024
54. Li M, Li H, Ruan Y, Wang T, Liu J. Stem cell therapy for diabetic erectile dysfunction in rats: a meta-analysis. PLoS One. 2016;11:4.
55. Shan H, Chen F, Zhang T, He S, Xu L, Wei A. Stem cell therapy for erectile dysfunction of cavernous nerve injury rats: a systematic review and meta-analysis. PLoS One. 2015;10(4):e0121428. doi:10.1371/journal.pone.0121428
56. Xia J, Minamino S, Kuwabara K, Arai S. Stem cell secretome as a new booster for regenerative medicine. Biosci Trends. 2019;13(4):299–307. doi:10.5582/bst.2019.01226
57. Liu S, Li R, Dou K, Li K, Zhou Q, Fu Q. Injectable thermo-sensitive hydrogel containing ADSC-derived exosomes for the treatment of cavernous nerve injury. Carbohydr Polym. 2023;300:120226. doi:10.1016/j.carbpol.2022.120226
58. An G, Guo F, Liu X, et al. Functional reconstruction of injured corpus cavernosa using 3D-printed hydrogel scaffolds seeded with HIF-1alpha-expressing stem cells. Nat Commun. 2020;11(1):2687. doi:10.1038/s41467-020-16192-x
59. Hansen ST, Lund M, Ostergaard LD, Lund L. Role of regenerative therapies on erectile dysfunction after radical prostatectomy. Int J Impot Res. 2021;33(4):488–496. doi:10.1038/s41443-020-00406-3
60. Zhu Y, Jiang T, Yao C, et al. Effects of stem cell-derived exosome therapy on erectile dysfunction: a systematic review and meta-analysis of preclinical studies. Sex Med. 2023;11(2):qfac019. doi:10.1093/sexmed/qfac019
61. He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8(1):237–255. doi:10.7150/thno.21945
62. Ouyang B, Sun X, Han D, et al. Human urine-derived stem cells alone or genetically-modified with FGF2 Improve type 2 diabetic erectile dysfunction in a rat model. PLoS One. 2014;9(3):e92825. doi:10.1371/journal.pone.0092825
63. Jeon SH, Zhu GQ, Bae WJ, et al. Engineered mesenchymal stem cells expressing stromal cell-derived factor-1 improve erectile dysfunction in streptozotocin-induced diabetic rats. Int J Mol Sci. 2018;19(12):3730. doi:10.3390/ijms19123730
64. Zhou J, Yin Y, Yang Y, et al. Knockdown of miR-423-5p simultaneously upgrades the eNOS and VEGFa pathways in ADSCs and improves erectile function in diabetic rats. J Cell Mol Med. 2021;25(20):9796–9804. doi:10.1111/jcmm.16927
65. Liu Q, Cui Y, Lin H, et al. MicroRNA-145 engineered bone marrow-derived mesenchymal stem cells alleviated erectile dysfunction in aged rats. Stem Cell Res Ther. 2019;10(1):398. doi:10.1186/s13287-019-1509-1
66. Li L, Zhang D, Li P, Damaser M, Zhang Y. Virus integration and genome influence in approaches to stem cell based therapy for andro-urology. Adv Drug Deliv Rev. 2015;82–83:12–21. doi:10.1016/j.addr.2014.10.012
67. Yan H, Rong L, Xiao D, et al. Injectable and self-healing hydrogel as a stem cells carrier for treatment of diabetic erectile dysfunction. Mater Sci Eng C Mater Biol Appl. 2020;116:111214. doi:10.1016/j.msec.2020.111214
68. Lin H, Dhanani N, Tseng H, et al. Nanoparticle improved stem cell therapy for erectile dysfunction in a rat model of cavernous nerve injury. J Urol. 2016;195(3):788–795. doi:10.1016/j.juro.2015.10.129
69. Zheng T, Zhang T, Zhang W, et al. Icariside II facilitates the differentiation of ADSCs to Schwann cells and restores erectile dysfunction through regulation of miR-33/GDNF axis. Biomed Pharmacother. 2020;125:109888. doi:10.1016/j.biopha.2020.109888
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.