Back to Journals » Journal of Pain Research » Volume 16
Bibliometric Analysis of Global Research on Transient Receptor Potential Vanilloid 1 in the Field of Pain
Received 24 February 2023
Accepted for publication 3 May 2023
Published 9 May 2023 Volume 2023:16 Pages 1517—1532
DOI https://doi.org/10.2147/JPR.S407384
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qi Fang
Sisi Wang,1 Wen Wang,2 Xiangming Ye1
1Center for Rehabilitation Medicine, Rehabilitation & Sports Medicine Research Institute of Zhejiang Province, Department of Rehabilitation Medicine, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Preventive Treatment Center, Quzhou Hospital of Traditional Chinese Medicine, Quzhou, Zhejiang, People’s Republic of China
Correspondence: Xiangming Ye, Department of Rehabilitation Medicine, Zhejiang Provincial People’s Hospital, 158 Shangtang Road, Gongshu District, Hangzhou City, Zhejiang, People’s Republic of China, Tel +86 571 87692748, Email [email protected]
Background: Transient Receptor Potential Vanilloid 1 (TRPV1) is a heat-activated cation channel modulated by inflammatory mediators, which is closely related to pain and serves as a potential analgesic target. However, the bibliometric analyses summarizing TRPV1 in the field of pain are scarce. This study aims to summarize the current status of TRPV1 in pain and the potential research direction.
Methods: Articles regarding TRPV1 in the pain field between 2013 and 2022 were extracted from the Web of Science core collection database on 31 December 2022. Scientometric software (VOSviewer and CiteSpace 6.1.R6) were used to perform bibliometric analysis. This study provided data on the trend of the annual outputs, countries/regions, institutions, journals, authors, co-cited references and keywords.
Results: A total of 2462 publications related to TRPV1 in the field of pain were extracted from 2013 to 2022, which were written by 12,005 authors of 2304 institutions, 68 countries/regions in 686 journals, with 48,723 citations totally. The number of publications has grown rapidly over the past 10 years. Most publications were from the USA and China; the Seoul Natl Univ was the most active institution; Tominaga M published the most papers and Caterina MJ was the most productive co-cited author; The top-contributing journal was Pain; The most cited references was the article authored by Julius D. “Neuropathic pain”, “inflammatory pain”, “visceral pain” and “migraine” were the most common types of pain in this field. The mechanism of TRPV1 in pain was one of the main research directions.
Conclusion: This study presented an overview of the major research directions of TRPV1 in the pain field by bibliometric methods over the past decade. The results could reveal the research trends and the hotspots in the field and provide helpful information for clinical treatments of pain.
Keywords: TRPV1, pain, VOSviewer, CiteSpace, bibliometric analysis
Introduction
Pain is newly defined as an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage.1 According to the duration, etiology and location of pain, it can be divided into acute and chronic pain, inflammatory and neuropathic pain, peripheral and central pain. Epidemiological researches showed that more than 20% of adults suffer from pain globally,2 which will not only induce both physically and psychologically unbearable suffering, but also cause huge economic problems in society worldwide.3 Therefore, it is an urgent problem to clarify the underlying mechanism of pain.
Transient receptor potential (TRP) channel is the main temperature and pain receptor in the human body.4,5 TRPV1, a member of TRP channel family, is a non-selective cation channel that transmits calcium ions.6 It was discovered by David Julius, the Nobel Prize winner in physiology or medicine in 2021.7 TRPV1 is mainly distributed in small and medium-sized nociceptive sensory neurons, which can be activated by heat and capsaicin to cause pain and is one of the targeted analgesic mechanisms recently.8–10 Therefore, TRPV1 is of great significance in the field of pain research. Over the past decade, a large number of articles have demonstrated that TRPV1 participates in the pain process.11–14 However, no systematic analyses of these publications have been performed. This study aims to conduct a bibliometric analysis specifically on the relationship between TRPV1 channels and pain, in order to comprehensively explore the research hotspots and trends in this field.
Bibliometrics is an interdisciplinary science that utilizes mathematical and statistical methods to quantitatively analyze all knowledge carriers. Some scholars apply bibliometrics to study the research progress of TRP channels and predict future development, but no bibliometric study has been conducted on TRPV1 in the pain field. In this study, we performed a bibliometric analysis to systematically evaluate the studies of TRPV1 in the pain field from 2013 to 2022 by VOSviewer and CiteSpace, comprehensively reveal the latest hot spots and trends in this field and provide reference for clinical and scientific research in the future.15,16
Material and Methods
Date Collection
Raw data were collected from the Science Citation Index Expanded (SCI-expanded) of the Web of Science Core Collection (WOSCC) database on 30 Dec 2022. Search strategy was as follows: TS = (“Transient potential vanilloid receptor 1” OR “Transient Receptor Potential Vanilloid 1” OR “TRPV1*” AND “Pain”). The publications time of the literature ranged from 2013 to 2022. Only original articles and reviews included, and no restrictions on language. After removing duplications, a total of 2462 records were retrieved, including 2096 articles and 366 reviews.
Visualized Analysis
GraphPad prism 9 was used to account the annual publication output. VOSviewer and CiteSpace software are widely used to visualize and analyze research trends in scientific literature. In this study, all downloaded publications were imported to VOSviewer to obtain the network visualization map (country/region, institution, author and cite-author, journal and cited-journal, keywords). In addition, CiteSpace (6.1.R6) software was employed to obtain the network visualization map (cited references and the citation burst of keywords), with the parameters were set as below: Time slicing: from 2013 to 2022; Years per slice: 1 year; Selection criteria: k = 25, and pruning (minimum spanning tree, pruning sliced networks). Other parameters were set according to the CiteSpace manual for different situations. Nodes and links compose of visualization knowledge maps. Each node in the map represents an element. The size of node represents the frequency of occurrence or citation. The thick lines between the nodes signify cooperation. The colors of nodes indicate different years. In VOSviewer, the circle and label form an element, and the size of the element depends on the degree of the node, the strength of the connection, and the amount of citation, etc. The color of the element represents the cluster to which it belongs.
Results
General Data and Annual Output
A total of 2462 publications related to TRPV1 in the pain field from 2013 to 2022. These publications were written by 12,005 authors from 2304 institutions in 68 countries/regions, and were published in 686 journals, with 48,723 citations totally. As shown in Figure 1A–C, the number of publications has shown an upward trend over the past decade, with the number bottoming out in 2014 and peaking at 302 in 2020. The citations of publications in 2013 increased from a total of 226 to 8853 citations in 2022 (an average of 19.79 times per year), and the numbers had a significant increase over time. Furthermore, articles account for approximately 85% in terms of paper type (Figure 1D), which indicating that more attention was paid to the original studies in the research of TRPV1 in the field of pain.
Distribution of Countries and Institutions
Geographically, all publications related to TRPV1 in the pain field in the last 10 years were distributed among 63 countries/regions. Table 1 lists the top 10 countries/regions ranked by the numbers of publications in the field. Among them, USA had the highest output with 743 papers (30.18% of 2462 papers), followed by Peoples R China (n=479, 19.46%) and Japan (n=250, 10.15%). Considering the citations, USA (20,789) also ranked first among the countries, followed by People’s R China (6760) and Germany (4834). Figure 2 shows the network map of countries with more than 30 publications. The three largest nodes respectively represented the USA, China and Japan for their huge number of publications. The USA had the strongest total link strength (TLS, TLS = 459), indicating the USA had the most frequent collaboration with other countries. And the closest cooperation was between the USA and China (TLS = 95).
 |
Table 1 The Top 10 Countries According to Publications from 2013 to 2022 |
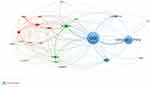 |
Figure 2 A country cooperation map related to TRPV1 research in field of pain from 2013 to 2022. |
A total of 2304 institutions made significant contributions in this field. The top 10 publications of institutions related to TRPV1 in the pain field are shown in Table 2. Most of them were located in the USA (n = 6) and followed by China (n = 2). Seoul Natl Univ (Korea, 56 publications) ranked first, Duke Univ (USA, 43 publications) and Washington Univ (USA, 40 publications) came second and third, respectively. Besides, the most citations was Duke Univ (USA, 1999 citations), followed by Harvard Med Sch (USA, 1100 citations) and Johns Hopkins Univ (USA, 1041 citations). Figure 3 shows the co-authorship network among institutions with 10 or more publications. The institutions formed four clusters with different colors and collaboration among institutions in the same region is more active.
 |
Table 2 The Top 10 Most Productive Institutions from 2013 to 2022 |
 |
Figure 3 An institution cooperation map related to TRPV1 in field of pain from 2013 to 2022. |
Authors and Co-Cited Authors
A total of 12,005 authors in the field of TRPV1 research in pain were analyzed. Table 3 shows the 10 most prolific authors and co-cited authors in this field. As shown in the data, Tominaga M, a professor of National Institute for Physiological Sciences in Japan, is one of the discoverers of TRPV1 (capsaicin receptor), mainly focused on the role of TRP channels in nociception,17–19 published the largest number of papers (24 publications, 577 citations), followed by Blumberg PM (21 publications, 221 citations) and Ferrer-Montiel A (21 publications, 402 citations). As for the co-cited authors, Caterina MJ ranked first with 1528 co-citations, followed by Szallasi A (560 co-citations) and Tominaga M (521 co-citations). Furthermore, VOSviewer software analyzed the information regarding authors and co-cited authors, then constructed a visual map to explore influential researchers and potential collaborators (Figure 4A and B). The 53 authors with more than 10 publications and the 88 co-cited authors with more than 100 co-citations formed several clusters, respectively. There was minor collaboration among clusters, but authors from the same cluster cooperated relatively closely.
 |
Table 3 The Top 10 Active Authors and Co-Cited Authors in Research of TRPV1 in the Field of Pain from 2013 to 2022 |
Journals and Co-Cited Journals
All 2462 articles were published in a total of 686 journals. The networks shown in Figure 5 reflect the collaboration among journals (Figure 5A) and cite-journals (Figure 5B). Table 4 shows the top 10 prolific journals and co-cited journals. The publishing countries of these journals were mostly located in England (n=5) and the USA (n=4). Among them, Pain (IF 2021=7.926, USA), the most authoritative journal in the field of pain, had the largest number of publications (85) and the most citations (2435), followed by Mol Pain with 76 publications and 1379 citations (IF 2021=3.37, England), Int J Mol Sci with 72 publications and 633 citations (IF 2021=6.208, England) ranked third. A total of 8010 journals were cited in the 2462 articles. The top 3 co-cited journals were Pain (IF 2021=7.926, 6874 co-citations), J Neurosci (IF 2021=6.709, 6047 co-citations) and Nature (IF 2021=69.504, 4510 co-citations), respectively. The co-citations of all of the listed journals were greater than 2000 and seven of the top 10 co-cited journals were top journal in their respective field.
 |
Table 4 The Top 10 Journals and Co-Cited Journals in Research of TRPV1 in the Field of Pain from 2013 to 2022 |
 |
Figure 5 Map of journal and co-cited journal related to TRPV1 in field of pain from 2013 to 2022. (A) Map of journals on TRPV1 in pain field. (B) Map of co-cited journals on TRPV1 in pain field. |
Co-Cited References
A total of 2462 articles were visualized and analyzed by CiteSpace. By analyzing the literature with high citation, the key knowledge base of the field can be obtained. The top 5 co-cited references are listed in Table 5 and the network of co-cited references is presented in Figure 6A. The most cited article is a review of TRP ion channels that can activate sensory neurons to produce acute or persistent pain, published in 2013 by Julius,20 the Nobel laureate, with 125 citations, indicating that the article has a greater influence and provides a theory foundation in this field. Other three co-cited references were all published in Nature: In 2013, Liao21 determined the structure of TRPV1 using electron cryo-microscopy. In the same year, Cao22 suggested a dual gating mechanism and accounted the reason for rich physiological modulation exhibited by TRPV1 and other TRP channels. In 2018, Vandewauw23 found that the initiation of the acute heat-evoked pain response in sensory nerve terminals depends on a triad of TRP ion channels: TRPM3, TRPV1, and TRPA1.
 |
Table 5 The Top 5 Co-Cited Reference in Research of TRPV1 in the Field of Pain from 2013 to 2022 |
The citation bursts of references refer to those that are frequently cited in a certain period of time. The top 25 References with the strongest citation bursts are shown in the Figure 6B. Among them, “Julius D, 2013, ANNU REV CELL DEV BI, V29, P355”20 had the highest burst strength with 29.11, followed by “Cao EH. 2013, NATURE, V504, P113”,22 with the burst of 18.84. And the reference “Vandewauw I, 2018, NATURE, V555, P662”23 ranked third with the burst strength of 18.38. Interestingly, the references with high burst strength were also the most frequently cited references, indicating the great impact of these papers in the field.
Furthermore, we performed a temporal co-citation analysis (Figure 7). We found the early research in this field primarily focused on “heat” (cluster #1), “TRPV1 antagonists” (cluster #5), and “pruritus” (cluster #6). However, these themes were gradually replaced by new themes over time. Recently, researchers had focused on “neuropathic pain” (cluster #0)”, indicating that “neuropathic pain” is a new hotspot and direction in the TRPV1-related pain field in recent years. Table 6 showed the top 5 most cited papers from research on TRPV1 in neuropathic pain. Among them, the most cited article is a research article, which confirms that TRPV1 channel is a potential detector of harmful stimuli and a biomarker of neuropathic pain.24
 |
Table 6 The Top 5 Co-Cited Reference in Research of TRPV1 on Neuropathic Pain |
Analysis of Keywords
By analyzing the keywords from publications of TRPV1 in the field of pain, we can inspect the research hotspots and predict the emerging trends during certain a period of time. 145 keywords with more than 30 occurrences were extracted from 2462 publications. The top 20 frequency keywords are shown in Table 7 and VOSviewer generates the network visualization map in Figure 8. The top 5 keywords were “trpv1” (605 times), “pain” (398 times), “capsaicin” (225 times), “trpa1” (145 times), “neuropathic pain” (131 times). Further analysis of the keywords shows that “neuropathic pain”, “inflammatory pain”, “migraine”, “visceral pain” and “osteoarthritis” are the most common pain types. The mechanism of “oxidative stress”, “substance p”, “calcitonin gene-related peptide”, “apoptosis” and “nerve growth factor” are most frequently listed (Table 8).
 |
Table 7 Top 20 Keywords in Terms of Frequency in Research of TRPV1 in the Field of Pain from 2013 to 2022 |
 |
Table 8 The Top 5 Pain Type and Mechanism Related to Natural Products in Cancer Research |
 |
Figure 8 Map of keywords related to TRPV1 in field of pain from 2013 to 2022. |
Furthermore, we also constructed a network map to visualize the clusters of keywords by Citespace (Figure 9). #0 labeling the “receptor” was the largest cluster, followed by “nerve injury” (cluster #1), and “ion channel” (cluster #2). Additionally, “postherpetic neuralgia” (cluster #3), “endocannabinoid system” (cluster #4), “irritable bowel syndrome” (cluster #5) and “vanilloid receptor” (cluster #6) were also the main research directions. Among them, several physiological processes including clusters #1, #3, and #5 were the research hotspot in this field since 2013.
The top 25 keywords with the strongest citation bursts from 2013 to 2022 are shown in Figure 10. Among them, “vanilloid receptor 1” was the first burst of keyword that began in 2013 and the most recent burst was observed in 2018, which had a duration with 5 years. “Animal model” ranked first with a strength of 8.11. Notably, “pharmacology”, “binding”, “mouse model”, “acid” and “allodynia” has burst recently and attracted widespread public attention hitherto.
 |
Figure 10 Top 25 keywords with the strongest citation bursts from 2013 to 2022. The red segment of the blue line denoted the burst duration of a keyword. |
Discussion
TRPV1 is an ion channel protein that is widely expressed in sensory nerve endings and plays a crucial role in sensing and transmitting pain signals. It can be activated by various stimuli, including heat, acid, and chemicals, resulting in calcium influx and changes in signal transduction that elicit pain responses. Moreover, TRPV1 can modulate neurotransmitter release to affect pain transmission and modulation. Therefore, it serves as a pivotal player in the regulation of nociception. Global trends in TRPV1 research are mainly focused on identifying novel therapeutic targets and assessing the therapeutic potential of TRPV1 antagonists for pain management.25–27 A comprehensive understanding of current trends in TRPV1 research related to pain will facilitate identification of potential partners and institutions, providing a new perspective for developing novel therapies and drugs that selectively target this receptor. We conducted this study to systematically reveal the research trends of TRPV1 in the field of pain. To the best of our knowledge, this is the first comprehensive bibliometric study in this field. In this study, we used biometric methods to study the papers related to TRPV1 in the pain filed between 2013 and 2022.
General Information, Countries/Regions and Institutions Analysis
The number of academic publications reflects the current pattern of TRPV1 research in the pain field, is one of the most significant indicators of bibliometrics. A total of 2642 publications were identified in this study. Figure 1 shows that the annual output generally maintained an increase trend in the past decade, with a linear increase in the average number of articles citations. From 2013 to 2020, the number of publications and citations in this field increased rapidly, indicating that TRPV1 in the pain field is widely concerned during this period. Then, the average number of publications has decreased year by year since 2020 and the potential reason may be related to the decline in the number of milestone scientific discoveries in this research field. Thus, more efforts should be taken to push forward further research in this field.
Research on TRPV1 in the field of pain has attracted the attention of 68 countries/regions worldwide. From the geographical distribution of countries, the USA had the largest number of publications and citations, indicating the great interest of American researchers in the field, and the USA was the research leader among the countries. It is worth noting that the analysis shows that the USA attaches great importance to academic cooperation with other countries, such as China and Japan. China ranked second among countries in the number of publications and citations. Interestingly, the closest cooperation was between the USA and China.
Furthermore, most of the 10 top institutions that published the most papers were from the USA, indicating that the American scientists made a huge contribution in this field. The Seoul Natl Univ in Korea was the most productive institution worldwide, which depended on the contribution of Lee J, the fourth most-published author, who focused on the discovery of novel TRPV1 agonists or antagonists and exploring their potential analgesic effects.28–30 In addition, Duke Univ ranked second among the top 10 most productive institutions with the most citations and had active academic collaboration with other institutions, which means the institution has a high academic reputation in this field and plays vital roles in the cooperation. However, global cooperation between institutions was characterized by geography, so efforts should be made to strengthen global cooperation to carry out higher quality academic results.
Journal and Cited Journal, Author and Cited Author, Cited-Reference Analysis
The research of TRPV1 in the pain field in the past ten years was mainly published in neuroscience and pain-related journals. Pain (IF 2021 =7.926), is an official publication of the international association for the study of pain and publishes original research on the nature, mechanisms and treatment of pain, ranked first both in publication journals and co-cited journals. In addition, for the cited journals, Nature (IF 2021 =69.504) was the core citation source journals of TRPV1 in pain research, and four articles among the top 5 co-cited references were published in Nature, indicating these journals have an essential influence in the field.
As for author analysis, Tominaga M was the most productive author published the largest number of papers. His articles focused on the mechanism of capsaicin receptor in pain.31,32 Caterina MJ ranked first among the top 10 co-cited authors, who had published two academic articles in top journals of Nature (in 1997) and Science (in 2000),9,33 mainly due to his pioneering discovery that the TRPV1 can be activated by thermal stimulation and is essential in thermal hyperalgesia. What cannot be ignored is that the corresponding author of the two articles is Nobel laureate Julius D. His paper entitled “TRP Channels and Pain” had the highest frequency among the top 5 co-cited references and the highest strength of bursts. The paper proved the TRP ion channels can activate sensory neurons to produce pain,20 providing a theoretical foundation for the research of TRPV1 in the pain field. Besides, the timeline view of co-cited references reveals that “neuropathic pain” (Cluster #0) is highlighted with the brightest color and largest nodes, containing the most publications. This suggests that the TRPV1 pathway plays a crucial role in regulating the underlying mechanism of neuropathic pain. In a word, these influential authors will be potential collaborators and these publications will provide in-depth insights for the further research of TRPV1 in pain field.
Keyword Analysis
Keywords can reflect a hot topic in academic field and are of great importance to researchers. In the research related to TRPV1 in the field of pain, the high-frequency keywords included “trpv1”, “pain”, “capsaicin”, “neuropathic pain”, “nociception”, “dorsal root ganglion”, “trp channels”, “spinal cord”, “inflammatory pain”, “itch”, “resiniferatoxin”, and “oxidative stress”. Most keywords are located in the middle of the density map, which also reflects the research hotspots in this field. Further analysis of keywords showed that “neuropathic pain”, “inflammatory pain” and “migraine” were the most studied pain types, “oxidative stress”, “apoptosis”, “substance p” (SP), “calcitonin gene-related peptide” (CGRP) and “nerve growth factor” (NGF) were the most widely studied mechanisms related to the TRPV1 in the pain field. As illustrated by the Keywords, this field is mainly concerned with the functional effects of TRP channel by multiple mechanisms such as “oxidative stress” and “apoptosis”, as well as regulating the expression of neuropeptides (such as SP and CGRP), and neurotrophic factor (such as NGF), which eventually leads to the occurrence of different pathological pains.34–38
In the cluster analysis of keywords, the research of TRPV1 in the pain field were divided into seven categories (Figure 7), describing the internal knowledge structure of certain research field, including “receptor”, “nerve injury”, “ion channel”, “postherpetic neuralgia”, “endocannabinoid system”, “irritable bowel syndrome” and “vanilloid receptor”. Endocannabinoids modulate a variety of fundamental physiological processes, including pain and inflammation, anxiety and depression, etc.39–41 Mechanism studies have proved that endocannabinoids are the endogenous ligands of the pain-mediating receptors - TRPV1. Various factors between the endocannabinoid system and the TRP channel, such as bradykinin, cytokines, SP, and NGF, can strongly sensitize the sensory nerves, leading to hyperalgesia by changing the threshold and sensitivity of the TRP channel to thermal and mechanical stimuli.42,43 Meanwhile, previous studies have confirmed that the endocannabinoid system is associated with various pain, including neuropathic pain, inflammatory pain and migraine.44–46 Therefore, the endocannabinoid system is considered to be a key mediator of TRPV1 in pain regulation. Recently, several studied have implicated that the endocannabinoid system is associated with pain-depression comorbidity.47,48 Nowadays, the comorbidity of depression and chronic pain is highly prevalent in patients. Thus, more and more researchers have begun to pay attention to psychological problems such as depression or anxiety caused by chronic pain. These results indicate that pain-depression comorbidity is expected to gradually become a new direction of TRPV1 in the field of pain. In addition, postherpetic neuralgia and irritable bowel syndrome are major clinical diseases, especially the former, which has an enormous impact on the life of patients. TRPV1 has been reported as a potential therapeutic target of postherpetic neuralgia, for the damage of TRPV1-positive sensory neurons and nerve terminals can induce thermal sensory impairment and tactile allodynia,49,50 which are the main clinical symptoms of postherpetic neuralgia.51 And “allodynia” is one of the current burst keywords, indicating that the role of TRPV1 in allodynia such as postherpetic neuralgia will be the hotspot and research trend in the future.
Strength and Limitations
Generally speaking, this study is the first bibliometric to systematically analyze TRPV1 in the field of pain over the past decade. In this review, the research hotspots and trends in this field are reviewed. In addition, various methods were used to analyze the data and provide comprehensive and objective guidance for the field.
However, there are some limitations in our study. First, we only extracted data from the WOSCC database, which makes our findings potentially not comprehensive. Second, some data were not standardized, which could lead to biased results. For example, “trpv1” and “transient receiver potential vanilloid 1” are regarded as different keywords by analysis software. In addition, research topics do not guarantee that each paper is entirely relevant to the topic, but the overall situation and trends in the field can still be described.
Conclusion
In conclusion, the study reveals an increase in TRPV1 research in the pain field from 2013 to 2022. Our results shows that USA is a leading contributor and main collaborating center in this area of research. Geographical location plays a significant role in global institutional cooperation, thus efforts should be made to strengthen international collaboration. Tominaga M and Caterina MJ ranked first among the top 10 most productive author and co-cited authors, respectively. They are potential collaborators in the future. Additionally, the current focus of TRPV1 hotspots in pain research is to explore its potential mechanisms in various types of pain, including neuropathic pain (such as postherpetic neuralgia), inflammatory pain, visceral pain and migraine. Related mechanisms include oxidative stress, endogenous cannabinoid system, neuropeptides (eg, SP and CGRP) and neurotrophic factors (eg, NGF). The global trend in this field still centers on understanding the mechanism and therapeutic targets of TRPV1. In summary, this study has provided potential collaborators, research trends and hotspots of TRPV1 in the field of pain, provides a new perspective for finding novel analgesic targets in clinical research, and finally provides a new therapy for clinical treatment of pain.
Data Sharing Statement
The raw data can be directly obtained from the Web of Science core collection database.
Acknowledgments
We are grateful to all study participants for their cooperation. This study was supported by the Zhejiang Provincial Natural Science Foundation [China] (No. LQ22H270014) and the “Seed” Fund of Zhejiang Provincial People’s Hospital (No. ZRY2022J018).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Raja SN, Carr DB, Cohen M, et al. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi:10.1097/j.pain.0000000000001939
2. Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health. 2011;11:770. doi:10.1186/1471-2458-11-770
3. Henschke N, Kamper SJ, Maher CG. The epidemiology and economic consequences of pain. Mayo Clin Proc. 2015;90(1):139–147. doi:10.1016/j.mayocp.2014.09.010
4. Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12(3):218. doi:10.1186/gb-2011-12-3-218
5. Premkumar LS, Abooj M. TRP channels and analgesia. Life Sci. 2012;92(8–9):415–424. doi:10.1016/j.lfs.2012.08.010
6. Moriello AS, De Petrocellis L, Vitale RM. Fluorescence-based assay for TRPV1 channels. Methods Mol Biol. 2023;2576:119–131. doi:10.1007/978-1-0716-2728-0_9
7. Hu J, Gao M, Zhang Y, et al. Novel piperazine urea derivatives as highly potent transient receptor potential vanilloid 1 (TRPV1) antagonists. Bioorg Chem. 2021;115:105229. doi:10.1016/j.bioorg.2021.105229
8. Ledford H, Callaway E. Medicine Nobel goes to scientists who discovered biology of senses. Nature. 2021;598(7880):246. doi:10.1038/d41586-021-01283-6
9. Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi:10.1038/39807
10. Katz B, Zaguri R, Edvardson S, et al. Nociception and pain in humans lacking functional TRPV1 channel. J Clin Invest. 2023;133(3):e153558. doi:10.1172/JCI153558
11. Iftinca M, Defaye M, Altier CAO. TRPV1-targeted drugs in development for human pain conditions. Drugs. 2021;81(1):7–27. doi:10.1007/s40265-020-01429-2
12. Akhilesh UA, Gadepalli A, Gadepalli A, et al. Unlocking the potential of TRPV1 based siRNA therapeutics for the treatment of chemotherapy-induced neuropathic pain. Life Sci. 2022;288:120187. doi:10.1016/j.lfs.2021.120187
13. Bogdan DM, Studholme K, DiBua A, et al. FABP5 deletion in nociceptors augments endocannabinoid signaling and suppresses TRPV1 sensitization and inflammatory pain. Sci Rep. 2022;12(1):9241. doi:10.1038/s41598-022-13284-0
14. Fang D, Kong LY, Cai J, et al. Interleukin-6-mediated functional upregulation of TRPV1 receptors in dorsal root ganglion neurons through the activation of JAK/PI3K signaling pathway: roles in the development of bone cancer pain in a rat model. Pain. 2015;156(6):1124–1144. doi:10.1097/j.pain.0000000000000158
15. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi:10.1007/s11192-009-0146-3
16. Chen C. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. JASIST. 2006;57(3):359–377. doi:10.1002/asi.20317
17. Takayama Y, Tominaga M. Involvement of TRPV1-ANO1 interactions in pain-enhancing mechanisms. Adv Exp Med Biol. 2018;1099:29–36. doi:10.1007/978-981-13-1756-9_3
18. Tominaga M. Activation and regulation of nociceptive transient receptor potential (TRP) channels, TRPV1 and TRPA1. Yakugaku Zasshi. 2010;130(3):289–294. doi:10.1248/yakushi.130.289
19. Takayama Y, Derouiche S, Maruyama K, Tominaga M. Emerging perspectives on pain management by modulation of TRP channels and ANO1. Int J Mol Sci. 2019;20(14):3411. doi:10.3390/ijms20143411
20. Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi:10.1146/annurev-cellbio-101011-155833
21. Liao M, Cao E, Julius D, et al. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504(7478):107–112. doi:10.1038/nature12822
22. Cao E, Liao M, Cheng Y, et al. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504(7478):113–118. doi:10.1038/nature12823
23. Vandewauw I, De Clercq K, Mulier M, et al. A TRP channel trio mediates acute noxious heat sensing. Nature. 2018;555(7698):662–666. doi:10.1038/nature26137
24. Marrone MC, Morabito A, Giustizieri M, et al. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat Commun. 2017;8:15292. doi:10.1038/ncomms15292
25. Weng HJ, Patel KN, Jeske NA, et al. Tmem100 is a regulator of TRPA1-TRPV1 complex and contributes to persistent pain. Neuron. 2015;85(4):833–846. doi:10.1016/j.neuron
26. Naik GG, Uniyal A, Chouhan D, et al. Natural products and some semi-synthetic analogues as potential TRPV1 ligands for attenuating neuropathic pain. Curr Pharm Biotechnol. 2022;23(6):766–786. doi:10.2174/1389201022666210719155931
27. Akhilesh T, Uniyal A, Gadepalli A, et al. Unlocking the potential of TRPV1 based siRNA therapeutics for the treatment of chemotherapy-induced neuropathic pain. Life Sci. 2022;288:120187. doi:10.1016/j.lfs.2021.120187
28. Lee H, Ahn S, Ann J, et al. Discovery of dual-acting opioid ligand and TRPV1 antagonists as novel therapeutic agents for pain. Eur J Med Chem. 2019;182:111634. doi:10.1016/j.ejmech.2019.111634
29. Kim C, Ann J, Lee S, et al. Discovery of 2- (3,5-difluoro-4 methylsulfonaminophenyl) propanamides as potent TRPV1 antagonists. Bioorg Med Chem Lett. 2018;28(14):2539–2542. doi:10.1016/j.bmcl.2018.05.043
30. Ann J, Kim HS, Thorat SA, et al. Discovery of Nonpungent Transient Receptor Potential Vanilloid 1 (TRPV1) agonist as strong topical analgesic. J Med Chem. 2020;63(1):418–424. doi:10.1021/acs.jmedchem.9b01046
31. Tominaga M, Caterina MJ, Malmberg AB, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–543. doi:10.1016/s0896-6273(00)80564-4
32. Tominaga M, Julius D. Capsaicin receptor in the pain pathway. Jpn J Pharmacol. 2000;83(1):20–24. doi:10.1254/jjp.83.20
33. Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi:10.1126/science.288.5464.306
34. Miller BA, Zhang W. TRP channels as mediators of oxidative stress. Adv Exp Med Biol. 2011;704:531–544. doi:10.1007/978-94-007-0265-3_29
35. Özdemir ÜS, Nazıroğlu M, Şenol N, Ghazizadeh V. Hypericum perforatum attenuates spinal cord injury-induced oxidative stress and apoptosis in the dorsal root ganglion of rats: involvement of TRPM2 and TRPV1 channels. Mol Neurobiol. 2016;53(6):3540–3551. doi:10.1007/s12035-015-9292-1
36. Zhu Y, Colak T, Fau - Shenoy M, et al. Nerve growth factor modulates TRPV1 expression and function and mediates pain in chronic pancreatitis. Gastroenterology. 2011;141(1):370–377. doi:10.1053/j.gastro.2011.03.046
37. Spekker EA-O, Körtési T, Vécsei L. TRP channels: recent development in translational research and potential therapeutic targets in migraine. Int J Mol Sci. 2022;24(1):700. doi:10.3390/ijms24010700
38. Ruiz-Cantero MC, Cortés-Montero E, Jain A, et al. The sigma-1 receptor curtails endogenous opioid analgesia during sensitization of TRPV1 nociceptors. Br J Pharmacol. 2023;180(8):1148–1167. doi:10.1111/bph.16003
39. Lowe H, Toyang N, Steele B, et al. The endocannabinoid system: a potential target for the treatment of various diseases. Int J Mol Sci. 2021;22(17):9472. doi:10.3390/ijms22179472
40. Blanton H, Reddy PH, Benamar K. Chronic pain in Alzheimer’s disease: endocannabinoid system. Exp Neurol. 2023;360:114287. doi:10.1016/j.expneurol.2022.114287
41. Fitzgibbon M, Finn DP, Roche M. High times for painful blues: the endocannabinoid system in pain-depression comorbidity. Int J Neuropsychoph. 2015;19(3):yv095. doi:10.1093/ijnp/pyv095
42. Storozhuk MV, Zholos AV. TRP channels as novel targets for endogenous ligands: focus on endocannabinoids and nociceptive signalling. Curr Neuropharmacol. 2018;16(2):137–150. doi:10.2174/1570159X15666170424120802
43. Muller C, Morales P, Reggio PH. Cannabinoid ligands targeting TRP channels. Front Mol Neurosci. 2019;11:487. doi:10.3389/fnmol.2018.00487
44. Maldonado R, Baños JE, Cabañero D. The endocannabinoid system and neuropathic pain. Pain. 2016;157 Suppl 1:S23–S32. doi:10.1097/j.pain.0000000000000428
45. Anand P, Whiteside G, Fowler CJ, et al. Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain Res Rev. 2009;60(1):255–266. doi:10.1016/j.brainresrev.2008.12.003
46. Greco R, Demartini C, Zanaboni AM, et al. The endocannabinoid system and related lipids as potential targets for the treatment of migraine-related pain. Headache. 2022;62(3):227–240. doi:10.1111/head.14267
47. Mecca CM, Chao D, Yu G, et al. Dynamic change of endocannabinoid signaling in the medial prefrontal cortex controls the development of depression after neuropathic pain. J Neurosci. 2021;41(35):7492–7508. doi:10.1523/JNEUROSCI.3135-20.2021
48. Wilkinson N. The ups and downs of endocannabinoid signaling in chronic pain-induced depression. J Neurosci. 2022;42(11):2143–2145. doi:10.1523/JNEUROSCI.2212-21.2022
49. Chen SR, Pan HL. Effect of systemic and intrathecal gabapentin on allodynia in a new rat model of postherpetic neuralgia. Brain Res. 2005;1042(1):108–113. doi:10.1016/j.brainres.2005.02.024
50. Wu CH, Lv ZT, Zhao Y, et al. Electroacupuncture improves thermal and mechanical sensitivities in a rat model of postherpetic neuralgia. Mol Pain. 2013;9:18. doi:10.1186/1744-8069-9-18
51. Nurmikko T, Bowsher D. Somatosensory findings in postherpetic neuralgia. J Neurol Neurosurg Psychiatry. 1990;52(2):135–141. doi:10.1136/jnnp.53.2.135
52. Gao Y, Cao E, Julius D, et al. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature. 2016;534(7607):347–351. doi:10.1038/nature17964
53. Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8(1):55–68. doi:10.1038/nrd2757
54. Kim YH, Back SK, Davies A, et al. TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord. Neuron. 2012;74(4):640–647. doi:10.1016/j.neuron.2012.02.039
55. Li Y, Adamek P, Zhang H, et al. The cancer chemotherapeutic paclitaxel increases human and rodent sensory neuron responses to TRPV1 by activation of TLR4. J Neurosci. 2015;35(39):13487–13500. doi:10.1523/JNEUROSCI.1956-15.2015
56. Basso L, Altier C. Transient receptor potential channels in neuropathic pain. Curr Opin Pharmacol. 2017;32:9–15. doi:10.1016/j.coph.2016.10.002
57. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi:10.1016/j.cell.2009.09.028
58. Moran MM, McAlexander MA, Bíró T, et al. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov. 2011;10(8):601–620. doi:10.1038/nrd3456
59. Gunthorpe MJ, Chizh BA. Clinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathway. Drug Discov Today. 2009;14(1–2):56–67. doi:10.1016/j.drudis.2008.11.005
60. Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: recent advances and setbacks. Brain Res Rev. 2009;60(1):267–277. doi:10.1016/j.brainresrev.2008.12.006
61. Karashima Y, Talavera K, Everaerts W, et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci USA. 2009;106(4):1273–1278. doi:10.1073/pnas.0808487106
62. Kremeyer B, Lopera F, Cox JJ, et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron. 2010;66(5):671–680. doi:10.1016/j.neuron.2010.04.030
63. Cavanaugh DJ, Chesler AT, Bráz JM, et al. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J Neurosci. 2011;31(28):10119–10127. doi:10.1523/JNEUROSCI.1299-11.2011
64. ONeill J, Brock C, Olesen AE, et al. Unravelling the mystery of capsaicin: a tool to understand and treat pain. Pharmacol Rev. 2012;64(4):939–971. doi:10.1124/pr.112.006163
65. Nilius B, Szallasi A. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev. 2014;66(3):676–814. doi:10.1124/pr.113.008268
66. Kaneko Y, Szallasi A. Transient receptor potential (TRP) channels: a clinical perspective. Br J Pharmacol. 2014;171(10):2474–2507. doi:10.1111/bph.12414
67. Usoskin D, Furlan A, Islam S, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18(1):145–153. doi:10.1038/nn.3881
68. Paulsen CE, Armache JP, Gao Y, et al. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015;520(7548):511–517. doi:10.1038/nature14367
69. Yang F, Zheng J. Understand spiciness: mechanism of TRPV1 channel activation by capsaicin. Protein Cell. 2017;8(3):169–177. doi:10.1007/s13238-016-0353-7
70. Moran MM, Szallasi A. Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. Br J Pharmacol. 2018;175(12):2185–2203. doi:10.1111/bph.14044
71. Gouin O, L’Herondelle K, Lebonvallet N, et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein Cell. 2017;8(9):644–661. doi:10.1007/s13238-017-0395-5
72. Moore C, Gupta R, Jordt SE, et al. Regulation of pain and itch by TRP channels. Neurosci Bull. 2018;34(1):120–142. doi:10.1007/s12264-017-0200-8
73. Garami A, Shimansky YP, Rumbus Z, et al. Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: insights from mathematical modeling and meta-analysis. Pharmacol Ther. 2020;208:107474. doi:10.1016/j.pharmthera.2020.107474.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





