Back to Journals » Infection and Drug Resistance » Volume 15
Beware of Brucella Spondylitis Following Vertebroplasty: An Unusual Case of Osteoporotic Vertebral Compression Fracture
Received 25 February 2022
Accepted for publication 12 May 2022
Published 18 May 2022 Volume 2022:15 Pages 2565—2572
DOI https://doi.org/10.2147/IDR.S363208
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Jie Wang, Qiang Zhang
Department of Orthopedics, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China
Correspondence: Qiang Zhang, Email [email protected]
Abstract: There are two main types of infectious spondylitis reported in literature following kyphoplasty or vertebroplasty: tuberculous spondylitis and pyogenic spondylitis; no cases of Brucella spondylitis have been reported to date. We present a unique case of Brucella spondylitis with intraspinal abscess following vertebroplasty. The patient suffered from osteoporotic vertebral compression fracture (OVCF) of the L4 vertebra and received vertebroplasty; he ate mutton while traveling in the epidemic area a week before vertebroplasty. Two weeks after the procedure, the patient had recurrent low back pain and was finally diagnosed with Brucella spondylitis around the L4 vertebra four months later. This case report provides a warning that eating any undercooked food that may cause bacteremia—especially in orthopedic patients with joint or osseous abnormalities and especially those with intraosseous devices or injected substances.
Keywords: vertebroplasty, complication, Brucella, spondylitis
Introduction
Kyphoplasty/vertebroplasty are effective procedures for treating OVCF and other bone-related diseases.1 The primary complications of this operation due to bone cement leakage include compression of the spinal cord or nerve root, pulmonary embolism, adjacent-level fractures.2 However, there are few reports of infectious spondylitis after kyphoplasty/vertebroplasty and none yet of Brucella spondylitis to date.3–5 We report unique Brucella spondylitis with intraspinal abscess, most probably due to locus minoris resistentiae following vertebroplasty.
Case Presentation
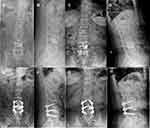
A 68-year-old female patient with lumbosacral pain caused by a fall was admitted to a local 3rd tier hospital without fever, fatigue, and sweating. Blood tests showed that white blood cell (WBC) count was 7.42x109/L and neutrophils accounted for 64.3%, erythrocyte sedimentation rate (ESR) was 10mm/hr, and C-reactive protein (CRP) was 3mg/L. X-ray showed a wedge-shaped change of the L4 vertebra (Figure 1A and B). Magnetic resonance imaging (MRI) demonstrated hypointense signal of L4 vertebra with a clear horizontal fracture line on T1-weighted imaging (T1WI), mixed intensity signal of L4 vertebra on T2-weighted imaging (T2WI), hyperintense signal, and a clear horizontal fracture line of L4 vertebra on Fat-suppressed T2-weighted images (FS-T2WI), quasi-circular areas of high-intensity signal around L5 was due to MRI artifact (Figure 1C–E). Diagnosis of fresh OVCF of L4 was explicitly based on trauma history and assistant examinations. The patient received vertebroplasty immediately due to unbearable pain. Her lumbosacral pain was relieved, and the spinal X-ray showed bone cement of L4 vertebra was uniformly dispersed when discharged from hospital three days following operation (Figure 2A and B). While there was recurrent pain in the lumbosacral region with intermittent fever and fatigue two weeks after the procedure, her core temperature was up to 37.9°C. This patient’s symptoms were progressively aggravated with radiation pain of both lower extremities, especially in the left lower extremity, during 4-month anti-inflammatory and analgesic treatment. Thus, she came to the orthopedic department of our hospital.
Medical history showed the patient ate mutton while traveling in the epidemic area one week before vertebroplasty. Due to severe low back pain, she was unable to stand. Percussion pain of the lower lumbar and paraspinal regions was obvious, radiating to both lower limbs. There was hyperesthesia on the posterolateral thigh and anterior medial leg of the lower extremities, with the left side being more affected than the right. Motion in the lumbar region was constrained both actively and passively. Muscle strength of bilateral quadriceps femoris and extensor pollicis longus was grade 4. The femoral nerve stretch test, straight leg raise test, and Braggard’s test were all positive on both sides. Thus, we suspect there was a great possibility of spinal infection, and further blood tests showed WBC 3.80x109/L with neutrophils accounting for 85.51%, ESR 32mm/hr, CRP 25.8mg/L, negative T-SPOT. TB assay (T-SPOT) and positive serum agglutination test (SAT) at a titer of 1:800.
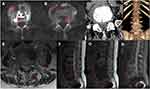
An X-ray, computerized tomography (CT) scan, and MRI of the spine were performed to determine the spinal segment affected. X-ray showed narrow intervertebral disc spaces at L3/4 and L4/5, and the osteophyte at the vertebra edge of L3-L5 proliferated obviously (Figure 2C and D). CT scan showed multiple destructive changes at L4 and L5 vertebra with paravertebral and intraspinal abscess formation around L4 vertebra (Figure 3A–D). MRI revealed hypointense signal of L3-L5 vertebra on T1WI of which T2WI demonstrated mixed intensity signal and FS-T2WI showed hyperintense signal (Figure 3E–H) consistent with infectious spondylitis with intraspinal abscess.
During hospitalization, antibiotics were administered, including rifampicin (0.6g po qd), doxycycline (0.1g po bid), and cefotaxime-sulbactam (2.25g ivgtt q8h). When empiric antibiotics were ineffective for two weeks, posterior focus debridement with decompression, fusion and internal fixation was performed, and large amounts of intraspinal and paraspinal purulent fluid were apparent during the procedure (Figure 4A), of which real-time polymerase chain reaction (Real-time PCR) test revealed Brucella melitensis (B. melitensis). The postoperative pathological results of the intervertebral disc of L3/4 and L4/5 (Figure 4B) demonstrated that organisms consistent with Brucella were present by Giemsa staining and Mycobacterium tuberculosis was not present by negative acid-fast staining (Figure 5A and B).
 |
Figure 4 Intraoperative purulent fluid is seen during revision surgery (A). Disc tissue and little bone cement from L3/4 and L4/5 levels were collected during revision surgery (B). |
After being discharged from the hospital ten days following the surgery, this patient experienced significant pain relief in the lumbosacral region and low extremities. The femoral nerve stretch test, straight leg raise test, and Braggard’s test on both sides all turned negative. The patient experienced a significant decline in infection indexes. X-ray showed postoperative changes of instrumentation placement (Figure 2E and F). Anti-brucellar medications were adjusted to rifampicin (0.6g po qd) and doxycycline (0.1g po bid) after discharge; the entire course of antibiotics treatment was half a year. Her discomforts wholly disappeared, and muscle strength of lower extremities was grade 5 at the last follow-up twelve months after discharge. The infection indexes all turned normal, and the titer of SAT dropped to 1:100. X-ray showed no abnormal changes in spinal instrumentation placement (Figure 2G and H); CT scan and MRI also demonstrated no abnormity (Figure 6A–H).
 |
Figure 6 No abnormalities on CT and MRI 12 months after discharge (A–H). |
Discussion
Vertebral infection following kyphoplasty or vertebroplasty is infrequent; Mycobacterium tuberculosis and Staphylococcus aureus are the most common pathogens.3–5 There are three types of causes of vertebral infection after kyphoplasty/vertebroplasty: (1) vertebral infection misdiagnosed as osteoporosis or the co-occurrence of osteoporosis and vertebral infection; (2) infection caused by operation; (3) infection caused by bacterial blood dissemination.3 Statistically, the shorter the time interval between the diagnosis of kyphoplasty/vertebroplasty and the diagnosis of postoperative infection, the more likely it is that infection has occurred prior to surgery; an infection caused by surgery and blood-borne dissemination of bacteria can also cause early infection, but it tends to be the leading cause of late infection.3,6
It is unclear whether the patient was misdiagnosed as OVCF with Brucella infection before vertebroplasty or spread through the blood after vertebroplasty and resulted in vertebral infection. She was admitted to the local hospital after experiencing low back pain following a trauma, and her core temperature and infection indexes (WBC, ESR, CRP) before vertebroplasty were normal. According to MRI, she was more inclined to be diagnosed with OVCF. However, vertebral osteoporotic compression fractures are more common in the thoracolumbar segment, while fractures of the L4 are relatively rare.7 In addition, the patient lacks CT images before PVP; even though X-ray and MRI can replace CT in most aspects of evaluating OVCF and reduce the cost to the patient, CT has guiding significance in the differential diagnosis of spinal infection and is capable of detecting early bone degradation.8 Therefore, for this atypical OVCF patient, further examination and evaluation should be performed before surgery.
The complications of kyphoplasty/vertebroplasty mainly include nerve injury, deep vein occlusion, and pulmonary embolism due to bone cement leakage; infectious complications are infrequent, and the primary infection types reported in the literature are tuberculous and pyogenic spondylitis to date.3–5 As far as we know, this study is the first report on Brucella spondylitis with abscess formation following vertebroplasty, a rare condition that the term locus minoris resistentiae can probably clarify.9–11
Primary sources of Brucella infection are cattle, sheep, and pigs; the transmission route is mainly direct contact with broken skin or mucous membranes and ingestion of contaminated food; B. melitensis is the most virulent species.12 Osteoarticular involvement in brucellosis is 10%~85%, and the spine is the most commonly affected site.13 An imaging characteristic of spinal infection is a narrowing of the intervertebral disc space, which is also a key to distinguishing spinal infection from OVCF or spinal tumor, while this change may not be evident at the beginning of the infection.14 Therefore, the possibility of Brucella spondylitis being misdiagnosed as OVCF cannot be excluded entirely. This patient ate mutton when traveling to the epidemic area one week before the first procedure, but she had no symptoms of infection before vertebroplasty, and blood tests showed the infectious indexes were all average; there was pain alleviation of two weeks following vertebroplasty. Thus, she was in the incubation period of brucellosis when receiving vertebroplasty.
The average incubation period of Brucella infection is two weeks, which can extend to several months or more than a year in some cases.15,16 When diagnosed with OVCF at the first hospital, this patient was most probably in the incubation period of brucellosis because her blood tests were regular, and she did not develop local or systemic symptoms until two weeks after vertebroplasty. The term locus minoris resistentiae refers to a region of low resistance, making it more vulnerable to be infected following accidental or surgical trauma,9–11 which explains why Brucella spondylitis with intraspinal abscess occurred at the surgical site of vertebroplasty in this case. This study also provides a warning that eating any undercooked food that may cause bacteremia—especially in orthopedic patients with joint or osseous abnormalities and especially those with intraosseous devices or injected substances.
Even though a positive blood bacterial culture is the gold standard for diagnosing brucellosis, cultures tend to be negative in the subacute and chronic phases of the illness.17 A titer of STA ≥ 1:160 is strongly indicative of brucellosis, but as China is a Brucella-endemic area, the threshold titer of SAT to be diagnostic is 1/320 or even 1/640; bacterial culture and STA tests were shown to have dropped at a positive rate with the progression of illness and application of antibiotics, whereas PCR and enzyme-linked immunosorbent assay (ELISA) kept high positive rates; specific Brucella can be analyzed using PCR.17,18 We cannot depend on SAT titer dynamic as the disease progress or antibiotics are administered, for it may be high even after recovery. Brucellosis may also be diagnosed by a rise in ESR and CRP levels.19 Patients with Brucella spondylitis were found to have non-caseous granuloma and Brucella positive by Giemsa staining in histopathological examinations.20
The principles of spinal infection treatment include lesion debridement, decompression, internal fixation, deformity correction, and bone graft fusion.20 Since bone cement of vertebroplasty is injected through the pedicle; its use affects the implantation of the pedicle. Nevertheless, considering the stability of the interbody fusion, we continue to use L4 bilateral pedicle screws. In addition to using shorter pedicle screws, we adjusted the direction of the screw placement during the procedure and avoided placing bone cement in the upper direction. Preoperative X-ray and CT scan revealed that the bone cement was mainly located in the lower and middle vertebrae of L4. Pedicle screws were implanted successfully, and vertebral body fixation was strengthened, beneficial to an interbody fusion. The fixation of the L4 vertebral body provides a more stable environment and promotes inflammatory absorption, focus repair, and interbody fusion stability.20
Due to the incubation period and early nonspecific symptoms of brucellosis, elderly patients diagnosed with OVCF who have an epidemic history of brucellosis should be well examined before and after vertebral augmentation. Brucellosis-related tests and inflammatory markers should be monitored preoperatively to exclude systemic or local infection; preoperative aspiration and pathological examination should be performed to exclude vertebral infection and metastatic tumor; infectious spondylitis should be considered when the postoperative fever of unknown origin or recurrent low back pain occurs. Clinicians should conduct adequate preoperative examinations, especially for atypical vertebral osteoporotic compression fractures. Therefore, preoperative evaluation and biopsy after vertebroplasty are required for pathological examination. Some clinicians believe that with the digitization of X-ray images and the upgrading of MRI equipment, their clarity has improved considerably, and they can replace CT in the following three aspects of the evaluation of OVCF: (1) The integrity of the posterior wall of the vertebral body; (2) the loss of the height of the vertebral body; (3) the anatomical morphology of the pedicle. However, CT scan is more sensitive than X-ray and MRI in detecting fine bone destruction caused by spinal infections.8 Furthermore, CT scan has a particular reference value in diagnosing vertebral osteoporosis.21 MRI is useful in the diagnosis of vertebral infection following vertebroplasty, and its sensitivity and specificity are over 90%.22 As far as the patient reported in this article is concerned, more important is monitoring postoperative MRI examination and blood tests, through which severe complications can be avoided.
Conclusion
In conclusion, clinicians should conduct adequate preoperative examinations, especially for atypical vertebral osteoporotic compression fractures. Eating any undercooked food that may cause bacteremia—especially in orthopedic patients with joint or osseous abnormalities and especially those with intraosseous devices or injected substances. The possibility of Brucella spondylitis should always be considered when recurrent low back pain occurs following vertebral augmentation, especially for elderly patients with an epidemic history.
Ethics Approval and Consent to Participate
Written informed consent was obtained from the patient and her family. The Ethics Committee of the Beijing Ditan Hospital of Capital Medical University approved this study.
Consent for Publication
The patient provided written informed consent for publication of this case report and accompanying images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work received no funding.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Long Y, Yi W, Yang D, Jiang N. Advances in vertebral augmentation systems for osteoporotic vertebral compression fractures. Pain Res Manag. 2020;2020:3947368. doi:10.1155/2020/3947368
2. Robinson Y, Tschöke SK, Stahel PF, Kayser R, Heyde CE. Complications and safety aspects of kyphoplasty for osteoporotic vertebral fractures: a prospective follow-up study in 102 consecutive patients. Patient Saf Surg. 2008;2:2. doi:10.1186/1754-9493-2-2
3. Abdelrahman H, Siam AE, Shawky A, Ezzati A, Boehm H. Infection after vertebroplasty or kyphoplasty. A series of nine cases and review of literature. Spine J. 2013;13(12):1809–1817. doi:10.1016/j.spinee.2013.05.053
4. Zou MX, Wang XB, Li J, Lv GH, Deng YW. Spinal tuberculosis of the lumbar spine after percutaneous vertebral augmentation (vertebroplasty or kyphoplasty). Spine J. 2015;15(6):e1–e6. doi:10.1016/j.spinee.2015.02.032
5. Ge CY, He LM, Zheng YH, et al. Tuberculous spondylitis following kyphoplasty: a case report and review of the literature. Medicine. 2016;95(11):e2940. doi:10.1097/MD.0000000000002940
6. Park JW, Park SM, Lee HJ, Lee CK, Chang BS, Kim H. Infection following percutaneous vertebral augmentation with polymethylmethacrylate. Arch Osteoporos. 2018;13(1):47. doi:10.1007/s11657-018-0468-y
7. Wang F, Tong T, Miao DC, Wang LF, Shen Y. Clinical correlation between osteoporotic thoracolumbar vertebral compression fractures and lumbar spondylolisthesis. Int Orthop. 2022;46(5):1095–1100. doi:10.1007/s00264-022-05327-y
8. Balériaux DL, Neugroschl C. Spinal and spinal cord infection. Eur Radiol. 2004;14(Suppl 3):E72–E83. doi:10.1007/s00330-003-2064-8
9. Bouvresse S, Chiras J, Bricaire F, Bossi P. Pott’s disease occurring after percutaneous vertebroplasty: an unusual illustration of the principle of locus minoris resistentiae. J Infect. 2006;53(6):e251–e253. doi:10.1016/j.jinf.2006.02.014
10. Farmand D, Valdez MC, Moosavi L, Cobos E. Locus minoris resistentiae: two cases of malignant metastasis and review of literature. J Investig Med High Impact Case Rep. 2021;9:2324709621997248. doi:10.1177/2324709621997248
11. Sherpa N, Shah R, Nordstrom B, Palmares C, Heidari A, Johnson R. Locus Minoris Resistentiae in Coccidioidomycosis: a Case Series. J Investig Med High Impact Case Rep. 2019;7:2324709619858110. doi:10.1177/2324709619858110
12. Adesokan HK, Alabi PI, Ogundipe MA. Prevalence and predictors of risk factors for Brucellosis transmission by meat handlers and traditional healers’ risk practices in Ibadan, Nigeria. J Prev Med Hyg. 2016;57(3):E164–E171.
13. Franc KA, Krecek RC, Häsler BN, Arenas-Gamboa AM. Brucellosis remains a neglected disease in the developing world: a call for interdisciplinary action. BMC Public Health. 2018;18(1):125. doi:10.1186/s12889-017-5016-y
14. Abe E, Yan K, Okada K. Pyogenic vertebral osteomyelitis presenting as single spinal compression fracture: a case report and review of the literature. Spinal Cord. 2000;38(10):639–644. doi:10.1038/sj.sc.3101057
15. Fiori PL, Mastrandrea S, Rappelli P, Cappuccinelli P. Brucella abortus infection acquired in microbiology laboratories. J Clin Microbiol. 2000;38(5):2005–2006. doi:10.1128/JCM.38.5.2005-2006.2000
16. Georghiou PR, Young EJ. Prolonged incubation in brucellosis. Lancet. 1991;337(8756):1543. doi:10.1016/0140-6736(91)93231-W
17. Li M, Zhou X, Li J, Sun L, Chen X, Wang P. Real-time PCR assays for diagnosing brucellar spondylitis using formalin-fixed paraffin-embedded tissues. Medicine. 2018;97(9):e0062. doi:10.1097/MD.0000000000010062
18. Xu N, Wang W, Chen F, Li W, Wang G. ELISA is superior to bacterial culture and agglutination test in the diagnosis of brucellosis in an endemic area in China. BMC Infect Dis. 2020;20(1):11. doi:10.1186/s12879-019-4729-1
19. Balın ŞÖ, Tartar AS, Akbulut A. The predictive role of haematological parameters in the diagnosis of osteoarticular brucellosis. Afr Health Sci. 2018;18(4):988–994. doi:10.4314/ahs.v18i4.19
20. Zhao R, Ding R, Zhang Q. Safety and efficacy of polyetheretherketone (PEEK) cages in combination with one-stage posterior debridement and instrumentation in Lumbar Brucella Spondylitis. Clin Neurol Neurosurg. 2020;199:106259. doi:10.1016/j.clineuro.2020.106259
21. Gausden EB, Nwachukwu BU, Schreiber JJ, Lorich DG, Lane JM. Opportunistic use of CT imaging for osteoporosis screening and bone density assessment: a qualitative systematic review. J Bone Joint Surg Am. 2017;99(18):1580–1590. doi:10.2106/JBJS.16.00749
22. Jeon I, Kong E, Yu D, Hong CP. Clinical and radiological analysis of pyogenic vertebral osteomyelitis immediately after successful antimicrobial therapy: considerations for assessing therapeutic response. Diagnostics. 2020;10(11):861. doi:10.3390/diagnostics10110861
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.