Back to Journals » Cancer Management and Research » Volume 10
Bevacizumab combined with chemotherapy vs single-agent therapy in recurrent glioblastoma: evidence from randomized controlled trials
Authors Chen Z , Xu N, Zhao C, Xue T, Wu X, Wang Z
Received 7 May 2018
Accepted for publication 2 June 2018
Published 23 July 2018 Volume 2018:10 Pages 2193—2205
DOI https://doi.org/10.2147/CMAR.S173323
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Zhouqing Chen,1,* Na Xu,2,* Chongshun Zhao,1 Tao Xue,1 Xin Wu,1 Zhong Wang1
1Department of Neurosurgery, The First Affiliated Hospital of Soochow Unicersity, Suzhou, Jiangsu Province 215006, China; 2State Key Laboratory of Medical Neurobiology, Institute of Brain Sciences and Collaborative Innovation Center for Brain Science, Fudan University, Shanghai 200032, China
*These authors contributed equally to this work
Background: Recent studies showed inconsistent results of bevacizumab combined with chemotherapy vs single-agent therapy in terms of their safety and efficacy for the treatment of recurrent glioblastoma. Therefore, we performed a meta-analysis to explore the value of bevacizumab combined with chemotherapy and single-agent therapy in recurrent glioblastoma treatment.
Methods: Databases such as MEDLINE, Embase, and Cochrane Library were searched for randomized controlled trials (RCTs) related to the topic of bevacizumab combined with chemotherapy and single-agent therapy as treatments for recurrent glioblastoma from January 1980 to April 2018. Subsequent articles were then sorted, evaluated, and analyzed.
Results: We pooled 1,169 patient cases from seven RCTs. Bevacizumab combined with chemotherapy showed a significantly improved progression-free survival (PFS) (HR=0.65; 95% CI 0.57–0.74; P<0.001) compared to single-agent therapy. In addition, the overall survival (OS) rate showed insignificant differences between the two groups (HR=0.96; 95% CI 0.83–1.12; P=0.622). Simultaneously, we found that bevacizumab combined with chemotherapy had a higher objective response rate (ORR) (OR=2.10; 95% CI 1.32–3.33; P=0.002), but also higher incidence of adverse events (AEs) (OR=1.85; 95% CI 1.26–2.71; P=0.002). However, in subgroup analysis, we found that AEs showed insignificant differences between the two treatment methods when bevacizumab was used as the single-agent therapy subgroup (P=0.058). In addition, in the subgroup with low corticosteroid use rate at baseline (N<50%), ORR (P=0.108) and AEs (P=0.134) showed insignificant differences between the two groups.
Conclusion: Bevacizumab combined with chemotherapy can significantly improve PFS and ORR, but did not prolong OS in these studies, and can even lead to higher odds of AEs. In addition, bevacizumab may play a dominant role and corticosteroid may be an unfavorable factor in the combination therapy of recurrent glioblastoma.
Keywords: bevacizumab, combination therapy, recurrent glioblastoma, meta-analysis
Introduction
Glioblastoma is a common primary brain tumor that is devastating for the nervous system. Survival rate of recurrent glioblastoma is extremely low, and the prognosis after recurrence is very severe with a short progression-free survival (PFS) period and overall survival (OS).1 Recurrent glioblastoma is different from newly diagnosed glioblastoma, it is a more complex disease characterized by poor physical condition, reoperation, multiple radiotherapy, and chemotherapy.2–4 However, chemotherapy remains crucial for patients who have relapsed after glioblastoma surgery.5,6
Glioblastoma is a primary brain tumor with dense and messy vessels. There is evidence that angiogenesis inhibitors can increase the PFS period in newly diagnosed or recurrent glioblastoma patients, which is presumably achieved by inhibiting the formation of vessels dependent on VEGF and vascular permeability of the tumor.7,8 Therefore, it is believed that angiogenesis inhibitors are beneficial in inhibiting the growth of glioblastoma and improving the efficacy of radiotherapy and chemotherapy.9,10
Bevacizumab is a humanized monoclonal antibody that targets VEGF-A and has been proposed as an available anti-angiogenic drug.3 Bevacizumab clears circulating VEGF and prevents VEGF from binding to receptors on the surface of endothelial cells, thereby inhibiting angiogenesis. Several Phase II clinical trials showed that bevacizumab has a marked response rate from 30% to 50% in patients with recurrent glioblastoma, prolonging PFS period and improving patients’ quality of life.11–13 However, these studies did not find a significant improvement in OS rates and long-term drug response rates. In some cases, researchers even imply that bevacizumab inhibited tumor growth in the short term, but promoted tumor growth in the long run.8,14 In recent years, many randomized controlled trials (RCTs) for bevacizumab combined with chemotherapy vs single-agent therapy in the treatment of recurrent glioblastoma have mushroomed, but the corresponding meta-analysis of these trials is rare.13,15–20 Therefore, it is necessary to further explore the efficacy and safety of combination therapy in recurrent glioblastoma.
In this meta-analysis, we pooled data from previous high-quality RCTs to investigate whether the value of bevacizumab combined with chemotherapy for recurrent glioblastoma is superior to single-agent therapy in terms of efficacy and safety, and to explore the potential factors that might influence the efficacy and safety of combination therapy.
Methods
Search strategy
Three major electronic databases including MEDLINE, Embase, and Cochrane databases were searched to identify relevant studies published from January 1980 to April 2018 by two independent investigators (ZQC and NX). The following search strategy was used for MEDLINE: (recurrent glioblastoma) AND (bevacizumab OR avastin) AND (RCT) NOT (animals). A similar search strategy was used for Embase and Cochrane databases. Reference lists of key articles were also screened from RCTs, post hoc analyses, reviews, comments, and meta-analyses to ensure no relevant articles were excluded.
Study selection and data collection
Only RCTs with recurrent glioblastoma patients treated with bevacizumab plus chemotherapy, bevacizumab, or chemotherapy alone were included in this meta-analysis. Studies where the intervention or control group did not receive chemotherapy or bevacizumab, but placebo, were excluded. Two independent investigators (ZQC and NX) scanned the titles and abstracts of all the studies to select applicable studies. Data on baseline characteristics of the included studies and outcome events were extracted independently by two investigators (ZQC and NX) (Table 1).
Outcomes of interest and quality assessment
The primary outcomes were the PFS and OS rates, secondary outcomes included objective response rate (ORR) and adverse events (AEs) (grade ≥3), the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 was used to classify and grade AEs. The risk of bias of the included trials was assessed independently by two investigators (ZQC and NX) using Cochrane Collaboration’s risk-of-bias tool. The risk-of-bias criteria included selection bias, performance bias, detection bias, attrition bias, reporting bias, and other potential biases. Each criterion was categorized as “low”, “unclear”, or “high” risk of bias.
Data synthesis and analysis
All data were calculated by two investigators (ZQC and NX) using STATA. HRs with 95% confidence interval (CI) were used to assess the time-to-event variables (OS and PFS), and the dichotomous outcomes (ORR and AEs) were analyzed as the ORs with 95% CI. All analyses were calculated using a random-effects model. In trials without direct HRs, Kaplan–Meier curves and follow-up period were used to calculate HRs.21 We used these methods to analyze a merged HR with 95% CI of the experimental group vs two control groups in one study. A P-value of <0.05 was considered statistically significant. Heterogeneity was tested with I2 statistics. High heterogeneity was defined as I2 values >50%. Sensitivity analysis of the effect of omitting each study, in turn, was performed to assess sources of heterogeneity.
Results
Study selection and characteristics
A total of 1,383 titles and abstracts were identified by searching MEDLINE, Embase, and Cochrane Library databases, from which we obtained 35 records without duplicates and irrelevant records. After excluding protocols, post hoc analyses studies, meta-analyses, comments, and reviews, we identified seven RCTs and ultimately used 1,169 patients for meta-analysis (Figure 1). The baseline characteristics of the included trials are shown in Table 1.
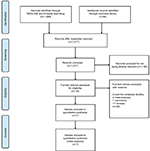  | Figure 1 The study search, selection, and inclusion process. Note: PRISMA adapted from Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009;6(6):e1000097. For more information, visit www.prisma-statement.org.27 |
PFS
Six RCTs presented prolonged median PFS from experimental group compared to control group, except one RCT which showed the same PFS in both groups, as shown in Table 1.13,15–20 Combination of bevacizumab and other chemotherapy agent improved PFS significantly compared with treatment with bevacizumab or other chemotherapy alone (HR 0.65; 95% CI 0.57–0.74; P<0.001). The heterogeneity test showed significant differences among studies (I2=66.1%, P=0.007) (Figure 2). To detect the source of the statistical heterogeneity, sensitivity analysis was performed. The sensitivity analysis showed that one trial was highly sensitive13 (Figure S1). After excluding this trial, the heterogeneity test showed insignificant differences among studies (I2=39.7%, P=0.141) (Figure S2). Combination of bevacizumab with chemotherapy also showed a significant PFS improvement when compared with bevacizumab or chemotherapy alone (HR 0.75; 95% CI 0.64–0.88; P<0.001).
  | Figure 2 The pooled HR of the progression-free survival outcomes. Note: The diamond indicates the estimated HR (95% CI) for all patients together. |
OS
All seven RCTs reported insignificant differences between experimental group and control group (Table 1).13,15–20 Figure 3 shows the insignificant differences between bevacizumab combined with chemotherapy and bevacizumab or chemotherapy alone (HR 0.96; 95% CI 0.83–1.12; P=0.622) in OS. Moderate heterogeneity was observed in pooled trial studies (I2=40.5%, P=0.121) (Figure 3). To detect the source of the statistical heterogeneity, sensitivity analysis was performed. The sensitivity analysis showed that all of the consolidated results were stable (Figure S3).
  | Figure 3 The pooled HR of the overall survival outcomes. Note: The diamond indicates the estimated relative risk (95% CI) for all patients together. |
ORR
In the meta-analysis of the seven trials, the bevacizumab combined with chemotherapy group showed significantly greater odds of ORR values (including complete response and partial response) (OR 2.1, 95% CI, 1.32–3.33, P=0.002) than bevacizumab or chemotherapy alone. However, moderate heterogeneity was observed in ORR (I2=51.0%, P=0.057) (Figure 4). To detect the source of the statistical heterogeneity, sensitivity analysis was performed. The sensitivity analysis showed that all of the consolidated results were stable (Figure S4).
AEs
Within the included studies, five presented the data of AEs.13,15,16,18,20 AEs of grade ≥3 are listed in Table 1. As shown in Figure 5, bevacizumab combined with chemotherapy was associated with a significantly higher odds of high grade AEs (OR 1.85; 95% CI 1.26–2.71; P=0.002). However, moderate heterogeneity was identified in AEs (I2=40.9%, P=0.148) (Figure 5). To detect the source of the statistical heterogeneity, sensitivity analysis was performed. The sensitivity analysis showed that all of the consolidated results were stable (Figure S5).
Subgroup analyses
Subgroup analysis was performed to examine the influence of bevacizumab use on the control group and the rate of corticosteroid drug use. We found that some results of the subgroup analysis were different from those of the overall analysis. For AEs, there were insignificant differences between the two arms when bevacizumab was used in the single-agent therapy subgroup (P=0.058). Additionally, in the low corticosteroid use rate at baseline (N<50%) subgroup, ORR (P=0.108) and AEs (P=0.134) showed insignificant differences between the two arms. Other results of subgroup analysis were similar to the overall analysis. Bevacizumab single-agent treatment control group showed a significant increase in PFS (P=0.001, Table 2) and ORR (P=0.019, Table 2). Non-bevacizumab single-agent treatment control group showed a dramatic PFS improvement (P<0.001, Table 2), better odds of ORR (P<0.001, Table 2), and higher odds of high grade AEs (AEs ≥3) (P<0.001, Table 2). In addition, when the rate of corticosteroid drug use (patients used corticosteroid drug/total patients) was less than 50%, PFS showed an apparent improvement (P<0.001; Table 2). However, when the rate of corticosteroid drug use was greater than 50%, PFS showed significant but little improvement (P=0.029; Table 2), better odds of ORR (P=0.016, Table 2), and greater odds of high grade AEs (AEs ≥3) (P=0.017, Table 2).
  | Table 2 Subgroup analysis of PFS, OS, ORR, and AEs Abbreviations: PFS, progression-free survival; OS, overall survival; ORR, object response rate; AEs, adverse events. |
Quality of the included studies
Full details about the risk of bias of the included studies are shown in Figure 6. For random sequence generation, the risk of bias of three trials was unclear. For allocation concealment, the risk of bias of all seven trials was unclear. For the blinding of outcomes assessment, the risk of bias was high in three studies and unclear in three studies. Apart from these three items, no high or unclear risk of bias was observed in any of the other items.
  | Figure 6 Risk of bias: a summary table for each risk of bias item for each study. |
Discussion
Based on the data gathered from seven published RCTs, our present meta-analysis showed that bevacizumab combined with chemotherapy was superior to single-agent therapy in terms of PFS and ORR as treatment for recurrent glioblastoma. However, combination therapy did not improve OS compared to single-agent therapy and could even lead to higher odds of AEs (grade ≥3). Analysis of the PFS and OS of the two subgroups (single-agent therapy [bevacizumab / non-bevacizumab] and corticosteroid use rate higher than 50%) obtained the same conclusion as the overall analysis. However, in the subgroup of corticosteroid use less than 50%, combination therapy did not offer a greater ORR. In the single-agent therapy using bevacizumab and the corticosteroid use lower than 50% subgroups, combination therapy did not show significantly higher odds of AEs compared with single-agent therapy.
In a previous meta-analysis,22 bevacizumab combined with chemotherapy for glioblastoma results were published. The meta-analysis of four RCTs included one newly diagnosed glioblastoma trial and three recurrent glioblastoma trials. In that meta-analysis, four clinical trials were included to assess efficacy and safety of combination therapy. Their results presented that, compared to single-agent therapy, bevacizumab combined with chemotherapy improved PFS significantly in both newly diagnosed glioblastoma and recurrent glioblastoma patients (pooled HRs, 0.57, P=0.0008; 0.70, P=0.0005). However, the bevacizumab combined with chemotherapy did not prolong OS significantly (pooled HRs, 1.02, P=0.91; 0.98, P=0.85). These conclusions are consistent with our analysis which found that combination therapy improved PFS but did not prolong OS. In addition, two other meta-analyses also compared PFS and OS for combination therapy and single-agent therapy.23,24 Each of these two studies included radiotherapy and O6-methylguanine-DNA methyltransferase methylation status respectively. In the same way, their research showed similar conclusions to ours. However, these two meta-analyses included only RCTs that analyzed newly diagnosed glioblastoma. Our study included all RCTs of recurrent glioblastoma to obtain more concrete clinical evidence of treatment of recurrent glioblastoma using combination therapy. Simultaneously, we performed sensitivity analysis and subgroup analysis to further explore the differences between combination therapy and single-agent therapy.
Two other important indicators in the prognosis of glioblastoma are ORR and AEs. ORR can effectively reflect the efficacy of combination therapy and AEs can effectively reflect the safety of combination therapy. In our seven pooled RCTs, ORR of the combined treatment group was not superior to the single-agent treatment group in only one clinical trial.16 In the two most recent RCTs,13,15 the ORR of the combination therapy group was significantly better than the single-agent group. In our pooled RCT studies, five studies included AEs assessment. However, use of combination therapy showed more serious AEs than the single-agent group (OR 1.85; 95% CI 1.26–2.71; P=0.002). Frequent AEs may be associated with increased toxicity due to use of combination therapy.
We realize that we found heterogeneity in the extraction of PFS, OS, ORR, and AEs. We detected the source of heterogeneity through sensitivity analysis and subgroup analysis. In the sensitivity analysis, OS, ORR, and AEs’ sensitivity were within the CI, whereas PFS analysis in a clinical trial13 was outside the CI and was considered highly sensitive. When we removed this study, the heterogeneity dropped from I2=66.1% to I2=39.7%, still indicating the superiority of the combination therapy compared to single-agent therapy. Why did the latest RCT study show higher sensitivity than other RCTs? In the clinical trial conducted by Wick et al in 2017, the PFS of combination therapy was significantly better than the single-agent therapy group. We suspect that this RCT study may be the only Phase III clinical trial which contained a larger sample size; other RCT studies were Phase II clinical trials. Improvements in drug production processes may also be a potential factor in improving efficacy.
Based on the baseline data from these RCTs, we found two key factors that probably influenced the results. One was whether bevacizumab was used as the single-agent therapy, the other one was whether more than 50% corticosteroid was used. We considered the two factors for subgroup analysis in our meta-analysis. We found that when bevacizumab was used in the single-agent therapy group, the AEs of the combined therapy group were insignificantly different from the single-agent group. Furthermore, the difference between the combination therapy and single-agent therapy groups became less obvious in ORR. It is not difficult to speculate that bevacizumab has a high contribution to ORR and AEs. When bevacizumab was not used in the single-agent therapy group, the difference between the two arms was significant, indicating that bevacizumab in the combination therapy group played a major role in treatment of recurrent glioblastoma. However, bevacizumab appears to be a double-edged sword that can bring about an increase in response rates and at the same time, raise the frequency of AEs. As Fine8 described in his article in the New England Journal of Medicine, although bevacizumab has its limitations, it is still an important therapeutic agent in the treatment of glioblastoma. In addition, in the corticosteroid used subgroup, we found that ORR and AEs of the combination therapy were not significantly different from single-agent therapy in the subgroup with corticosteroid use rate less than 50%. These results gave us insight that corticosteroid use may enhance the efficacy of combination therapy. Furthermore, corticosteroid use also increased the frequency of AEs, at the same time reducing the difference in PFS between the combination therapy and single-agent therapy groups. This finding showed that the use of corticosteroid may have the potential to shorten PFS in the combined treatment. In fact, this result was not surprising. Corticosteroid itself had the effect of reducing inflammation and brain edema, at the same time bringing about many AEs.25,26 In order to confirm this point of view, larger scale Phase III clinical trials and additional meta-analyses are needed.25
This meta-analysis has some advantages. The seven RCTs included were large-scale, multicenter, Phase II or III trials that were well-performed.13,15–20 The assessment of efficacy and safety of bevacizumab combination therapy of glioblastoma was highly reliable. In addition, we included sensitivity analysis and subgroup analysis to further explore the factors affecting the efficacy and safety of combination therapy.
The present meta-analysis still has several limitations that should be noted. First of all, we performed this analysis based on limited data. Only one of the seven RCTs was a Phase III clinical trial, which means that in order to obtain more conclusive results, we need more comprehensive, multicenter, large-sample randomized controlled clinical trials. Secondly, the chemotherapeutic drugs of combination therapy and single-agent therapy were not completely consistent. The dose and course of medication were not exactly the same. Lastly, there was a certain degree of heterogeneity in these RCTs’ data, and the results inevitably have a certain degree of bias. However, these seven RCT studies are still reliable, and our results will be helpful for physicians in making informed decisions regarding the treatment of glioblastoma.
Conclusion
The present meta-analysis indicated that bevacizumab combined with chemotherapy can significantly improve PFS and ORR, but did not prolong OS and can potentially lead to more incidents of AEs. In addition, bevacizumab may play a dominant role and corticosteroid may be an unfavorable factor in combination therapy as a treatment for recurrent glioblastoma. These conclusions provide concrete evidence for further research on bevacizumab combination therapy for recurrent glioblastoma.
Acknowledgments
We thank all the participants for their support of this research. In addition, we are particularly grateful for Weiwei Lin’s (from the University of Pittsburgh School of Pharmacy) assistance in the completion of the language modification process of this article.
This work was supported by the National Natural Science Foundation of China (no 81571115).
Author contributions
ZW was the principal investigator. ZQC and NX designed the study and developed the analysis plan. XW and TX analyzed the data and performed meta-analysis. ZQC and NX contributed to writing of the article. CSZ revised the manuscript and performed language polishing. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Burri SH, Gondi V, Brown PD, Mehta MP. The Evolving Role of Tumor Treating Fields in Managing Glioblastoma: Guide for Oncologists. Am J Clin Oncol. 2018;41(2):191–196. | ||
de Pascalis I, Morgante L, Pacioni S, et al. Endothelial trans-differentiation in glioblastoma recurring after radiotherapy. Mod Pathol. Epub 2018. | ||
Poulsen HS, Urup T, Michaelsen SR, Staberg M, Villingshøj M, Lassen U. The impact of bevacizumab treatment on survival and quality of life in newly diagnosed glioblastoma patients. Cancer Manag Res. 2014;6:373–387. | ||
Omuro A, Beal K, Mcneill K, et al. Multicenter Phase IB Trial of Carboxyamidotriazole Orotate and Temozolomide for Recurrent and Newly Diagnosed Glioblastoma and Other Anaplastic Gliomas. J Clin Oncol. 2018;36(17):1702–1709. | ||
Shergalis A, Bankhead A. 3rd, Luesakul U, Muangsin N, Neamati N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol Rev. 2018;70(3):412–445. | ||
Bronnimann C, Izquierdo C, Cartalat S, et al. Rechallenge with bevacizumab in patients with glioblastoma progressing off therapy. J Neurooncol. 2018;138(1):141–145. | ||
das S, Marsden PA. Angiogenesis in glioblastoma. N Engl J Med. 2013;369(16):1561–1563. | ||
Fine HA. Bevacizumab in glioblastoma--still much to learn. N Engl J Med. 2014;370(8):764–765. | ||
Hundsberger T, Reardon DA, Wen PY. Angiogenesis inhibitors in tackling recurrent glioblastoma. Expert Rev Anticancer Ther. 2017;17(6):507–515. | ||
Field KM, Jordan JT, Wen PY, Rosenthal MA, Reardon DA. Bevacizumab and glioblastoma: scientific review, newly reported updates, and ongoing controversies. Cancer. 2015;121(7):997–1007. | ||
Jakobsen JN, Hasselbalch B, Stockhausen MT, Lassen U, Poulsen HS. Irinotecan and bevacizumab in recurrent glioblastoma multiforme. Expert Opin Pharmacother. 2011;12(5):825–833. | ||
Olson JJ, Nayak L, Ormond DR, et al. The role of targeted therapies in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014;118(3):557–599. | ||
Wick W, Gorlia T, Bendszus M, et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N Engl J Med. 2017;377(20):1954–1963. | ||
Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. | ||
Balana C, de Las Penas R, Sepúlveda JM, et al. Bevacizumab and temozolomide versus temozolomide alone as neoadjuvant treatment in unresected glioblastoma: the GENOM 009 randomized phase II trial. J Neurooncol. 2016;127(3):569–579. | ||
Cloughesy T, Finocchiaro G, Belda-Iniesta C, et al. Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase II Study of Onartuzumab Plus Bevacizumab Versus Placebo Plus Bevacizumab in Patients With Recurrent Glioblastoma: Efficacy, Safety, and Hepatocyte Growth Factor and O6-Methylguanine-DNA Methyltransferase Biomarker Analyses. J Clin Oncol. 2017;35(3):343–351. | ||
Weathers SP, Han X, Liu DD, et al. A randomized phase II trial of standard dose bevacizumab versus low dose bevacizumab plus lomustine (CCNU) in adults with recurrent glioblastoma. J Neurooncol. 2016;129(3):487–494. | ||
Field KM, Simes J, Nowak AK, et al. Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol. 2015;17(11):1504–1513. | ||
Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. | ||
Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. | ||
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. | ||
Yang SB, Gao KD, Jiang T, Cheng SJ, Li WB, Wb L. Bevacizumab combined with chemotherapy for glioblastoma: a meta-analysis of randomized controlled trials. Oncotarget. 2017;8(34):57337–57344. | ||
Li Y, Hou M, Lu G, Ciccone N, Wang X, Zhang H. The Prognosis of Anti-Angiogenesis Treatments Combined with Standard Therapy for Newly Diagnosed Glioblastoma: A Meta-Analysis of Randomized Controlled Trials. PLoS One. 2016;11(12):e0168264. | ||
du C, Ren J, Zhang R, et al. Effect of Bevacizumab Plus Temozolomide-Radiotherapy for Newly Diagnosed Glioblastoma with Different MGMT Methylation Status: A Meta-Analysis of Clinical Trials. Med Sci Monit. 2016;22:3486–3492. | ||
Alkhalili K, Zenonos G, Fernandez-Miranda JC. Do Corticosteroids Compromise Survival in Glioblastoma? Neurosurgery. 2016;79(4):N15–N16. | ||
Pitter KL, Tamagno I, Alikhanyan K, et al. Corticosteroids compromise survival in glioblastoma. Brain. 2016;139(Pt 5):1458–1471. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009;6(6):e1000097. |
Supplementary materials
  | Figure S1 Progression-free survival sensitivity analysis showed that first trial (Wick et al1 2017) was highly sensitive and the remaining randomized controlled trials were within the CI. |
  | Figure S2 The pooled HR of the progression-free survival outcomes after exclusion of highly sensitive trial (Wick et al1 2017). Note: The diamond indicates the estimated HR (95% CI) for all patients together. |
  | Figure S3 Sensitivity analysis of overall survival showed that all of the consolidated results were stable. |
  | Figure S4 Sensitivity analysis of object response rate showed that all of the consolidated results were stable. |
  | Figure S5 Sensitivity analysis of adverse events (grade ≥3) showed that all of the consolidated results were stable. |
References
Wick W, Gorlia T, Bendszus M, et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N Engl J Med. 2017;377(20):1954–1963. | ||
Balana C, de Las Penas R, Sepúlveda JM, et al. Bevacizumab and temozolomide versus temozolomide alone as neoadjuvant treatment in unresected glioblastoma: the GENOM 009 randomized phase II trial. J Neurooncol. 2016;127(3):569–579. | ||
Cloughesy T, Finocchiaro G, Belda-Iniesta C, et al. Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase II Study of Onartuzumab Plus Bevacizumab Versus Placebo Plus Bevacizumab in Patients With Recurrent Glioblastoma: Efficacy, Safety, and Hepatocyte Growth Factor and O6-Methylguanine-DNA Methyltransferase Biomarker Analyses. J Clin Oncol. 2017;35(3):343–351. | ||
Weathers SP, Han X, Liu DD, et al. A randomized phase II trial of standard dose bevacizumab versus low dose bevacizumab plus lomustine (CCNU) in adults with recurrent glioblastoma. J Neurooncol. 2016;129(3):487–494. | ||
Field KM, Simes J, Nowak AK, et al. Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol. 2015;17(11):1504–1513. | ||
Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. | ||
Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



