Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Beta2-Microglobulin as Predictive Biomarkers in the Prognosis of Hepatocellular Carcinoma and Development of a New Nomogram
Authors Lin Q , Jiang Z, Mo D, Liu F, Qin Y , Liang Y, Cheng Y, Huang H, Fang M
Received 29 June 2023
Accepted for publication 4 October 2023
Published 11 October 2023 Volume 2023:10 Pages 1813—1825
DOI https://doi.org/10.2147/JHC.S425344
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Qiumei Lin,1,* Zongwei Jiang,1,* Dan Mo,2,* Fengfei Liu,1 Yuling Qin,1 Yihua Liang,1 Yuchen Cheng,3 Hao Huang,1 Min Fang1,4
1Department of Clinical Laboratory, Guangxi Medical University Cancer Hospital, Nanning, People’s Republic of China; 2Department of Breast, Guangxi Zhuang Autonomous Region Maternal and Child Health Care Hospital, Nanning, 530025, People’s Republic of China; 3Department of Clinical Laboratory, Wuzhou Maternal and Child Health-Care Hospital, Wuzhou, People’s Republic of China; 4Engineering Research Center for Tissue & Organ Injury and Repair Medicine, Guangxi Medical University Cancer Hospital, Nanning, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hao Huang; Min Fang, Email [email protected]; [email protected]
Background: Accurate prognosis is crucial for improving hepatocellular carcinoma (HCC) patients, clinical management, and outcomes post-liver resection. However, the lack of reliable prognostic indicators poses a significant challenge. This study aimed to develop a user-friendly nomogram to predict HCC patients’ post-resection prognosis.
Methods: We retrospectively analyzed the data from 1091 HCC patients, randomly split into training (n=767) and validation (n=324) cohorts. Receiver operating characteristic (ROC) curves determined the optimal cut-off value for alpha1-microglobulin (α 1MG) and Beta2-microglobulin (β 2MG). Kaplan–Meier analysis assessed microglobulin’s impact on survival, followed by Cox regression to identify prognostic factors and construct a nomogram. The predictive accuracy and discriminative ability of the nomogram were measured by the concordance index (C-index), calibration curves, area under the ROC curve (AUC), and decision curve analysis (DCA), and were compared with the BCLC staging system, Edmondson grade, or BCLC stage plus Edmondson grade.
Results: Patients with high β 2MG (≥ 2.395mg/L) had worse overall survival (OS). The nomogram integrated β 2MG, BCLC stage, Edmondson grade, microvascular invasion (MVI), and serum carbohydrate antigen 199 (CA199) levels. C-index for training and validation cohorts (0.712 and 0.709) outperformed the BCLC stage (0.660 and 0.657), Edmondson grade (0.579 and 0.564), and the combination of BCLC stage with Edmondson grade (0.681 and 0.668), improving prognosis prediction. Calibration curves demonstrated good agreement between predicted and observed survival. AUC values exceeded 0.700 over time, highlighting the nomogram’s discriminative ability. DCA revealed superior overall net income compared to other systems, emphasizing its clinical utility.
Conclusion: Our β 2MG-based nomogram accurately predicts HCC patients’ post-resection prognosis, aiding intervention and follow-up planning. Significantly, our nomogram surpasses existing prognostic indicators, including BCLC stage, Edmondson grade, and the combination of BCLC stage with Edmondson grade, by demonstrating superior predictive performance.
Keywords: hepatocellular carcinoma, nomogram, microglobulin, prognosis
Introduction
Hepatocellular carcinoma (HCC) is a widespread solid malignancy and the third leading cause of cancer-related deaths worldwide.1,2 Its estimated annual incidence is projected to exceed 1,000,000 cases by 2025.3 Resection is the most common cure for HCC worldwide, notably, according to the Chinese official guidelines and the clinical experience of our center and other country centers, intermediate and advanced-stage HCC patients could undergo resection, which is associated with good survival.4–7
However, the high recurrence and metastasis rate of HCC after operation is still a big challenge for patients’ survival, and the prognosis remains poor.8,9 The inherent heterogeneity of HCC poses significant challenges in accurately predicting prognoses and devising suitable interventions for affected individuals.10 Thus, it is imperative to construct a model identifying high-risk patients accurately for adjuvant therapy to improve overall survival (OS).
The Barcelona Clinical Liver Cancer (BCLC) staging system stands as the predominant clinical framework employed for prognosticating HCC outcomes.11 Nonetheless, within tertiary medical centers, a notable proportion of patients, up to 50%, veer away from the treatment recommendations outlined by the BCLC.12 Alpha-fetoprotein (AFP) is the most extensively used biomarker for determining the prognosis of HCC.13 Unfortunately, AFP is negative in approximately 30–40% of HCC patients.14,15 Therefore, there exists a critical need to identify a practical and reliable prognostic model that can offer more precise predictions of individual HCC patient outcomes.
Alpha1-microglobulin (α1MG) is a ubiquitous protein, that has reductase activity and the binding characteristics of free radicals and heme,16,17 but its direct involvement in tumor-related processes remains less explored. β2MG, as a member of the major histocompatibility complex class I (MHC-I) and a vital component of the human immune system, assumes a pivotal role in maintaining the physiological equilibrium of the body and exerting its influence on diverse processes occurring in the glomerulus and other nucleated cells.18,19 The role of β2MG in cancer is diverse, with effects primarily mediated through the immune system, as well as in cell proliferation, invasion and metastasis.20–22 The expression of β2MG increased in the progression of human prostate cancer, breast cancer, and renal cell carcinoma, bladder cancer.23–27 Additionally, it has been observed that an elevated serum β2MG level is not only a significant predictor of OS in patients with plasmacytoma, but also a marker of central nervous system metastasis in small cell lung cancer.28,29 However, there has been no reported evidence regarding the association between α1MG or β2MG and HCC to date.
This study reveals a strong relationship between increased serum β2MG levels and a poor prognosis in HCC patients for the first time. By incorporating β2MG and other readily accessible clinicopathological features, we successfully developed and validated a novel prognostic nomogram specifically tailored for HCC patients undergoing hepatectomy. This nomogram not only simplifies data acquisition but also exhibits robust predictive efficacy.
Materials and Methods
Population and Information Gathering
The study encompassed a period spanning from October 2013 to December 2021. 1976 patients diagnosed with HCC underwent curative resection at the Guangxi Medical University Cancer Hospital (Nanning, China). The following inclusion and exclusion standards were applied to select participants for this study. The inclusion standards for this study were as follows: (1) individuals who had not undergone any prior anti-cancer treatment, (2) no other concurrent malignancies, and (3) comprehensive laboratory, pathological, and follow-up data are available. On the other hand, the exclusion standards consisted of (1) individuals who had received relevant antitumor therapy such as chemotherapy or radiotherapy (n=125), (2) absence of a clear and definitive pathological diagnosis (n=68), (3) presence of malignant tumors other than hepatocellular carcinoma (HCC) (n=132), (4) cases of recurrent HCC (n=115), and (5) comprehensive laboratory, pathological, and follow-up data are not available (n=445). Finally, this study retrospectively covered 1091 patients in total.
Random assignment divided the patients into two groups: the training cohort (n = 767) and the validation cohort (n = 324). Data collection was by two independent investigators, QML and ZWJ, with validation by a third investigator, MD. General information like age and sex, serum AFP, CA199, α1MG and β2MG levels, and histopathological information like node number, differentiation, and vascular infiltration were all clinical factors that were gathered. The flow of the research program is depicted in Figure 1.
 |
Figure 1 The flow of study. Abbreviations: HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; β2MG, beta2-microglobulin; ROC, receiver operating characteristic. |
The Barcelona Clinic Liver Cancer (BCLC) staging system was used to determine the tumor stage.30,31 Microvascular invasion (MVI) is defined as the presence of nests of over 50 cells within the endothelial vessel lumen when examined microscopically.32
Follow-Up
Patients were followed up, either by phone or through an outpatient surveillance system, to determine their condition or the date of death if the patient had already died. Patients underwent regular monitoring at specific intervals following their surgery. The OS was determined by calculating the time between the surgical intervention and either the date of death or the date of the last follow-up. The last follow-up date of this study was in September 2022.
Statistical Analysis
The statistical analyses were performed using IBM SPSS Statistics software version 26.0 and R version 4.2.1. To determine the optimal cutoff values for α1MG and β2MG, we conducted receiver operating characteristic (ROC) curve analysis. To compare categorical variables between the training and validation cohorts, the chi-square test was utilized. Survival curves were plotted using the Kaplan-Meier method and compared using the Log rank test. In the subsequent multivariable Cox regression analysis, only variables with a significance level of p < 0.05 in the univariate analysis were included. Independent prognostic factors were determined based on their statistical significance (p < 0.05) in the multivariable Cox regression analysis. Based on the multivariable analysis-determined independent prognostic factors, we constructed a predictive nomogram using the rms package in R to predict individual OS at 1, 3, and 5 years. Calibration curves were produced to visually evaluate the congruence between the actual OS outcomes and the OS predictions derived from the nomogram. DCA analysis was performed via the R package “stdca.R”. The predictive accuracy and discriminative ability of the nomogram were assessed through various measures, including the C-index, ROC curves, decision curves, and DCA analysis, and compared with other systems. Statistical tests were conducted utilizing a two-sided approach, wherein a significance level of p < 0.05 denoted statistical significance.
Results
Patient Characteristics
Patients included in the analysis were randomly divided into two groups in a 7:3 ratio, with 767 from the training cohort and 324 from the validation cohort. The baseline characteristics of all patients recruited are summarized in Table 1. Most of the enrollees were male (n = 956; 87.63%), and 754 (69.11%) patients were aged ≥45 years old. No significant differences were observed between the training and validation cohorts concerning age, BCLC stage, liver cirrhosis, Edmondson grade, node number, MVI, CEA, AFP, α1MG, and β2MG (p = 0.062–0.897), except for sex and CA199 (p = 0.002–0.036).
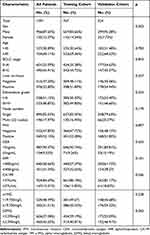 |
Table 1 Patient Demographics and Clinical Characteristics |
A Higher Concentration of Microglobulin is Related to the Poor Prognosis of HCC
In order to evaluate the prognostic value of microglobulin in HCC, we compared the survival differences among patients with different concentrations of microglobulin by drawing the Kaplan-Meier survival curve. The results showed that in the training cohort, the OS of the ɑ1MG≥19.750mg/L group was significantly shortened (p = 0.027, Figure 2A), and the β2MG≥2.395mg/L group was also significantly correlated with the poor overall survival (p < 0.001, Figure 2B). In the validation cohort, there was no significant difference in OS between the two groups of α1MG (p = 0.168, Figure 2C), and the β2MG≥2.395mg/L group was still significantly related to the poor OS (p = 0.012, Figure 2D). Kaplan -Meier survival curve of ɑ1MG showed different results in training and validation cohorts, which may be caused by insufficient samples in the validation cohort.
Univariate Analysis and Multivariate Analysis
Besides β2MG, the univariate Cox regression analysis also revealed that BCLC stage [p < 0.001, hazard ratio (HR)=2.862], Edmondson grade (p < 0.001, HR=1.751), node number (p = 0.001, HR=1.556), MVI (p < 0.001, HR=2.485), AFP (p < 0.001, HR=1.622), CA199 (p = 0.010, HR=1.451), α1MG (p = 0.028, HR=1.282), and β2MG (p < 0.001, HR=1.595) were all identified as risk factors for the OS of HCC patients. Furthermore, the multivariate Cox regression analysis confirmed that BCLC stage (p < 0.001, HR=2.185), Edmondson grade (p = 0.001, HR=1.477), MVI (p < 0.001, HR=1.906), CA199 (p = 0.015, HR=1.428), and β2MG (p = 0.001, HR=1.444) were independent risk factors for the OS of HCC patients. The study’s data underwent univariate and multivariate Cox regression analyses, and the results are presented in Table 2.
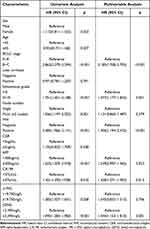 |
Table 2 Univariate and Multivariable Analysis for Overall Survival of the Training Cohort |
Prognostic Nomogram for OS
Based on multivariate Cox regression analysis in the training cohort, a nomogram was constructed for OS prediction and involved all independent prognostic factors that were mentioned above in the training cohort (Figure 3A) and validation cohort (Figure 3B). This nomogram aimed to predict the OS of HCC patients who underwent resection at 1, 3, and 5 years. The constructed model demonstrated a high level of accuracy in predicting the OS rate of HCC, as indicated by a C-index of 0.712 (95% CI, 0.698–0.726). The performance of the nomogram was evaluated using calibration curves and AUC values. Figure 4A and B display calibration curves for predicting 1-year, 3-year, and 5-year OS, indicating good calibration. In the training cohort, the area under the receiver operating characteristic curve (AUC) values for predicting 1-year, 3-year, and 5-year OS were 0.763, 0.732, and 0.710, respectively (Figure 4C). In the validation cohort, the corresponding AUC values for predicting 1-year, 3-year, and 5-year OS were 0.811, 0.714, and 0.711, respectively (Figure 4D). Both the calibration curves and ROC curves demonstrated effective prediction of the 1-, 3-, and 5-year OS rates. In conclusion, the nomogram’s accuracy, as indicated by the C-index and AUC values, suggests its potential as a useful tool in clinical practice for prognostic assessment in HCC patients.
The Decision Curve Analysis
The decision curve analysis was conducted to assess the net benefit of the models in predicting OS of HCC patients. Based on Figure 5A, it is clear that the nomogram model (represented by the red line) exhibited the highest net benefit when compared to the BCLC stage model (blue line). The higher position of the red line (nomogram) compared to the blue line (BCLC stage) indicates that the nomogram model offers a greater net benefit across a range of threshold probabilities. This suggests that the nomogram model is more effective in predicting OS and provides better decision-making support for treatment strategies in HCC patients. The results of the analysis indicated that the nomogram outperformed not only the BCLC stage model but also the Edmondson grade model and the combined BCLC stage and Edmondson grade model in predicting OS. In the validation cohort, this superiority was seen across a larger range of threshold probabilities (Figure 5B). The decision curves demonstrated the higher net clinical benefit associated with the nomogram, further supporting its superior predictive performance in determining the OS of HCC patients.
 |
Figure 5 Decision curve analysis for 5-year survival predictions. (A) The decision curve of the training cohort; (B) The decision curve of the validation cohort. |
Risk Stratification of OS
Cut-off values for total points were determined using the X-tile program to stratify patients in both the training and validation cohorts into three risk groups based on OS.33 The three risk categories were defined as follows: low-risk group (3.00–93.00 points), intermediate-risk group (96.00–125.00 points), and high-risk group (127.00–160.00 points). In the training cohort, the low-risk group had an OS rate of 80.4%, the intermediate-risk group had an OS rate of 50.4%, and the high-risk group had an OS rate of 21.3%. This difference in OS rates among the three risk groups was statistically significant (p < 0.001), as shown in Figure 6A. Similarly, in the validation cohort, the low-risk group had an OS rate of 72.5%, the intermediate-risk group had an OS rate of 49.7%, and the high-risk group had an OS rate of 20.8%. Again, this difference in OS rates among the risk groups was statistically significant (p < 0.001), as depicted in Figure 6B. In both the training and validation cohorts, the classification scheme based on total points accurately distinguished survival outcomes between the three risk categories. Furthermore, the risk stratification provided by this scheme holds significant value in predicting and monitoring the prognosis of patients with HCC.
 |
Figure 6 Survival graph for all three groups based on the predictor from the nomogram model. (A) Kaplan-Meier curve in the training cohort. (B) Kaplan-Meier curve in the validation cohort. |
Discussion
This study presents, for the very first time, a remarkable correlation between increased serum β2MG levels and an adverse prognosis in individuals diagnosed with HCC. Through the integration of β2MG and other easily obtainable clinicopathological characteristics, we have effectively constructed and validated a pioneering prognostic nomogram designed specifically for HCC patients undergoing hepatectomy. This nomogram not only streamlines data collection but also demonstrates exceptional predictive accuracy.
Currently, the prognostic value of serum β2MG has been extensively studied in various types of lymphoproliferative disorders, such as non-gastric mucosa-associated lymphoid tissue lymphoma,34 mucosa-associated lymphoid tissue lymphoma,35 extranodal natural killer/T-cell lymphoma,36,37 Hodgkin’s lymphoma,38 follicular lymphoma,39 diffuse large B-cell lymphoma,40,41 and multiple myeloma.42 The robustness of serum β2MG as a prognostic indicator has led to its incorporation into prognostic staging systems for multiple myeloma and follicular lymphoma.39,42 However, the prognostic value of serum β2MG in HCC has not been reported. Our study is the first to reveal a correlation between elevated serum β2MG levels and poor prognosis in HCC patients, suggesting that β2MG holds promise as a prognostic marker for HCC. β2MG plays a crucial role in tumor progression by exerting significant influence on the immune microenvironment, maintaining stem cell-like tumor populations, enhancing migration and invasion capabilities, promoting proliferation, and acting as a signaling and growth-promoting factor for bone metastasis.21–23,43 Although β2MG has been associated with human tumors, its significance in HCC has not been fully investigated. Therefore, further research is warranted to elucidate the precise mechanisms by which β2MG promotes HCC.
We constructed a nomogram based on β2MG, CA199, and clinical characteristics to predict the prognosis of HCC patients. Taking the BCLC stage, Edmondson grade, and the combination of the BCLC stage with Edmondson grade as comparatives, the nomogram exhibited a higher C-index (0.712) compared to the BCLC stage (0.660), Edmondson grade (0.579), and the combination of BCLC stage with Edmondson grade (0.681). The results suggested the nomogram had a superior predictive performance than the BCLC stage, Edmondson grade, and the combination of the BCLC stage with Edmondson grade. Such a superior predictive performance attributed to hurdle the limitations of the BCLC staging system, making the nomogram an effective tool for determining disease prognosis benefitting improving the OS.44 Moreover, we compared our nomogram with other similar models for HCC patients published in the previous literature. These models include a nomogram based on NLR (neutrophil to lymphocyte ratio), GMWG (the geometric mean of GGT and white WBC count), and tumor size with a C-index value of 0.70,45 a prognostic model developed by parameters of Aspartate Transaminase/Alanine Transaminase Ratio and clinical features with a C-index value of 0.680,46 and a prognostic model constructed by platelet count and clinical features with a C-index value of 0.660.47 Our model achieved a C-index of 0.712 (95% CI, 0.698–0.726), indicating higher predictive accuracy compared to these previously published models. In recent years, several scholars, including Long et al,48 Tang et al,49 and Chen et al,50 have developed prognostic nomograms for predicting OS in HCC patients using costly genomic data. However, it is worth noting that their respective nomogram models exhibited lower C-index values and incurred higher costs compared to our developed nomogram. Specifically, the C-index values reported in their studies were 0.69, 0.66, and 0.703, respectively, whereas our nomogram outperformed them in terms of predictive accuracy and discriminative ability. These results indicated that our predictive model based on β2MG was able to reliably predict the prognosis of patients with HCC after liver resection using inexpensive and easily accessible variables. Additionally, our strategy successfully divided HCC patients into high-risk, intermediate-risk, and low-risk groups, leading to personalized adjuvant therapy for high-risk patients to achieve improved survival outcomes.
In conclusion, the exceptional predictive performance above all indicated we successfully developed and validated a groundbreaking prognostic nomogram specifically designed for HCC patients undergoing liver resection by integrating β2MG, CA199, and pathological features. Furthermore, this study had the key advantage of introducing a novel prognostic indicator, ie, β2MG, for predicting HCC. Given the ease of obtaining β2MG and CA199 levels through routine preoperative laboratory tests, our nomogram had the potential for daily practice in the clinic, providing a useful tool for clinical decision-making and personalized adjuvant therapy management for HCC patients. However, our study also had some limitations. For example, due to its retrospective nature, selection bias was inevitable. Besides, our study only assessed the prognostic value of the nomogram for predicting OS in HCC patients and did not evaluate its impact on predicting disease-free survival (DFS). The combination of OS and DFS in the nomogram would have better clinical utility. Additionally, the lack of validation using an independent dataset poses a limitation to our study, as it hinders the confirmation of the external validity of our findings. Therefore, the prognostic nomogram we constructed will need to be validated in the future on a larger scale and in multicenters to be more consistent with clinical application.
Conclusion
In summary, our study not only establishes a significant association between elevated serum β2MG levels and an unfavorable prognosis in HCC patients but also presents a novel prognostic nomogram tailored specifically for HCC patients undergoing hepatectomy. This nomogram not only simplifies data acquisition but also demonstrates exceptional predictive efficacy. Our findings hold substantial promise for advancing clinical practice and optimizing patient care in the field of HCC management. However, the mechanism by which β2MG promotes the progression of HCC and multi-center validation still requires further research.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the author Qiumei Lin (Email: [email protected]) upon reasonable request.
Ethics Statement
This research was approved by the Medical Ethics Committee of Guangxi Medical University Cancer Hospital. (LW2023088) and conducted following the ethical principles outlined in the Helsinki Declaration of 1964 and its subsequent amendments, or other ethical standards with equivalent requirements. To ensure patient confidentiality, the identities of the individuals included in this study were anonymized using computer-generated ID numbers, and thus, patient consent was waived.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study received funding from various sources, including the National Science Foundation of China (81760530), the National Science Foundation of Guangxi (2022GXNSFAA035510), and the Postdoctoral Science Foundation of China (2021M693803).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yang C, Zhang H, Zhang L, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023;20(4):203–222.
2. Lee HM, Lidofsky SD, Taddei TH, Townshend-Bulson LJ. Attacking the public health crisis of hepatocellular carcinoma at its roots. Hepatology. 2023;77(4):1456–1459. doi:10.1002/hep.32741
3. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat RevDis Prim. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
4. Kim H, Ahn SW, Hong SK, et al. Survival benefit of liver resection for Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma. Br J Surg. 2017;104(8):1045–1052. doi:10.1002/bjs.10541
5. Zhong JH, Lu SD, Wang YY, Ma L, Li LQ. Intermediate-stage HCC--upfront resection can be feasible. Nat Rev Clin Oncol. 2015;12(5):295. doi:10.1038/nrclinonc.2014.122-c3
6. Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260(2):329–340. doi:10.1097/SLA.0000000000000236
7. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
8. Nakayama H, Okamura Y, Higaki T, Moriguchi M, Takayama T. Effect of blood product transfusion on the prognosis of patients undergoing hepatectomy for hepatocellular carcinoma: a propensity score matching analysis. J Gastroenterol. 2023;58(2):171–181. doi:10.1007/s00535-022-01946-9
9. Jeng LB, Li TC, Wang J, Teng CF. Increased plasma levels of monocyte chemoattractant protein-1 in patients with hepatitis B virus pre-S2 gene deletion mutation predict a higher risk of hepatocellular carcinoma recurrence after curative surgical resection. Cancer. 2023;129(17):2621–2636. doi:10.1002/cncr.34815
10. Lin WP, Xing KL, Fu JC, et al. Development and validation of a model including distinct vascular patterns to estimate survival in hepatocellular carcinoma. JAMA network open. 2021;4(9):e2125055. doi:10.1001/jamanetworkopen.2021.25055
11. He Z, She X, Liu Z, et al. Advances in post-operative prognostic models for hepatocellular carcinoma. J Zhejiang Univ Sci B. 2023;24(3):191–206. doi:10.1631/jzus.B2200067
12. Golfieri R, Bargellini I, Spreafico C, Trevisani F. Patients with Barcelona clinic liver cancer stages B and C hepatocellular carcinoma: time for a subclassification. Liver Cancer. 2019;8(2):78–91. doi:10.1159/000489791
13. Dupuy M, Iltache S, Rivière B, et al. Plasma hPG(80) (Circulating Progastrin) as a novel prognostic biomarker for hepatocellular carcinoma. Cancers. 2022;14(2):402. doi:10.3390/cancers14020402
14. Liu L, Wang Q, Zhao X, et al. Establishment and validation of nomogram model for the diagnosis of AFP-negative hepatocellular carcinoma. Front Oncol. 2023;13:1131892. doi:10.3389/fonc.2023.1131892
15. Huang C, Fang M, Feng H, et al. N-glycan fingerprint predicts alpha-fetoprotein negative hepatocellular carcinoma: a large-scale multicenter study. Int J Cancer. 2021;149(3):717–727. doi:10.1002/ijc.33564
16. Kristiansson A, Davidsson S, Johansson ME, et al. α(1)-Microglobulin (A1M) protects human proximal tubule epithelial cells from heme-induced damage in vitro. Int J Mol Sci. 2020;21(16):5825. doi:10.3390/ijms21165825
17. Bergwik J, Kristiansson A, Welinder C, et al. Knockout of the radical scavenger α(1)-microglobulin in mice results in defective bikunin synthesis, endoplasmic reticulum stress and increased body weight. Free Radic Biol Med. 2021;162:160–170. doi:10.1016/j.freeradbiomed.2020.02.019
18. Gao Y, Hong Y, Huang L, et al. β2-microglobulin functions as an endogenous NMDAR antagonist to impair synaptic function. Cell. 2023;186(5):1026–1038.e1020. doi:10.1016/j.cell.2023.01.021
19. Sivanathan PC, Ooi KS, Mohammad Haniff MAS, et al. Lifting the veil: characteristics, clinical significance, and application of β-2-microglobulin as biomarkers and its detection with biosensors. ACS Biomater Sci Eng. 2022;8(8):3142–3161. doi:10.1021/acsbiomaterials.2c00036
20. Josson S, Nomura T, Lin JT, et al. β2-microglobulin induces epithelial to mesenchymal transition and confers cancer lethality and bone metastasis in human cancer cells. Cancer Res. 2011;71(7):2600–2610. doi:10.1158/0008-5472.CAN-10-3382
21. Huang WC, Wu D, Xie Z, et al. beta2-microglobulin is a signaling and growth-promoting factor for human prostate cancer bone metastasis. Cancer Res. 2006;66(18):9108–9116. doi:10.1158/0008-5472.CAN-06-1996
22. Li D, Zhang Q, Li L, et al. β2-microglobulin maintains glioblastoma stem cells and induces M2-like polarization of tumor-associated macrophages. Cancer Res. 2022;82(18):3321–3334. doi:10.1158/0008-5472.CAN-22-0507
23. Huang WC, Havel JJ, Zhau HE, et al. Beta2-microglobulin signaling blockade inhibited androgen receptor axis and caused apoptosis in human prostate cancer cells. Clin Cancer Res. 2008;14(17):5341–5347. doi:10.1158/1078-0432.CCR-08-0793
24. Bunning RA, Haworth SL, Cooper EH. Serum beta-2-microglobulin levels in urological cancer. J Urol. 1979;121(5):624–625. doi:10.1016/S0022-5347(17)56910-4
25. Nomura T, Huang WC, Zhau HE, et al. Beta2-microglobulin promotes the growth of human renal cell carcinoma through the activation of the protein kinase A, cyclic AMP-responsive element-binding protein, and vascular endothelial growth factor axis. Clin Cancer Res. 2006;12(24):7294–7305. doi:10.1158/1078-0432.CCR-06-2060
26. Li K, Du H, Lian X, et al. Characterization of β2-microglobulin expression in different types of breast cancer. BMC Cancer. 2014;14(1):750. doi:10.1186/1471-2407-14-750
27. Chai D, Li K, Du H, et al. β2-microglobulin has a different regulatory molecular mechanism between ER(+) and ER(-) breast cancer with HER2(). BMC Cancer. 2019;19(1):223. doi:10.1186/s12885-019-5410-1
28. Jung SH, Kim K, Yoon SE, et al. Validation of the revised diagnostic criteria for primary plasma cell leukemia by the Korean Multiple Myeloma Working Party. Blood Cancer J. 2022;12(11):157. doi:10.1038/s41408-022-00755-w
29. Pedersen AG, Bach FW, Nissen M, Bach F. Creatine kinase BB and beta-2-microglobulin as markers of CNS metastases in patients with small-cell lung cancer. J Clin Oncol. 1985;3(10):1364–1372. doi:10.1200/JCO.1985.3.10.1364
30. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
31. EAftSot L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
32. Cong WM, Bu H, Chen J, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22(42):9279–9287. doi:10.3748/wjg.v22.i42.9279
33. Zhou Y, Tang L, Chen Y, Zhang Y, Zhuang W. An immune panel signature predicts prognosis of lung adenocarcinoma patients and correlates with immune microenvironment. Front Cell Dev Biol. 2021;9:797984. doi:10.3389/fcell.2021.797984
34. Yoo C, Yoon DH, Yoon S, et al. Prognostic impact of β2-microglobulin in patients with non-gastric mucosa-associated lymphoid tissue lymphoma. Leuk Lymphoma. 2015;56(3):688–693. doi:10.3109/10428194.2014.917640
35. Udzura M, Kobayashi H, Taguchi Y, Sekino H. Intrasellar intercarotid communicating artery associated with agenesis of the right internal carotid artery: case report. Neurosurgery. 1988;23(6):770–773. doi:10.1227/00006123-198812000-00019
36. Takeuchi M, Miyoshi H, Asano N, et al. Human leukocyte antigen class II expression is a good prognostic factor in adult T-cell leukemia/lymphoma. Haematologica. 2019;104(8):1626–1632. doi:10.3324/haematol.2018.205567
37. Li H, Shao G, Zhang Y, et al. Nomograms based on SUVmax of (18)F-FDG PET/CT and clinical parameters for predicting progression-free and overall survival in patients with newly diagnosed extranodal natural killer/T-cell lymphoma. Cancer Imaging. 2021;21(1):9. doi:10.1186/s40644-020-00379-y
38. Vassilakopoulos TP, Nadali G, Angelopoulou MK, et al. The prognostic significance of beta(2)-microglobulin in patients with Hodgkin’s lymphoma. Haematologica. 2002;87(7):701–708; discussion 708.
39. Federico M, Bellei M, Marcheselli L, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27(27):4555–4562. doi:10.1200/JCO.2008.21.3991
40. Chen Y, Neelapu S, Feng L, et al. Prognostic significance of baseline peripheral absolute neutrophil, monocyte and serum β2-microglobulin level in patients with diffuse large b-cell lymphoma: a new prognostic model. Br J Haematol. 2016;175(2):290–299. doi:10.1111/bjh.14237
41. López-Guillermo A, Colomo L, Jiménez M, et al. Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J Clin Oncol. 2005;23(12):2797–2804. doi:10.1200/JCO.2005.07.155
42. Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33(26):2863–2869. doi:10.1200/JCO.2015.61.2267
43. Hofbauer D, Mougiakakos D, Broggini L, et al. β(2)-microglobulin triggers NLRP3 inflammasome activation in tumor-associated macrophages to promote multiple myeloma progression. Immunity. 2021;54(8):1772–1787.e1779. doi:10.1016/j.immuni.2021.07.002
44. Erstad DJ, Tanabe KK. Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma. Ann Surg Oncol. 2019;26(5):1474–1493. doi:10.1245/s10434-019-07227-9
45. Ren L, Chen D, Xu W, et al. Predictive potential of Nomogram based on GMWG for patients with hepatocellular carcinoma after radical resection. BMC Cancer. 2021;21(1):817. doi:10.1186/s12885-021-08565-2
46. Mo Q, Liu Y, Zhou Z, et al. Prognostic value of aspartate transaminase/alanine transaminase ratio in patients with hepatitis B virus-related hepatocellular carcinoma undergoing hepatectomy. Front Oncol. 2022;12:876900. doi:10.3389/fonc.2022.876900
47. Shim JH, Jun MJ, Han S, et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg. 2015;261(5):939–946. doi:10.1097/SLA.0000000000000747
48. Long J, Zhang L, Wan X, et al. A four-gene-based prognostic model predicts overall survival in patients with hepatocellular carcinoma. J Cell Mol Med. 2018;22(12):5928–5938. doi:10.1111/jcmm.13863
49. Tang B, Zhu J, Li J, et al. The ferroptosis and iron-metabolism signature robustly predicts clinical diagnosis, prognosis and immune microenvironment for hepatocellular carcinoma. Cell Commun Signal. 2020;18(1):174. doi:10.1186/s12964-020-00663-1
50. Chen B, Yang Z, Lang Z, et al. M6A-related lncRNAs predict clinical outcome and regulate the tumor immune microenvironment in hepatocellular carcinoma. BMC Cancer. 2022;22(1):867. doi:10.1186/s12885-022-09925-2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



