Back to Journals » OncoTargets and Therapy » Volume 11
β-Catenin-driven adrenocortical carcinoma is characterized with immune exclusion
Authors Liu S , Ding G, Zhou Z, Feng C
Received 15 December 2017
Accepted for publication 1 March 2018
Published 9 April 2018 Volume 2018:11 Pages 2029—2036
DOI https://doi.org/10.2147/OTT.S159979
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Carlos E Vigil
Shenghua Liu,1,2,* Guanxiong Ding,1,2,* Zhongwen Zhou,1,2 Chenchen Feng1,2
1Department of Urology, Huashan Hospital, Shanghai, China; 2Fudan Institute of Urology, Shanghai, China
*These authors contributed equally to this work
Aim: Adrenocortical carcinoma (ACC) is characterized by overexpressed CTNNB1, which is reported to modulate immune exclusion. Cross talk between CTNNB1 and cancer immunity in ACC remains unclear.
Materials and methods: In silico reproduction of TCGA-ACC dataset (N = 92) and external validation using tissue samples were performed (N = 16). Expression data of CTNNB1, PD-1, and PD-L1 were extracted in silico and tumor-infiltrating lymphocytes (TILs) were profiled using code provided by Tumor IMmune Estimation Resource (TIMER). In-house formalin-fixed paraffin-embedded ACC samples were processed using immunohistochemical (IHC) staining for CTNNB1, CD45, PD-1, and PD-L1.
Results: Increased CTNNB1 expression was significantly associated with worsened overall survival (OS) (P = 0.006). CD8+ cells were significantly associated with better OS (P = 0.02). Higher PD-L1 (P = 0.019), but not PD-1 expression (P = 0.325), was associated with better OS. CTNNB1 overexpression was significantly associated with increased tumor purity (r = 0.356, P = 0.002) and fewer TILs (r = -0.833, P = 0.029), decreased infiltrating CD8+ cells (P = 0.033), and increased infiltrating B cells (P = 0.026). CTNNB1 expression was negatively correlated with PD-L1 expression (r = -0.308, P = 0.006) but not with PD-1 expression (P = 0.067), which were externally validated (P = 0.032 for PD-L1 and P = 0.400 for PD-1). The Cox regression model encompassing gender, B cells, CD8+ cells, PD-L1, CTNNB1, and Ki-67 revealed that only Ki-67 overexpression remained significantly associated with OS (P < 0.001), while CTNNB1 showed marginal significance (P = 0.06). CTNNB1-overexpressed patients were more likely to have cortisol excess (P = 0.003).
Conclusion: ACC with CTNNB1 overexpression is associated with poor prognosis and decreased immunity. Our findings suggest that CTNNB1-targeting therapy may overcome immune exclusion in ACC.
Keywords: adrenocortical carcinoma, CTNNB1, tumor-infiltrating lymphocyte, PD-L1, prognosis
Introduction
Adrenocortical carcinoma (ACC) is a rare and aggressive disease. Though curable at its early stage by surgery, there is a dearth of definitive treatment for advanced or metastatic disease.1 Unfortunately, with the absence of specific cancer-related early symptoms, about 70% of patients are diagnosed with stage III or IV diseases. Driving genetic alterations have recently been identified owing to the second-generation sequencing technique, showing driver genes including CTNNB1, TP53, CDKN2A, RB1, MEN1, and newly identified PRKAR1A, RPL22, TERF2, and CCNE1.2,3
CTNNB1 encodes β-catenin, an essential component of the canonical Wnt/β-catenin pathway, which plays an important role in a variety of biological processes that involve cell growth and differentiation, including but not limited to cell–cell adhesion, development, and cardiac physiology.4–6 Such biological advantage can also be taken in case of cancer. It has been estimated that ~10% of all tissue samples sequenced from all cancers display mutations in the CTNNB1 gene.7 Alterations such as gain or loss of functional mutation at different sites of the gene, as well as overexpression, result toward enhanced and/or increased gene product that vitalizes cancer cells. As one of the driver genes in ACC, CTNNB1 mutation is reported to be present in up to 70% of cases and to exert pro-tumorigenic function via activation of Wnt/β-catenin pathway.8
Recent development in immune checkpoint inhibitors (ICIs) appears promising, despite moderate response rate. Of note, anti-PD-1/PD-L1 treatment has been proven efficacious in several types of solid and hematological tumors, including melanoma of the skin, non-small-cell lung cancer, kidney cancer, bladder cancer, head and neck cancers, and Hodgkin’s lymphoma.9 Five PD-1/PD-L1 blockade agents and one anti-CTLA4 agent are now US FDA approved and are being tested further in more types of cancer for safety and efficacy. Identifying more cancer types that can potentially benefit from ICI and developing sensitive and specific biomarkers for potential responder are the two current hotspots for research work.
Our previous findings shed light on a possible immune-modulating role of CTNNB1 in ACC.10,11 In the current study, we aim to evaluate association between CTNNB1 expression and cancer immunity in silico followed by external validation. Our findings may not only complement the current understanding of biology of ACC but also contribute to development of novel targeted and immune therapies for ACC.
Materials and methods
Data mining of TCGA-ACC dataset
The TCGA ACC (Provisional) dataset was selected on the cBioportal online platform. The CTNNB1 mRNA expression level of all cases was queried. The clinical information including age, gender, tumor stage, excessive hormone status, survival period, and follow-up time was extracted. To obtain the immunity profile of TCGA-ACC dataset, an online analytical tool named “Tumor IMmune Estimation Resource (TIMER)” was used. TIMER is a web resource for systematical evaluations of the clinical impact of different immune cells in diverse cancer types.12 The immune estimate of TCGA-ACC dataset was obtained and plotted from TIMER platform. The gene module on TIMER provided the correlation of selected gene expression with various immune infiltration levels. The survival module illustrated the Kaplan–Meier curve and provided the Cox regression analysis between overall survival (OS) and clinical factors, immune infiltrates, or gene expression level.
Patients and ACC specimen
Sixteen tumor samples from ACC patients, who had undergone surgical resection between January 2009 and August 2017, were included. HE-stained sections were reviewed by an independent pathologist for confirmation. Clinicopathological parameters such as age, gender, stage, metastasis status, and Ki-67 score were extracted from the patient medical records and pathological reports. Endocrine activity was not included as over half of the cases were incidentalomas and diagnosis of ACC was made only after resection. Survival data were not collected as over half of the patients were lost to follow-up. Signed informed consent was obtained from each patient whose sample was analyzed in the current study and the study protocol was approved by the local ethical committee (Huashan Institutional Review Board).
Immunostaining and pathological evaluation
A standard immunohistochemical (IHC) protocol was followed and 2 μm unstained tumor sections from formalin-fixed paraffin-embedded tissue were deparaffinized in xylene and rehydrated through graded alcohols. After antigen retrieval, the sections were stained with primary antibodies, PD-L1 (28-9; Abcam; dilution: 1:200), CD45 (EP322Y; Abcam; dilution: 1:250), and β-catenin (E247; Abcam; dilution: 1:250), followed by antibody localization using the Dako Envision + HRP-labeled polymer (DAKO). Staining was visualized by 5-minute incubation with diaminobenzidine.
Positivity for PD-L1 and PD-1 was defined as positive staining in both tumor cells and tumor-infiltrating lymphocytes (TILs). Expression of PD-L1 on tumor cell was scored as 0 (absence of staining), 1 (1%–10% of staining), 2 (10%–50% of staining), and 3 (>50% of staining). The extent of staining in TILs was recorded as absent (0), focal (1), mild (2), moderate (3), and strong (4) with score 0 or 1 considered negative. IHC expression of β-catenin in tumor cell was evaluated and scored as percentage of cytoplasmic and/or nuclear positivity multiplied by intensity (weak, 1; moderate, 2; and strong, 3), as described previously. All sections were reviewed by two independent pathologists until consensus was made.
Statistical analysis
The chi-square test was used to compare categorical variables. The Student’s t-test and Mann–Whitney tests were used for continuous parametric variables. The Spearman’s correlation coefficient was applied to measure the correlation between two groups. The Cox regression model was used to test the contribution of individual factor to survival in a multivariate analysis. The P-value of <0.05 was accepted as statistically significant.
Results
Patient characteristics
The TCGA-ACC cohort included 92 cases. For comparison of the CTNNB1 expression in patients with different clinicopathological parameters, we studied distribution of the cases within top and bottom 30% of CTNNB1 expression. Demographic and clinicopathological parameters of those patients are summarized in Table 1. Female patients and hormone excess were associated with higher CTNNB1 expression. Demographic and clinicopathological parameters of the 16 ACC patients in the validation cohort are summarized in Table 2. Expression of CTNNB1 was not associated with any parameter in this cohort.
Prognostic role of CTNNB1, TILs, and PD-1/PD-L1 in ACC
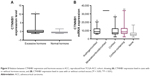
Overactive canonical WNT signaling featuring gain-of-function alteration in CTNNB1 has been identified as one of the driver genetic events in ACC. When CTNNB1 expression level was divided into top 30% versus lower 30%, we found that higher CTNNB1 expression was significantly associated with worsened OS (Figure 1A). Accordingly, increased infiltrating CD8+ cells (top 30% vs bottom 30%) was significantly associated with improved prognosis (Figure 1B). We then explored whether PD-L1 expression was associated with survival in TCGA cohort and found that higher PD-L1 expression was significantly associated with improved survival (Figure 1C), while PD-1 expression was not (Figure 1D).
In order to elucidate the independent impact of CTNNB1 expression on survival, we included factors unevenly distributed among patients with different CTNNB1 expressions (Table 3). We found that Ki-67, the only clinically validated prognostic marker, remained the only significant factor in the multivariate model, leaving marginal significance for CTNNB1 expression. Effects of CD8+ cells, B cells, or gender on survival was lost in the Cox model (Table 3).
  | Table 3 Cox regression analysis of factors contributing to overall survival |
Association of CTNNB1 with cancer immunity in ACC
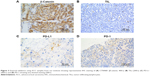
Cancer-intrinsic β-catenin has been shown to play a role in immune modulation.13 We therefore hypothesized that immunity in ACC could be linked to CTNNB1 expression. We analyzed correlation between CTNNB1 expression and TIL abundance indicated by decreased tumor purity. We found that higher CTNNB1 expression was significantly associated with increased purity (Figure 2A). Cases with higher CTNNB1 expression showed significantly decreased infiltration of CD8+ cells (Figure 2B, left panel) and increased infiltration of B cells (Figure 2B, right panel). CTNNB1 expression was also negatively correlated with decreased PD-L1 level and was not correlated with PD-1 expression (Figure 2C). Expressions of CTNNB1 and Ki-67 were not correlated in the TCGA cohort, nor was the correlation observed in our validation cohort (Figure 2D, Table 2). Owing to the rarity of the disease, we collected 16 samples of ACC as validation cohort. Using continuous IHC expression (Figure 3A–D), we validated that CTNNB1 expression was significantly associated with decreased PD-L1 expression (r = −0.752, P = 0.002) and was not associated with PD-1 expression (P = 0.302). Indeed, PD-1 was barely expressed in ACC (Figure 3D).
CTNNB1 and PD-1/PD-L1 in ACC
As higher PD-L1 was associated with worsened prognosis in many cancers, we hypothesized that our contradictory findings were due to increased TILs with concomitant increased PD-L1 expression in ACC. Of note, increased purity was significantly associated with decreased PD-L1 expression (Figure 4A). Specifically, PD-L1 expression was associated with increased CD8+ cells, neutrophils, and B cells (Figure 4B–D). In our validation cohort, we found that CTNNB1 expression was significantly correlated with decreased TILs (r = −0.650, P = 0.006). Also, TIL abundance was significantly associated with PD-L1 (r = −0.635, P = 0.015) but not with PD-1 expression (P = 0.303). Nonetheless, we used a combined scoring system including PD-L1 positivity in both tumor cells and TILs, and we observed majority of the tumor cells expressed PD-L1 (Figure 4B).
CTNNB1 was associated with increased cortisol level
Over 50% of ACC cases were characterized with hormone excess. There is a dearth of study showing association between CTNNB1 and hormone status. Given the aforementioned findings, we hypothesized that decreased TILs in ACC with higher CTNNB1 expression could result from excessive hormone levels, especially cortisol. We found that cases with excessive hormone demonstrated significantly higher CTNNB1 expression (Figure 5A). In the analyses with breakdown of hormone types, we found that excessive cortisol-containing cases showed significantly higher CTNNB1 expression (Figure 5B). Cases with excessive hormone types other than cortisol demonstrated CTNNB1 expression level not significantly different from cases without (“None” in Figure 5B, data not shown). There were three patients in our validation cohort who exhibited excessive cortisol and data for the rest were missing.
Discussion
While the prognostic role of CTNNB1 expression in ACC has been reported in several studies,14,15 its role in cancer immune modulation remains unstudied. We have here demonstrated that higher CTNNB1 expression is not only associated with poor OS but also with impaired immune response, both revealed in silico and in our in-house validation cohort. Previous study has demonstrated that PD-L1 positivity in either tumor cell membrane or TIL is not significantly associated with higher stage at diagnosis, higher tumor grade, excessive hormone secretion, or OS in ACC.16 The discrepancy with our findings could result from the dichotomized designation for positivity therein.
We suggest that the most important finding in the current study is the negative association between CTNNB1 and TIL counts, including decreased infiltration of CD8+ cells. This possibly puts ACC in a newly recognized cancer entity in which the immune escape is β-catenin-driven. While Wnt/β-catenin pathway plays an important role in T-cell immunity, the regulatory mechanism has long been studied solely within T cell itself.17 How cancer-intrinsic β-catenin is involved in immune exclusion is a newfound that has recently come into view.18 Spranger et al have presented the first evidence of an inverse relationship between melanoma intrinsic β-catenin signaling and intratumoral T-cell infiltration and have used autochthonous mouse models to prove the causal effect by CTNNB1.13,19 Despite the rapid development of ICIs, only a limited percentage (<~40%) of patients with a certain type of cancer respond to the therapy and there are certain tumors that do not even respond at all. Understanding the mechanisms leading to a non-T-cell-inflamed microenvironment are crucial for the development of novel treatment modalities to expand the fraction of patients benefiting from immunotherapy. Here we have for the first time revealed this correlation in both TCGA and external validation cohorts in ACC. The findings not only hold promise to optimize ICI in ACC but can also be extrapolated to other cancers which are β-catenin-driven.
ACC is also characterized with hormone excess. Our findings indicate that CTNNB1 overexpression is associated with excessive cortisol, the most common type of hormone excess. We, however, did not include the hormone status in the Cox model, considering that mixed hormonal types could confound the interpretation. How CTNNB1 is associated with hormone excess, in particular cortisol excess, warrants further study.
CD8+ T cells play critical role in cancer immunity. Brownlie et al has reported resistance to TGF-β suppression and improved antitumor responses in CD8+ T cells lacking PTPN22.20 De Meulenaere et al has reported that CD8+ T lymphocytes constitute an independent prognostic marker in patients diagnosed with oropharyngeal squamous cell carcinoma.21 In our study, CD8+ cells are significantly associated with improved OS. Also, lower CD8+ counts are identified in cases with higher CTNNB1 expression. Both findings support the anticancer role of CD8+ cells in ACC.
Another intriguing finding in the current study is increased B-cell counts in ACC with higher CTNNB1 expression. Recent studies suggest that B cells likely play a dual role in cancer immunity.22 Distinct subsets of B cells dynamically help shape the tumor microenvironment in both a pro- and antitumorigenic manner. On one hand, B cells exert pro-tumorigenic effect via contributing to an angiogenic and pro-inflammatory microenvironment, and by directly or indirectly suppressing T-cell activation.23 On the other hand, B cells exert anticancer effect via production of cytokines coordinating other immune cells, particularly through enhancement of cytotoxic T-cell activity.24 The sophisticated coordination between TIL subtypes can be reflected in our finding that B cells are increased in CTNNB1 overexpressed cases while not contributing to prognosis. Though it appears that B cells exert pro-tumorigenic role in ACC, our results solely serve as an observation in a cross-sectional perspective.
The implication of our findings lies in two aspects. First, we have provided clue for a pro-tumorigenic pathway in ACC with CTNNB1 activation. How CTNNB1 is mechanistically associated with immune escape warrants further elaboration. Second, association between CTNNB1 and immunity supports application of WNT inhibitor in ACC; for instance, dual inhibition of CTNNB1 and PD-L1 using a novel compound BBI-801, which is well tolerated with no signs of toxicity observed.25 Our study has limitations. First, the in silico reproduction should be validated in a larger cohort. However, as a rare disease, multicenter involvement is required for validation with enough power. Second, the heterogeneous detection method for CTNNB1 and PD-L1 in TCGA, our cohort, and other reports makes it biased for horizontal comparison of the results. In vitro studies also provide limited translational merit as autochthonous mouse model for ACC is lacking. In a rare and lethal disease like ACC, perhaps only patients enrolling the trial could answer the question.
Acknowledgment
This study was supported in part by the National Natural Science Foundation of China (Grant No. 81502189).
Disclosure
The authors report no conflicts of interest in this work.
References
Xu Y, Qi Y, Zhu Y, Ning G, Huang Y. Molecular markers and targeted therapies for adrenocortical carcinoma. Clin Endocrinol (Oxf). 2014;80(2):159–168. | ||
Zheng S, Cherniack AD, Dewal N, et al. Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell. 2016;30(2):363. | ||
Assie G, Letouze E, Fassnacht M, et al. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet. 2014;46(6):607–612. | ||
Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16(1):51–59. | ||
Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121(11):3529–3537. | ||
Hertig CM, Butz S, Koch S, Eppenberger-Eberhardt M, Kemler R, Eppenberger HM. N-cadherin in adult rat cardiomyocytes in culture. II. Spatio-temporal appearance of proteins involved in cell-cell contact and communication. Formation of two distinct N-cadherin/catenin complexes. J Cell Sci. 1996;109(Pt 1):11–20. | ||
Forbes SA, Bindal N, Bamford S, et al. COSMIC: mining complete cancer genomes in the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2011;39(Database issue):D945–D950. | ||
Bonnet S, Gaujoux S, Launay P, et al. Wnt/beta-catenin pathway activation in adrenocortical adenomas is frequently due to somatic CTNNB1-activating mutations, which are associated with larger and nonsecreting tumors: a study in cortisol-secreting and -nonsecreting tumors. J Clin Endocrinol Metab. 2011;96(2):E419–E426. | ||
Kythreotou A, Siddique A, Mauri FA, Bower M, Pinato DJ. Pd-L1. J Clin Pathol. 2018;71(3):189–194. | ||
Guo T, Feng C. Pembrolizumab for advanced urothelial carcinoma. N Engl J Med. 2017;376(23):2303–2304. | ||
Feng C, Chen S. MP37-02 NUTLIN-3A as a novel anticancer agent for adrenocortical carcinoma with CTNNB1 mutation. J Urology. 2017;197(4):e475. | ||
Li B, Severson E, Pignon JC, et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17(1):174. | ||
Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–235. | ||
Rubin B, Regazzo D, Redaelli M, et al. Investigation of N-cadherin/beta-catenin expression in adrenocortical tumors. Tumour Biol. 2016;37(10):13545–13555. | ||
Gaujoux S, Grabar S, Fassnacht M, et al. Beta-catenin activation is associated with specific clinical and pathologic characteristics and a poor outcome in adrenocortical carcinoma. Clin Cancer Res. 2011;17(2):328–336. | ||
Fay AP, Signoretti S, Callea M, et al. Programmed death ligand-1 expression in adrenocortical carcinoma: an exploratory biomarker study. J Immunother Cancer. 2015;3:3. | ||
Lu YC, Robbins PF. Cancer immunotherapy targeting neoantigens. Semin Immunol. 2016;28(1):22–27. | ||
Spranger S, Gajewski TF. A new paradigm for tumor immune escape: beta-catenin-driven immune exclusion. J Immunother Cancer. 2015;3:43. | ||
Hu-Lieskovan S, Homet Moreno B, Ribas A. Excluding T cells: is beta-catenin the full story? Cancer Cell. 2015;27(6):749–750. | ||
Brownlie RJ, Garcia C, Ravasz M, Zehn D, Salmond RJ, Zamoyska R. Resistance to TGFbeta suppression and improved anti-tumor responses in CD8+ T cells lacking PTPN22. Nat Commun. 2017;8(1):1343. | ||
De Meulenaere A, Vermassen T, Aspeslagh S, et al. Tumor PD-L1 status and CD8+ tumor-infiltrating T cells: markers of improved prognosis in oropharyngeal cancer. Oncotarget. 2017;8(46):80443–80452. | ||
Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol. 2017;14(8):662–674. | ||
de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7(5):411–423. | ||
Rubtsov AV, Rubtsova K, Kappler JW, Jacobelli J, Friedman RS, Marrack P. CD11c-expressing B cells are located at the T cell/B cell border in spleen and are potent APCs. J Immunol. 2015;195(1):71–79. | ||
Li Y, Gao Y, Wang Y, et al. In vivo delivery of asymmetric gene-silencing RNAs targeting CTNNB1 and PD-L1 show a broad spectrum of potent antitumor activities in preclinical cancer models. Cancer Res. 2017;77(13 Supplement):LB-069. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.