Back to Journals » Journal of Pain Research » Volume 16
Best Practices for Dorsal Root Ganglion Stimulation for Chronic Pain: Guidelines from the American Society of Pain and Neuroscience
Authors Chapman KB , Sayed D , Lamer T , Hunter C, Weisbein J , Patel KV, Dickerson D, Hagedorn JM , Lee DW, Amirdelfan K, Deer T , Chakravarthy K
Received 21 May 2022
Accepted for publication 17 January 2023
Published 14 March 2023 Volume 2023:16 Pages 839—879
DOI https://doi.org/10.2147/JPR.S364370
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Ellen M Soffin
Kenneth B Chapman,1– 3 Dawood Sayed,4 Tim Lamer,5 Corey Hunter,6 Jacqueline Weisbein,7 Kiran V Patel,1– 3 David Dickerson,8,9 Jonathan M Hagedorn,10 David W Lee,11 Kasra Amirdelfan,12 Timothy Deer,13 Krishnan Chakravarthy14,15
1The Spine & Pain Institute of New York, New York, NY, USA; 2Department of Anesthesiology, Zucker School of Medicine at Hofstra Northwell, Manhasset, NY, USA; 3Department of Anesthesiology, NYU Langone Medical Center, New York, NY, USA; 4Department of Anesthesiology, The University of Kansas Medical Center (KUMC), Kansas City, KS, USA; 5Department of Anesthesiology and Perioperative Medicine, Division of Pain Medicine, Mayo Clinic, Rochester, MN, USA; 6Ainsworth Institute of Pain Management, New York, NY, USA; 7Napa Valley Orthopedic Medical Group, Napa, CA, USA; 8Department of Anesthesiology, Critical Care and Pain Medicine, NorthShore University Health System, Evanston, IL, USA; 9Department of Anesthesia & Critical Care, University of Chicago, Chicago, IL, USA; 10iSpine Pain Physicians, Maple Grove, MN, USA; 11Fullerton Orthopedic Surgery Medical Group, Fullerton, CA, USA; 12IPM Medical Group, Inc., Walnut Creek, CA, USA; 13The Spine and Nerve Center of the Virginias, Charleston, WV, USA; 14Department of Anesthesiology and Pain Medicine, University of California San Diego Health Sciences, San Diego, CA, USA; 15VA San Diego Healthcare System, San Diego, CA, USA
Correspondence: Kenneth B Chapman, NYU Langone Medical Center, Zucker School of Medicine at Hofstra/Northwell, Pain Medicine at Staten Island University Hospital, 1360 Hylan Boulevard, Staten Island, NY, 10305, USA, Email [email protected]
Abstract: With continued innovations in neuromodulation comes the need for evolving reviews of best practices. Dorsal root ganglion stimulation (DRG-S) has significantly improved the treatment of complex regional pain syndrome (CRPS), and it has broad applicability across a wide range of other conditions. Through funding and organizational leadership by the American Society for Pain and Neuroscience (ASPN), this best practices consensus document has been developed for the selection, implantation, and use of DRG stimulation for the treatment of chronic pain syndromes. This document is composed of a comprehensive narrative literature review that has been performed regarding the role of the DRG in chronic pain and the clinical evidence for DRG-S as a treatment for multiple pain etiologies. Best practice recommendations encompass safety management, implantation techniques, and mitigation of the potential complications reported in the literature. Looking to the future of neuromodulation, DRG-S holds promise as a robust intervention for otherwise intractable pain.
Keywords: dorsal root ganglion, neurostimulation, chronic pain, best practice, guidelines
Introduction
With the introduction of new medical therapies, there is an inevitable growth of fundamental knowledge and improvements in outcomes. Dorsal root ganglion stimulation (DRG-S) is a novel form of neuromodulatory therapy, that, rather than placing the electrical field over the dorsal columns of the spinal cord, as with conventional, tonic spinal cord stimulation (t-SCS), is placed near the cell nuclei of the afferent neurons of the dorsal root ganglia (DRG). Given its novelty, the corresponding body of evidence is evolving compared to longer-employed therapies. The pivotal ACCURATE study, published in 2017, demonstrated superiority of DRG-S to t-SCS in the treatment of complex regional pain syndrome (CRPS) and causalgia.1 Additional studies have shown results differing from past t-SCS work, from potential treatment indications to functional and psychological outcomes. Supporting basic science work is also demonstrating that on a cellular, mechanistic level, DRG-S is functioning in a different manner.
Stimulating at the DRG is currently accepted as an alternative form of neuromodulation used for complex regional pain syndrome I and II and dermatomal pain syndromes, although additional benefits compared to t-SCS are becoming apparent. Targeting the somata of the pseudo-unipolar afferent nerve fibers allows access to all nerve fiber types. Surrounded by dura mater and only partially by a thin layer of cerebrospinal fluid (CSF), DRG-S utilizes a fraction of the energy required. Both modalities utilize a tonic waveform; however, DRG-S has efficacy when delivered in a subthreshold, paresthesia-free manner, at frequencies as low as 4 Hz, and when delivered intermittently.2–4 None of these are possible with conventional t-SCS.
Early basic science work identified filtering of afferent fibers at the DRG as the basic mechanism of action.5–7 However, filtering afferent signaling does not explain the effects of DRG-S on broad, multi-dermatomal conditions with a single lead placement.8,9 These effects suggest orthodromic effects from neurostimulation, an accepted mechanism of t-SCS, but rarely discussed with DRG-S. Single lead coverage of low back pain led to a theory that stimulating the DRG of the cutaneous branches of a dermatome led to inhibition at points of convergence centrally in the dorsal horn (DH).10 Additional antidromic effects from DRG-S on the sympathetic nervous system, namely its ability to influence neuroinflammation and tissue perfusion in peripheral vascular disease, are only beginning to be explored.11–14
While these potential clinical benefits, alternative mechanisms, and potential expanded indications are worthy of excitement, a corresponding growth of prospective randomized research is still developing. This best-practice manuscript is meant to serve as a guide to the current state of the use of DRG-S, utilizing peer-reviewed literature, a reliance on clinical evidence, experience, and expert opinion, to assist practitioners in the application of this therapy in the safest, most efficacious manner, and to ensure the highest level of patient care. This builds on previous work by Deer et al, with an update of recent evidence.15
Anatomy and Function of the DRG
The DRG is the bundle of cell bodies of sensory afferent neurons situated at the intervertebral foramen of each of the 31 pairs of human spinal nerves. Sensory information travels centrally along a mixed spinal nerve to the DRG, where afferent axons bifurcate to give rise to a stem axon and a central axon, the T-junction.16
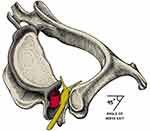
At the DRG, the perineurium encasing the spinal nerve root is replaced by a thicker layer of dura mater, with the arachnoid space starting at the proximal segment of the DRG.17,18 Thereafter, the dorsal and ventral roots travel in the subarachnoid space to their appropriate vertebral levels to split into nerve rootlets, entering at separate levels of the spinal cord. See Figure 1.
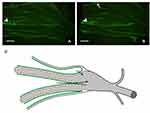 |
Figure 1 Representative sections through immune-stained lumbar DRG showing the CSF occupying sub-arachnoid space (SAS) stopping at the DRG as a fold-like recess (A and B arrows). The SAS is in direct contact with DRG between the spinal nerve roots (A and Barrow heads) and in the angle between the dorsal root and surface of DRG . The scheatic drawing (C) summarizes the position of the CSF delimited by the arachnoid (dotted green line) in relation to DRG. Continuous black and dashed red lines indicate dura and pia mater, respectively. Reprinted from Ann Anat, 205, Joukal M, Klusáková I, Dubový P. Direct communication of the spinal subarachnoid space with the rat dorsal root ganglia. 9–15, Copyright 2016, with permission from Elsevier.18 |
The DRG also contains non-neuronal cells and tissues vital to supporting and protecting the functions of the nucleus. Each neuronal cell body is encased by satellite glial cells. These cells support the somata and have interconnecting gap junctions which are involved with the sensitization of adjacent neurons.19,20 See Figure 2. In addition to connective tissue, there is a group of immune cells consisting mainly of macrophages and lymphocytes, blood vessels, and bundles of sympathetic nerve fibers.21
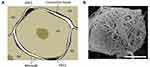 |
Figure 2 (A) Schematic drawing of a DRG neuron cell bodies (N1-5) which are enveloped by a sheath of satellite glial cells (SGC 1+2, black and separated by connective tissue). (B) Scanning electron micrograph showing an intricate meshwork of sympathetic fibers forming a terminal Dogiel’s nest around the cell body of a rat DRG neuron. Scale bar, 10 μm. Images reprinted from Nascimento AI, Mar FM, Sousa MM. The intriguing nature of dorsal root ganglion neurons: linking structure with polarity and function. Prog Neurobiol. 2018;168:86–103.21 |
Function of Somata Within the DRG
The DRG has an active role in the processing of stimuli as well as up- and down-regulation of pain. After inflammation, partial nerve injury, or direct compression of the DRG,22,23 a portion of its afferent cell bodies may become sufficiently hyperexcitable and generate ectopic action potentials (APs).24 These ectopic APs are relayed to the T-junction where they may collide with incoming APs to decrease neural input.25 Action potentials can also be initiated in adjacent, unaffected C-fibers in the DRG and propagate centrally to cause signs and symptoms associated with neuropathic pain.21,23,26 Pain-transmitting C-fibers are particularly prone to the effects of ectopic APs.5,7,27 See Figure 3A.
APs are initiated in the peripheral terminals of DRG neurons, although there are some small-fiber afferent sympathetic fibers that are capable of sending APs back down the same axon. This process is known as the dorsal root reflex (DRR).28 The DRR is utilized in peripheral vascular innervation, where the release of substance P and calcitonin gene-related peptide (CGRP) from the peripheral terminals contributes to vasodilation and neurogenic inflammation.29 This system allows for the effects of antidromic propagation of APs with DRG-S. See Figure 3B and C.
By increasing or decreasing afferent signaling through ectopic APs, the DRG is positioned to serve as the “gatekeeper” of information from the external and internal environments to the CNS.
DRG Size
The size of the DRG is correlated to the number of neurons it contains and varies depending on its vertebral level.30 In the cervical region, DRG size increases from the very small and absent in >70% at the level C1 to the largest at the C8 level.31 The C8 DRG also has a larger volume (177 mm3) than the adjacent T1 DRG (144 mm3), and close to double the volume of the C5 DRG despite a smaller receptive field.30 Similarly, the lumbar DRG size increases from the L1 to L5 levels, from approximately 4 mm × 4.5 mm at L1 to 5.5 mm × 10 mm at L5. Conversely, the size of sacral DRG decreases from S1 to S4 with the S1 DRG measuring roughly 6.5×13 mm compared to 5×3 mm at S4.32
Foramina
The DRG and nerve root exit the spinal canal through the intervertebral foramina, which also contain arteries, veins, ligaments, and epidural fat. The anterior border of the foramen is formed by the posterior aspect of the adjacent vertebral bodies, intervertebral disc, and the lateral expansion of the posterior longitudinal ligament. The dorsal border is the superior and inferior articular processes and facet joint. The roof and floor of the neural foramen are formed by the pedicles of the respective levels.
The size of the lumbar foramen ranges in transverse and sagittal measurement from 8.5 mm wide and 18 mm tall at L1 to 10×22.5 mm at L5.33
The DRG usually lies in the superior portion of the foramen close to the rostral pedicle.34 In the thoracic and lumbar levels, the DRG is positioned in close approximation to the vertebral pedicle, with the L5 DRG found intraforaminal in roughly 75% of cases, with only 6% found extraforaminal.35 In contrast, the sacral DRG was more likely to be found within the spinal canal than the neuroforamen. The S1 DRG is intraforaminal in up to 60% of cases, at S2 DRG the majority are in the spinal canal (50–85%), and at the S3 and S4 all the DRG are within the canal.35,36
The neural tissue and vessels are suspended in the foramen by transforaminal and intraforaminal ligaments. The 5 transforaminal ligaments are thick, rigid, and often unyielding, and their function is to support the intraforaminal structures. Measuring 2–5 mm and crisscrossing the foramen, these ligaments occupy a significant portion of the foraminal area. The finer intraforaminal ligaments suspend the neural and vascular tissues and prevent them from contacting the transforaminal ligaments and periosteum, enabling electrophysiological transmissions.37 These ligaments in addition to bony overgrowth may be potential obstacles to DRG-S lead placement. See Figure 4.
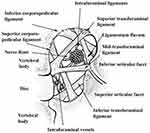 |
Figure 4 The various lumbar intervertebral and intraforaminal ligaments, nerve root, and vessels. The thicker transforaminal ligaments may impede foraminal access. Reproduced with permission from Akdemir G. Thoracic and lumbar intraforaminal ligaments. J Neurosurg Spine. 2010;13(3):351–355.37 |
Vasculature of the DRG
Blood supply to the DRG is provided by the spinal branch of the dorsal trunk of the segmental arteries. The radiculomedullary branches provide blood supply to the spinal nerve, running alongside the ventral aspect of the DRG and nerve roots.38 After piercing the DRG, the vessel forms a subcapsular capillary plexus, then penetrates deeper, branching into intraganglionic vessels, forming a dense capillary network, that directly interacts with sensory neurons.39 Capillaries in the DRG are fenestrated and lack a blood–brain barrier, allowing blood-borne molecules to directly enter the DRG and interact with neuronal and non-neuronal cells.40 The regulation of blood flow to the DRG is achieved by muscular sphincters which adjust flow to functional and metabolic demands.41
Peripheral nerves generally have a tight blood–nerve interface, similar to the blood–brain barrier, especially in comparison to the intensely vascularized and highly permeable vascular interface of the DRG.39 This permits a three-fold increase of blood flow within the DRG as compared to a peripheral nerve.42
This porous barrier allows for systemic states such as diabetes mellitus and permits blood-borne toxic substances such as chemotherapeutic agents access to sensitive neuronal tissue. The alpha-motoneurons which are housed within the tight blood–brain barrier of the CNS, and not the DRG, are less susceptible to such injury. This microstructural feature, specific for the DRG, likely explains why anti-neoplastic and anti-HIV agents preferentially induce a peripheral sensory polyneuropathy.39
Multiple changes in the DRG are seen in diabetic peripheral neuropathy (DPN): changes in microvascular blood flow,43 histologic changes such as thickening of the perineural cell basement membrane,44 changes in metabolic and immunologic processes, and a severity-dependent decrease in DRG volume.45–50 These pathophysiological changes seen in DPN support the notion that DPN originates at the DRG rather than the peripheral nerve itself.51,52
These observations suggest the DRG plays a central and primary role in the pathogenesis of sensory polyneuropathies, further supporting its role as a therapeutic target.
Relation to the Sympathetic Chain
The sympathetic chain serves as a conduit for small fiber nerves to travel broad distances to attach to blood vessels or to enter remote mixed nerve roots to get to their end organ targets. DRGs at the levels of T1–L2 contain sympathetic afferent fibers. The DRG offers distinct characteristics that could make it an optimal target for neuromodulation.
Sensory information is relayed through somatic afferent C- and Aδ-fibers, passing through the DRG to the dorsal horn. Sympathetic fibers are relayed to the hypothalamus, which controls the autonomic response. Efferent signals travel down the dorsal longitudinal fasciculus to reach sympathetic preganglionic neurons in the intermediolateral nucleus of the spinal cord. Preganglionic fibers exit through the ventral root and enter the sympathetic chain through the white ramus communicans (WRC) to synapse onto sympathetic postganglionic neurons located in the paravertebral sympathetic ganglia. The postganglionic fibers, which are unmyelinated C-fibers, exit the sympathetic chain through the grey ramus communicans (GRC) to join the mixed spinal nerve at that level, and go on to their targets.53
Sympathetic tone is predicated on the frequency of AP firing, with lower frequencies seen in basal tone, and higher frequencies in excitatory states, such as vasoconstriction.54 In turn, antidromic propagation of APs at very low frequencies could provide a normalizing effect on the vasculature and decrease neurogenic inflammation.14,28,29,53,55,56
Sympathetic preganglionic WRC neurons can synapse with anywhere from 1:4 to 1:32 postganglionic GRC neurons,57 and as such the sympathetic nervous system has been described as an amplifier of neural transmission. This feature allows for potential diffuse messaging and neuromodulation from a single DRG.58
Brief History of DRG-S Technology
The DRG was identified as a target for dermatomal pain control since the first reported ganglionectomy in the 1970s.59 Until 2010, given the risk of deafferentation pain, the most promising DRG-involved pain control technique pulsed radiofrequency, which ultimately only demonstrated modest efficacy in the cervical spine.60
Prior to 2011, applying a dosed electrical field over the DRG was reported for pain in only two case reports and involved use of a t-SCS system. In 1995, Wright and Collition placed SCS leads at the L2 DRG based on Nakamura et al’s concept of sympathetic convergence and reported satisfactory results over 8 months.61,62 In 2011, the second case report described an SCS lead over the C2 DRG for post-herpetic neuralgia (PHN).63 Alo et al described lumbar and sacral nerve root stimulation with leads placed transforaminally or sacrally via the sacrococcygeal hiatus, where they likely captured the DRG that lie within the sacral canal.32,64
The DRG emerged as a potential target for neuroelectrical modulation secondary to work beginning in the 1980s.65,66 It was in September 2004 that the first patent was filed for a novel device to stimulate the DRG specifically.67 See Figure 5. At this time, t-SCS on the dorsal columns was the standard and sole option, relying on paresthesia that was prone to position-related changes in stimulation. It was technologically primitive by today’s standards, combined with less robust lead configurations leading to less coverage for low back and even less so for the distal extremities. DRG-S was conceptualized to meet some of these shortcomings.
 |
Figure 5 An original patent image demonstrating an implantation concept with an electrode being implanted into a dorsal root ganglia. Reproduced from Kim DH, Imran MA. Method and system for stimulating a dorsal root ganglion. 2009:1–72. Available from: https://patents.google.com/patent/US7580753B2/en. Creative Commons.67 |
Applying a lead on the DRG required several modifications from the existing t-SCS systems. These included a smaller lead diameter, increased flexibility, a reduced contact size, and a novel technique for device implantation. In addition, device programming needed to allow finer adjustments as dosing needs were substantially lower than previously utilized.
The proof-of-concept, first-in-human work was performed on three patients in 2008. This experience helped mold the next phase of lead and delivery engineering, IPG modification, and lead placement methodology. Once a reproducible device was available, a pilot study was conducted in the spring of 2009 that demonstrated a statistically significant pain improvement over 72 hours.68 The next phase of research was moved to Europe and Australia in 2010. Two small multicenter studies demonstrated safety and efficacy of targeting the DRG for foot pain and FBSS,69,70 and this led to regulatory approvals in Europe and Australia in 2011.
In 2012, Deer et al published the 2009 study data, where 8 of 10 trialed subjects achieved pain relief while reporting no adverse events, using average settings of 68 Hz, pulse width of 200 µs, and 0.800 mA.71 Shortly after that publication, in a multicenter European and Australian prospective trial of DRG-S, Liem et al again demonstrated safety and efficacy of DRG-S in 32 patients followed for 6 months.72 Eighty-nine percent of patients with neuropathic foot pain achieved significant pain relief, an area that is often challenging to cover with t-SCS.
Given the results of early studies, a prospective, multicenter, randomized comparative effectiveness trial of 152 subjects diagnosed with CRPS I and II of the lower extremities, comparing DRG-S to t-SCS, named the ACCURATE trial, was initiated.1 As with the Liem trial, this trial included pain conditions or painful regions such as the foot, knee, and inguinal areas that have historically been more challenging to treat, even with t-SCS, and used average settings of approximately 20 Hz, 300 µs, and 0.900 mA. Further programming advancements followed, as by 2019 it was identified that DRG-S was effective without the need for paresthesia recruitment, and by the next year it was revealed that DRG-S was effective at frequencies as low as 4 Hz and when dosed intermittently.2–4
DRG-S Mechanism of Action
DRG-S places an electrical field over the nuclei of primary afferent neurons, allowing for modulation of prior to signal propagation within the spinal cord and potentially inhibiting at synapses with second-order neurons in the DH.
Pre-clinical and clinical work is beginning to unravel mechanistic and therapeutic mechanisms underlying DRG-S, and differences compared to t-SCS are becoming evident. On the cellular level, the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) is elevated in t-SCS but not with DRG-S, neither in the dorsal horn or in the DRG itself.73,74 Furthermore, Aδ-fiber stimulation at 1 Hz causes long-term depression in the DH,75 a likely mechanism underpinning the delayed washout of DRG-S at 1 Hz compared to 20 Hz and 1000 Hz,76 and clinically reflected in the ability to cycle DRG-S.
Impeding the transduction of afferent pain signaling and of ectopic AP transmission is the most commonly accepted mechanism of DRG-S, and this has been supported in basic science research.5,7,22 However, the effects of DRG-S on broad, multi-dermatomal conditions with a single lead placement would depend on the orthodromic propagation of stimulation, an accepted mechanism of t-SCS, but rarely discussed regarding DRG-S.8,10,77,78
It has been postulated that DRG-S activates Aδ-, Aβ-, and C-fiber low threshold mechanoreceptor (LTMRs) fibers, which utilize the endogenous opioid system (EOS) to modulate the touch and pain processes at frequencies clinically utilized with DRG-S.75,79,80 This mechanism is thought to underlie the ability of DRG-S to modulate nociceptive or mixed-pain syndromes.
Additionally, DRG-S has demonstrated promising antidromic effects on the sympathetic nervous system in the treatment of peripheral vascular disease, blood pressure reduction, as well as decreasing neuroinflammation.11,13,14,81
Narrative Literature Review of Peer-Reviewed Clinical Findings in Dorsal Root Ganglion Stimulation
Methods
Development Process
As part of its mission to improve patient care and access to advanced neuromodulation techniques, the American Society of Pain and Neuroscience formed the Best Practices Work Group, consisting of ASPN members who were chosen for their clinical expertise, familiarity with the current peer-reviewed literature, research capabilities, diversity of practice, and previous publications. All authors disclosed any conflicts of interest and recused themselves from any section impacted. The primary author served as a conflict-free referee of any bias in the paper.
DRG-S has been approved in the United States since 2016 for the diagnosis of CRPS I and causalgia. The ACCURATE study remains the lone multicenter, randomized controlled DRG-S trial. Many of the remaining reports averaged results of pooled diagnoses and non-standardized lead configurations, making interpretation of diagnosis-specific results and recommendations for DRG-S best practices challenging. Nonetheless, the available data per condition from these papers demonstrate fragmented evidence of very good outcomes for a wide variety of diagnoses. Successful DRG-S relies on lead placement, and we see mixed results from inaccurate lead placement.82 Currently, only a handful of prospective papers are diagnosis and lead placement specific, and these tended to have small numbers.9,77 Currently, a post-approval real-world study is ongoing in the United States, the TARGET study, with FDA monitoring to identify best uses of the therapy and continuing improvements in safety.83
In the last year there have been three systematic reviews on DRG-S demonstrating CRPS and causalgia as the only diagnoses to potentially qualify for grade A evidence with high certainty.84–86 To reduce redundancy, we will present the literature in a narrative format, based on the current body of evidence, per diagnosis/body part.
Literature Search, Evidence Ranking, and Consensus Development
A comprehensive search of several databases from 2010 to August 2022, limited to English language and excluding animal studies, was made. The databases included Ovid MEDLINE® and Epub ahead of print, in-process, and other non-indexed citations, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an experienced librarian with input from three of the study investigators (JMH, TJL, KBC). Controlled vocabulary supplemented with keywords was used to search for studies describing DRG-S. Additional keywords included “ganglia”, “spinal ganglia”, “neuromodulation”, “electrostimulation”, “neurostimulation”, “dorsal root”, and “drg”. For the purposes of grading, we defined a case report as any study involving ≤2 patients and a case series as any study with ≥3 patients.
Identified peer-reviewed literature was critiqued using the United States Preventive Services Task Force (USPSTF) criteria for quality of evidence,87 with modifications for neuromodulation studies (see Table 1). After USPSTF letter grading was assigned, the working subgroup then assigned the “level of certainty regarding benefit” as described in Table 2.
 |
Table 1 Quality of Evidence Ranking Using United States Preventative Services Task Force Criteria Modified for DRG-S |
 |
Table 2 Levels of Certainty Regarding Net Benefit |
Work groups were convened to evaluate the literature and examine the evidence for the topics developed by the lead authors in outline form. Recusal was performed for any section where the author had a direct conflict of interest. After the literature search was completed and separated by body part treated, outcomes were collected and cited. Diagnosis-specific data from publications with multiple diagnoses were extracted when available. If data per diagnosis were not provided, the data were excluded.
As a consensus guideline, this document provides recommendations regarding practices for DRG-S, and should not be construed as a standard of care. Best practices were based on several factors, including peer-reviewed evidence, and, regardless of the strength of evidence, requires interpretation for clinical application.
Results
Complex Regional Pain Syndrome (CRPS) Type I
The treatment of CRPS and causalgia with DRG-S is accepted as a potential first-line neuromodulatory therapy, and the majority of DRG-S literature is focused on the treatment of CRPS type I and II. Aside from the ACCURATE study, there are 9 case reports and a presence in virtually all the DRG-S publications.1,88–94 Published in 2017, the ACCURATE multicenter prospective, randomized, controlled trial evaluated the safety and efficacy of DRG-S compared to traditional SCS in subjects with CRPS type I or causalgia at three and 12 months. The percentage of patients who achieved greater than 50% reduction in VAS at three months with DRG-S was 81.2% versus 55.7% in the SCS arm and 74.2% in the DRG-S group versus 53.0% with SCS at 12 months. DRG-S demonstrated superiority compared to SCS for CRPS I and II at 3 (p < 0.0004) and 12 months (p < 0.0004). DRG-S subjects also experienced significantly less paresthesia in non-painful regions.1
A number of case reports have reported significant pain relief in patients with CRPS with utilization of DRG-S88,90–92 One case report described improvements in the physical manifestations of CRPS in a patient with leg pain after a spinal cord injury.93 Patient preference for DRG-S is suggested for treating CRPS to have a preference for DRG-S over SCS when trialed with both treatment options89 or after inadequate relief with SCS.94,95
Utilizing a multi-waveform SCS system, Al Kaisy et al retrospectively compared t-SCS, 1K SCS, and burst-SCS on the DRG.96 CRPS was the indication in 19 out of 39 (49%) patients. Thirty-two of 39 patients proceeded to implant after trial (82%) with 28/32 responders (87.5%) at 6 weeks and 21/32 (66%) at a mean of 18 months' follow-up. A burst protocol was preferred in the majority, 78% (25/32), of patients. As the leads and SCS system used in this study were not designed to treat the DRG, the inability to fine-tune frequency and amplitude may have played a role in this preference over t-SCS.
Causalgia and Post-Surgical Pain Etiologies
The benefits of DRG-S for post-surgical pain syndromes (PSPS) and causalgia include better dermatomal coverage, proximity to the sympathetic chain, and utilizing points of convergence in the DH for broader coverage.10,14 The evidence regarding DRG-S for PSPS/causalgia (including post-hernia, post-arthroplasty, failed back surgery syndrome, post-amputation, post-abdominal, and pelvic surgery) is reported in 3 prospective and 3 retrospective case series, a subset of the ACCURATE RCT, and 11 case reports.97–106
Given the limited, low-quality evidence SCS has demonstrated for the following indications, compared to the positive results in the DRG-S studies, there is an opportunity for DRG-S to be considered as a first-line treatment option for intractable, non-responsive PSPS and causalgia.
Groin, Post-Herniorrhaphy, and Abdominal Wall Pain
Post-herniorrhaphy pain stems from distal dermatomal receptive fields, an optimal condition for DRG-S to provide improved paresthesia-free coverage.4,107 Given the limited evidence for SCS and PNS for inguinal and post-herniorrhaphy pain108–113 and positive results from the prospective and retrospective case series, and case reports discussed below, DRG-S is positioned as a reasonable consideration for first-line application.101,105,114
Morgalla et al reported on a prospective case series on 34 consecutive patients who previously had undergone inguinal hernia repair with PSPS from ilioinguinal or iliohypogastric nerve injury.101 Thirty of 34 trialed patients had >50% pain improvement and underwent implantation. Most patients (25/30) had implantation at L1 and L2 on the effected side. At three months' follow-up, VAS decreased a mean 75.5% (30/30 patients) which was maintained at 63.5% at three years (11/30 reached this time period). At three years, 72.7% of patients (8/11) reported more than a 50% decrease in pain. Patients had improvements in Beck Depression Inventory (BDI), Pain Disability Index (PDI), Pain Catastrophizing Scale (PCS), and Brief Pain Inventory (BPI), along with 73.3% reducing their opioid requirements after implantation.
In a retrospective review, Schu et al reported on 29 patients with chronic groin pain.105 Within this cohort, ten of 13 patients trialed with DRG-S for post-herniorrhaphy chronic pain went on to implantation, with a mean follow-up time of 17.4 weeks. Eighty percent of patients reported >50% VAS reduction with a mean VAS reduction of 76.8%. Five patients had follow-up longer than six months, with the mean follow-up time 30.6 weeks and reduction in VAS of 74.3%.
In 2014, Zuidema et al reported on a 3-patient case series using paresthesia mapping and leads placed at the T12, L2, and T11 and L2 levels. At between 2 and 3 months patients had between 90% and 100% pain relief.114 Finally, it is worth noting that within the ACCURATE trial 14 patients were diagnosed with causalgia in an ilioinguinal nerve distribution.1
Mol et al retrospectively followed 5 patients suffering from anterior cutaneous nerve entrapment syndrome (ACNES) treated with DRG-S for 12 months.115 These patients with non-surgical caused nerve entrapment pain in anterior abdominal wall had 3 patients sustaining >50% pain relief, one 37% relief, and one patient without improvement at 12 months' follow-up. Another case series included 9 patients treated for ACNES with DRG-S followed for 3–30 months.116 At 3 months 8 of 9 experienced >50% pain relief, with four of nine reaching 30 months with maintained improvements. In another case report Akuamoah et demonstrated impressive improvements with DRG-S placed for hernia pain after an extreme lateral interbody fusion (XLIF) surgery, and then later adding leads for the same failed XLIF.117 VAS improved from hernia/flank pain from a VAS of 10 to 1.5 at 18 months and the T12 and S1 leads improved low back and leg pain from a 9/10 to a 3.5/10, ODI from 78 to 29, and EQ-5D from −0.11 to 0.59 at 6 months post lead addition.
Post-Surgical Joint Pain
Post-surgical joint pain or causalgia of the post-surgical joint is a PSPS that has garnered little attention with SCS compared with DRG-S.118–120 There have been 2 prospective studies, 1 retrospective, 1 case series, 3 case reports, and 1 subset analysis of the ACCURATE study representing 66 patients with post joint replacement pain.99,102,104,121–124 The evidence DRG-S has demonstrated in post-surgical joint pain/causalgia compared to SCS places DRG-S as the authors' preferred neuromodulatory option in refractory post-surgical joint pain/causalgia.
Hunter et al included 12 post-total knee arthroplasty (TKA) patients in a retrospective review from a larger cohort of 217 patients.104 During the trial period, the post-TKA patients experienced an approximately 70% decrease in their pain. Eight patients would go on to receive an implantation of a permanent system. The authors recommended ipsilateral L3 and L4 DRG stimulation for optimal results. Long-term results were not described.
Morgalla et al trialed 62 patients, with 51 proceeding to implant to treat causalgia of multiple regions, including 30 trialed with causalgia of the knee with 27 proceeding to implant.121 Other regions included hand, foot, leg, and PLP. Twenty-five patients reached 3 years' follow-up. Results were not separated per body part; however, overall improvements included a 50% reduction in pain (VAS of 8 to 4), BPI decreased 53% (6.8 to 3.2), BPI-PI 68% (5.7 to 1.8), and Beck Depression Index (BDI) decreased 24% (~10.5 to 8).
Kretzchmar et al retrospectively reported on 23 patients following DRG-S for post-surgical pain, including 11 post-knee surgeries and 2 post-total hip replacements.99 The authors did not report the data for these patients separately. At 36 months, the cohort demonstrated a VAS improvement of 73% (55 to 15), SF-12 MCS improvement of 25% (34 to 44), PCS improvement of 18% (42 to 50), and at 12 months a Quality-of-Life Impairment by Pain Inventory (QLIP) improvement of 102% (18 to 37). Leads for knee pain were placed at the L3 and L4 levels, and, for the hip, one patient was implanted at the L1, L2, and L3 levels and one patient at the L5 and S1 levels.
Martin et al reported on 12 patients implanted with a DRG-S, five of which had post-surgical knee pain.102 The average follow-up for all patients was 34 months (range 15 to 78 months). Of the five post-surgical patients, 3 have a single L3 lead, 1 a single L4 lead, and 1 patient has L3 and L4 leads. The average pain relief for the five post-surgical patients was 72.7% at an unknown mean time period.
A recent case series and a case report by Chapman et al, totaling 5 patients, reported on joint pain deemed not primarily neuropathic in nature treated with DRG-S.123 Joints included the hip, knee, and ankle. There were impressive improvements in VAS, quality of life as measured by the EQ-5, as well as joint-specific testing. Joint-specific questionnaires demonstrated impressive improvements.
In a paper detailing 3 novel implant techniques utilized on two patients suffering from post-TKR knee pain, Schultheis et al demonstrated a reduction in NRS of 50% over 12 months with leads at L3 and L4.124 There were improvements across multiple domains including multiple functional, psychological, and sleep domains, including a 24% improvement in PDI.
Failed Back Surgery Syndrome and Non-Surgical Low Back Pain
DRG-S for failed back surgery syndrome (FBSS) and non-surgical low back pain (NSLBP) has shown limited but impressive results in the treatment of pain, function, affect, and quality of life. SCS remains the gold standard neuromodulatory therapy for FBSS and NSLBP, although evidence is accumulating to consider DRG-S as a second-line therapy, or after failed SCS cases, as evidenced in the following reports:
Weiner et al carried out a prospective study using a wireless SCS system on the DRG for FBSS in 2016.106 They reported on 11 patients who underwent unilateral DRG-S ranging from L1 to L5 for 45 days. Stimulation settings of 500 µs pulse width with 100 Hz frequency were used, which are levels incongruent with today’s practice. Yet the overall pain reduction was 59.9%, with L2 experiencing the most significant relief at 73%.
One year later, Huygen et al published a prospective study evaluating L2 or L3 DRG-S for LBP secondary to FBSS in 12 patients.82 At least one lead was placed at the L2 or L3 and additional leads to provide paresthesia coverage in additional painful areas. Included were patients with non-radiating LBP and patients with low back and lumbar radicular pain. Average pain relief was 45.5% at 12 months, and more than half of the patients reported at least 50% pain improvement. Mood and quality of life were also improved with DRG-S at 12 months.
Based on the results of the previous study, Kallewaard et al followed up with a prospective study of 13 patients treated with DRG-S for axial LBP secondary to FBSS after lumbar discectomy.77 Eleven patients with non-radiating pain had a successful trial and were implanted. At one year, average pain was reduced by 72% (NRS 7.2 to 2.3), and quality of life measured with EQ-5D improved 38% (0.61 to 0.84), disability measured with the ODI improved by 58% (42.1 to 21.5), and a 94% improvement in mood was seen as measured with POMS (16.4 to 1).
Non-Surgical Low Back Pain
DRG-S for the treatment of NSLBP was studied by Kallewaard et al in a prospective, single-arm case series for discogenic LBP treated at the bilateral L2 level.9 L2 DRG-S for discogenic pain only was based on the notion of sympathetic convergence at the L2 level in conjunction with the results of Huygen et al.82 Fifteen patients who had a negative response to medial branch blocks and a concordant discogram were successfully trialed and proceeded to permanent implant. Patients reported a mean NRS reduction of 63.4% (n=15) at six months and 68.3% (n=14) at 12 months (p < 0.001). Impressively, the cohort demonstrated a 141% increase in mood (EQ-5D 0.34 to 0.82), a 58% improvement in ODI (46.1% to 19.2%), and a 101% improvement in POMS (19.2 to −0.11) at the 12-month endpoint.
Reports Containing Mixed FBSS and NSLBP
Outcomes of FBSS and NSLBP treated with DRG-S have been studied in several additional reports. The combined outcomes of the following papers demonstrate further lower-quality evidence for the use of DRG-S to treat both FBSS and NSLBP:
Chapman et al described outcomes of DRG-S at the T12 level for axial low back pain regardless of surgical intervention.8 As prior studies utilized L2 placement based on sympathetic convergence, the authors provided a review of literature on the potential mechanisms underlying efficacy of DRG-S at T12.10 The cohort included 7 patients with a history of lumbar fusion, 5 with a laminectomy/discectomy, and 5 patients with NSLBP, with 4 having failed prior stimulation. Ten patients also had S1 leads placed for lumbar radicular pain or sacroiliac joint pain. Patients experienced a 76% reduction in VAS (9.3 to 2.2), and a 78% improvement in ODI, a 180% improvement in EQ-5 (0.30 to 0.84), a 93% improvement in psychological status as measured by the MCS (SF-36 30.8 to 59.5), and a 108% improvement in PCS (SF-36 23.8 to 49.4). Moreover, 70% of patients reduced opioid consumption, with two stopping completely.
Another study measuring the effects of frequency on outcomes included 20 patients titrated to 4 Hz.2 Their cohort included 20 patients, of which 8 had a history of prior lumbar fusion, 2 with laminectomy/discectomy, and 10 treated for NSLBP. All patients had bilateral T12 leads to cover lower back pain and a combination of unilateral or bilateral S1 or other lumbar leads to cover lower extremity pain. Frequency was titrated from a mean of 16 Hz to 4 Hz over a mean period of 80 days with pre-DRG-S, pre-titration, and post-titration outcomes measured. Efficacy was maintained at 4 Hz, as their improvements from baseline of 77% in VAS (8.8 to 2.0), 72% (67 to 19) in disability, and a 145% improvement in mood (EQ-5D 0.33 to 0.81) were maintained at 366 days post-implant. The cohort also maintained an opioid reduction of 87 to 43 MME and a reduction in the number of interventional procedures performed over the equivalent pre-DRG-S period from 0.5 to 0.04 per month. The article was published with a sister review of literature article on the mechanisms underlying stimulation at 4 Hz.79
In additional work from this group, measuring changes in quantitative sensory testing (QST) measurements, 11 patients were followed: 6 with a history of FBSS and 5 with NSLBP.125 Leads were placed at T12 and S1 for all patients, with 2 patients requiring leads at L4. Quantitative sensory testing was performed in patients before trial lead placement and either before trial lead removal or at the one-month follow-up after permanent implant. Patients experienced a 77% reduction in VAS, a 62% improvement in ODI, an 80% improvement in EQ-5, a 24% improvement psychological status as measured by the MCS (SF-36 42 to 52), and a 104% improvement in PCS (SF-36 23 to 47). The paper also demonstrated a normalization of pressure pain threshold, mechanical detection threshold, and conditioned pain modulation, signs of DRG-S improving both the nociceptive and neuropathic components of LBP.
Huygen et al followed 56 patients treated with DRG-S for 1 year with mixed diagnoses. Twenty-five of these patients were treated for FBSS.126 Lead locations were not mentioned. Baseline NRS improved from 8 to 3.9 in FBSS patients at 1 year. The overall cohort also contained 24 patients with CRPS I and II, post-amputation pain, radicular pain, and others. At 12 months for the overall cohort, improvements included VAS reduction of 49% (8.0 to 4.1), BPI Pain Intensity improved 26%, BPI Pain severity improved 26%, mood as measured by POMS improved 52% (27.8 to 13.3), and EQ-5 improved 72% (0.36 to 0.62).
n summary, DRG-S has demonstrated an improvement in pain, function, affect, and quality of life for FBSS and NSLBP. Although yet to be reproduced in a larger-scale study, DRG-S shows concordant improvements in functional and psychological measures as compared to pain to a degree that is worthy of further studies.
Peripheral Neuropathy
The ability to directly modulate the Aδ and C small fibers that innervate the skin and subcutaneous tissue, at level Aβ collaterals, and taking advantage of convergence in the DH, would appear to make DRG-S an optimal therapy for pain associated with peripheral neuropathic (PN). There are only two small prospective studies and one retrospective study, and seven case reports of application of DRG-S for varying causes of PN.58,127–134 Reports included diabetic PN, painful small-fiber PN, idiopathic PN, polysensory PN, hereditary sensory and autonomic PN, PN associated with Lyme’s disease, and chemotherapeutic agent-induced PN. Follow-up in most cases was as short as 6 weeks to 6–12 months. Leads were typically placed at L4-S1, with less than half of patients implanted with S1 leads. Our authors agree that S1 lead placement is integral to success with distal lower extremity neuropathic pain, and there may be potential for improved results with an addition of an S1 lead.
Eldabe et al retrospectively reported on treating DPN, with 10 patients trialed with DRG-S, 7 undergoing device implant, and 2 patients directly converted without a trial undergoing device explant.127 In the remaining 5 patients, 4 reached 12-month follow-up, and there was a 64% reduction in VAS. Aside from one failure secondary to inability to place leads, placement was from primarily L5 to L2. The two additional failures had only single level leads placed at L5, and the explanted patient also only had a single L5 lead placed. Based on convergence at the S1 level, the authors concur that a single level S1 lead is often adequate for the treatment of peripheral diabetic neuropathy.
Koetsier et al in a prospective study treating polyneuropathy reported 8 of 9 patients having a successful trial, with 7 patients implanted.131 Leads were placed at the L5 in 95% of cases and S1 in 44% of cases. At 6 months' follow-up they found a 57% improvement in VAS, a 50% improvement in anxiety and depression as measured by HADS, a 60% improvement in BPI pain intensity, and a 20% improvement in quality of life as measured by the EQ-5D.
Falowski et al retrospectively evaluated 8 patients with DRG-S placed at L4-S1 for diabetic neuropathy and polyneuropathy.129 This small cohort demonstrated an 80% reduction in pain at 6 weeks, with seven out of eight patients decreasing their opioids or discontinuing them. Ho et al described a series on painful hereditary and idiopathic axonal polyneuropathy.132 Three of four patients trialed went to implantation with bilateral L5 and S1 leads placed. There was an average pain reduction of 74% at 6 months.
Post-Amputation
Eldabe et al published a retrospective study on eight patients utilizing DRG stimulation for PLP. Leads were placed at relevant DRG based on patient-reported pain distributions.103 The average follow-up was nine months, and the average VAS decreased from 83.5 mm to 38.9 mm (53.4%).
Hunter et al published a retrospective case series on 4 patients using selective radiofrequency stimulation to for predicting targets for neuromodulation in patients with post-amputation pain.135 Lead locations were in the range L3-5, and patients experienced 60%, 85%, 90%, and 90% relief after the trial period. No implant results were given.
Post-Herpetic Neuralgia
PHN is a dermatomal pain syndrome that has yielded mixed results when treated with DRG-S. Varicella zoster infection-induced apoptosis of afferent cell bodies within the DRG reduces the somata targeted by DRG-S, and likely decreasing resultant potential efficacy.
In literature, there are 8 reports with patients treated with DRG-S for PHN. Seven of 8 reports detailed trial and implant rates totaling 20 trialed patients, with 10 patients proceeding to device implant.71,121,136–141 Outcomes related to treatment were generally not detailed, aside from a patient with a 37.5% reduction in NRS at 12 months and one explanted due to lack of pain relief,136 and 3 patients in a case series with >50% pain relief at 12–18 months' follow-up.140 DRG-S was effective in a case of subacute pain from Herpes Zoster outbreak at 2 months' follow-up.142 Mansano et al document a case of DRG-S of the Gasserian ganglion for trigeminal neuralgia and a novel implant technique utilizing intraoral puncture with maxillary fixation.141 Previous attempts at this type of target were complicated by high migration rates, fractures from biting, and irritating tonic suprathreshold stimulation.
Our authors conclude that evidence of DRG-S efficacy in PHN is relatively poor and is dependent on the ability to capture unaffected somata. Additionally, the placement of leads at levels adjacent to the affected DRG may inhibit pain through collateral Aβ fibers, a theory that needs elucidation.
Pelvic and Visceral Pain
The evidence for DRG-S for chronic abdominal or pelvic pain is reported in 1 case series and 4 case reports.143–147 The first-described use of DRG-S for chronic pelvic pain was published in 2016. In their case report, the authors described a case involving the use L1 and L2 DRG-S for chronic pelvic girdle pain. At 6 months, the patient reported a decrease in NRS from 7/10 at baseline to 4/10.143
Hunter et al followed with a case report of seven patients who underwent bilateral L1 and bilateral S2 DRG-S for chronic pelvic pain of varying etiologies. At the time of publication, three patients had follow-up beyond six months post-implantation and were continuing to report pain reduction greater than 50%.144 In another case report, Zuidema et al reported on the use of S3 DRG-S for refractory perineal pain after right-sided Bartholin’s cyst surgical resection.98 At two weeks' follow-up, VAS improved almost 89%.
Two additional case reports with different pelvic pain etiologies have also been published. Giordano reported four-month results of bilateral L1 and S2 DRG-S for coccyx and pelvic pain, and the patient was reporting 70–80% relief.145 Similarly, Hassanain reported the 12-month results of a patient with testicular pain who had 70–80% pain relief.146
Kloosterman et al described the use of bilateral T11 DRG-S for pain following Roux-en-Y gastric bypass surgery.97 At six-month follow-up, the patient continued to report approximately 90% pain relief, and improvements in ODI (78% to 10%) and SF-36 (mental: 20.9 to 58 and physical: 15.5 to 58.1). Justiz et al utilized DRG-S at the bilateral T10 levels for the treatment of chronic abdominal pain due to hereditary pancreatitis, with VAS improving from 8 to 0 at 6-month follow-up.147
Mixed Etiologies
The initial study targeting the DRG was a prospective, multicenter, single-arm, pilot study including ten subjects with chronic intractable neuropathic pain of the trunk and/or limbs and followed for four weeks upon which the leads were removed.71 Patients received an average of 2.9 leads attached to an external generator. At baseline, the overall mean VAS improved 70% during the four-week study. The average decrease in back pain was 84%, and leg and foot pain improved 80% and 70%, respectively. It was noted that there were no changes in stimulation related to body position.
A follow-up 32-subject study treating a variety of painful etiologies treated with DRG-S and followed for six months was undertaken by Liem et al.72 At all time periods, more than half of subjects reported pain relief of at least 50%. At six months post-implantation, average overall pain ratings were 58% lower than at baseline (p < 0.001), and the proportion of subjects experiencing at least a 50% reduction in pain in the back, leg, and foot areas were 57%, 70%, and 89%, respectively.
Wensing et al followed 25 patients treated with DRG-S for post-surgical pain of multiple diagnosis for 2 years.100 Improvements were seen from a baseline VAS of 76 to 38 and 46, at 1 and 2 years, respectively. There were corresponding improvements in EQ-5 from a baseline 0.48 to 0.70 and 0.68 at 1 and 2 years. Improvements in physical function were measured with pain severity (6.9 at baseline, 4.4 at 1 year, 4.5 at 2 years) and interference (4.6 at baseline, 3.0 at 1 year, and 3.5 at 2 years). Although three patients underwent device explant over 2 years, 71% of patients were highly satisfied with the therapy at the endpoint.
DRG-S was utilized in a case of post-stroke lower extremity pain after a medullary infarction with 100% reduction in pain who was weaned off 32 mg hydromorphone at 6 months' follow-up.148
Piedade et al also studied the effects of frequency, where 19 patients were randomized to 20 Hz, 40 Hz, 60 Hz, 80 Hz, and sham.149 CRPS I and II were the most frequent pain etiology. Pre DRG-S mean VAS was 8.6 and reduced to 3.9 with therapy. Pain intensity returned to 3.7 at 20 Hz but increased with higher frequencies reaching 5.8 at 80 Hz. Significant differences among the groups were shown over VAS, MPQ, EQ-5D, and BDI, and it was concluded that 20 Hz was a more optimal frequency.
Sympathetically Mediated Conditions
The effects of DRG-S on the sympathetic nervous system (SNS) have been of brought to light in recent works.11,12,14,81,150,151 Anatomic proximity, access to Aδ- and C-fibers and direct antidromic propagation to the sympathetic chain positions DRG-S optimally to modulate signaling within the sympathetic nervous system. See Figure 6.
Sverrisdottir at al. studied 14 patients with a variety of diagnoses who were implanted with DRG-S at pathology-specific levels (in the entire cohort, leads were implanted from C6 to L5 to evaluate the effects of DRG-S on the SNS).11 Blood pressures, heart rate, and sympathetic activity in muscle measured by multiunit postganglionic sympathetic nerve activity (MSNA) were taken at 3 and 6 months post device implant. Findings included greater systolic blood pressure with left-sided compared to right-sided leads, and normalization of blood pressure in all hypertensive patients. These findings were maintained over 2 years as well as MME decrease from 88 to 50. MSNA decreased 13%. The authors concluded that blood pressure improvements were related to the DRG-S ability to decrease sympathetic outflow.
Chapman et al reported on 3 patients treated for peripheral vascular disease with DRG-S.14 Results included a resolution of dry gangrene with recorded pre- and post-DRG-S pulse volume recordings with impressive results. An additional case detailed a patient trialed with both SCS and DRG-S with comparative PtCO2 improvements. SCS improved to levels consistent with the literature,152,153 whereas DRG-S improved transcutaneous oxygen monitoring levels to near normal values.
In a case report, Hagedorn et al used DRG-S to treat erythromelalgia-related pain of the bilateral feet.154 Erythromelalgia is a neurovascular disorder classically characterized by erythema, warmth, and episodic burning pain in the feet, hands, and face. It is believed to be due to a defect in Aδ- and C-sympathetic afferent neurons. At 3 months post implant, the patient reported an 80% improvement in pain, as well as improved sleep and standing tolerance.
Neurogenic inflammation is a process by which the inflammatory process occurs through antidromic signaling along afferent sympathetic nerves. In two related articles, Gravius et al and Kinfe et al investigated the effects of single ipsilateral L4 DRG-S on chronic post-surgical knee pain in 12 patients for 3 months.12,81 Specific details on prior surgical procedures were not provided. Additionally, measured were effects on QST and associated inflammatory biomarkers and gene transcription. Ipsilateral L4 DRG-S was found to provide pain relief and improved functional status in this small patient cohort. In addition, the transcription of 21 different genes was changed after DRG-S, indicating the potential relationship between DRG-S, pain control, and inflammatory mediators. This correlation was also demonstrated in the rodent model.155
Potential for Less Habituation
In an ACCURATE sub-study analysis, Levy et al evaluated therapy habituation with DRG-S versus t-SCS at 12 months for CRPS type I and II.156 In both groups, reported percentage pain relief was higher at end of trial than all other follow-up time periods. Notably, the percentage pain relief with DRG-S was maintained at 1, 3, 6, 9, and 12 months, whereas, in the SCS group, percentage pain relief was significantly less at 9 and 12 months post-implantation, leading authors to conclude that therapy habituation is less likely with DRG-S as compared to SCS.
This concept was supported by a real-world pooled data retrospective analysis of 249 implants which demonstrated only 10 explants over a mean 27-month follow-up, with only 7 explanted for inadequate pain relief.157 Only one patient underwent explant for infection (0.4%), and there was an overall explant rate of 2% per year. Patients who suffered complications were more likely to undergo explant. The authors offer hypothesis for their findings and why their findings differed from previous publications.
Summary
The described evidence demonstrates the effectiveness of DRG-S in the treatment for pain in the setting of CRPS, causalgia, and PSPS; see Table 3. This growing knowledge base has led to the expanded use and a growing body of lower-level evidence in the treatment of a wide variety of pain conditions. Promising outcomes have been presented in not just focal pain syndromes, but also more diffuse dermatomal conditions. DRG-S also appears to not only improve pain, but have concordant improvements in function, mood, and quality of life.
 |
Table 3 ASPN Best Practices Guidelines for DRG Stimulation Evidence Ranking |
Best Practices and Pearls of Expert Opinion
Safety
Preoperative Evaluation and Selection of Anesthetic Technique
The American Society of Anesthesiologists' statement on anesthetic care for interventional pain procedures illustrates the balance between comfort and safety, and recommends patients be able to provide feedback during procedures that carry risk of injury to neural structures.158 This aligns with the manufacturers' DRG-S Physician Implant Manual which states: “The patient must be awake and conversant during portions of the procedure to minimize the likelihood of nerve damage”.159 Although sedation is commonly utilized during DRG-S placement, it is vital to maintain an appropriate anesthetic level to allow patient interaction and feedback. Inhaled or intravenous general anesthetics may be necessary for some patients, or secondary to surgeon preference, and this requires a form of intraoperative neuromonitoring (IONM). The goal of either anesthetic technique is to minimize potential patient harm, and optimize patient experience while placing DRG-S leads.160,161
Preoperative evaluation of relevant patient factors and foreseeable procedural challenges influence the specific sedation and anesthesia plan for individuals undergoing DRG-S placement. Choice of anesthetic and monitoring should be discussed prior to the day of the procedure, and implanter and patient preferences should be aligned. Discussions regarding patient positioning, procedural pain tolerance, and reinforcement in your confidence in the patient’s ability to tolerate the experience are paramount. This pre-operative discussion of the anesthetic and IONM plan minimizes communication-related errors before they occur.
Intraoperative Neuromonitoring
Improving outcomes and decreasing complications with implantable therapies is a necessary and vital process. DRG-S lead placement utilizes a semi-rigid introducer sheath to steer the lead into the intervertebral foramen.162 Given the sensitive nature of the DRG, the potential for foraminal stenosis and the presence of the spinal cord in cases above L1, protection from neural insult during placement is vital.163,164 Thus, DRG-S lead placement should be performed either with the patient awake and alert or through utilizing IONM, although these practices have not yet been universally adopted. This likely carried over from both SCS habits and infrastructural limitations required for IONM, and is ultimately secondary to a lack of definitive guidance.15
Electrophysiologic Neuromonitoring (SSEP and EMG)
Since its first use with SCS in 1986, IONM has transformed into a mechanism to assure safe SCS lead positioning in the anesthetized patient, particularly for paddle placement.165 IONM assesses the functional integrity of specific neural structures and pathways based on their functionality, with the most critical for DRG-S placement being somatosensory evoked potentials (SSEP) and electromyography (EMG).161 SSEP is a non-continuous method of monitoring the integrity of the neural circuit, by stimulating a peripheral nerve and sensing at the somatosensory cortex through scalp electrodes.166,167 These recordings require signal averaging, so there is a time delay until signal interpretation and surgeon notification of changes.167
Rates of device-related adverse events with DRG-S range from 3.1% to 36.8%, with neurologic complications responsible for only a small percentage of the total.1,168,169 Evidence is suggesting that IONM may improve the safety profile compared to monitored anesthesia care. In two retrospective studies utilizing IONM with DRG-S, a total of 182 DRG-S cases elicited 16 IONM alerts, with three patients experiencing a transient paresthesia in the post-operative period and no serious neurologic complications.160,161 Anesthetics and other factors can impact the reliability of IONM,169–171 see Table 4. Importantly, both SSEP and spontaneous EMG allow the physician to detect abnormalities early, determine the cause, and avoid or prevent further neurological insult.
 |
Table 4 Operative Effects on Intraoperative Neuromonitoring |
Of note, IONM does not exclude the possibility of central nervous system injury; thus, safe, skillful technique remains requisite. IONM changes necessitate ceasing surgical manipulation while physiological and pharmacological concerns are evaluated, and recent procedural steps reviewed and potentially reversed. Surgery may commence when baseline signals have returned.
Awake, Responsive Patient
A fully awake patient provides optimal neuromonitoring, as a patient reporting a sudden onset of neurologic symptoms such as radicular pain or paresthesia provides real-time clinical feedback. Anesthetic choice is patient- and provider-specific, which may be a limiting factor for this technique. A responsive patient negates the reliance on additional equipment and input from an electrophysiologic technician, at the potential expense of patient comfort. DRG-S lead placement may cause transient discomfort, particularly when passing through the foraminal ligaments. Delivery of a patient-specific anesthetic plan should be decided by the perioperative team and expectations established. Sedation can be titrated with short-acting benzodiazepines (ie, midazolam) and opioids (ie, fentanyl), and/or dexmedetomidine to maintain responsiveness. Administration of the alpha-agonist dexmedetomidine in procedural sedation demonstrated its efficacy in awake placement of SCS paddles placed through laminotomy approach, as well as for DRG-S.172,173 Propofol or ketamine are options, but should be utilized in a limited fashion and can be used without issue after lead placement, as neural injury is unlikely at that point. Unequivocally, as the patient is the monitor of neurological function, the patient needs to remain communicative and responsive to painful stimuli when IONM is not in use.
Safety Summary
Regardless of anesthetic method, the placement of DRG-S leads is considered safe overall, and complication rates are comparable to SCS when proper techniques are followed.161 Although predominantly transient, neurologic injury is one of the most devastating complications of DRG-S. Injuries may present as transient radiculopathy or neuritis, but can include more severe symptomatology, such as weakness, paralysis, and incontinence. Potential underlying causes include direct trauma, bleeding, or infection, and early identification of pending neurological injury is crucial for patient safety and outcomes.
Given the relative paucity of data comparing a general anesthetic with IONM during DRG-S placement as compared to a responsive patient with moderate sedation, formal recommendations cannot be made at this time. Further studies are required on this topic.
Technique
The traditional approach for DRG-S lead placement used an oblique angle, contralateral to the target foramen, entering skin two levels below the target foramen at the lateral border of the pedicle.162,174 The practice of anchoring DRG-S leads to the deep fascia during implant was recently found to reduce migration risk to ~1% per lead.175 Lead fracture was not increased by the use of an anchor in this study, as was a common belief at the time, but remained at ~4%.
Fracture rates were blamed on the integrity of the 1.0 mm DRG-S lead, which is significantly thinner and flexible when compared to a t-SCS lead. However, it was noted in a study on multifidus muscle stimulation, where SCS-sized leads were placed in a similar lateral oblique approach, that ~45% of leads demonstrated signs of fracture before 1 year. The authors hypothesized that these fractures were secondary to traversing the paraspinal muscles and resultant entrapment of the lead in the superficial plane.176,177 To potentially reduce the risk of lead fracture and migrations, this led to a novel ipsilateral, paramedian approach for DRG-S lead placement and anchoring to be presented.178 The ipsilateral technique maximizes an entry parallel to the spinous process and avoidance of paraspinal musculature and fascia and is discussed in the following text.
Ipsilateral Lead Placement Using a Paramedian Approach
This novel technique utilizes a paramedian approach, akin to SCS entry, designed to avoid the paraspinal musculature, and emphasizes cutting down to fascia during implantation. Placing the lead ipsilaterally holds several advantages, including ease and safety of placement given the paramedian trajectory and allowing for lateralization of leads in the epidural space and subfascial pocket. Additionally, reducing the angle of entry from midline often minimizes the need for 90 cm leads in lumbar cases.
The Tuohy needle entry at the skin is between the pedicle and the spinous process (SP) using a vertical plane trajectory, ipsilateral to the target foramen. To optimize placement:
- Align the rostral border of the lamina below the target interlaminar space using a cephalocaudal tilt on the fluoroscopy unit.
- With the laminar border aligned, rotate the fluoroscope 5–10° to the side of needle entry; this maximizes visualization of the working surface of the lamina and shifts the spinous process (SP) out of the planned trajectory.
- Advance towards the rostromedial lamina with needle position over the lamina throughout, until contacting periosteum. This guides needle depth prior to entering the ligamentum flavum.
- Redirect the Tuohy needle to the lower portion of the midline interlaminar space; maintaining a shallow angle maximizes clearance rostrally for the introducer sheath bevel. Load the introducer sheath with guidewire after epidural access.
- Next, align the inferior endplate of the level of the target DRG in midline view to maximize the infra-pedicular view. Rotate the needle’s bevel and curved introducer sheath toward the target and advance the sheath.
- Maintain dorsal position of introducer sheath throughout. Pass guidewire through the foramen, modifying deployment location should it deflect. Using small movements while stabilizating the sheath limits risks. Avoid passing the introducer sheath through the foramen.
- After the guidewire passes through the foramen, exchange the guidewire with the lead. A lateral fluoroscopic view confirms the appropriate foraminal plane. When adequate anterior-posterior (AP) and lateral position is obtained, create the “S” tension loop in the traditional manner. See Figure 7.
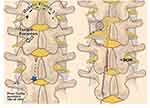 |
Figure 7 Anatomic landmarks for medialized ipsilateral approach. The blue star (*) denotes the puncture site. The light blue circle represents the traditional Tuohy needle puncture site. Medial approach ipsilateral “S” loop placed. For implant, the skin is incised from the infra-pedicular line to the Tuohy. Reproduced with permission from Chapman KB, Spiegel MA, Dickerson DM, et al. A paramedian approach for dorsal root ganglion stimulation placement developed to limit lead migration and fracture. Pain Pract. 2021;21(8):991–1000. © 2021 World Institute of Pain.178 |
The approach to implanting leads using the paramedian approach has been detailed for adjacent level lead placement and bilateral lead placement.177 When using either this or the traditional contralateral approach, it is vital to anchor leads and dissect to the deep fascia to minimize lead migration and fracture.
Alternative Techniques
Given the incidence of spinal surgery and central stenosis in the lumbar spine, the traditional anterograde placement of DRG-S leads may not be possible. Such anatomical variations may exclude DRG-S as a modality using the anterograde approach. However, in the appropriate patient, there still may be several options for placement for the experienced implanter. The following techniques have been described in literature: a transgrade, or retrograde contralateral technique,96,179 an outside-in, or transforaminal technique,124,180 and open surgical approaches.124,181 A summary of these techniques is demonstrated in Figures 8 and 9.
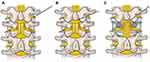 |
Figure 8 Schematic representation of transgrade DRG lead placement. (A) depicts the initial position where the Tuohy needle enters the skin. (B) depicts the Tuohy needle contacting the lamina prior to walking off and entering the epidural space. The red cross indicates graphic target for Tuohy needle. (C) depicts the Touhy needle within the epidural space with the introducer sheath and lead positioned along the superior aspect of the contralsteral foramen, one level below. Reprinted with permission from Chapman KB, Ramsook RR, Groenen PS, Vissers KC, van Helmond N. Lumbar transgrade dorsal root ganglion stimulation lead placement in patients with post-surgical anatomical changes: a technical note. Pain Pract. 2020;20(4):399–404.© 2019 World Institute of Pain.179 |
 |
Figure 9 Outside in approach: (A) Far oblique view with Tuohy needle making contact with the SAP. (B) AP view with Tuohy needle contacting SAP. (C) Tuohy needle retracted slightly with introducer and lead loaded and directed ventrally to the foramen. (D) Lead advanced alone into. (E) Lead advanced and Tuohy retracted slightly. (F) Loops made in soft tissue by advancing the introducer slightly and advancing lead. Then rotating introducer caudally and repeating. (G and H) Final AP and lateral views of lead position. Reproduced with permission from Chapman KB, Nagrani S, Patel KV, Yousef T, van Helmond N. Lumbar dorsal root ganglion stimulation lead placement using an outside-in technique in 4 patients with failed back surgery syndrome: a case series. A&A Pract. 2020;14(10):e01300.180. Available from: https://journals.lww.com/aacr/Abstract/2020/08000/Lumbar_Dorsal_Root_Ganglion_Stimulation_Lead.14.aspx.180 |
Sacral Anatomy Relevant to DRG-S Placement
A detailed understanding of sacral anatomy can reduce multiple common issues encountered in sacral DRG-S lead placement. The sacrum is formed by the fusion of five progressively smaller sacral vertebrae and their costal elements, stabilizing the lordotic lumbar spine at the lumbosacral angle, which measures roughly 35.182,183 Two ridges run lateral to the midline sacral crest called the intermediate crests, formed from the fused articular processes of the sacral vertebrae. They are largest at the S1 level, where they may partially obstruct the medial aspect of the S1 posterior sacral foramen (PSF) in the AP view. This ridge narrows and forms the sacral cornua caudally; it is a landmark for the sacrococcygeal hiatus, and lies just medial to the S4 PSF.
The sacral canal measures approximately 30 mm in width and 20 mm in height at the S1 level, and decreases in diameter caudally to the sacral hiatus.184–186 The thecal sac ends at approximately the S2 level.
The sacral foramina have an anterior and posterior component. The anterior sacral foramen (ASF) communicates with the sacral canal through the intervertebral foramina, which is bordered rostrally and caudally by the pedicles and laterally by the fused winged sacral transverse processes.187 The PSF is an opening on the dorsal aspect of the sacrum which allows the sensory fibers to exit. The 4 PSF and ASF vary in size widths and depths, with the S1 PSF measuring 12×10 mm followed by a smaller 8 mm × 8 mm S2 PSF.184,185 The rostral border of the S1 PSF lies approximately 2.5 cm from the superior margin of the sacrum and medial border 2 cm from midline, and 2.5 cm from the PSIS.188 In the lateral view, the ASF lies at the level of the remnant of the fused sacral intervertebral discs.
The ASF fan out inferolaterally, with the DRG and nerve root exiting the sacral canal at approximately 30° degrees ventrally and 15 caudad to the transverse plane, and are accompanied by foraminal vessels. Intraforaminal instrumentation should be minimized as the DRG is sensitive to both physical and chemical stimuli.189,190 This avoids the potential for neuritis, paresthesia, or weakness, all of which are commonly self-limited.191–193
Accessing the sacral foramen can be challenging, as the PSF is smaller than the ASF and its view is often obscured under fluoroscopy. Ipsilateral oblique techniques have been used to improve foraminal visualization,194 but it places the DRG directly in the trajectory of the Tuohy needle and introducer sheath.195 A recently described technique using measurements for sacral lead placement was designed to avoid these issues.196 See Figure 10.
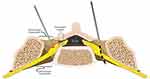 |
Figure 10 Axial view of the S1 with needle placement from the oblique, and AP/medial approach. Reprinted from Chapman KB, van Helmond N, Kallewaard JW, et al. An anatomy-informed, novel technique for S1 dorsal root ganglion stimulation lead placement. Pain Med. 2022;23(10):1750–1756. Creative Commons.196 |
Sacral DRG-S Technique
A 35° cephalad tilt with the fluoroscopic unit is required to compensate for the lumbosacral junction and convex sacrum, as the distances are measured horizontal to the surface of the sacrum. Draw lines over the aligned sacral promontory and the midline with a skin marker. The foramen falls approximately under the L5/S1 facet joint. Our initial target for the S1 foramen is the medial aspect of the PSF, measuring 2.0 cm from midline and 3.0 cm from the squared sacral promontory. See Figure 11.
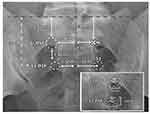 |
Figure 11 Sacral measurements under fluoroscopy. Sacral endplate aligned to ~35. Reprinted from Chapman KB, van Helmond N, Kallewaard JW, et al. An anatomy-informed, novel technique for S1 dorsal root ganglion stimulation lead placement. Pain M. 2022;23(10):1750–1756. Creative Commons.196 |
There are several principles to placement:
- Minimize instrumentation after posterior foraminal access.
- Passing the lead can often be performed without the introducer.
- Intra-canal loops can be challenging to place, and should be performed cautiously, in both the awake and IONM patient.
Use a 22g Quincke “finder” needle to enter the PSF, and, after confirmation, remove and replace with the Tuohy needle. Contact periosteum at the medial lip of the foramen and “walk off”, stopping as you penetrate the PSF’s ligaments. Limit passing the Tuohy needle beyond the posterior plate of the sacrum and check lateral views to assure placement.
Advance the introducer sheath loaded until the first indicator line on the sheath reaches the hub. Rotate the bevel of the needle to open inferolaterally and pass the lead through intraforaminal space with minimal sheath advancement. The final position should have the distal contact of the lead nearing the anterior border of the sacral vertebral body.
To create the sacral loop, with the introducer sheath within the Tuohy, rotate the needle bevel and introducer rostrally. Apply gentle caudad pressure on the Tuohy hub and advance the introducer sheath several millimeters rostrally. As the tip appears in the cephalad direction, slowly continue advancing the introducer sheath and lead rostrally. Once the lead bows in the rostral direction, retract the stylet 3–4 cm and attempt to advance the lead. If manipulation of the introducer sheath within the canal is required, slow movement combined with a responsive patient or IONM is imperative. Avoid blindly maneuvering the introducer sheath within the foramen.
Once the cephalad loop is created, retract the introducer sheath to within the Tuohy needle, then rotate caudad, and advance lead. The lead advancing slightly into the S1 foramen may be acceptable given the foraminal diameter. In the event intra-canal loop placement is not possible, extra-canal loop can be placed to reinforce sacral leads prior to anchor placement.
Cervical and Upper Thoracic Placement
To date, cervical and thoracic DRG-S above the level of T10 is off-label in the United States, but is approved in many areas of the world, and is performed off-label in the USA. Potential applications published in literature include CRPS of the upper extremity, intercostal neuralgia, post-surgical neuropathic pain in the hand, thorax, breast, and shoulder, peripheral vascular disease (PVD), and PHN. Benefits of DRG-S in the cervical and thoracic spine are similar to those demonstrated in the lumbar region. As the spinal cord lies ventrally, lead placement at these levels holds inherent risk, which can be limited with safety monitoring precautions as mentioned in this paper and should be reserved for experienced implanters.
The smaller Aδ- and C-fibers reach their end organ targets through the sympathetic chain. Lead placement directed at the stellate ganglion, with its ability to amplify sympathetic signaling, has the potential to have profound results. Along these lines, the most targeted levels in the cervical spine are C8 and C7, respectively for neuropathic pain. Propagation into the sympathetic chain allows for multi-dermatomal coverage for neuropathic pain syndromes, colloquially described as “cross-talk”.
The normal spine transitions from thoracic kyphosis to a cervical lordosis. Recent cervical magnetic resonance imaging (MRI) should be considered prior to DRG-S, particularly in those over the age of 40, or with a history of cervical spinal disease. The average healthy adult cervical spinal canal diameter from C3-C7 measures approximately 13 mm, with roughly 8 mm or 60% taken by the spinal cord itself.197,198 Disc herniations, osteophytes, and ligamentous hypertrophy may further decrease this diameter and limit the ability and potentially increase risks of placement. The larger curved introducer sheath protrudes 8 mm from the shaft, and thus ventral rotation can lead to contact, displacement, or trauma to neural structures.
The cervical ventral and dorsal roots exit the spinal canal over the pedicle and enter a bony groove formed by the short transverse process of the cervical vertebrae. The foramina of the cervical spine are distinct from the lumbar spine in that there is a medial and lateral zone, with the medial zone the opening of the spinal canal at the level of the pedicle, and the lateral zone the bony transverse process and groove.199 The bony projection of the lateral foramina travels at a 45-degree angle ventrally and protects its contents with the bony anterior and posterior tubercles.200 The vertebral artery transects this bony lateral foramen through the transverse foramen. The DRG lies outside the spinal canal within the lateral foramen dorsal to the vertebral artery. See Figure 12. The relation of the DRG to the vertebral artery within the bony groove makes it imperative not to pass the introducer sheath outside of the medial foramen. The vertebral arteries enter the transverse foramen at the level of C6, travels within the bony transverse processes, and above C1 heads centrally to join the contralateral vertebral artery to form the basilar artery of the brainstem. Injury to this artery can potentially lead to paralysis, stroke, or death.
In some settings, it may be prudent to access the foramen with the shorter bevel sheath. Bevel control is imperative as rotation of the bevel ventrally can lead to contact with the cord. Guidewire utilization can limit sheath manipulation during lead placement. The pliability of the guidewire and lead are less likely to cause vascular insult should contact occur.
The extra-canicular location of the DRG at these levels are lateral to the pedicle and lie in the oblique plane as described above. Thus, programming should occur using contacts over this region.
Cervical and thoracic DRG-S lead placement should be reserved for experienced implanters who are well versed with DRG-S. The procedure must be performed with either the awake patient or using IONM, with small, precise movements, with constant reevaluation of neurologic status.
As such, when it comes to choosing the optimal patient for cervical DRG-S, the most common diagnosis is neuropathic pain in the hand, as the C6-8 levels may carry less inherent risk. The potential to influence the sympathetic nervous system at these levels may allow for multi-dermatomal coverage with a single lead placement. Placement above C5 is generally not recommended or supported in the current evidence. Fortunately, because of the mechanisms discussed, placement at C6 or below, usually provides adequate coverage for the entire upper limb. The eventual development of a paddle lead for DRG may make higher cervical placement more accessible for conditions such as occipital nerve pain or lower facial pain.
Upper Thoracic Lead Placement
The steep angulation of the mid-thoracic spinous processes can make lead placement challenging. The same considerations for cervical lead placement hold true for thoracic placement regarding IONM and the avoidance of rotating the bevel ventrally.
Prone positioning on an operating table creates a cervical kyphosis, which can be exaggerated by the placement of a pillow under the chest. Kyphosis maximizes the interlaminar window; however, it increases the angle of epidural access. Experience can predicate whether an in-plane or out-of-plane approach is taken to achieve a shallow epidural access.
Level of Lead Placement by Diagnosis
Both DRG-S and SCS utilize orthodromic and segmental antidromic mechanisms; however, success of DRG-S has a greater dependance on the level of placement per condition.78 DRG-S utilizes dermatomal signal filtering mechanisms for better dermatomal coverage, as well as the potential for sympathetic amplification, and convergence within the DH for broader effects.10,79 Utilizing alternative mechanisms, DRG-S is demonstrating effects on not only neuropathic pain, but on more mixed-type pain syndromes.
For dermatomal neuropathic pain, the lead should be placed at the effected dermatomal level. This utilizes local effects of DRG-S on filtering of incoming signaling and additionally through traditional Aβ gating mechanisms.
Multi-dermatomal neuropathic pain can be covered by individual leads, but strategic single lead placement can be as effective. Mechanistically, activation of Aβ fibers at the DRG propagate APs into the DH and dorsal columns, where they can inhibit through Aβ collaterals at adjacent levels in a manner similar to SCS. The authors concur that the level with the most potential for single-lead, multi-dermatomal coverage is at the S1 levels. A case series by Skaribas et al detailed coverage of the entire foot with S1 placement,201 as further evidenced in multiple patients from other case reports and series.2,8,58,128 See Table 5.
 |
Table 5 Level of Lead Placement per Diagnosis and per Body Region |
For mixed-pain syndromes lead placement is more specific, requiring leads placed at each DRG innervating the source of pain, whether bone, muscle, or skin. This is secondary to the more specific pain transmitting Aδ- and C-fibers, which travel in Lissauer’s tract to specific spinal segments to synapse with second-order neurons. Here, accompanying Aδ- and C-fiber LTMRs modulate sensation through pre- and post-synaptic inhibition. Stimulated Aβ fibers enter at the corresponding spinal segment, eventually joining the inhibitory collateral network of the dorsal columns.80 Additionally, capitalizing at points of convergence within the spinal cord may offer multi-dermatomal mixed-pain syndromes such as low back pain.8 See Figure 13.
 |
Figure 13 Demonstrates collateral network of Aβ, Aδ, and C-fibers as they travel in Lissauer’s tract to a specific segment of the dorsal horn. |
Salvage Therapy
The term “salvage” describes the reintroduction of an alternate form of neuromodulation to recapture therapeutic efficacy. The distinct and alternative mechanisms of action specific to DRG-S allow for its effective application as a salvage therapy, reported in multiple papers.8,88,94,139,202–204
The efficacy of DRG-S as a salvage modality was demonstrated in a retrospective review of 60 patients who lost efficacy or failed SCS and were trialed with DRG-S with 56 implanted at a mean 34 months' follow-up. The cohort demonstrated a 55% improvement in VAS (8.7 ±1.2 to 3.8 ±2.1), 52% in ODI (64 ±14% to 31 ±18%), and 44% improvement in EQ-5D-5L scores (0.40 ±0.15 to 0.71 ±0.15) at last follow-up. There were two patients explanted for lack of pain relief over that period, at 21 and 28 months after implant. CRPS (n=28) and FBSS (n=24) were the most common diagnoses. T12 and S1 were the most common leads placed. Failed t-SCS, burst-SCS, and HF-SCS were salvaged.205
Device Programming
The consequence of the DRG’s minimal CSF buffer and significantly lower energy delivery requirements is the resultant narrower therapeutic window. This can lead to loss of therapeutic benefit with understimulation and range from paresthesia, to painful paresthesia, to motor stimulation with overstimulation. As such, finer control than the 0.10 mA and 10 Hz steps available with SCS systems is necessary. Recent bench work is demonstrating safety and efficacy of the burst waveform on the DRG,206,207 and two small studies have used paresthesia-free waveforms with SCS leads on the DRG successfully.95,208 Given the limited data for these non-conventional programming approaches, the following discussion refers exclusively to the FDA-approved DRG-S specific Proclaim system (Abbott, Chicago, IL, USA).
DRS-S Specific Technology
Both t-SCS and DRG-S utilize a tonic waveform; however, when placed over the DRG, there are clinical effects not seen when applied over the dorsal columns.2,3,107 Commonly applied DRG-S settings in literature hover near 20 Hz, 300 µs pulse width, and 0.5 mA amplitude. We have witnessed DRG-S progress from higher-frequency settings similar to t-SCS71 to the lowest frequency of the device’s programming capabilities, 4 Hz.2 The fractional charge delivery of DRG-S directly modulates Aδ- and C-fibers in addition to afferent Aβ fibers and makes for a more specific form of neuromodulation.
The 6.1×5×1.3 cm commercial DRG BluetoothTM enabled IPG can power 4 leads simultaneously. The characteristic that sets the Proclaim IPG from SCS is its ability to deliver a more specific, finely tuned delivery of electrical current, with ranges demonstrated in Table 6. The Proclaim IPG’s initial charge is 3.0 V, and it has a proposed lifespan of 6.5 years using a two-lead configuration.159 However, a recent evaluation of IPG battery consumption of 106 consecutive patients implanted with a mean 3.7 leads per patient for a mean 22 months used only 0.013 V, or less than 5% of the charge until the elective replacement indicator alarm is activated.209 These details on charge delivery and battery usage reveal that 6.5 years using 2 leads was likely an underestimation of potential IPG lifespan.
 |
Table 6 Operating Parameters of DRG-S and SCS |
The 1.0 mm commercially available slim tip lead is available in 50 cm or 90 cm lengths, each with four 1.25 mm metallic contacts spaced by 5 mm spanning its distal 2.5 cm. SCS leads can be utilized on the DRG with systems capable of subthreshold parameters, although lead diameter and technical challenges to placement are of concern.
The patient programmer is an application which gives the patient control of their device based on programs set forth by the clinician programmer. This gives the patient control over several functions, including turning their device on and off and changing to “surgery mode” or “MRI mode” if necessary. Most importantly, it allows one to toggle between different pre-determined programs, and once within a program it allows the patient to adjust the strength of stimulation per lead in picoamps. This empowers the patient to self-regulate should subtle changes occur over time.
The clinician programmer is the master controller for the implanted DRG-S system, controlling settings on the patient programmer. Fifteen individual programs can be created and stored, utilizing varying contact configurations, each with adjustable parameters of frequency, pulse width, amplitude, and “on/off” paradigms. System data can be queried, including patient demographics, impendence levels of electrodes per lead, and the total remaining charge on the IPG. “MRI mode” and “surgery mode” regulation is also available.
Programming
Paralleling cardiac pacemakers, remote programming has now been introduced to neuromodulation. This technology allows patients greater access to reprogramming other than those determined by geographic limitations. After patient consent, remote reprogramming can be performed through a wireless connection between the clinician and patient virtually.210
When programming, there are several general principles:
- Program delivery should be below sensory perception.
- Deliver the lowest possible efficacious dose, which in turn conserves energy.
- Total charge delivered/second is measured in microcoulombs per second (µC/s), and is the product of frequency (Hz) × pulse width (pW) × amplitude (mA).
- Adjustment pW for dermatomal coverage – although paresthesia-based dermatomal coverage may not be necessary.107
- Dosing paradigms based on “on/off” paradigms.
- Avoid overstimulation through patient education.
The DRG-S system utilizes a tonic waveform delivered through a 4-contact lead. Effective at paresthesia and paresthesia-free levels, device programming should start at subthreshold levels given patient preference for paresthesia-free pain relief.211,212 Incremental steps in picoamps allows the ability to fine-tune the electrical field over the DRG with a tonic waveform, and results in a far lower total charge per second for efficacy.79
Ideally, programming is performed with the guidance of recent imaging. As the DRG is most commonly located under the pedicle,213 programming begins with paresthesia mapping with electrodes under the pedicle.
Electrical current travels from anode (+) to cathode (-), and their configuration shapes the electric field. The energy required for depth of penetration is proportional to the distance and number of the anode and cathode. Although multiple derivations with 4 contacts are possible, the most basic for DRG-S is the “skipped bipole” configuration, which would create an electrical field over 1 cm in length, displaced toward the cathode. A simple “extended guarded array” configuration of (+, -, -, +) can span over 2 cm, at the expense of increased electrical output.
Using the three adjustable parameters, attempt to allocate dermatomal paresthesia in the pain distribution. Dermatomal coverage may not be achieved in some cases, and the closest possible distribution is acceptable. Dermatomal coverage does not predicate pain relief as seen with t-SCS.
The need for intraoperative paresthesia mapping is at the discretion of the provider. A lead placed with two contacts within the foramen in the AP and lateral fluoroscopic view is often adequate. Initial post-operative programming should be delayed until any anesthetic effects have dissipated. Waiting this period also allows any fluid in the epidural space and foramen to dissipate as well. Saline, heme, or serous fluid increases impedance and can lead to overstimulation if the patient is programmed before these fluids dissipate.
After selecting the electrode array, the following approach can be utilized:
- Increase mA to the threshold
Paresthesia is typically achieved in lumbar DRG at <1.0 mA and is dependent on the distance of the electrical field generated from the DRG. Inability to capture paresthesia would indicate that the chosen contacts are outside the field of neural tissue. Sacral leads may require slightly higher mA given the larger anterior sacral foraminal diameter and an often perpendicularly placed lead relative to the laterally spanning DRG.33,214 Additionally, variables such as facet arthropathy, osteophytes, and disc material that can cause foraminal size to rapidly decrease are absent in the sacrum.
Pulse width (pW) is the duration of the pulse applied, and initial programming can be started at 260 µs. As with SCS, adjustment of the pW can be used to capture different nerve fibers within the electrical field. Interestingly, when applied to the DRG, we see decreasing pW extending dermatomal paresthesia coverage. The opposite often occurs with SCS. Given that the mA is relatively fixed to subthreshold levels, pW is the variable most likely used to achieve a change in dermatomal coverage.
DRG-S has efficacy at the range of frequencies available with the Proclaim IPG. Although 20 Hz is the default frequency and the rate most published on, efficacy has been demonstrated at as low as 4 Hz, and at much higher levels.2,72,79 With no demonstrable differences in clinical outcomes within this range, the four-fold decrease in charge delivered/second favors using very low frequencies. Of note, lowering frequency may require a small increase in mA to recapture subthreshold levels.
If unable to achieve paresthesia, distribute the electrical field across different contacts of the array. If unable to achieve paresthesia with multiple contact distributions, an evaluation for lead migration should be made and confirmed with imaging.
Patient education on the use of the patient programmer and understanding of appropriate therapeutic levels of stimulation is paramount to success and limits reprogramming visits. A patient that comprehends that stimulation levels should be maintained just below sensory perception prevents the patient from dropping their stimulation significantly and falling outside the therapeutic window. The level at which DRG-S loses efficacy below paresthesia threshold is not yet known, thus educating the patient to keep their amplitude 1 step (0.025 mA) below their sensing stimulation assures coverage.
If the patient has inadequate pain relief with their current program/programs and is within the therapeutic window, then attempts at reprogramming can be made.
Options for reprogramming are:
- Modifying the contacts chosen to “shape” the electrical field. The electrical field is based on the configuration of electrodes used.
Or
Dosing Strategies
As with pharmaceuticals, the concept of lowest therapeutic dose for efficacy minimizes risk potential. Discontinuous stimulation is another means to reduce total charge delivered/s to neural tissue.
The tonic low-frequency waveform has not displayed efficacy on the DC.215,216 Burst DR-SCS on the other hand has demonstrated efficacy when cycled, resulting in an extension of battery life to 10 years.217,218 DRG-S has similarities with Burst DR-SCS in regard to its effects on BOLD,219,220 its efficacy at subthreshold levels, and clinical observations of efficacy minutes to hours after lead pull suggesting long-term potentiation.220 These similarities led to a study comparing DRG-S when applied continuously, to one minute “on”/one minute “off”, and one minute “on”/two minutes “off” dosing paradigms, demonstrating no clinical difference in VAS, ODI, or EQ-5 noted between the groups.3 Although the optimal dosing parameters remain unknown, implementing dosing paradigms decreases the total electricity delivered to neural tissue and should be considered when programming.
Utilizing intermittent dosing and very low frequencies delivers the smallest possible electrical charge to the nervous system without altering outcomes. Applying very-low-frequency stimulation on the DRG delivered with cycling paradigms can significantly decrease total charge delivered per second to neural tissue compared to traditional and high-frequency SCS.2,3
Additional Programming Considerations
As previously reviewed, loss of efficacy, or habituation, has not yet been demonstrated with DRG-S.156,157 Given such, if patients implanted with DRG-S present with worsening pain, efforts should be made at reprogramming and, if available, review of recent imaging to evaluate lead location. Device evaluation and reprogramming efforts should be performed prior to alternative options such as medication changes or interventional procedures.
Complications in Literature: Mitigation
As with many new therapies, a developing understanding of the potential adverse events and factors to mitigate these negative events grows. Complications such as lead migration and fracture soon after became events that some have tried to use to define this therapy. Issues that mirrored the early stages of SCS were evidenced by Racz et al in 1989, where they reported on 26 patients experiencing 18 migrations and 6 lead fractures,221 and it was not until 2004 that the midline anchoring technique was introduced.222
Assessing the rates of DRG-S complications requires a thorough understanding of the hardware, technology, and techniques used for placement. As such, infection rates seen in earlier European DRG-S studies compared to US studies demonstrate substantially higher rates.72,82,223 North et al in 2020 in the PROMISE RCT demonstrated that the incidence of infection for subjects trialed with SCS >10 days was 24.1% vs 1.4% for subjects trialed ≤10 days, and early European DRG-S studies used extended trials of up to 30 days.224 Similarly, earlier studies used paresthesia-based programming with frequencies similar to SCS, leading to a battery depletion time which was similar to an SCS non-rechargeable IPG.72,225,226 And the initial lead iteration contained a “ball tip” lead that was prone to dislocation, adding to the hardware complications. Lead migration and fracture mitigation via lead anchoring is discussed below.
Our growing understanding of the optimal level/s for lead placement per condition is still evolving, and, if suboptimal, outcomes will likely correspond. This was evidenced when Huygen et al placed L2 DRG leads for low back pain of multiple origins including 5 patients with foot pain.82 Additionally, there was a lack of S1 lead placement in virtually all the early prospective studies, a junctional lead location believed to be a level of convergence for lower extremity neuropathic pain.1,72,201
Migration and Lead Fracture
Akin to early SCS adoption, the occurrence of lead fractures and migrations with DRG-S frequency was unnecessarily high.227,228 A recent pooled analysis of 249 implants (756 leads) with a mean follow-up of 27 months demonstrated that anchoring leads reduced lead migration and fracture rate from 8.4% to 1.4% and 3.1% to 1.9% per lead implant, respectively.175 This study also demonstrated that anchoring leads did not increase fracture rate, as some practitioners believed, but rather lowered rates.
Two factors have been associated with lead fracture: Entrapment within the superficial fascial plane, and tension from the oblique trans-paraspinal muscle approach.177,229 This has led to the introduction of a new implant technique using a paramedian, ipsilateral approach.178
The authors recommend conscientious anchoring below the fascial planes, when possible, to reduce migration and lead fracture.
Neural Insult
Given the sensitive nature of the DRG, insult to the DRG can lead to complications such as neuritis or weakness, both usually transient although long-term injury may rarely occur.227 It is established that neural injury can be limited though monitoring neural function, either through the awake patient or IONM. General anesthetics should be avoided, including intravenous general anesthetics such as propofol without IONM while leads are being placed. Minimize foraminal instrumentation with the semi-rigid introducer sheath and rotating its bevel ventrally in the canal. Consider repositioning the introducer sheath or re-accessing the epidural space at a different location rather than making multiple repeated failed attempts at foraminal passage.
The authors recommend monitoring for neural function through the awake patient or through IONM.
Electromagnetic Energy Related
The BluetoothTM technology implanted in the current DRG-S header is sensitive to strong electromagnetic fields. Excessive exposure may cause an inability to sync the IPG to programmer requiring IPG replacement.
Magnetic Resonance Imaging
The current Proclaim IPG is MRI-conditional using 50 cm leads for head and extremity MRI using prescribed parameters when placed below the T10 level.230 Placing the device into “MRI mode” restricts energy transmission into the IPG and potential damage to the BluetoothTM technology.
As stated in the commercial IPG MRI procedural manual, the following potential adverse events may occur in the MRI environment: Lead electrode heating resulting in tissue damage or serious patient injury; IPG heating resulting in tissue damage in the implant pocket, patient discomfort, or both; induced currents on leads resulting in overstimulation or shocking sensations; damage to the IPG or leads causing the system to fail to deliver stimulation or causing the system to deliver overstimulation; damage to the functionality or mechanical integrity of the IPG resulting in the inability to communicate with the IPG; movement or vibration of the IPG or leads.230
Of note, the controller that enables “MRI mode” is required to take the device out of “MRI mode”. A separate device or clinician programmer cannot perform this function if it did not initiate placement into “MRI mode”.
Electrocautery-Based Radiofrequency Energy
Radiofrequency energy is in the spectrum of electromagnetic energy. Thus, the use of electrocautery and radiofrequency energy can render the IPG unable to connect to the programming device. “Surgery mode” was designed to limit this potential. Traditional precautions include limiting electrocautery use, placement of the grounding pad adjacent to procedural site, and use of bipolar cautery devices. In addition to losing ability for device connection, there is a possibility that motor or painful stimulation may occur if the device remains in the “on mode”.231,232
The authors recommend ensuring patient and clinician awareness of various safety steps in protecting the patient and DRG-S device during MRI and radiofrequency energy exposure.
Future Work
Expanded Indications
The evidence stated in our narrative review provides ample opportunity for expansion of indications. The greatest opportunity is a greater understanding and utilization in what is appearing to be the greatest difference from other forms of neuromodulation, and that is the treatment of chronic mixed nociceptive/neuropathic pain conditions such as post-joint replacement pain. Modulation of the sympathetic nervous system would allow for greater treatment of non-pain related conditions, including cardiac, vascular, and neuroinflammatory conditions.
Programming and Software Improvements
Programming Improvements
The current optimal programming is still unknown. We have seen the potential for very low frequency and intermittent dosing with this therapy; however, a standardization of programming should be developed as our understanding improves. Regarding programming parameters, of interest would be the ability to stimulate at fractional levels, as evidence supports DRG-S efficacy at less than 1 Hz.75,233
As our understanding of neuronal messaging improves so to can our ability deliver stimulation parameters to achieve the desired outcomes. The tonic waveform available on the DRG at subthreshold levels provides efficacy at low energy levels. However, the application of alternative waveforms on the DRG is yet to be studied. Potential application of a closed loop feedback on the DRG may be of interest as patients utilizing paresthesia-free therapy may fall too far below threshold and lose benefit, particularly in the population that is less tech savvy.
Alternative Waveforms
In the rodent model, passive recharge burst waveform demonstrated efficacy at both 20 and 40 Hz interburst frequency.234 In addition, Franken et al demonstrated that tonic waveform and burst on the DRG were equally effective in rodents.207 In humans, Al-Kaisy et al used an active recharge burst on the DRG with safety and efficacy.96 High-frequency stimulation also has been efficacious on the DRG.208 These studies deem further work on the clinical application of alternative waveforms on the DRG worthy.
Hardware/IPG
Opportunities for improvements in future generations of IPG include conditional MRI compatibility, miniaturization of the IPG, and alternative lead designs. The current commercial system is MRI-conditional for head and extremity using 50 cm leads, full body MRI conditionality. Additionally, the fractional delivery requirements with DRG-S allow for a potentially smaller IPG.
Improvements in lead design and integrity could be beneficial, in addition to alternative placement options. These possibilities include a paddle lead for surgical placement, a lead designed for outside in placement, or a version of a self-anchoring lead such as a tined lead. Improvements in current lead fixation methods could be an easier-to-apply, radiopaque anchor, or a self-deploying anchoring system.
Conclusion
DRG-S is a relatively novel neuromodulation technique designed to target focal neuropathic pain. As our understanding of the therapy evolves, the scope of therapeutic application and evolution of technique similarly expands. As with many novel therapies, the learning curve involves not only learning of potential therapeutic applications and mastery of technique, but also an in-depth awareness of potential complications.
This work highlights some of the unique characteristics of the DRG and its attractiveness as a target for neuromodulation. As the quantity of evidence-based literature is still limited, this piece was designed to serve as a guideline for safe application of the therapy to the best of our current knowledge. We hope the potential this therapy holds inspires readers to collect data and pursue further research required to support or dispute our current understanding of DRG-S.
Abbreviations
ACNES, anterior cutaneous nerve entrapment syndrome; AP, action potential; ASF, anterior sacral foramen; ASPN, American Society of Pain and Neuroscience; BDI, Beck Depression Inventory; BPI, Brief Pain Inventory; CGRP, calcitonin gene-related peptide; CNS, central nervous system; CRPS, complex regional pain syndrome; CSF, cerebrospinal fluid; DH, dorsal horn; DPN, diabetic peripheral neuropathy; DRG, dorsal root ganglia; DRG-S, dorsal root ganglion stimulation; DRR, dorsal root reflex; EMG, electromyography; EOS, endogenous opioid system; EQ-5D, EuroQOL health-related quality of life instrument; FBSS, failed back surgery syndrome; GABA, gamma-aminobutyric acid; HADS, Hospital Anxiety and Depression Scale; HIV, human immunodeficiency virus; IONM, intraoperative neuromonitoring; IPG, implantable pulse generator; LBP, low back pain; LTMR, low-threshold mechanoreceptor; MCS, mental component scale (of SF-36 family of scales); MME, morphine milligram equivalent; MRI, magnetic resonance imaging; MSNA, multiunit postganglionic sympathetic nerve activity; NRS, numeric rating scale; NSLBP, non-surgical low back pain; ODI, Oswestry Disability Index; PCS, Pain Catastrophizing Scale; PCS, physical component scale (of SF-36 family of scales); PDI, Pain Disability Index; PHN, postherpetic neuralgia; PLP, phantom limb pain; PN, peripheral neuropathy; POMS, Profile of Mood Scale; PSF, posterior sacral foramen; PSPS, post-surgical pain syndromes; PVD, peripheral vascular disease; pW, pulse width; QLIP, Quality of Life Impairment by Pain Inventory; QST, quantitative sensory testing; RF, radiofrequency; SAP, superior articular process; SCS, spinal cord stimulation; SNS, sympathetic nervous system; SP, spinous process; SSEP, somatosensory evoked potentials; TKA, total knee arthroplasty; t-SCS, tonic spinal cord stimulation; USPSTF, United States Preventive Services Task Force; VAS, visual analog scale.
Acknowledgments
Third-party editing assistance was provided by Allison Foster, PhD, of Foster Medical Communications.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Dr. Deer and Dr. Chakravarthy both served as senior corresponding authors.
Disclosure
Kenneth B Chapman has research funded by Abbott. Krishnan Chakravarthy is a consultant to Medtronic, Biotronik, SI Bone, Vertos, and Vivex Biologics, a shareholder in Mainstay Medical, Aya Biosciences, Higgs Boson Health, Oska Wellness, Rune Labs, UMEHEAL, Yantra Biomedical, and Nalu Medical, and founder of Accufix Medical, Douleur Therapeutics, and NXTSTIM. Jacqueline Weisbein is a consultant for Abbott, Medtronic, Saluda, Biotronik, and SI Bone. Kiran V Patel is a consultant and speaker for Abbott. Jonathan M Hagedorn is consultant for Abbott, Boston Scientific, Medtronic, Nevro, and Saluda. Timothy Deer is a consultant for Abbott, Ethos, Medtronic, Boston Scientific, Nevro, Nalu, Tissue Tech, Spinal Simplicity, and Saluda, has equity in Saluda, SPR, Spinethera, Nalu, Cornerloc, Ethos, PainTEQ, Spinal Simplicity, and Vertos, and has research funding with Boston Scientific, Abbott, and Saluda. He also is a research investigator for Abbott, Avanos, Mainstay Medical, and PainTEQ. He reports a patent pending to Abbott. Dawood Sayed reports personal fees from Abbott, Medtronic, Merit, and Boston Scientific, outside the submitted work. Corey Hunter reports personal fees from Abbott, during the conduct of the study; grants and/or personal fees from Saluda, Nalu, Biotronik, Boston Scientific, PainTEQ, outside the submitted work. David Dickerson reports grants and/or personal fees from Abbott, SPR Therapeutics, Pfizer, Nalu, Myovant Biosciences, and Vertos Medical, outside the submitted work. David W Lee is a consultant for Abbott, Medtronic, Boston Scientific, Mainstay Medical, Petal Surgical. The authors report no other conflicts of interest in this work.
References
1. Deer TR, Levy RM, Kramer J, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. J Pain. 2017;158(4):669–681. doi:10.1097/j.pain.0000000000000814
2. Chapman KB, Yousef TA, Vissers KC, van Helmond N, Stanton-Hicks M. Very low frequencies maintain pain relief from dorsal root ganglion stimulation: an evaluation of dorsal root ganglion neurostimulation frequency tapering. Neuromodulation. 2021;24(4):746–752. doi:10.1111/ner.13322
3. Chapman KB, Tupper C, Yang AH, van Helmond N, Yousef T. Intermittent dorsal root ganglion stimulation is as efficacious as standard continuous dosing in treating chronic pain: results from a randomized controlled feasibility trial. Neuromod Technol Neural Interface. 2021. doi:10.1016/j.neurom.2021.10.008
4. Deer TR, Levy RM, Kramer J, et al. Comparison of paresthesia coverage of patient’s pain: dorsal root ganglion vs. spinal cord stimulation. An ACCURATE study sub-analysis. Neuromodulation. 2019;22(8):930–936. doi:10.1111/ner.12920
5. Gemes G, Koopmeiners A, Rigaud M, et al. Failure of action potential propagation in sensory neurons: mechanisms and loss of afferent filtering in C-type units after painful nerve injury. J Physiol. 2013;591(4):1111–1131. doi:10.1113/jphysiol.2012.242750
6. Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromod Technol Neural Interface. 2015;18(1):24–32. doi:10.1111/ner.12247
7. Chao D, Zhang Z, Mecca CM, Hogan QH, Pan B. Analgesic dorsal root ganglionic field stimulation blocks conduction of afferent impulse trains selectively in nociceptive sensory afferents. Pain. 2020;161(12):2872–2886. doi:10.1097/j.pain.0000000000001982
8. Chapman KB, Groenen PS, Patel KV, Vissers KC, van Helmond N. T12 dorsal root ganglion stimulation to treat chronic low back pain: a case series. Neuromod Technol Neural Interface. 2020;23(2):203–212. doi:10.1111/ner.13047
9. Kallewaard JW, Edelbroek C, Terheggen M, Raza A, Geurts JW. A prospective study of dorsal root ganglion stimulation for non-operated discogenic low back pain. Neuromod Technol Neural Interface. 2020;23(2):196–202. doi:10.1111/ner.12937
10. Chapman KB, Groenen PS, Vissers KC, van Helmond N, Stanton-Hicks MD. The pathways and processes underlying spinal transmission of low back pain: observations from dorsal root ganglion stimulation treatment. Neuromod Technol Neural Interface. 2021;24(4):610–621. doi:10.1111/ner.13150
11. Sverrisdottir YB, Martin SC, Hadjipavlou G, et al. Human dorsal root ganglion stimulation reduces sympathetic outflow and long-term blood pressure. JACC Basic Transl Sci. 2020;5(10):973–985. doi:10.1016/j.jacbts.2020.07.010
12. Kinfe TM, Asif M, Chakravarthy KV, et al. Unilateral L4-dorsal root ganglion stimulation evokes pain relief in chronic neuropathic postsurgical knee pain and changes of inflammatory markers: part II whole transcriptome profiling. J Transl Med. 2019;17(1). doi:10.1186/s12967-019-1952-x
13. Pan B, Zhang Z, Chao D, Hogan QH. Dorsal root ganglion field stimulation prevents inflammation and joint damage in a rat model of rheumatoid arthritis. Neuromodulation. 2018;21(3):247–253. doi:10.1111/ner.12648
14. Chapman KB, Kloosterman J, Schor JA, Girardi GE, van Helmond N, Yousef TA. Objective improvements in peripheral arterial disease from dorsal root ganglion stimulation: a case series. Ann Vasc Surg. 2021;74:519.e7–519.e16. doi:10.1016/j.avsg.2021.01.069
15. Deer TR, Pope JE, Lamer TJ, et al. The neuromodulation appropriateness consensus committee on best practices for dorsal root ganglion stimulation. Neuromod Technol Neural Interface. 2019;22(1):1–35. doi:10.1111/ner.12845
16. Kostelic JK, Haughton VM, Sether LA. Lumbar spinal nerves in the neural foramen: MR appearance. Radiology. 1991;178(3):837–839. doi:10.1148/radiology.178.3.1994428
17. Tubbs RS, Lobashevsky A, Oakes P, et al. Meningeal relationships to the spinal nerves and rootlets: a gross, histological, and radiological study with application to intradural extramedullary spinal tumors. Child’s Nerv Syst. 2015;31(5):675–681. doi:10.1007/s00381-015-2648-z
18. Joukal M, Klusáková I, Dubový P. Direct communication of the spinal subarachnoid space with the rat dorsal root ganglia. Ann Anat. 2016;205:9–15. doi:10.1016/j.aanat.2016.01.004
19. Lemes JBP, de Campos Lima T, Santos DO, et al. Participation of satellite glial cells of the dorsal root ganglia in acute nociception. Neurosci Lett. 2018;676:8–12. doi:10.1016/j.neulet.2018.04.003
20. Matsuda S, Kobayashi N, Terashita T, et al. Phylogenetic investigation of Dogiel’s pericellular nests and Cajal’s initial glomeruli in the dorsal root ganglion. J Comp Neurol. 2005;491(3):234–245. doi:10.1002/cne.20713
21. Nascimento AI, Mar FM, Sousa MM. The intriguing nature of dorsal root ganglion neurons: linking structure with polarity and function. Prog Neurobiol. 2018;168:86–103. doi:10.1016/j.pneurobio.2018.05.002
22. Devor M. Ectopic discharge in Aβ afferents as a source of neuropathic pain. Exp Brain Res. 2009;196(1):115–128. doi:10.1007/s00221-009-1724-6
23. Ma C, LaMotte RH. Multiple sites for generation of ectopic spontaneous activity in neurons of the chronically compressed dorsal root ganglion. J Neurosci. 2007;27(51):14059–14068. doi:10.1523/JNEUROSCI.3699-07.2007
24. Liu C-N, Wall PD, Ben-Dor E, Michaelis M, Amir R, Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000;85(3):503–521. doi:10.1016/S0304-3959(00)00251-7
25. Wang W, Gu J, Li Y-Q, Tao Y-X. Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain? Mol Pain. 2011;7(1):16. doi:10.1186/1744-8069-7-16
26. Djouhri L. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26(4):1281–1292. doi:10.1523/JNEUROSCI.3388-05.2006
27. Du X, Hao H, Gigout S, et al. Control of somatic membrane potential in nociceptive neurons and its implications for peripheral nociceptive transmission. Pain. 2014;155(11):2306–2322. doi:10.1016/j.pain.2014.08.025
28. Lobanov OV, Peng YB. Differential contribution of electrically evoked dorsal root reflexes to peripheral vasodilatation and plasma extravasation. J Neuroinflammation. 2011;8(1):20. doi:10.1186/1742-2094-8-20
29. Sorkin LS, Eddinger KA, Woller SA, Yaksh TL. Origins of antidromic activity in sensory afferent fibers and neurogenic inflammation. Semin Immunopathol. 2018;40(3):237–247. doi:10.1007/s00281-017-0669-2
30. West CA, McKay Hart A, Terenghi G, Wiberg M. Sensory neurons of the human brachial plexus: a quantitative study employing optical fractionation and in vivo volumetric magnetic resonance imaging. Neurosurgery. 2012;70(5):1183–1194. doi:10.1227/NEU.0b013e318241ace1
31. Tubbs RS, Loukas M, Slappey JB, Shoja MM, Oakes WJ, Salter EG. Clinical anatomy of the C1 dorsal root, ganglion, and ramus: a review and anatomical study. Clin Anat. 2007;20(6):624–627. doi:10.1002/ca.20472
32. Ebraheim NA, Lu J. Morphometric evaluation of the sacral dorsal root ganglia. A cadaveric study. Surg Radiol Anatomy. 1998;20(2):105–108.
33. Torun F, Dolgun H, Tuna H, Attar A, Uz A, Erdem A. Morphometric analysis of the roots and neural foramina of the lumbar vertebrae. Surg Neurol. 2006;66(2):148–151. doi:10.1016/j.surneu.2006.02.041
34. Mandell JC, Czuczman GJ, Gaviola GC, Ghazikhanian V, Cho CH. The lumbar neural foramen and transforaminal epidural steroid injections: an anatomic review with key safety considerations in planning the percutaneous approach. Am J Roentgenol. 2017;209(1):W26–W35. doi:10.2214/AJR.16.17471
35. Moon HS, Kim YD, Song BH, Cha YD, Song JH, Lee MH. Position of dorsal root ganglia in the lumbosacral region in patients with radiculopathy. Korean J Anesthesiol. 2010;59(6):398–402. doi:10.4097/kjae.2010.59.6.398
36. Vialle E, Vialle R, Contreras W, Jacob C. Anatomical study on the relationship between the dorsal root ganglion and the intervertebral disc in the lumbar spine. Revista Brasileira de Ortopedia (English Edition). 2015;50(4):450–454. doi:10.1016/j.rboe.2015.06.013
37. Akdemir G. Thoracic and lumbar intraforaminal ligaments. J Neurosurg Spine. 2010;13(3):351–355. doi:10.3171/2010.3.SPINE09799
38. Gilchrist RV. Anatomy of the intervertebral foramen. Pain Physician. 2002;4;5(10;4):372–378. doi:10.36076/ppj.2002/5/372
39. Jimenez-Andrade JM, Herrera MB, Ghilardi JR, Vardanyan M, Melemedjian OK, Mantyh PW. Vascularization of the dorsal root ganglia and peripheral nerve of the mouse: implications for chemical-induced peripheral sensory neuropathies. Mol Pain. 2008;4:1–8. doi:10.1186/1744-8069-4-10
40. Kiernan JA. Vascular permeability in the peripheral autonomic and somatic nervous systems: controversial aspects and comparisons with the blood-brain barrier. Microsc Res Tech. 1996;35(2):122–136. doi:10.1002/(SICI)1097-0029(19961001)35:2<122::AID-JEMT3>3.0.CO;2-S
41. Kobayashi T, Storrie B, Simons K, Dotti CG. A functional barrier to movement of lipids in polarized neurons. Nature. 1992;359(6396):647–650. doi:10.1038/359647a0
42. Sasaki H, Schmelzer JD, Zollman PJ, Low PA. Neuropathology and blood flow of nerve, spinal roots and dorsal root ganglia in longstanding diabetic rats. Acta Neuropathol. 1997;93(2):118–128. doi:10.1007/s004010050592
43. Zochodne DW, Ho LT, Allison JA. Dorsal root ganglia microenvironment of female BB Wistar diabetic rats with mild neuropathy. J Neurol Sci. 1994;127(1):36–42. doi:10.1016/0022-510x(94)90132-5
44. Johnson PC. Thickening of the human dorsal root ganglion perineurial cell basement membrane in diabetes mellitus. Muscle Nerve. 1983;6(8):561–565. doi:10.1002/mus.880060805
45. Jende JME, Kender Z, Rother C, et al. Diabetic polyneuropathy is associated with pathomorphological changes in human dorsal root ganglia: a study using 3T MR neurography. Front Neurosci. 2020;14. doi:10.3389/fnins.2020.570744
46. Sango K, Mizukami H, Horie H, Yagihashi S. Impaired axonal regeneration in diabetes. perspective on the underlying mechanism from in vivo and in vitro experimental studies. Front Endocrinol. 2017;8:12. doi:10.3389/fendo.2017.00012
47. Warzok R, Wattig B, Rudel J, Schwanengel H, Timmel A. [Morphology of diabetic neuropathy]. Zentralblatt Fur Allg Pathol u Pathol Anat. 1987;133(2):119–126. German.
48. Wattig B, Warzok R, von Zglinicki T, Röder H, Radzewitz B. [Experimental diabetic neuropathy. Morphometric studies of spinal ganglia cells in short-term streptozotocin-induced diabetes]. Zentralblatt Fur Allg Pathol u Pathol Anat. 1987;133(2):127–132. German.
49. Novak P, Pimentel DA, Sundar B, Moonis M, Qin L, Novak V. Association of statins with sensory and autonomic ganglionopathy. Front Aging Neurosci. 2015;7:191. doi:10.3389/fnagi.2015.00191
50. Sidenius P, Jakobsen J. Reduced perikaryal volume of lower motor and primary sensory neurons in early experimental diabetes. Diabetes. 1980;29(3):182–186. doi:10.2337/diab.29.3.182
51. Zochodne DW. Is early diabetic neuropathy a disorder of the dorsal root ganglion? A hypothesis and critique of some current ideas on the etiology of diabetic neuropathy. J Peripheral Nervous Syst. 1996;1(2):119–130.
52. Kobayashi M, Zochodne DW. Diabetic neuropathy and the sensory neuron: new aspects of pathogenesis and their treatment implications. J Diabetes Investig. 2018;9(6):1239–1254. doi:10.1111/jdi.12833
53. Glatte P, Buchmann SJ, Hijazi MM, Illigens BM-W, Siepmann T. Architecture of the cutaneous autonomic nervous system. Front Neurol. 2019;10. doi:10.3389/fneur.2019.00970
54. Johnson JM, Yen TC, Zhao K, Kosiba WA. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am J Physiol. 2005;288(4):57–4. doi:10.1152/ajpheart.00849.2004
55. Wang J, Ren Y, Zou X, Fang L, Willis WD, Lin Q. Sympathetic influence on capsaicin-evoked enhancement of dorsal root reflexes in rats. J Neurophysiol. 2004;92(4):2017–2026. doi:10.1152/jn.00145.2004
56. Musa R, Qurie A. Raynaud disease (Raynaud Phenomenon, Raynaud Syndrome). StatPearls Publishing; 2020. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29763008.
57. Wolf GA. The ratio of preganglionic neurons to postganglionic neurons in the visceral nervous system. J Comp Neurol. 1941;75(2):235–243. doi:10.1002/cne.900750204
58. Chapman KB, van Roosendaal B-KW, van Helmond N, Yousef TA. Unilateral dorsal root ganglion stimulation lead placement with resolution of bilateral lower extremity symptoms in diabetic peripheral neuropathy: a case report. Cureus. 2020. doi:10.7759/cureus.10735
59. Pope JE, Deer TR, Kramer J. A systematic review: current and future directions of dorsal root ganglion therapeutics to treat chronic pain. Pain Med. 2013;14(10):1477–1496. doi:10.1111/pme.12171
60. Van Zundert J, Patijn J, Kessels A, Lamé I, van Suijlekom H, Van kleef M. Pulsed radiofrequency adjacent to the cervical dorsal root ganglion in chronic cervical radicular pain: a double blind sham controlled randomized clinical trial. Pain. 2007;127(1):173–182. doi:10.1016/j.pain.2006.09.002
61. Hassenbusch SJ, Stanton-Hicks J, Covington EC. Spinal cord stimulation versus spinal infusion for low back and leg pain. Acta Neurochirurgica. Supplement. 1995;64(6):109–115. doi:10.1007/978-3-7091-9419-5_24
62. Nakamura SI, Takahashi K, Takahashi Y, Yamagata M, Moriya H. The afferent pathways of discogenic low-back pain. Evaluation of L2 spinal nerve infiltration. J Bone J Surg. 1996;78-B(4):606–612. doi:10.1302/0301-620x.78b4.0780606
63. Lynch PJ, McJunkin T, Eross E, Gooch S, Maloney J. Case report: successful epiradicular peripheral nerve stimulation of the C2 dorsal root Ganglion for postherpetic neuralgia. Neuromodulation. 2011;14(1):58–61. doi:10.1111/j.1525-1403.2010.00307.x
64. Alo KM, Yland MJ, Redko V, Feler C, Naumann C. Lumbar and sacral nerve root stimulation (NRS) in the treatment of chronic pain: a novel anatomic approach and neuro stimulation technique. Neuromodulation. 1999;2(1):23–31. doi:10.1046/j.1525-1403.1999.00023.x
65. Bevan BYS, Yeats J. Protons activate a cation conductance in a sub-population of rat dorsal root ganglion neurones. J Physiol. 1991;433(1):145–161. doi:10.1113/jphysiol.1991.sp018419
66. Devor M, Wall PD. Cross-excitation in dorsal root ganglia of nerve-injured and intact rats. J Neurophysiol. 1990;64(6):1733–1746. doi:10.1152/jn.1990.64.6.1733
67. Kim DH, Imran MA. Method and system for stimulating a dorsal root ganglion. 2009:1–72.
68. Grigsby E, Deer T, Weiner RL, Wilcosky B, Kramer J. Prospective, multicenter clinical trial studying dorsal root ganglion stimulation in the treatment of back pain. In:
69. Russo MA, Brooker C, Verrills P, Cousins MJ, Van Buyten J-P, Liem L. Dorsal root ganglion stimulation for the treatment of chronic neuropathic foot pain. In:
70. Liem L, Russo MA, Smet I, Huygen FJ. Dorsal root ganglion stimulation for the treatment of failed back surgery syndrome. In:
71. Deer TR, Grigsby E, Weiner RL, Wilcosky B, Kramer JM. A prospective study of dorsal root ganglion stimulation for the relief of chronic pain. Neuromod Technol Neural Interface. 2013;16(1):67–72. doi:10.1111/ner.12013
72. Liem L, Russo M, Huygen FJPM, et al. A multicenter, prospective trial to assess the safety and performance of the spinal modulation dorsal root ganglion neurostimulator system in the treatment of chronic pain. Neuromod Technol Neural Interface. 2013;16(5):471–482. doi:10.1111/ner.12072
73. Koetsier E, Franken G, Debets J, et al. Mechanism of dorsal root ganglion stimulation for pain relief in painful diabetic polyneuropathy is not dependent on GABA release in the dorsal horn of the spinal cord. CNS Neurosci Ther. 2019;26:1–8. doi:10.1111/cns.13192
74. Franken G, Douven P, Debets J, Joosten EAJ. Conventional dorsal root ganglion stimulation in an experimental model of painful diabetic peripheral neuropathy: a quantitative immunocytochemical analysis of intracellular γ-aminobutyric acid in dorsal root ganglion neurons. Neuromodulation. 2021;24(4):639–645. doi:10.1111/ner.13398
75. Sandkühler J, Chen JG, Cheng G, Randić M. Low-frequency stimulation of afferent Aδ-fibers induces long-term depression at primary afferent synapses with substantia gelatinosa neurons in the rat. J Neurosci. 1997;17(16):6483–6491. doi:10.1523/JNEUROSCI.17-16-06483.1997
76. Koetsier E, Franken G, Debets J, et al. Dorsal root ganglion stimulation in experimental painful diabetic polyneuropathy: delayed wash-out of pain relief after low-frequency (1Hz) stimulation. Neuromod Technol Neural Interface. 2020;23(2):177–184. doi:10.1111/ner.13048
77. Kallewaard JW, Nijhuis H, Huygen F, et al. Prospective cohort analysis of DRG stimulation for failed back surgery syndrome pain following lumbar discectomy. Pain Pract. 2019;19(2):204–210. doi:10.1111/papr.12734
78. Smits H, van Kleef M, Holsheimer J, Joosten EAJ. Experimental spinal cord stimulation and neuropathic pain: mechanism of action, technical aspects, and effectiveness. Pain Pract. 2013;13(2):154–168. doi:10.1111/j.1533-2500.2012.00579.x
79. Chapman KB, Yousef TA, Foster A, D. Stanton-Hicks M, van Helmond N. Mechanisms for the clinical utility of low-frequency stimulation in neuromodulation of the dorsal root ganglion. Neuromod Technol Neural Interface. 2021;24(4):738–745. doi:10.1111/ner.13323
80. Arcourt A, Gorham L, Dhandapani R, et al. Touch receptor-derived sensory information alleviates acute pain signaling and fine-tunes nociceptive reflex coordination. Neuron. 2017;93(1):179–193. doi:10.1016/j.neuron.2016.11.027
81. Gravius N, Chaudhry SR, Muhammad S, et al. Selective L4 dorsal root ganglion stimulation evokes pain relief and changes of inflammatory markers: part I profiling of saliva and serum molecular patterns. Neuromodulation. 2019;22(1):44–52. doi:10.1111/ner.12866
82. Huygen F, Liem L, Cusack W, Kramer J. Stimulation of the L2-L3 dorsal root ganglia induces effective pain relief in the low back. Pain Pract. 2018;18(2):205–213. doi:10.1111/papr.12591
83. Devices AM. TARGET Post-Approval Study (TARGET PAS). Available from: Clinicaltrials.gov.
84. Deer TR, Hunter CW, Mehta P, et al. A systematic literature review of dorsal root ganglion neurostimulation for the treatment of pain. Pain Med. 2020;21(8):1581–1589. doi:10.1093/PM/PNAA005
85. Stelter B, Karri J, Marathe A, Abd-Elsayed A. Dorsal root ganglion stimulation for the treatment of non-complex regional pain syndrome related chronic pain syndromes: a systematic review. Neuromodulation. 2021;2020. doi:10.1111/ner.13361
86. Nagpal A, Clements N, Duszynski B, Boies B. The effectiveness of dorsal root ganglion neurostimulation for the treatment of chronic pelvic pain and chronic neuropathic pain of the lower extremity: a comprehensive review of the published data. Pain Med. 2020. doi:10.1093/pm/pnaa369
87. Harris RP, Helfand M, Woolf SH, et al. Current methods of the U.S. preventive services task force: a review of the process. Am J Prev Med. 2001;20(3):21–35. doi:10.1016/S0749-3797(01)00261-6
88. Goebel A, Lewis S, Phillip R, Sharma M. Dorsal root ganglion stimulation for complex regional pain syndrome (CRPS) recurrence after amputation for CRPS, and failure of conventional spinal cord stimulation. Pain Pract. 2018;18(1):104–108. doi:10.1111/papr.12582
89. van Bussel CM, Stronks DL, Huygen FJPM. Dorsal column stimulation vs. dorsal root ganglion stimulation for complex regional pain syndrome confined to the knee: patients’ preference following the trial period. Pain Pract. 2018;18(1):87–93. doi:10.1111/papr.12573
90. Van Buyten J-P, Smet I, Liem L, Russo M, Huygen F. Stimulation of dorsal root ganglia for the management of complex regional pain syndrome: a prospective case series. Pain Pract. 2015;15(3):208–216. doi:10.1111/papr.12170
91. Garg A, Danesh H. Neuromodulation of the cervical dorsal root ganglion for upper extremity complex regional pain syndrome—case report. Neuromodulation. 2015;18(8):765–768. doi:10.1111/ner.12307
92. Pinckard-Dover H, Palmer A, Petersen EA. A review of neuromodulation for treatment of complex regional pain syndrome in pediatric patients and novel use of dorsal root ganglion stimulation in an adolescent patient with 30-month follow-up. Neuromodulation. 2020;2020. doi:10.1111/ner.13257
93. Dombovy-Johnson ML, Hagedorn JM, Lamer TJ. Dorsal root ganglion stimulation for complex regional pain syndrome in spinal cord injury. Pain Med. 2021;22(5):1224–1227. doi:10.1093/pm/pnaa452
94. Smith GL, Petersen EA, Paul C, Goree JH. Transgrade dorsal root ganglion stimulation as a salvage technique for three different anatomical barriers: a case series. Neuromodulation. 2020;2020. doi:10.1111/ner.13276
95. Pendem K, Jassal N. Dorsal root ganglion stimulation as treatment for complex regional pain syndrome of the foot refractory to spinal cord stimulation: a case report. Cureus. 2021;13(1):4–6. doi:10.7759/cureus.12753
96. Al-Kaisy A. Effectiveness of “transgrade” epidural technique for dorsal root ganglion stimulation. A retrospective, single-center, case series for chronic focal neuropathic pain. Pain Physician. 2019;6(22):601–611. doi:10.36076/ppj/2019.22.601
97. Kloosterman JR, Yang A, van Helmond N, Chapman KB. Dorsal root ganglion stimulation to treat persistent abdominal pain after bypass surgery. Pain Med. 2020;21(1):201–203. doi:10.1093/pm/pnz193
98. Zuidema X, Breel J, Wille F. S3 dorsal root ganglion/nerve root stimulation for refractory postsurgical perineal pain: technical aspects of anchorless sacral transforaminal lead placement. Case Rep Neurol Med. 2016;2016:1–3. doi:10.1155/2016/8926578
99. Kretzschmar M, Reining M, Schwarz MA. Three-year outcomes after dorsal root ganglion stimulation in the treatment of neuropathic pain after peripheral nerve injury of upper and lower extremities. Neuromod Technol Neural Interface. 2021;24(4):700–707. doi:10.1111/ner.13222
100. Wensing AG, Breel JS, Hollmann MW, Wille F. Prospective observational cohort study on dorsal root ganglion stimulation in chronic postsurgical pain: results of patient-reported outcomes at two years. Neuromod Technol Neural Interface. 2022;1–8. doi:10.1016/j.neurom.2021.11.005
101. Morgalla MH, Bolat A, Fortunato M, Lepski G, Chander BS. Dorsal root ganglion stimulation used for the treatment of chronic neuropathic pain in the groin: a single-center study with long-term prospective results in 34 cases. Neuromod Technol Neural Interface. 2017;20(8):753–760. doi:10.1111/ner.12713
102. Martin SC, Macey AR, Raghu A, et al. Dorsal root ganglion stimulation for the treatment of chronic neuropathic knee pain. World Neurosurg. 2020;143(143):e303–e308. doi:10.1016/j.wneu.2020.07.102
103. Eldabe S, Burger K, Moser H, et al. Dorsal root ganglion (DRG) stimulation in the treatment of phantom limb pain (PLP). Neuromodulation. 2015;18(7):610–616. doi:10.1111/ner.12338
104. Hunter CW, Sayed D, Lubenow T, et al. DRG FOCUS: a multicenter study evaluating dorsal root ganglion stimulation and predictors for trial success. Neuromodulation. 2019;22(1):61–79. doi:10.1111/ner.12796
105. Schu S, Gulve A, ElDabe S, et al. Spinal cord stimulation of the dorsal root ganglion for groin pain-a retrospective review. Pain Pract. 2015;15(4):293–299. doi:10.1111/papr.12194
106. Weiner RL, Yeung A, Garcia CM, Perryman LT, Speck B. Treatment of FBSS low back pain with a novel percutaneous DRG wireless stimulator: pilot and feasibility study. Pain Medicine. 2016;17(10):1911–1916. doi:10.1093/pm/pnw075
107. Verrills P, Mitchell B, Vivian D, Cusack W, Kramer J. Dorsal root ganglion stimulation is paresthesia-independent: a retrospective study. Neuromodulation. 2019;22(8):937–942. doi:10.1111/ner.12921
108. Yakovlev AE, Al Tamimi M, Barolat G, et al. Spinal cord stimulation as alternative treatment for chronic post-herniorrhaphy pain. Neuromod Technol Neural Interface. 2010;13(4):288–291. doi:10.1111/j.1525-1403.2010.00276.x
109. Elias M. Spinal cord stimulation for post-herniorrhaphy pain. Neuromodulation. 2000;3(3):155–157. doi:10.1046/j.1525-1403.2000.00155.x
110. Richter B, Novik Y, Bergman JJ, Tomycz ND. The efficacy of BurstDR spinal cord stimulation for chronic abdominal pain: a clinical series. World Neurosurg. 2020;138:77–82. doi:10.1016/j.wneu.2020.02.075
111. Reverberi C, Bonezzi C, Demartini L. Peripheral subcutaneous neurostimulation in the management of neuropathic pain: five case reports. Neuromodulation. 2009;12(2):146–155. doi:10.1111/j.1525-1403.2009.00201.x
112. Stinson LW, Roderer GT, Cross NE, Davis BE. Peripheral subcutaneous electrostimulation for control of intractable post-operative inguinal pain: a case report series. Neuromodulation. 2001;4(3):99–104. doi:10.1046/j.1525-1403.2001.00099.x
113. Lepski G, Vahedi P, Tatagiba MS, Morgalla M. Combined spinal cord and peripheral nerve field stimulation for persistent post-herniorrhaphy pain. Neuromodulation. 2013;16(1):84–89. doi:10.1111/j.1525-1403.2012.00463.x
114. Zuidema X, Breel J, Wille F. Paresthesia mapping: a practical workup for successful implantation of the dorsal root ganglion stimulator in refractory groin pain. Neuromodulation. 2014;17(7):665–669. doi:10.1111/ner.12113
115. Mol FMU, Roumen RMH. DRG spinal cord stimulation as solution for patients with severe pain due to anterior cutaneous nerve entrapment syndrome: a case series. Neuromodulation. 2018;21(3):317–319. doi:10.1111/ner.12692
116. Bral P, Smet I, Jerjir A, Devos M, Van Buyten JP. Dorsal root ganglion stimulation for patients with refractory pain due to anterior cutaneous nerve entrapment syndrome: a case series. Pain Pract. 2021;1–7. doi:10.1111/papr.13086
117. Akuamoah LA, Tupper C, Nagrani S, Chapman KB. Dorsal root ganglion stimulation to treat focal postsurgical and diffuse chronic pain: a case report. A&A Pract. 2022;16(5):e01589. doi:10.1213/xaa.0000000000001589
118. Urits I, Markel M, Vij N, et al. Use of spinal cord stimulation for the treatment of post total knee arthroplasty pain. Best Pract Res Clin Anaesthesiol. 2020;34(3):633–642. doi:10.1016/j.bpa.2020.07.006
119. Granville M, Berti A, Jacobson RE. Use of spinal cord stimulation in elderly patients with multi-factorial chronic lumbar and non-radicular lower extremity pain. Cureus. 2017;9(11). doi:10.7759/cureus.1855
120. Lowry AM, Simopoulos TT. Spinal cord stimulation for the treatment of chronic knee pain following total knee replacement. Pain Physician. 2010;13(3):251–256. doi:10.36076/ppj.2010/13/251
121. Morgalla MH. Dorsal root ganglion stimulation (DRGS) for the treatment of chronic neuropathic pain: a single-center study with long-term prospective results in 62 cases. Pain Physician. 2018;1(21;1):E377–E387. doi:10.36076/ppj.2018.4.E377
122. Victor S, Burnett C, Lange R, Pohler K. Dorsal root ganglion stimulator for avascular necrosis of the Hip. Baylor Univ Med Cent Proc. 2018;31(4):532–533. doi:10.1080/08998280.2018.1483149
123. Chapman KB, Tupper C, Vissers KC, van Helmond N, Yousef,T. Dorsal root ganglion stimulation for the treatment of joint pain with predominantly nociceptive characteristics: a case series. Pain Pract. 2022. doi:10.1111/papr.13180
124. Schultheis BC, Willie C, Ross-Steinhagen NE, De Ridder D, Vancamp T, Weidle PA. Alternative dorsal root ganglion neuromodulation electrode implantation: a report of 2 cases with 3 different techniques. J Neurol Surg. 2021;4:1–6.
125. Chapman KB, van Roosendaal B-K, Yousef TA, Vissers KC, Hel N. Dorsal root ganglion stimulation normalizes measures of pain processing in patients with chronic low-back pain: a prospective pilot study using quantitative sensory testing. Pain Pract. 2021;21(5):568–577. doi:10.1111/papr.12992
126. Huygen FJPM, Liem L, Nijhuis H, Cusack W, Kramer J. Evaluating dorsal root ganglion stimulation in a prospective Dutch cohort. Neuromod Technol Neural Interface. 2019;22(1):80–86. doi:10.1111/ner.12798
127. Eldabe S, Espinet A, Wahlstedt A, et al. Retrospective case series on the treatment of painful diabetic peripheral neuropathy with dorsal root ganglion stimulation. Neuromod Technol Neural Interface. 2018;21(8):787–792. doi:10.1111/ner.12767
128. Groenen PS, Van helmond N, Chapman KB. Chemotherapy-induced peripheral neuropathy treated with dorsal root ganglion stimulation. Pain Med. 2019;20(4):857–859. doi:10.1093/pm/pny209
129. Falowski S, Pope JE, Raza A. Early US experience with stimulation of the dorsal root ganglia for the treatment of peripheral neuropathy in the lower extremities: a multicenter retrospective case series. Neuromodulation. 2019;22(1):96–100. doi:10.1111/ner.12860
130. Maino P, Koetsier E, Kaelin-Lang A, Gobbi C, Perez R. Efficacious dorsal root ganglion stimulation for painful small fiber neuropathy: a case report. Pain Physician. 2017;20(3):E459–E463.
131. Koetsier E, van Kuijk SMJ, Melli G, et al. Dorsal root ganglion stimulation for the management of intractable painful polyneuropathy: a prospective pilot study. Neuromodulation. 2020;2020. doi:10.1111/ner.13336
132. Ho KWD, Rempe T, Jerath N, Antony A. Dorsal root ganglion stimulation as a potentially effective treatment for painful hereditary and idiopathic axonal polyneuropathy: a retrospective case series. Neuromodulation. 2020;23(2):234–238. doi:10.1111/ner.12924
133. Grabnar M, Kim C. Dorsal root ganglion stimulation for treatment of chemotherapy-induced neuropathy. Am J Phys Med Rehabil. 2020;2020–2022. doi:10.1097/phm.0000000000001542
134. Karri J, Bruel B. Dorsal root ganglion stimulation for post-lyme disease chronic peripheral neuropathic pain. Neuromodulation. 2021;24(4):794–795. doi:10.1111/ner.13136
135. Hunter CW, Yang A, Davis T. Selective radiofrequency stimulation of the dorsal root ganglion (DRG) as a method for predicting targets for neuromodulation in patients with post amputation pain: a case series. Neuromodulation. 2017;20(7):708–718. doi:10.1111/ner.12595
136. Piedade GS, Vesper J, Chatzikalfas A, Slotty PJ. Cervical and high-thoracic dorsal root ganglion stimulation in chronic neuropathic pain. Neuromodulation. 2019;22(8):951–955. doi:10.1111/ner.12916
137. Anthony CL, Tora MS, Bentley JN, Texakalidis P, Boulis NM. Dorsal root ganglion stimulation for thoracic neuralgia: a report of six cases. Cureus. 2019;11(5):3–9. doi:10.7759/cureus.4615
138. Liem L, Russo M, Huygen FJPM, et al. One-year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromod Technol Neural Interface. 2015;18(1):41–49. doi:10.1111/ner.12228
139. Papa A, Saracco E, Di Dato MT, et al. Dorsal root ganglion stimulation for the management of chronic neuropathic pain: a retrospective case series during four years follow-up in a single center. Open Pain J. 2020;13(1):35–41. doi:10.2174/1876386302013010035
140. Kim JH, Apigo A, Fontaine C. Dorsal root ganglion stimulation for refractory post-herpetic neuralgia. Pain Pract. 2021;4–8. doi:10.1111/papr.13017
141. Williams BA, Ibinson JW, Bonant S, Gilbert KL, Piva SR. A tale of two cohorts: the trials and tribulations of ever-changing orthopedic / acute pain medicine hospital practices during the execution of military-funded clinical-translational research (2013–2021). Pain Med. 2022;23(1):1–28. doi:10.1093/pm/pnab267
142. Roybal AE, Sivanesan E, Chen Y. Case report: dorsal root ganglion (DRG) stimulation for acute neuropathic pain from acute herpes zoster infection. SAGE Open Medical Case Reports. 2021;9:2050313X2110622. doi:10.1177/2050313x211062297
143. Rowland DCL, Wright D, Moir L, FitzGerald JJ, Green AL. Successful treatment of pelvic girdle pain with dorsal root ganglion stimulation. Br J Neurosurg. 2016;30(6):685–686. doi:10.1080/02688697.2016.1208810
144. Hunter CW, Yang A. Dorsal root ganglion stimulation for chronic pelvic pain: a case series and technical report on a novel lead configuration. Neuromodulation. 2019;22(1):87–95. doi:10.1111/ner.12801
145. Giordano NL, Van helmond N, Chapman KB. Coccydynia treated with dorsal root ganglion stimulation. Case Rep Anesthesiol. 2018;2018:1–4. doi:10.1155/2018/5832401
146. Hassanain M, Murphy P. Dorsal root ganglion stimulation for the treatment of bilateral intractable chronic testicular pain. Neuromodulation. 2019;22(1):115–116. doi:10.1111/ner.12805
147. Justiz R, Smith N. Thoracic DRG for chronic abdominal pain secondary to hereditary pancreatitis.
148. Kretzschmar M, Reining M. Dorsal root ganglion stimulation for treatment of central poststroke pain in the lower extremity after medullary infarction. Pain. 2021;162(11):2682–2685. doi:10.1097/j.pain.0000000000002439
149. Piedade GS, Gillner S, Mcphillips PS, Vesper J, Slotty PJ. Frequency dependency of therapeutic efficacy in dorsal root ganglion stimulation for neuropathic pain. Acta Neurochirurgica. 2022;1:3. doi:10.1007/s00701-022-05161-6
150. Parker T, Divanbeighi AP, Huang Y, Aziz TZ, Sverrisdottir YB, Green AL. Dorsal root ganglion stimulation: a new target for autonomic neuromodulation? Clin Auton Res. 2021;31(1):135–137. doi:10.1007/s10286-020-00751-9
151. Kuwabara Y, Salavatian S, Howard-Quijano K, Yamaguchi T, Lundquist E, Mahajan A. Neuromodulation with thoracic dorsal root ganglion stimulation reduces ventricular arrhythmogenicity. Front Physiol. 2021;12:1–10. doi:10.3389/fphys.2021.713717
152. Petrakis IE, Sciacca V. Spinal cord stimulation in diabetic lower limb critical ischaemia: transcutaneous oxygen measurement as predictor for treatment success. Eur J Vasc Endovasc Surg. 2000;19(6):587–592. doi:10.1053/ejvs.1999.1036
153. Ubbink DT, Vermeulen H. Spinal cord stimulation for non-reconstructable chronic critical leg ischaemia. Cochrane Database Syst Rev. 2013;2013(2). doi:10.1002/14651858.CD004001.pub3
154. Hagedorn JM, Canzanello N, Lamer TJ. Dorsal root ganglion stimulation for erythromelalgia related foot pain: a case report and review of the literature. Pain Pract. 2021;21(6):698–702. doi:10.1111/papr.12998
155. Yu G, Segel I, Zhang Z, Hogan QH, Pan B. Dorsal root ganglion stimulation alleviates pain-related behaviors in rats with nerve injury and osteoarthritis. Anesthesiology. 2020;133(2):408–425. doi:10.1097/ALN.0000000000003348
156. Levy RM, Mekhail N, Kramer J, et al. Therapy habituation at 12 months: spinal cord stimulation versus dorsal root ganglion stimulation for complex regional pain syndrome type I and II. J Pain. 2020;21(3–4):399–408. doi:10.1016/j.jpain.2019.08.005
157. Chapman KB, Yang A, Mogilner AY, et al. Dorsal root ganglion stimulation device explantation: a multicenter pooled data analysis. Pain Pract. 2022;22(5):522–531. doi:10.1111/papr.13113
158. Comittee on Pain Management. Statement on Anesthetic Care During Interventional Pain Procedures for Adults. The American Society of Anesthesiologists; 2019.
159. Abbott. ProclaimTM DRG Implantable Pulse Generator Clinician’s Manual Model 3664. Abbott; 2018.
160. Falowski SM, Deer T, Tubic G, Mehta P. Multicenter retrospective analysis of dorsal root ganglion stimulator placement using intraoperative neuromonitoring in asleep patients during early periods of adoption. Neuromodulation. 2020;2020. doi:10.1111/ner.13286
161. Hagedorn JM, Deer TR, Falowski SM, et al. An observational study of intraoperative neuromonitoring as a safety mechanism in placement of percutaneous dorsal root ganglion stimulation and spinal cord stimulation systems. J Pain Res. 2020;13:3349–3353. doi:10.2147/JPR.S289416
162. Vancamp T, Levy RM, Peña I, Pajuelo A. Relevant anatomy, morphology, and implantation techniques of the dorsal root ganglia at the lumbar levels. Neuromodulation. 2017;20(7):690–702. doi:10.1111/ner.12651
163. Wall PD, Devor M. Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain. 1983;17(4):321–339. doi:10.1016/0304-3959(83)90164-1
164. Darby SA, Frysztak RJ. Neuroanatomy of the Spinal Cord.
165. Mammis A, Mogilner AY. The use of intraoperative electrophysiology for the placement of spinal cord stimulator paddle leads under general anesthesia. Neurosurgery. 2012;70(2 Suppl Operative):230–236. doi:10.1227/neu.0b013e318232ff29
166. Gonzalez AA, Jeyanandarajan D, Hansen C, Zada G, Hsieh PC. Intraoperative neurophysiological monitoring during spine surgery: a review. Neurosurg Focus. 2009;27(4):1–10. doi:10.3171/2009.8.FOCUS09150
167. Park J-H. Intraoperative neurophysiological monitoring in spinal surgery. World J Clin Cases. 2015;3(9):765. doi:10.12998/wjcc.v3.i9.765
168. Huygen FJPM, Kallewaard JW, Nijhuis H, et al. Effectiveness and safety of dorsal root ganglion stimulation for the treatment of chronic pain: a pooled analysis. Neuromodulation. 2020;23(2):213–221. doi:10.1111/ner.13074
169. Deer T, Pope J, Hunter C, et al. Safety analysis of dorsal root ganglion stimulation in the treatment of chronic pain. Neuromodulation. 2020;23(2):239–244. doi:10.1111/ner.12941
170. Deletis V, Sala F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: a review focus on the corticospinal tracts. Clin Neurophysiol. 2008;119(2):248–264. doi:10.1016/j.clinph.2007.09.135
171. Charalampidis A, Jiang F, Wilson JRF, Badhiwala JH, Brodke DS, Fehlings MG. The use of intraoperative neurophysiological monitoring in spine surgery. Global Spine J. 2020;10(1_suppl):104S–114S. doi:10.1177/2192568219859314
172. ter Bruggen FFJA, Eralp I, Leliveld L, Jansen C, Stronks DL, Huygen F. Dexmedetomidine as a sedative in the awake implantation of a neuromodulative system. Pain Pract. 2017;17(2):208–213. doi:10.1111/papr.12425
173. Vanhauwaert DJ, Couvreur T, Vandebroek A, De Coster O, Hanssens K. Conscious sedation using dexmedetomidine during surgical paddle lead placement improves outcome in spinal cord stimulation: a case series of 25 consecutive patients. Neuromodulation. 2020;2020. doi:10.1111/ner.13124
174. Verrills P. Dorsal root ganglion stimulation for pain control. In: Krames E, Hunter PP, Rezai AR, editors. Neuromodulation: Comprehensive Textbook of Principles, Technologies, and Therapies. Academic Press; 2018:683–692.
175. Chapman KB, Mogilner AY, Yang AH, et al. Lead migration and fracture rate in dorsal root ganglion stimulation using anchoring and non-anchoring techniques: a multicenter pooled data analysis. Pain Practice. 2021;21(8):859–870. doi:10.1111/papr.13052
176. Deckers K, De Smedt K, Mitchell B, et al. New therapy for refractory chronic mechanical low back pain—restorative neurostimulation to activate the lumbar multifidus: one year results of a prospective multicenter clinical trial. Neuromodulation. 2018;21(1):48–55. doi:10.1111/ner.12741
177. Chapman KB, Patel KV, van Helmond N, Chang Chien GC. Dorsal root ganglion stimulation lead fracture within the superficial fascial layers in four cases. A Case Rep. 2020;14(11):1–4. doi:10.1213/XAA.0000000000001307
178. Chapman KB, Spiegel MA, Dickerson DM, et al. A paramedian approach for dorsal root ganglion stimulation placement developed to limit lead migration and fracture. Pain Pract. 2021;21(8):991–1000. doi:10.1111/papr.13063
179. Chapman KB, Ramsook RR, Groenen PS, Vissers KC, van Helmond N. Lumbar transgrade dorsal root ganglion stimulation lead placement in patients with post-surgical anatomical changes: a technical note. Pain Pract. 2020;20(4):399–404. doi:10.1111/papr.12859
180. Chapman KB, Nagrani S, Patel KV, Yousef T, van Helmond N. Lumbar dorsal root ganglion stimulation lead placement using an outside-in technique in 4 patients with failed back surgery syndrome: a case series. A&A Pract. 2020;14(10):e01300. doi:10.1213/XAA.0000000000001300
181. Piedade GS, Cornelius JF, Chatzikalfas A, Vesper J, Slotty PJ. Open microsurgical dorsal root ganglion lead placement. Neuromodulation. 2019;22(8):956–959. doi:10.1111/ner.12905
182. Maurice Abitbol M. Evolution of the lumbosacral angle. Am J Phys Anthropol. 1987;72(3):361–372. doi:10.1002/ajpa.1330720309
183. Pal GP. Weight transmission through the sacrum in man. J Anat. 1989;162:9–17.
184. Saluja S, Agarwal S, Tuli A, Raheja S, Tigga SR, Paul S. Morphometric analysis of the Sacrum and its surgical implications. J Clin Diagnostic Res. 2018;12(6):AC01–AC06. doi:10.7860/JCDR/2018/33991.11661
185. Arman C, Naderi S, Kiray A, et al. The human sacrum and safe approaches for screw placement. J Clin Neurosci. 2009;16(8):1046–1049. doi:10.1016/j.jocn.2008.07.081
186. Xu R, Ebraheim NA, Robke J, Huntoon M, Yeasting RA. Radiologic and anatomic evaluation of the anterior sacral foramens and nerve grooves. Spine. 1996;21(4):407–410. doi:10.1097/00007632-199602150-00001
187. Whelan MA, Palmer Gold R. Computed tomography of the sacrum. I. Normal anatomy. Am J Neuroradiol. 1982;3(5):547–554.
188. Cha YD, Choi JK, Yang CW, Lim HK, Heo GA, Kim BG. Relationship between first dorsal sacral foramen and lumbar facet joint connecting line in South Korea populations. Med (United States). 2017;96(29). doi:10.1097/MD.0000000000007544
189. Kobayashi S, Yoshizawa H, Yamada S. Pathology of lumbar nerve root compression Part 1: intraradicular inflammatory changes induced by mechanical compression. J Orthop Res. 2004;22(1):170–179. doi:10.1016/S0736-0266(03)00131-1
190. Lin X-Y, Yang J, Li H-M, Hu S-J, Xing J-L. Dorsal root ganglion compression as an animal model of sciatica and low back pain. Neurosci Bull. 2012;28(5):618–630. doi:10.1007/s12264-012-1276-9
191. Khan Z, Shankar H. Lumbar 5 nerve root injury following dorsal root ganglion stimulator lead placement. Neuromodulation. 2020;23(2):258–259. doi:10.1111/ner.12945
192. Yuan AS, Almodovar JL, Erekson E. Neurologic injury after sacral neuromodulation. Female Pelvic Med Reconstruct Surg. 2019;25(2):e45–e46. doi:10.1097/SPV.0000000000000701
193. Swinn M, Schott G, Oliver S, Kitchen N, Fowler C. Leg pain after sacral neuromodulation: anatomical considerations. BJU International. 1999;84(9):1113–1115. doi:10.1046/j.1464-410x.1999.00419.x
194. Kang RA, Sim WS, Choi JW, et al. Comparison between anteroposterior and oblique “Scotty dog” approach during S1 transforaminal epidural steroid injection: a randomized controlled trial. Medicine. 2020;99(43):e22895. doi:10.1097/MD.0000000000022895
195. Fish DE, Lee PC, Marcus DB. The S1 “scotty dog”: report of a technique for S1 transforaminal epidural steroid injection. Arch Phys Med Rehabil. 2007;88(12):1730–1733. doi:10.1016/j.apmr.2007.07.041
196. Chapman KB, van Helmond N, Kallewaard JW, et al. An anatomy-informed, novel technique for S1 dorsal root ganglion stimulation lead placement. Pain Med. 2022;23(10):1750–1756. doi:10.1093/pm/pnac062
197. Morishita Y, Naito M, Wang JC. Cervical spinal canal stenosis: the differences between stenosis at the lower cervical and multiple segment levels. Int Orthop. 2011;35(10):1517–1522. doi:10.1007/s00264-010-1169-3
198. Ulbrich EJ, Schraner C, Boesch C, et al. Normative MR cervical spinal canal dimensions. Radiology. 2014;271(1):172–182. doi:10.1148/radiol.13120370
199. Tanaka N, Fujimoto Y, An HS, Ikuta Y, Yasuda M. The anatomic relation among the nerve roots, intervertebral foramina, and intervertebral discs of the cervical spine. Spine. 2000;25(3):286–291. doi:10.1097/00007632-200002010-00005
200. Ebraheim NA, An HS, Xu R, Ahmad M, Yeasting RA. The quantitative anatomy of the cervical nerve root groove and the intervertebral foramen. Spine. 1996;21(14):1619–1623. doi:10.1097/00007632-199607150-00001
201. Skaribas IM, Peccora C, Skaribas E. Single S1 dorsal root ganglia stimulation for intractable complex regional pain syndrome foot pain after lumbar spine surgery: a case series. Neuromod Technol Neural Interface. 2019;22(1):101–107. doi:10.1111/ner.12780
202. Ghosh P, Gungor S. Utilization of concurrent dorsal root ganglion stimulation and dorsal column spinal cord stimulation in complex regional pain syndrome. Neuromodulation. 2020;2020. doi:10.1111/ner.13144
203. Yang A, Hunter CW. Dorsal root ganglion stimulation as a salvage treatment for complex regional pain syndrome refractory to dorsal column spinal cord stimulation: a case series. Neuromodulation. 2017;20(7):703–707. doi:10.1111/ner.12622
204. Piedade GS, Vesper J, Slotty PJ. Synergetic efficacy of simultaneous DRG- and traditional spinal cord stimulation. Acta Neurochir. 2020;162(2):257–260. doi:10.1007/s00701-019-04166-y
205. Deer TR, Russo MA, Grider JS, et al. The neurostimulation appropriateness consensus committee (NACC): recommendations for surgical technique for spinal cord stimulation. Neuromodulation. 2022;25(1):1–9. doi:10.1016/j.neurom.2021.10.015
206. Franken G, Debets J, Joosten EAJ. Dorsal root ganglion stimulation in experimental painful diabetic peripheral neuropathy: burst vs. conventional stimulation paradigm. Neuromodulation. 2019;22(8):943–950. doi:10.1111/ner.12908
207. Franken G, Debets J, Joosten EAJ. Nonlinear relation between burst dorsal root ganglion stimulation amplitude and behavioral outcome in an experimental model of painful diabetic peripheral neuropathy. Neuromodulation. 2020;23(2):158–166. doi:10.1111/ner.13070
208. Billet B, Hanssens K, De Coster O, et al. Wireless high-frequency dorsal root ganglion stimulation for chronic low back pain: a pilot study. Acta Anaesthesiol Scand. 2018;62(8):1133–1138. doi:10.1111/aas.13138
209. Chapman KB, Tupper C, Amireh A, van Helmond N, Yousef T. Impact of lowering frequency of dorsal root ganglion stimulation on implantable pulse generator consumption. Reg Anesth Pain Med. 2022;rapm-2022–103644. doi:10.1136/rapm-2022-103644
210. Deer TR, Esposito MF, Cornidez EG, Okaro U, Fahey ME, Chapman KB. Teleprogramming service provides safe and remote stimulation options for patients with DRG-S and SCS implants. J Pain Res. 2021;14:3259–3265. doi:10.2147/jpr.s332966
211. Kapural L, Yu C, Doust MW, et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain. Anesthesiology. 2015;123(4):851–860. doi:10.1097/ALN.0000000000000774
212. Deer T, Slavin KV, Amirdelfan K, et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromod Technol Neural Interface. 2018;21(1):56–66. doi:10.1111/ner.12698
213. Martin S, Hadjipavlou G, Garcia Ortega R, et al. The importance of the location of dorsal root ganglion stimulator electrodes within the nerve root exit foramen. Neuromodulation. 2020;23(2):245–251. doi:10.1111/ner.12959
214. Arora S, Verma M, Kaur S, Chhabra S, Jain P. Measurement of sacral parameters of surgical importance in North Indian population. J Clin Diagnostic Res. 2018;12(2):AC05–AC09. doi:10.7860/JCDR/2018/28616.11185
215. Wolter T, Winkelmüller M. Continuous versus intermittent spinal cord stimulation: an analysis of factors influencing clinical efficacy. Neuromodulation. 2012;15(1):13–20. doi:10.1111/j.1525-1403.2011.00410.x
216. Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery. 2006;58(3):481–496. doi:10.1227/01.NEU.0000192162.99567.96
217. Deer T, Pope J, Hayek S, et al. Neurostimulation for the treatment of axial back pain: a review of mechanisms, techniques, outcomes, and future advances. Neuromod Technol Neural Interface. 2014;17:52–68. doi:10.1111/j.1525-1403.2012.00530.x
218. Vesper J, Slotty P, Schu S, et al. Burst SCS microdosing is as efficacious as standard burst SCS in treating chronic back and leg pain: results from a randomized controlled trial. Neuromodulation. 2019;22(2):190–193. doi:10.1111/ner.12883
219. Pawela CP, Kramer JM, Hogan QH. Dorsal root ganglion stimulation attenuates the BOLD signal response to noxious sensory input in specific brain regions: insights into a possible mechanism for analgesia. Neuroimage. 2017;147:10–18. doi:10.1016/j.neuroimage.2016.11.046
220. Parker T, Huang Y, Raghu ALB, FitzGerald JJ, Green AL, Aziz TZ. Dorsal root ganglion stimulation modulates cortical gamma activity in the cognitive dimension of chronic pain. Brain Sci. 2020;10(2):95. doi:10.3390/brainsci10020095
221. Racz GB, McCarron RF, Talboys P. Percutaneous dorsal column stimulator for chronic pain control. Spine. 1989;14(1):1–4. doi:10.1097/00007632-198901000-00001
222. Mironer Y, Brown C, Satterthwaite J, Cohen M, Tonder LM, Grumman S. A new technique of “midline anchoring” in spinal cord stimulation dramatically reduces lead migration. Neuromod Technol Neural Interface. 2004;7(1):32–37. doi:10.1111/j.1525-1403.2004.04004.x
223. Moman RN, Peterson AA, Maher DP, et al. Infectious complications of dorsal root ganglion stimulation: a systematic review and pooled analysis of incidence. Neuromodulation. 2021. doi:10.1111/ner.13473
224. North R, Desai MJ, Vangeneugden J, et al. Postoperative infections associated with prolonged spinal cord stimulation trial duration (PROMISE RCT). Neuromodulation. 2020;23(5):620–625. doi:10.1111/ner.13141
225. Costandi S, Mekhail N, Azer G, et al. Longevity and utilization cost of rechargeable and non-rechargeable spinal cord stimulation implants: a comparative study. Pain Pract. 2020;20(8):937–945. doi:10.1111/papr.12926
226. Eldabe S, Copley S, Gulve A, et al. A prospective long-term follow-up of dorsal root ganglion stimulation for the management of chronic intractable pain. Pain. 2021. doi:10.1097/j.pain.0000000000002405
227. Sivanesan E, Bicket MC, Cohen SP. Retrospective analysis of complications associated with dorsal root ganglion stimulation for pain relief in the FDA MAUDE database. Reg Anesth Pain Med. 2019;44(1):100–106. doi:10.1136/rapm-2018-000007
228. Horan M, Jacobsen AH, Scherer C, et al. Complications and effects of dorsal root ganglion stimulation in the treatment of chronic neuropathic pain: a nationwide cohort study in Denmark. Neuromodulation. 2020;2020. doi:10.1111/ner.13171
229. Chauhan G, Roth B, Mekhail NA. Dorsal root ganglion stimulation lead fractures: potential mechanisms and ways to avoid. BMJ Case Rep. 2021;14(5):e241353. doi:10.1136/bcr-2020-241353
230. Abbott. MRI procedure information clinician’s manual. 2020.
231. Abbott. Important medical device advisory NM implantable pulse generator (IPG) inoperable when exposed to monopolar electrosurgery; 2017. Available from: https://www.neuromodulation.abbott/content/dam/bss/divisionalsites/nm/pdfs/guides/ProclaimElite_Infinity_ImportantMedicalAdvisory_PhysicianLetter_June2017.pdf.
232. Chapman KB, Schirripa F, Yousef T, Deygoo J, van Helmond N. Lumbar radiofrequency ablation interfering with S1 dorsal root ganglion stimulation systems: experience from two cases. Pain Pract. 2020;20(7):780–786. doi:10.1111/papr.12901
233. Ikeda H, Asai T, Randić M, Murase K. Robust suppression of afferent-induced excitation in the rat spinal dorsal horn after conditioning low-frequency stimulation. J Neurophysiol. 1999;82(4):1957–1964. doi:10.1152/jn.1999.82.4.1957
234. Yu G, Segel I, Tran H, et al. Analgesic effects of tonic and burst dorsal root ganglion stimulation in rats with painful tibial nerve injury. Neuromodulation. 2021;2021:1–10. doi:10.1111/ner.13472
235. Sievert H, Piedade GS, McPhillips P, Vesper J, Slotty PJ. The role of periradicular infiltration in dorsal root ganglion stimulation for chronic neuropathic pain. Acta Neurochir. 2021;163(8):2135–2140. doi:10.1007/s00701-021-04745-y
236. Lo Bianco G, Papa A, Gazzerro G, et al. Dorsal root ganglion stimulation for chronic postoperative pain following thoracic surgery: a pilot study. Neuromodulation. 2020;2020. doi:10.1111/ner.13265
237. Purves D. Neuroscience.
238. Morgalla MH. Dorsal root ganglion stimulation for the treatment of persistent post-mastectomy pain: case report. Neuromodulation. 2019;22(1):117–118. doi:10.1111/ner.12894
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.