Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Benefits of Budesonide/Glycopyrronium/Formoterol Fumarate Dihydrate on COPD Exacerbations, Lung Function, Symptoms, and Quality of Life Across Blood Eosinophil Ranges: A Post-Hoc Analysis of Data from ETHOS
Authors Bafadhel M, Rabe KF , Martinez FJ , Singh D, Darken P , Jenkins M, Aurivillius M, Patel M, Dorinsky P
Received 14 May 2022
Accepted for publication 13 November 2022
Published 6 December 2022 Volume 2022:17 Pages 3061—3073
DOI https://doi.org/10.2147/COPD.S374670
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Min Zhang
Mona Bafadhel,1 Klaus F Rabe,2 Fernando J Martinez,3 Dave Singh,4 Patrick Darken,5 Martin Jenkins,6 Magnus Aurivillius,7 Mehul Patel,6 Paul Dorinsky8
1Department of Immunobiology, School of Immunology and Microbial Sciences, Faculty of Life Sciences and Medicine, King’s College London, London, UK; 2LungenClinic Grosshansdorf and Christian-Albrechts University Kiel, Airway Research Center North, Member of the German Center for Lung Research (DZL), Grosshansdorf, Germany; 3Joan and Sanford I. Weill Department of Medicine, Weill Cornell Medicine, New York, NY, USA; 4Medicines Evaluation Unit, University of Manchester, Manchester University NHS Foundation Hospitals Trust, Manchester, UK; 5AstraZeneca, Gaithersburg, MD, USA; 6AstraZeneca, Cambridge, UK; 7AstraZeneca, Gothenburg, Sweden; 8Formerly of AstraZeneca, Durham, NC, USA
Correspondence: Mona Bafadhel, Department of Immunobiology, School of Immunology and Microbial Sciences, Faculty of Life Sciences and Medicine, King’s College London, 5th floor, Tower Wing, Guy’s Hospital, Great Maze Pond, London SE1 9RT, UK, Tel +44 0207 188 8717, Email [email protected]
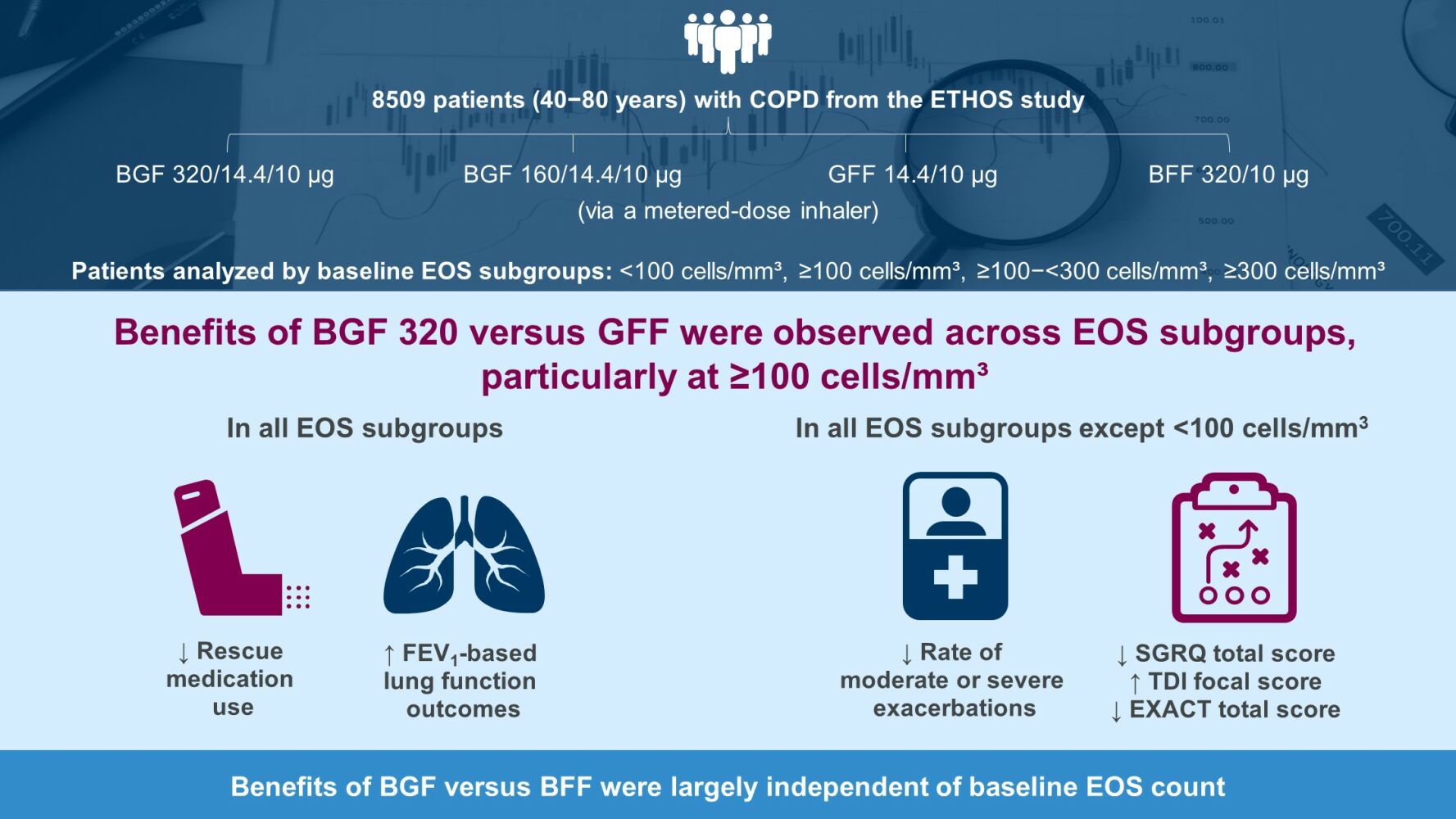
Purpose: Blood eosinophil (EOS) count can guide treatment decisions for chronic obstructive pulmonary disease (COPD). In the 52-week ETHOS study (NCT02465567), budesonide/glycopyrronium/formoterol fumarate dihydrate (BGF) triple therapy at two inhaled corticosteroid doses reduced moderate/severe exacerbation rates and improved lung function, symptoms, and disease-related quality of life (QoL) versus dual therapy with glycopyrronium/formoterol fumarate dihydrate (GFF) or budesonide/formoterol fumarate dihydrate (BFF) in patients with moderate-to-very severe COPD. This subgroup analysis evaluated treatment benefits in ETHOS by baseline EOS count.
Methods: Patients (40− 80 years) with a COPD history were randomly assigned 1:1:1:1 to receive BGF 320/14.4/10 μg, BGF 160/14.4/10 μg, GFF 14.4/10 μg, or BFF 320/10 μg via a metered-dose inhaler. This post-hoc analysis assessed endpoints by baseline EOS count using Global Initiative for Obstructive Lung Disease thresholds (< 100, ≥ 100, ≥ 100−< 300, ≥ 300 cells/mm3), and investigated continuous relationships between treatment effects and EOS count on exacerbations, symptoms, disease-related QoL, lung function, and safety.
Results: In the modified intention-to-treat population (n=8509), 82.6% had EOS counts ≥ 100 cells/mm3. BGF 320 reduced moderate/severe exacerbation rates versus GFF in the ≥ 100, ≥ 100−< 300, and ≥ 300 subgroups; treatment differences increased with EOS count. BGF 320 improved rescue medication use and lung-function outcomes across all subgroups, and St George’s Respiratory Questionnaire total score, Transition Dyspnea Index focal score, and Exacerbations of Chronic Pulmonary Disease Tool total score in all except the < 100 subgroup versus GFF. Benefits of BGF 320 versus BFF were generally consistent across subgroups. Safety data were comparable across subgroups.
Conclusion: Benefits of BGF versus GFF were observed across EOS counts, particularly at ≥ 100 cells/mm³; versus BFF, benefits were largely independent of EOS. These findings confirm that benefits of ICS-containing triple therapy are not restricted to EOS counts ≥ 300 cells/mm³, supporting recommendations to consider triple therapy in patients with an exacerbation history and EOS counts ≥ 100 cells/mm³.
Keywords: eosinophils, inhaled corticosteroids, triple therapy
Graphical Abstract:
Introduction
For patients with chronic obstructive pulmonary disease (COPD) and a history of exacerbations, treatment with triple therapy containing an inhaled corticosteroid (ICS), long-acting β2-agonist (LABA), and long-acting muscarinic antagonist (LAMA) is recommended as an escalation step from ICS/LABA or LAMA/LABA dual therapy.1 Blood eosinophil (EOS) count can help predict ICS response in patients with COPD, even in those who have not had an exacerbation in the previous year.2 Previous analyses have consistently shown a continuous relationship between EOS counts and ICS-containing treatment effects, with responsiveness across a broad range of EOS counts that increases at higher EOS counts.2–6 Not only has the relationship between EOS count and exacerbations2–5 and mortality6 been well characterized, but greater efficacy on lung function following ICS treatment has also been observed at higher baseline EOS counts.2,7 In addition, limited data suggest that greater benefits on symptoms and disease-related quality of life (QoL) may be achieved by the administration of ICS therapy in patients with higher EOS counts.4,5,7
The Global Initiative for Obstructive Lung Disease (GOLD) report currently recommends that the addition of ICS therapy be considered for patients on LAMA/LABA therapy who have exacerbations and EOS counts ≥100 cells/mm3.1 A threshold of >300 cells/mm3 is used to identify patients most likely to benefit from initiating ICS use, while the use of ICS is not recommended with EOS counts <100 cells/mm³ given the limited evidence of relevant treatment effects.1,3
In the 52-week ETHOS study (NCT02465567), triple therapy with a budesonide/glycopyrronium/formoterol fumarate dihydrate (BGF) metered-dose inhaler at two ICS dose levels reduced moderate and/or severe exacerbation rates and improved lung function and symptoms versus dual therapy with glycopyrronium/formoterol fumarate dihydrate (GFF) or budesonide/formoterol fumarate dihydrate (BFF) in patients with moderate-to-very severe COPD and at least one exacerbation in the previous year.8–10 The large sample size of ETHOS presents an opportunity to evaluate EOS count data with respect to treatment benefits on exacerbation rates, symptoms, disease-related QoL, and lung function, and to compare findings with observations from other studies. Thus, we present subgroup analyses assessing the efficacy and safety of BGF versus GFF and BFF in patients enrolled in the ETHOS study by EOS count (<100, ≥100, ≥100–<300, and ≥300 cells/mm3) and describe the continuous relationship between treatment effects and increasing EOS count.
Methods
Study Design and Population
Details of the primary ETHOS study design have been published previously.8,11 In brief, ETHOS was a Phase 3, randomized, double-blind, parallel-group study conducted in 26 countries. Patients with moderate-to-very severe COPD were assigned in a 1:1:1:1 ratio to receive BGF 320/14.4/10 µg (hereafter referred to as BGF 320), BGF 160/14.4/10 µg (hereafter referred to as BGF 160), GFF 14.4/10 µg, or BFF 320/10 µg. All treatments were administered twice daily over 52 weeks via a single metered-dose inhaler.
Patients between 40 and 80 years of age who had an established clinical history of COPD with a post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity ratio <0.70 and FEV1 25–<65% predicted normal were eligible for inclusion. In addition, eligible patients were required to have a COPD Assessment Test score of ≥10 at screening, two or more inhaled maintenance therapies for at least 6 weeks prior to screening (if on maintenance ICS at screening [Visit 1], ICS was continued throughout the screening period), a smoking history of ≥10 pack-years, and a documented history of moderate or severe exacerbations in the 12 months prior to screening (≥1 moderate or severe if post-bronchodilator FEV1 <50% of predicted normal or ≥2 moderate or ≥1 severe if post-bronchodilator FEV1 ≥50% of predicted normal). Patients who had a current diagnosis of asthma, COPD due to α1 antitrypsin deficiency, or any clinically significant uncontrolled conditions other than COPD were excluded.
Blood EOS counts were measured at the screening (Visit 1) and randomization (Visit 4) visits. Baseline EOS count was recorded as the average of available EOS counts over these visits. For these post-hoc analyses, patients were assessed in four subgroups based on baseline EOS count: <100 cells/mm3, ≥100 cells/mm3, ≥100–<300 cells/mm3, and ≥300 cells/mm3.
The ETHOS study was performed in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with International Council for Harmonisation/Good Clinical Practice and applicable regulatory requirements. The study protocol and informed consent form were approved by the appropriate institutional review board, independent ethics committee, or health authority, and written informed consent was obtained from all patients before screening.
Endpoints
Primary and secondary endpoint data from the overall ETHOS population have been reported previously.8 Exacerbation endpoints included in this subgroup analysis were the annual rate of moderate or severe COPD exacerbations (the primary endpoint in ETHOS), and the annual rate of severe exacerbations (a secondary endpoint in ETHOS). Additionally, secondary COPD symptom and disease-related QoL endpoints were assessed using the European regulatory approach (over 24 weeks unless otherwise specified) in this subgroup analysis and included changes from baseline in average daily rescue medication use, St George’s Respiratory Questionnaire (SGRQ) total score, Exacerbations of Chronic Pulmonary Disease Tool (EXACT) total score over 52 weeks, and Transition Dyspnea Index (TDI) focal score. Lung function (morning pre-dose trough FEV1 and FEV1 area under the concentration–time curve from 0−4 h [AUC0−4]) was also analyzed in a subset of patients who participated in a pulmonary function test sub-study of ETHOS.9
Safety was assessed via adverse-event monitoring. Cardiovascular and cerebrovascular events, pneumonia cases, and cause-specific deaths were reviewed by an independent data monitoring committee and an independent clinical endpoint committee throughout the study.
Statistical Analysis
All subgroup analyses of the primary and secondary symptom-related endpoints used on-treatment data from the modified intention-to-treat (mITT) population of ETHOS (all randomized and treated patients with data obtained before discontinuation of treatment). Safety was assessed in the safety population (all randomized and treated patients); patients with no post-randomization safety assessments were excluded.
The focus of this analysis was on BGF 320, but results for BGF 160 are included for completeness. Treatment comparisons in each subgroup were made for both BGF doses versus GFF and BFF. Exacerbation rates were analyzed using negative binomial regression, with adjustment for ICS use at screening, baseline post-bronchodilator percent predicted FEV1, baseline COPD exacerbation history (1/≥2), log baseline EOS count, and geographic region. Differences between treatments in rescue medication use, SGRQ total score, TDI focal score, and EXACT total score were analyzed using a linear repeated measures model in each subgroup. Models for SGRQ total score and TDI focal score were adjusted for baseline score, treatment, visit, treatment-by-visit interaction, ICS use at screening, baseline post-bronchodilator percent predicted FEV1, and percent reversibility to bronchodilator. Analysis of daily rescue medication use and EXACT total score used similar models with adjustment for 4-weekly time intervals rather than visit. P-values for these post-hoc analyses were not adjusted for multiplicity and are provided for information in the Figures. The analyses were not prospectively powered to achieve statistical significance, and interpretation of the data focused on observing the magnitude and direction of the treatment differences in the subgroups. The subgroup analyses were supplemented by a generalized additive model investigating the relationship of endpoints to EOS count as a continuous variable.
Results
Study Population
Overall, 8588 patients were randomized, and the mITT population included 8509 patients. The safety population included 8529 patients. The mean age of patients in the mITT population was 64.7 years (standard deviation 7.6) and 59.7% were male. Baseline demographics were similar across EOS count subgroups, with the inherent exception of baseline EOS count (Table 1). Within the mITT population, 17.3% of patients had EOS <100 cells/mm³, 82.6% had EOS ≥100 cells/mm3, 67.9% had EOS ≥100−<300 cells/mm³, and 14.7% had EOS ≥300 cells/mm³. EOS variability in the safety population during the ETHOS study is shown in Supporting Table S1. Within the 52-week study period, 40% of patients had both a minimum EOS count of <100 cells/mm3 and a maximum EOS count of ≥100 cells/mm³, and 5.9% of patients had a minimum EOS count of <100 cells/mm3 and a maximum EOS count of >300 cells/mm3. A higher proportion of patients receiving GFF had a maximum EOS count >300 cells/mm3 (34.9%) compared with patients receiving BGF 320 (30.1%), BGF 160 (30.6%), and BFF (28.9%). Additionally, a smaller proportion of patients receiving GFF had a maximum EOS count <100 cells/mm3 (3.8%) compared with patients receiving BGF 320 (4.7%), BGF 160 (4.9%), and BFF (4.9%).
 |
Table 1 Demographics and Baseline Characteristics by EOS Subgroup (mITT Population) |
COPD Exacerbations
BGF 320 reduced the rate of moderate/severe exacerbations versus GFF in the ≥100, ≥100−<300, and ≥300 cells/mm³ subgroups (Figure 1). Rate reductions for BGF 320 versus GFF increased with increasing EOS counts, with a 22% reduction for patients in the EOS ≥100−<300 cells/mm³ subgroup and a 52% reduction for patients with EOS ≥300 cells/mm³ (Figure 1). Rate reductions for BGF 320 versus BFF were largest in the <100 cells/mm³ subgroup (22%; Figure 1) but treatment differences overall were generally seen across the range of EOS counts (Figure 2A). Similar treatment benefits were observed with BGF 160 versus GFF and BFF (Figures 1 and 2A).
 |
Figure 2 Efficacy endpoints by baseline EOS count (mITT population). (A) Annual rate of moderate/severe exacerbationsa; (B) Annual rate of severe exacerbations; (C) Change from baseline in rescue medication use over 24 weeks; (D) Change from baseline in SGRQ total score over 24 weeks; (E) Change from baseline in FEV1 AUC0−4 over 24 weeks; (F) Change from baseline in morning pre-dose trough FEV1 over 24 weeks. Data are from generalized additive models. Banded areas indicate 95% Bayesian credible intervals. aFrom N Engl J Med, Rabe KF, et al. Triple Inhaled Therapy at Two Glucocorticoid Doses in Moderate-to-Very-Severe COPD, 383(1):35–48. Copyright © 2020 Massachusetts Medical Society.8 Reprinted with permission from Massachusetts Medical Society. Abbreviations:AUC0–4, area under the concentration–time curve from 0−4 h; BFF, budesonide/formoterol fumarate dihydrate; BGF, budesonide/glycopyrronium/formoterol fumarate dihydrate; COPD, chronic obstructive pulmonary disease; EOS, blood eosinophil; FEV1, forced expiratory volume in 1 s; GFF, glycopyrronium/formoterol fumarate dihydrate; mITT, modified intention-to-treat; SGRQ, St George’s Respiratory Questionnaire. |
Reductions in the rate of severe exacerbations for BGF 320 versus GFF were greatest in the ≥300 cells/mm³ subgroup (57%; Figure 3), and treatment differences tended to increase with increasing EOS count (Figure 2B). Rate reductions for BGF 320 versus BFF were largest in the <100 cells/mm³ subgroup (39%; Figure 3), but rate reductions were observed across all EOS subgroups.
Symptoms and Disease-Related QoL
BGF 320 reduced rescue medication use versus dual therapies in patients across all EOS subgroups (Figure 4). BGF 320 reduced rescue medication use versus GFF in all four EOS subgroups, with treatment differences increasing with baseline EOS count (Figures 2C and 4). Treatment differences between BGF 320 and BFF were greatest for patients with baseline EOS <100 cells/mm3 but were seen across the range of EOS counts (Figure 4).
BGF 320 improved SGRQ total score versus GFF in the ≥100, ≥100−<300, and ≥300 cells/mm³ subgroups, with the greatest treatment benefits for BGF 320 observed in the ≥300 cells/mm³ subgroup (Figure 5). Treatment differences between BGF 320 and GFF in SGRQ total score also tended to increase with increasing EOS count (Figure 2D). Compared with BFF, BGF 320 improved SGRQ total score in all subgroups, with the largest treatment benefits observed in the ≥300 cells/mm³ subgroup (Figure 5).
Improvements in EXACT total score were observed with BGF 320 versus GFF and BFF in the ≥100, ≥100−<300, and ≥300 cells/mm³ subgroups (Supporting Figure S1), and treatment differences generally increased with higher baseline EOS counts (Supporting Figure S2A). TDI focal score also improved with BGF 320 versus GFF in the ≥100, ≥100−<300, and ≥300 cells/mm³ subgroups (Supporting Figure S3), with the difference between treatments increasing with baseline EOS count (Supporting Figure S2B). Improvements with BGF 320 versus BFF in EXACT total score (Supporting Figure S1) and TDI focal score (Supporting Figure S2) were observed in all EOS subgroups.
Lung Function
Improvements in morning pre-dose trough FEV1 (Figure 6A) and FEV1 AUC0–4 (Figure 6B) over 24 weeks were observed with BGF 320 versus GFF in all subgroups, though improvements were smaller in the <100 cells/mm³ subgroup. Compared with BFF, BGF 320 improved trough FEV1 (Figure 6A) and FEV1 AUC0–4 (Figure 6B) in all subgroups. Improvements versus GFF generally increased with higher EOS counts (Figure 2E and F) and were greatest in patients with EOS ≥300 cells/mm3 (Figure 6A and B).
Safety
Safety data by baseline EOS subgroup and treatment group are presented in Table 2, and further data on specific TEAEs during the treatment period are presented in Supporting Table S2. Within each EOS subgroup, the incidence of treatment-emergent adverse events (TEAEs) was generally comparable across treatments. The incidence of confirmed major adverse cardiovascular events (MACE), confirmed pneumonia, overall deaths, and cardiovascular and respiratory deaths was similar across EOS subgroups (Table 2), but the incidence of confirmed MACE was highest in patients receiving GFF (23.5−39.8 per 1000 patient-years) and lowest in patients with EOS counts ≥300 cells/mm³ receiving BGF 320 (3.6 per 1000 patient-years). The incidence of cardiovascular death in patients receiving GFF with EOS counts ≥100 cells/mm3, ≥100–300 cells/mm3, and ≥300 cells/mm3 was greater than double that of patients receiving either dose of BGF (Supporting Table S2).
 |
Table 2 Adverse Events During the 52-Week Treatment Period by Treatment Group and EOS Subgroup (Safety Populationa) |
Discussion
In the ETHOS study, triple therapy with both doses of BGF showed benefits on exacerbations, symptoms, and disease-related QoL compared with LAMA/LABA therapy (GFF), and these benefits increased with EOS count, further demonstrating that EOS count can help predict an ICS versus non-ICS effect as observed versus LAMA/LABA dual therapy. Treatment benefits of BGF compared with ICS/LABA therapy (BFF) were also observed, and these benefits were generally consistent across the range of EOS counts. Consistent with the current GOLD recommendations,1 the strongest benefits of ICS-containing triple therapy were observed in patients with EOS ≥300 cells/mm3. However, it is important to note that patients with EOS counts ≥100−<300 cells/mm³ also experienced clear and consistent benefits with BGF 320 compared with both dual therapies on exacerbations, symptoms, disease-related QoL, and lung function, suggesting that patients with EOS counts in this range also benefit from triple therapy.
In the EOS <100 cells/mm3 subgroup, treatment benefits of BGF 320 compared with GFF were observed for rescue medication use. However, overall, this subgroup did not experience greater benefits of ICS-containing triple therapy compared with LAMA/LABA for other endpoints, and the precise EOS count threshold at which treatment benefit began varied between endpoints. Patients with lower EOS counts have less type 2 inflammation, which can explain a lower response to ICS.12–14 Furthermore, lower blood and sputum EOS counts are associated with a different microbiome, characterized by an increased presence of Haemophilus influenzae that further upregulates the burden of neutrophilic airway inflammation present in COPD.15–18 Lower EOS counts therefore seem to mark a COPD subgroup with a microbiome and airway inflammation profile that responds less to ICS treatment.
The findings of the current analysis are consistent with previously published clinical trial analyses.2,5,19 For example, in the 24-week KRONOS study in 1902 patients with symptomatic COPD, reductions in exacerbation rates with BGF triple therapy versus dual therapy with GFF increased as EOS count increased, particularly at EOS counts above 100 cells/mm³.2 Improvements in lung function with triple therapy in KRONOS were driven by patients with baseline EOS counts ≥150 cells/mm³, with the greatest improvements observed above 250 cells/mm³, while the treatment benefits of BGF compared with BFF on lung function occurred across a broad range of EOS counts. Furthermore, in the 52-week IMPACT trial of 10,355 patients with symptomatic COPD and at least one moderate or severe exacerbation in the previous year, Pascoe et al reported that the magnitude of treatment benefits with ICS-containing regimens compared with a LAMA/LABA was largest in patients with an EOS count of ≥100 cells/mm³.5
Overall, safety data were comparable across the EOS subgroups. However, it is notable that incidence of cardiovascular death was higher in patients receiving GFF versus BGF in each EOS subgroup except the <100 cells/mm3 subgroup. These findings extend upon previous analyses of MACE and mortality in ETHOS, which report higher incidence of MACE and cardiovascular death with GFF versus BGF,6,20 with this difference being more pronounced with increasing EOS for non-fatal myocardial infarction and cardiovascular death but not for non-fatal stroke.20 However, these findings should be interpreted with caution due to the low number of events in each subgroup.
The findings from the ETHOS study indicate not only that exacerbation rates are reduced but also that symptoms, disease-related QoL, and lung function improve with ICS-containing triple therapy, particularly at EOS counts greater than 100 cells/mm3, and show clear evidence of treatment benefits for patients with EOS counts between 100 and 300 cells/mm3. Thus, the current analyses add to this body of evidence2,5 by analyzing treatment effects across a broad range of clinically relevant endpoints using EOS count thresholds in line with the existing GOLD recommendations.1
The current findings should be considered in light of the strengths and limitations of the ETHOS study. The strengths of the ETHOS study include its large sample size. While the patient population was large (n = 8509), the percentages of patients with baseline EOS counts ≥300 cells/mm³ and <100 cells/mm³ were relatively small (14.7% and 17.3%, respectively) by virtue of the natural distribution of EOS.21 This led to greater uncertainty in the estimates for these subgroups. However, given the large overall sample size, both groups still were of reasonable absolute sizes. A further limitation of this study is that ETHOS was not designed or prospectively powered to achieve statistical significance in these post-hoc subgroup analyses. As such, the unadjusted p-values should be interpreted with caution, and interpretation of the results should instead focus on the magnitude and direction of the treatment differences in the subgroups.
It is also important to note that patients were grouped by baseline EOS count, per measurements at screening and randomization. However, it has been shown that EOS levels can vary over time,22 and data modeling indicates that the relationship between EOS count and ICS effectiveness is continuous.3 Thus, basing clinical decisions on precise EOS cut-off values may be over-simplistic, especially given important factors such as smoking status and its impact on blood EOS.1,23
Conclusions
In conclusion, treatment benefits for BGF 320 over dual therapy with LAMA/LABA on moderate or severe COPD exacerbation rates, symptoms, disease-related QoL, and lung function were observed across a range of EOS counts, particularly above 100 cells/mm3, and these benefits generally increased with higher baseline EOS count. Benefits of BGF compared with ICS/LABA dual therapy were largely independent of EOS. Overall, these findings confirm those of previous studies,2,5,19 that patients with EOS counts ≥300 cells/mm3 experience the greatest benefits of ICS-containing triple therapy on exacerbation rates, symptoms, disease-related QoL, and lung function. Importantly, this study provides further evidence that patients with EOS counts ≥100−<300 cells/mm³ also experience clear benefits of ICS-containing therapy on these same outcomes.
Abbreviations
AUC0–4, area under the concentration–time curve from 0−4 h; BFF, budesonide/formoterol fumarate dihydrate; BGF, budesonide/glycopyrronium/formoterol fumarate dihydrate; CAT, COPD Assessment Test; CI, confidence interval; COPD, chronic obstructive pulmonary disease; EOS, blood eosinophil; EXACT, Exacerbations of Chronic Pulmonary Disease Tool; FEV1, forced expiratory volume in 1 s; GFF, glycopyrronium/formoterol fumarate dihydrate; GOLD, Global Initiative for Obstructive Lung Disease; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist, LAMA, long-acting muscarinic antagonist; LSM, least square mean; MACE, major adverse cardiovascular event; mITT, modified intention-to-treat; QoL, quality of life; RR, rate ratio; SD, standard deviation; SGRQ, St George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index; TEAE, treatment-emergent adverse event.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data-sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Ethics Approval and Informed Consent
The study protocol and informed consent form were approved by the appropriate institutional review board, independent ethics committee, or health authority (Supporting Table S3), and written informed consent was obtained from all patients before screening.
Acknowledgments
Medical writing support, under the direction of the authors, was provided by Sara Cameron, MPhil, and Daniel Spindlow, MSc, CMC Connect, a division of IPG Health Medical Communications, funded by AstraZeneca in accordance with Good Publication Practice (GPP 2022) guidelines.24 Dave Singh is supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC).
Author Contributions
All authors made a significant contribution to the work reported. MB and DS contributed to the study conception or design and the interpretation of data. KFR, FJM, PDa, and PDo contributed to the study conception or design, analysis of data and interpretation of data. MJ and MP contributed to the analysis of data and interpretation of data. MA contributed to the study conception or design, acquisition of data, and analysis of data. All authors took part in drafting, revising, or critically reviewing the article, gave final approval of the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
This study was sponsored by AstraZeneca. The study sponsor was involved in the design and conduct of the study, in the data analysis and interpretation, and in the review of the manuscript for accuracy. Decisions on final content were made by the authors. Medical writing support for development of this manuscript was funded by AstraZeneca.
Disclosure
MB reports grants from AstraZeneca, and honoraria from AstraZeneca, Chiesi, and GlaxoSmithKline; and is on the scientific advisory board for Albus Health and ProAxsis. KFR reports grants and personal fees from AstraZeneca and Boehringer Ingelheim; and personal fees from Berlin Chemie, Chiesi Pharmaceuticals, GlaxoSmithKline, Novartis, Regeneron, Roche, and Sanofi, outside the submitted work. FJM reports grants, personal fees, and non-financial support from AstraZeneca during the conduct of the study; grants, personal fees, and non-financial support from AstraZeneca, Boehringer Ingelheim, Bioscale/Proterrix Bio, Chiesi, CSL Behring, Gala, GlaxoSmithKline, Metronic, Novartis, Polarean, Pulmatrix, Pulmonx, Sanofi/Regeneron, Sunovion, Teva, Theravance/Viatris, and Verona; grants and personal fees from AstraZeneca, Chiesi, GlaxoSmithKline, and Sanofi/Regeneron. He is also a COPD teleconsultant for Bayer. DS reports personal fees from AstraZeneca during the conduct of the study; and personal fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Epiendo, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Mundipharma, Novartis, Peptinnovate, Pfizer, Pulmatrix, Sanofi, Synairgen, Teva, Theravance, and Verona, outside the submitted work. PDa, MJ, MA, and MP are employees of AstraZeneca and hold stock and/or stock options in the company. PDo is a former employee of AstraZeneca and previously held stock and/or stock options in the company. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease. 2022 GOLD Report. Global strategy for the diagnosis, management and prevention of COPD. Available from: https://goldcopd.org/2022-gold-reports-2/.
2. Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–758. doi:10.1016/S2213-2600(18)30327-8
3. Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med. 2018;6(2):117–126. doi:10.1016/S2213-2600(18)30006-7
4. Siddiqui SH, Guasconi A, Vestbo J, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(4):523–525. doi:10.1164/rccm.201502-0235LE
5. Pascoe S, Barnes N, Brusselle G, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med. 2019;7(9):745–756. doi:10.1016/S2213-2600(19)30190-0
6. Martinez FJ, Rabe KF, Ferguson GT, et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. A randomized, double-blind, multicenter, parallel-group study. Am J Respir Crit Care Med. 2021;203(5):553–564. doi:10.1164/rccm.202006-2618OC
7. Mathioudakis AG, Bikov A, Foden P, et al. Change in blood eosinophils following treatment with inhaled corticosteroids may predict long-term clinical response in COPD. Eur Respir J. 2020;55(5):1902119. doi:10.1183/13993003.02119-2019
8. Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi:10.1056/NEJMoa1916046
9. Rabe KF, Martinez FJ, Singh D, et al. Improvements in lung function with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler versus dual therapies in patients with COPD: a sub-study of the ETHOS trial. Ther Adv Respir Dis. 2021;15:17534666211034329. doi:10.1177/17534666211034329
10. Martinez FJ, Rabe KF, Ferguson GT, et al. Benefits of budesonide/glycopyrrolate/formoterol fumarate (BGF) on symptoms and quality of life in patients with COPD in the ETHOS trial. Respir Med. 2021;185:106509. doi:10.1016/j.rmed.2021.106509
11. Rabe KF, Martinez FJ, Ferguson GT, et al. A Phase III study of triple therapy with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler 320/18/9.6 μg and 160/18/9.6 μg using co-suspension delivery technology in moderate-to-very severe COPD: the ETHOS study protocol. Respir Med. 2019;158:59–66. doi:10.1016/j.rmed.2019.08.010
12. Kolsum U, Damera G, Pham T-H, et al. Pulmonary inflammation in patients with chronic obstructive pulmonary disease with higher blood eosinophil counts. J Allergy Clin Immunol. 2017;140(4):1181–1184. doi:10.1016/j.jaci.2017.04.027
13. Higham A, Beech A, Wolosianka S, et al. Type 2 inflammation in eosinophilic chronic obstructive pulmonary disease. Allergy. 2021;76(6):1861–1864. doi:10.1111/all.14661
14. George L, Taylor AR, Esteve‐Codina A, et al. Blood eosinophil count and airway epithelial transcriptome relationships in COPD versus asthma. Allergy. 2020;75(2):370–380. doi:10.1111/all.14016
15. Dicker AJ, Huang JT, Lonergan M, et al. The sputum microbiome, airway inflammation, and mortality in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2021;147(1):158–167. doi:10.1016/j.jaci.2020.02.040
16. Wang Z, Locantore N, Haldar K, et al. Inflammatory endotype-associated airway microbiome in COPD clinical stability and exacerbations: a multicohort longitudinal analysis. Am J Respir Crit Care Med. 2020;203(12):1488–1502. doi:10.1164/rccm.202009-3448OC
17. Beech A, Lea S, Li J, Jackson N, Mulvanny A, Singh D. Airway bacteria quantification using polymerase chain reaction combined with neutrophil and eosinophil counts identifies distinct COPD endotypes. Biomedicines. 2021;9(10):1337. doi:10.3390/biomedicines9101337
18. Beech AS, Lea S, Kolsum U, et al. Bacteria and sputum inflammatory cell counts; a COPD cohort analysis. Respir Res. 2020;21(1):289. doi:10.1186/s12931-020-01552-4
19. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391(10125):1076–1084. doi:10.1016/S0140-6736(18)30206-X
20. Bafadhel M, Rabe KF, Singh D, Jenkins M, Dorinsky P, Patel M. The relationship between eosinophils and reduction in major adverse cardiac events in ETHOS. Eur Respir J. 2021;58(suppl65):RCT208.
21. Kolsum U, Southworth T, Jackson N, Singh D. Blood eosinophil counts in COPD patients compared to controls. Eur Respir J. 2019;54(4):1900633. doi:10.1183/13993003.00633-2019
22. Schumann DM, Tamm M, Kostikas K, Stolz D. Stability of the blood eosinophilic phenotype in stable and exacerbated COPD. Chest. 2019;156(3):456–465. doi:10.1016/j.chest.2019.04.012
23. Hartl S, Breyer M-K, Burghuber OC, et al. Blood eosinophil count in the general population: typical values and potential confounders. Eur Respir J. 2020;55(5):1901874. doi:10.1183/13993003.01874-2019
24. DeTora LM, Toroser D, Sykes A, et al. Good publication practice (GPP) guidelines for company-sponsored biomedical research: 2022 Update. Ann Intern Med. 2022;175(9):1298–1304. doi:10.7326/M22-1460
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





