Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Benefit of Prompt Vs Delayed Initiation of Triple Therapy Following an Exacerbation in Patients with COPD in Japan: A Retrospective Cohort Study
Authors Czira A , Akiyama S , Ishii T, Wood RP , Camidge LJ, Wallis H , Jennison T, Wild RAC , Yarita M, Hashimoto K , Rothnie KJ , Ismaila AS
Received 2 June 2023
Accepted for publication 17 October 2023
Published 8 December 2023 Volume 2023:18 Pages 2933—2953
DOI https://doi.org/10.2147/COPD.S419119
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Alexandrosz Czira,1 Shoko Akiyama,2 Takeo Ishii,2 Robert P Wood,3 Lucinda J Camidge,3 Hannah Wallis,3 Thomas Jennison,3 Rosie AC Wild,3 Masao Yarita,2 Kenichi Hashimoto,2 Kieran J Rothnie,1 Afisi S Ismaila4,5
1Value Evidence and Outcomes, R&D Global Medical, GSK, Brentford, Middlesex, UK; 2Value Evidence and Outcomes, Japan Medical and Development, GSK, Tokyo, Japan; 3Real-World Evidence, Adelphi Real World, Bollington, UK; 4Value Evidence and Outcomes, R&D Global Medical, GSK, Collegeville, PA, USA; 5Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON, Canada
Correspondence: Kieran J Rothnie, Value Evidence and Outcomes, R&D Global Medical, GSK, Brentford, Middlesex, TW8 9GS, UK, Tel +44 7920 369573, Email [email protected]
Purpose: There is currently limited evidence for the optimal timing of triple therapy initiation in Japan, which is crucial for optimizing strategies for the effective treatment of chronic obstructive pulmonary disease (COPD). This study assessed the impact of prompt vs delayed initiation of triple therapy following a COPD exacerbation on clinical and economic outcomes in patients in Japan.
Patients and Methods: Retrospective cohort study of patients in the Medical Data Vision Co., Ltd. database initiating triple therapy as single-inhaler triple therapy (fluticasone furoate/umeclidinium/vilanterol or budesonide/glycopyrronium/formoterol) or multiple-inhaler triple therapy within 180 days of a moderate-to-severe exacerbation (index). For the main analysis, patients were categorized as prompt or delayed initiators, initiating triple therapy within 0– 30 days or 31– 180 days of index, respectively. Inverse probability of treatment weighting based on propensity scores was used to adjust for measured confounders between prompt and delayed cohorts.
Results: For the main analysis, 610 (60.3%) and 402 (39.7%) patients were prompt and delayed initiators, respectively. The rate of subsequent moderate-to-severe exacerbations following index exacerbation was numerically lower in prompt vs delayed initiators (weighted rate ratio 0.95, 95% confidence interval [CI]: 0.74– 1.21; P = 0.6603). Time-to-first subsequent moderate-to-severe exacerbation increased significantly in prompt vs delayed initiators (weighted hazard ratio 0.77, 95% CI: 0.64– 0.93; P = 0.0053). In patients indexed on a severe exacerbation, delayed initiation resulted in significantly higher 90-day all-cause readmissions vs prompt initiation (42.1% vs 30.6%; P = 0.0329 [weighted estimates]). Weighted healthcare resource utilization rates were numerically lower in prompt vs delayed initiators, and weighted direct costs (all cause and COPD-related) were significantly lower in prompt initiators.
Conclusion: This real-world study demonstrated that earlier initiation of triple therapy resulted in several benefits in clinical outcomes for COPD and may also reduce the economic burden of COPD management in Japan.
Plain Language Summary: Patients with chronic obstructive pulmonary disease (COPD) can experience flare-ups in their symptoms, known as exacerbations. If exacerbations continue despite patients taking either one or a combination of two respiratory medications, guidelines suggest that they should take a combination of three treatments, known as triple therapy. In Japan, the best timing for patients to start triple therapy following an exacerbation is currently unknown, therefore this study compared the impact on clinical and economic outcomes of patients starting triple therapy 0– 30 days (prompt initiators) or 31– 180 days (delayed initiators) after their first exacerbation. When looking at clinical outcomes for COPD, we found that prompt initiators had a significantly longer time period from starting triple therapy to experiencing their next moderate-to-severe exacerbation, and those who had an initial severe exacerbation had significantly fewer hospital readmissions during the 90 days that followed their initial exacerbation than delayed initiators. When looking at economic outcomes for COPD, we found that medical costs were significantly lower in prompt versus delayed initiators. Findings of this study suggest that starting triple therapy earlier following an exacerbation may lead to improved clinical outcomes for patients with COPD in Japan and can reduce the cost of managing this disease.
Keywords: healthcare resource utilization, hospital readmissions, direct medical costs, inverse probability of treatment weighting, single inhaler triple therapy, multiple inhaler triple therapy
Introduction
The high prevalence, morbidity, and mortality associated with chronic obstructive pulmonary disease (COPD) presents a significant burden to healthcare systems worldwide.1 In Japan, the prevalence of COPD is estimated to be at least 8.6% in individuals aged ≥40 years, and up to 10.3% in patients aged ≥60 years.2 In patients with a history of smoking or respiratory symptoms in Japan, the prevalence of COPD is 22%.3,4 One study estimated the average annual cost per COPD patient aged <65 years to be 438,975 Japanese Yen (¥); 4389 United States [US] dollars ($), increasing to ¥467,871 ($4678) for patients aged ≥65 years.3
COPD exacerbations are a key driver of the clinical and economic burden of the disease.5–9 Effective treatment, including early intervention, is vital to mitigate the impact of COPD on global healthcare resource utilization (HCRU), costs, and patient quality of life.1,6,8–10 The Global Initiative for Chronic Obstructive Lung Disease11 guidelines recommend a stepwise approach to treatment from monotherapy with a long-acting β2-agonist (LABA) or a long-acting muscarinic antagonist (LAMA), to dual therapy with LABA/LAMA or an inhaled corticosteroid (ICS)/LABA), then further escalation to triple therapy with ICS/LAMA/LABA if patients continue to experience exacerbations.11 Notably, the latest Japanese Respiratory Society guidelines recommend the addition of ICS only for patients with COPD with concomitant asthmatic features and/or with frequent exacerbations as well as blood eosinophilia.12
Triple therapy can be administered as multiple-inhaler triple therapy (MITT), using two (ICS/LABA + LAMA or LABA/LAMA + ICS) or three (ICS + LAMA + LABA) different inhaled devices, or single-inhaler triple therapy (SITT) with all three components in one device.13,14 Currently, there are several single and dual therapy drugs available in Japan for the treatment of COPD which can be combined to form MITT,15–18 as well as two SITT devices.19,20 SITT with fluticasone furoate, umeclidinium, and vilanterol (FF/UMEC/VI) was approved in Japan in March 2019 for the long-term, once-daily, maintenance treatment of patients with COPD.19 SITT with budesonide, glycopyrronium, and formoterol (BUD/GLY/FOR) was approved for use in Japan in June 2019.20
Evidence on the timing of triple therapy initiation is vital to optimizing future treatment strategies for effective management of symptoms and mitigation of disease progression in COPD patient populations. Previous studies in the US and United Kingdom (UK) have shown that prompt initiation (≤30 days) of SITT8 or MITT6,21 following an index exacerbation,8,21 or index COPD-related hospitalization/emergency department visit,6 is associated with reduced COPD exacerbations and medical costs compared with delayed initiation (31–180 days post-index). A similar study was conducted in Japan, but findings were limited by low MITT use at the time of the study.22 There is therefore currently limited evidence to inform the optimal timing of triple therapy initiation in Japan.6,11,22
This real-world study was performed to evaluate the impact of prompt vs delayed initiation of triple therapy following a moderate-to-severe exacerbation (index exacerbation) on clinical and economic outcomes for patients with COPD in Japan.
Materials and Methods
Study Design and Data Source
This retrospective, weighted, cohort study was based on health insurance claims (outpatient and inpatient) data provided by Medical Data Vision Co., Ltd. (MDV; Tokyo, Japan). The MDV data cover a broad range of health data in Japan, including around 26% of acute care hospitals nationally (over 460 facilities), representing approximately 40 million patients, making it one of the largest Japanese healthcare datasets.23,24 Data are available on administrative claims, while Diagnosis Procedures Combination (DPC) data from hospitals participating in the DPC system (approximately 374) are also available for inpatient data. DPC hospitals in Japan provide a range of medical care, including acute-phase inpatient care.24
The index date was defined as the first and/or earliest date of exacerbation (moderate or severe) occurring within the indexing period of April 1, 2010 and March 31, 2020 (Figure 1). The baseline period was defined as the 12 months prior to the index date. The follow-up period spanned from the index date until the earlier of the study period end (March 31, 2021) or end of data availability per-patient (ie, last/most recently recorded diagnosis or medical claim). The minimum follow-up (from and including the index date) was 12 months.
 |
Figure 1 Study Design. Abbreviation: HCRU, healthcare resource utilization. |
An intention-to-treat approach was adopted, whereby patients who discontinued triple therapy treatment within the follow-up period were observed and outcomes continued to be assessed until end of follow-up. Triple therapy was defined as either MITT or SITT use. A MITT regimen was defined as the concomitant use of two different inhalers that form a triple therapy, namely: ICS/LABA + LAMA or LABA/LAMA + ICS or three different inhalers in the form of ICS + LAMA + LABA. SITT use was defined as a prescription for a single device inhaler of either FF/UMEC/VI or BUD/GLY/FOR.
Study Population
Patients with COPD recorded in the MDV database initiating triple therapy within 180 days of an index exacerbation were included in the study.
Eligible patients were required to have ≥1 inpatient and/or ≥2 outpatient diagnoses of COPD (International Classification of Diseases version 10 code of J42, J43, or J44) at ≥40 years of age at any timepoint in their medical history; ≥1 moderate-to-severe COPD exacerbation within the indexing period; ≥1 pharmacy claim for SITT, or ≥1 day overlapping pharmacy claims for MITT on, or within, 180 days of the index date; ≥1 diagnosis or medical claim record in the 12 months prior to the index date, in addition to ≥1 diagnosis or medical claim record in the 12–18 months prior to the index date; ≥12 months of data availability following, and including, the index date up to the earlier of: last/most recent diagnosis or medical claim record, or March 31, 2021 (ie, end of study period). Patients were excluded if they experienced ≥1 moderate-to-severe exacerbation during the baseline period, or ≥1 diagnostic code of any medical condition incompatible with a COPD diagnosis at any point in their medical history prior to, or including, the month of index. Conditions that were incompatible with a COPD diagnosis included any condition related to lung or bronchial developmental abnormalities, degenerative processes (cystic fibrosis, pulmonary fibrosis), pulmonary resection, or any other significant respiratory disorders other than COPD that can interfere with a clinical COPD diagnosis or substantially change the natural history of the disease.
For the main analysis, patients were categorized as prompt initiators if triple therapy was initiated within 0–30 days of the index date, and as delayed initiators if triple therapy was initiated 31–180 days following the index date. The definition of prompt initiation is consistent with other previously published or ongoing GSK studies with similar research objectives.6 Throughout the manuscript, data are reported according to these definitions of prompt and delayed initiators, unless otherwise specified. For the sensitivity analysis, however, triple therapy initiation within 0–14 days was considered prompt, and initiation within 15–180 days was considered delayed.
Study Outcomes
The primary objective of this study was to compare the rate of subsequent moderate-to-severe exacerbations among Japanese patients with COPD receiving prompt (0‒30 days) vs delayed (31‒180 days) initiation of triple therapy following an initial moderate-to-severe exacerbation in patients with no prior exacerbations in the 12 months prior to the index exacerbation.
The secondary objectives were to compare patients with COPD receiving prompt (0‒30 days) vs delayed (31‒180 days) initiation of triple therapy in relation to: time-to-first subsequent moderate-to-severe exacerbation; 30-day, 60-day, and 90-day all-cause and COPD-related hospital readmissions (following an initial severe exacerbation); time-to-first hospital readmission (following an initial severe exacerbation); all-cause and COPD-related HCRU; and direct medical costs.
Exacerbations, hospital readmissions, HCRU, and associated direct medical costs were evaluated during the follow-up period. Each endpoint was assessed after initiation of triple therapy onwards (so as not to introduce immortal time bias), with the exception of hospital readmissions, which were assessed from the index date (for only those patients indexed on a severe exacerbation, ie, hospital admission) to ensure readmissions were assessed at fixed time points following discharge from the index admission.
Data Analysis
Inverse probability of treatment weighting (IPTW) based on propensity scores was used to adjust for measured confounders between prompt and delayed cohorts. Covariates considered for inclusion in the propensity score model included demographic variables such as age at index, clinical characteristics such as most recent body mass index (BMI) measurement prior to index, medication use during baseline, COPD-related HCRU and medical costs during baseline, and exacerbations during baseline (Supplemental Table 1). Selection of covariates for inclusion in the model was mainly based on background knowledge of the association of pre-treatment variables with the outcomes of interest.
Patient Characteristics at Baseline
Baseline patient demographics and clinical characteristics were described for both prompt and delayed initiators. Baseline characteristics considered as covariates for the comparison of endpoints between prompt and delayed initiators were assessed pre- and post-weighting using standardized mean differences (SMDs), with values between −10% and 10% indicative of an adequately balanced covariate.
Exacerbations
Exacerbations were compared between prompt and delayed cohorts using unweighted and weighted (post-IPTW) rate ratios (RRs) obtained from negative binomial regression. All analyses were conducted for moderate-to-severe exacerbations and for moderate and severe exacerbations separately. Moderate exacerbations were defined as an outpatient diagnosis record for either COPD (International statistical Classification of Diseases, 10th revision [ICD]-10 of J42, J43, and J44), COPD exacerbation (J44.1), acute lower respiratory tract infection (J44.0), or chronic respiratory failure (J96.1), combined with at least one outpatient pharmacy claim of a systemic corticosteroid (SCS; including oral corticosteroids) and at least one outpatient pharmacy claim for an antibiotic within the same calendar month as the outpatient diagnosis. Severe exacerbations were defined as an inpatient diagnosis record for either COPD, COPD exacerbation, acute lower respiratory tract infection, or chronic respiratory failure, combined with an inpatient pharmacy claim of SCS and an inpatient pharmacy claim for antibiotic. Pharmacy claims for SCS and antibiotics should be no greater than one day apart or observed on the same day and should occur within one day of the hospital admission (in the case of severe exacerbations).
Rates of exacerbations (frequency of exacerbations per person-year) were calculated as the number of episodes divided by person-years of observation. Time-to-first subsequent moderate-to-severe exacerbations were assessed with Kaplan–Meier (KM) survival analysis, and compared between prompt and delayed cohorts using unweighted and weighted (post-IPTW) Cox proportional hazards regression.
Hospital Readmissions
The proportion of patients with 30-day, 60-day, and 90-day all-cause and COPD-related hospital readmissions were reported unweighted and weighted (post-IPTW), and all-cause and COPD-related hospital readmissions were compared between prompt and delayed cohorts using odds ratios and the P-value obtained from an unweighted and weighted (post-IPTW) logistic regression. Time-to-first hospital readmission (all-cause and COPD-related) was assessed with KM survival analysis, and compared between prompt and delayed cohorts using unweighted and weighted (post-IPTW) Cox proportional hazards regression.
HCRU and Direct Medical Costs
All-cause and COPD-related HCRU (limited to hospitalizations and outpatient visits only) were calculated as the number of events divided by person-years of observation. HCRU was compared between prompt and delayed cohorts using unweighted and weighted (post-IPTW) RRs obtained from negative binomial regression.
All-cause and COPD-related direct medical costs (total medical costs, hospitalization costs, and outpatient costs) were calculated and reported per person per year, by dividing the costs incurred over the follow-up period by the person-years of observation. All costs were inflated to March 2021 Japanese Yen using the consumer price index of medical care in Japan on a month-by-month basis.25 Costs were reported using mean, SD, median, 25th and 75th percentiles, and minimum and maximum values, and compared between prompt and delayed cohorts using estimates from an unweighted and weighted (post-IPTW) generalized linear model with log link and gamma distribution.
Results
Patient Attrition
A total of 1012 patients met the eligibility criteria and were included in the analysis (Figure 2). Of these, 975 (96.3%) initiated MITT and 37 (3.7%) initiated SITT (majority FF/UMEC/VI 34 [3.4%]) (Supplemental Table 2).
 |
Figure 2 Patient Attrition. Abbreviations: COPD, chronic obstructive pulmonary disease; MITT, multiple-inhaler triple therapy; SITT, single-inhaler triple therapy. |
For the main analysis, initiation of triple therapy was prompt (0–30 days) in 610 (60.3%) patients and delayed (31–180 days) in 402 (39.7%) patients. A total of 190 (57.8%) prompt initiators and 139 (42.2%) delayed initiators were indexed on a severe exacerbation. For the sensitivity analysis, initiation of triple therapy was prompt (0–14 days) in 495 (48.9%) patients and delayed (15–180 days) in 517 (51.1%) patients. The distribution of time to initiation of triple therapy is shown in Supplemental Figure 1. The total number of patients who initiated triple therapy on day 0 was 273 (27.0%); 178 (65.2%) were indexed on a moderate exacerbation and 95 (34.8%) were indexed on a severe exacerbation.
Patient Characteristics at Baseline
Demographics and Clinical Characteristics
Baseline demographics and clinical characteristics for all triple therapy initiators are shown in Table 1. The overall cohort had a mean age (SD) of 71.04 (11) and 70% were male. For most baseline characteristics, values and/or proportions were similar regardless of timing of triple therapy initiation.
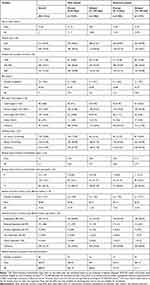 |
Table 1 Demographics and Clinical Characteristics at Baseline Across the Main and Sensitivity Analyses |
Comorbidities
Baseline comorbidities (including suspected cases in addition to confirmed diagnoses) for all triple therapy initiators are shown in Table 2. Patients had a mean (SD) of 6.46 (3.12) baseline comorbidities, and the most frequent comorbidities included cancer (64%), diabetes (61%), and hypertension (57%). Slight differences were observed between prompt and delayed initiators in relation to current asthma diagnosis (92% prompt; 82% delayed; Table 2).
 |
Table 2 Comorbidities at Baseline Across the Main and Sensitivity Analyses |
Treatment History
Treatment patterns at baseline for all triple therapy initiators are shown in Table 3. A mean (SD) of 2.28 (1.66) medication classes/treatment strategies were received within the 12-month baseline period. During this period, the most common treatments were SCS (59%), short-acting β2-agonist (SABA; 49%), leukotriene receptor agonist (33%), and methylxanthines (30%). Means and percentages were similar regardless of timing of triple therapy initiation, with numerically higher numbers of patients prescribed SABA, and ICS/LABA/LAMA among prompt initiators, compared to delayed initiators. A slightly higher number of delayed patients received SCS compared to prompt. Immediately prior to index, the most common treatments were ICS/LABA (42%), LAMA (21%), and LABA/LAMA (15%), and no treatment (18%).
 |
Table 3 Treatment Patterns at Baseline Across the Main and Sensitivity Analyses |
HCRU and Direct Medical Costs
HCRU and direct costs at baseline for all triple therapy initiators are shown in Table 4. Though standardized mean differences of >10% were observed for a number of variables for unweighted data (data not shown), for HCRU and length of stay data, weighted data were adequately balanced. For cost data, weighted data were adequately balanced, except for “year of indexing 2020” where there were fewer patients in the prompt cohorts across total COPD-related costs and COPD-related follow-up medications.
 |
Table 4 HCRU and Direct Costs at Baseline Across the Main and Sensitivity Analyses |
Rate of Subsequent Exacerbation Following Triple Therapy
For the main analysis, although absolute SMDs of >10% were observed for several baseline covariates included in the propensity model for unweighted data, weighted data were adequately balanced with the exception of “year of indexing 2020”, where there were fewer patients in the delayed cohort. Weighted baseline covariates were adequately balanced for the sensitivity analysis.
The rate of subsequent exacerbations following an index exacerbation was generally similar or numerically lower in prompt initiators of triple therapy vs those with delayed initiation (Figure 3A). No significant differences were observed in rate of exacerbations between prompt and delayed initiators in the main analysis. Unweighted RRs were 0.93, 95% confidence interval (CI): 0.73–1.17; P = 0.5424 for moderate-to-severe exacerbations; 0.98, 95% CI: 0.73–1.31; P = 0.9026 for moderate exacerbations, and 0.77, 95% CI: 0.57–1.04; P = 0.093 for severe exacerbations. After weighting, RRs were broadly similar to unweighted estimates; 0.95, 95% CI: 0.74–1.21; P = 0.6603 for moderate-to-severe exacerbations; 1.01, 95% CI: 0.76–1.34; P = 0.9719 for moderate exacerbations, and 0.72, 95% CI: 0.52–1.01; P = 0.0545 for severe exacerbations. Incidence rates of moderate exacerbations were similar between prompt and delayed subgroups (Supplemental Table 3).
In the sensitivity analysis, moderate-to-severe and moderate exacerbation rates before and after weighting in patients who initiated therapy promptly were numerically higher, while severe exacerbation rates were numerically lower vs those with delayed initiation (Figure 3B). No significant differences were observed in rate of exacerbations between prompt and delayed initiators in the sensitivity analysis. Unweighted RRs were 1.06, 95% CI: 0.84–1.35; P = 0.617 for moderate-to-severe exacerbations; 1.12, 95% CI: 0.83–1.50; P = 0.4613 for moderate exacerbations, and 0.89, 95% CI: 0.66–1.20; P = 0.4395 for severe exacerbations. After weighting, RRs were 1.06, 95% CI: 0.83–1.36; P = 0.6535 for moderate-to-severe exacerbations; 1.13, 95% CI: 0.84–1.53; P = 0.4162 for moderate exacerbations, and 0.89, 95% CI: 0.64–1.23; P = 0.4991 for severe exacerbations.
Time-to-First Subsequent Exacerbation Following Triple Therapy
Absolute SMDs of >10% were observed for a number of baseline covariates included in the propensity model for unweighted data. For the severe exacerbation analysis, weighted baseline covariates were adequately balanced. For the moderate-to-severe and moderate exacerbation analyses, however, an SMD of >10% was observed for “year of indexing 2020”, where there were fewer patients in the prompt cohort.
Time-to-first subsequent moderate-to-severe exacerbation increased significantly among prompt initiators vs delayed initiators. The unweighted hazard ratio11 was 0.78, 95% CI: 0.66–0.93; P = 0.0064 and the weighted HR was 0.77, 95% CI: 0.64–0.93; P = 0.0053 (Figure 4). The unweighted median time-to-first subsequent moderate-to-severe exacerbation was 47.2 months for prompt initiators and 29.4 months for delayed initiators; the weighted median was 45.7 months for prompt initiators and 27.7 months for delayed initiators (Supplemental Table 4). KM plots are shown in Supplemental Figure 2. Results were also significant for those who experienced a moderate exacerbation (unweighted HR 0.73, 95% CI: 0.59–0.90; P = 0.0032; weighted HR 0.77, 95% CI: 0.62–0.97; P = 0.0235). The unweighted median time-to-first subsequent moderate exacerbation was 83.2 months for prompt initiators and 53.4 months for delayed initiators; the weighted median was 83.2 months for prompt initiators and 56.4 months for delayed initiators (Figure 4, Supplemental Table 4). Median time-to-first subsequent exacerbation was not reached for severe exacerbations.
All-Cause and COPD-Related Hospital Readmission Following Triple Therapy
For these analyses, although absolute SMDs of >10% were observed for a number of baseline covariates included in the propensity model for unweighted data, weighted data were adequately balanced.
Among patients indexed on a severe exacerbation, delayed triple therapy initiation resulted in numerically higher 90-day all-cause readmissions in the delayed cohort compared with prompt initiation prior to weighting, but statistical significance was not achieved (40.3% vs 31.6%; P = 0.1036). After weighting, delayed initiators had significantly higher 90-day all-cause readmissions compared with prompt initiators (42.1% vs 30.6%; P = 0.0329; Figure 5A).
Readmissions were also numerically increased in the delayed cohort for 60-day all-cause (unweighted 31.7% vs 26.3% [P = 0.2909]; weighted 32.6% vs 26.0% [P = 0.1994]), 60-day COPD-related (unweighted 25.2% vs 24.7% [P = 0.927]; weighted 26.4% vs 25.0% [P = 0.7707]), and 90-day COPD-related (unweighted 32.4% vs 28.4% [P = 0.4409]; weighted 32.8% vs 27.6% [P = 0.3173]), compared with prompt initiation, but statistical significance was not achieved (Figure 5A and B). For 30-day readmissions, delayed initiators experienced slightly reduced readmissions compared to prompt initiators both before and after weighting; statistical significance was not achieved (Figures 5A and B).
Time-to-first hospital readmission was numerically longer with prompt vs delayed treatment initiation after an initial severe exacerbation, but no HRs (unweighted or weighted) were significant (Figure 5C). KM plots are shown in Supplemental Figure 3. The weighted median time-to-readmission was 4.2 months in delayed initiators vs 10.3 months in prompt initiators for all-cause readmissions (unweighted 5.0 vs 10.1 months), and 7.3 vs 14.1 months in prompt initiators for COPD-related readmissions (unweighted 8.4 vs 14.1 months).
All-Cause and COPD-Related HCRU and Direct Medical Costs Following Triple Therapy
For these analyses, although absolute SMDs of >10% were observed for a number of baseline covariates included in the propensity model for unweighted data, for HCRU and length of stay data, weighted data were adequately balanced. For cost data, weighted data were adequately balanced with the exception of “year of indexing 2020”, where there were fewer patients in the prompt cohorts across total COPD-related costs and COPD-related follow-up medications.
Among patients who initiated therapy promptly, unweighted and weighted HCRU rates were numerically lower compared with those with delayed initiation for all-cause and COPD-related hospitalizations; however, statistical significance was not reached (Figure 6A). Prompt initiators had a lower rate of hospital stay days than delayed initiators (weighted RR 0.67, 95% CI: 0.46–0.98; P = 0.0395 [unweighted RR 0.72, 95% CI: 0.50–1.03; P = 0.0731, not significant] for all cause hospital stays, and weighted RR 0.54, 95% CI: 0.35–0.86; P = 0.0088 [unweighted RR 0.57, 95% CI: 0.38–0.87; P = 0.0088] for COPD-related hospital stays; Figure 6B).
All-cause costs per person-year were significantly lower in prompt initiators compared with delayed initiators for medication (unweighted P = 0.0044; weighted P = 0.0032); outpatient stays (unweighted P = 0.0044; weighted P = 0.0324) and total costs (unweighted P = 0.0086; weighted P = 0.0203) (Figure 7A). For COPD-related costs per person-year among prompt initiators, significantly lower total costs (weighted P = 0.0356; unweighted P = 0.0038) and medication costs (weighted P = 0.0338; unweighted P = 0.0336) were observed compared with those with delayed initiation (Figure 7B); unweighted outpatient costs were also significantly lower (P = 0.011) but the difference between prompt and delayed initiators became non-significant after weighting (P = 0.0638).
Discussion
In this real-world study from Japan, prompt initiation (≤30 days) of triple therapy following a first exacerbation was associated with numerically lower subsequent moderate-to-severe and severe exacerbations compared with delayed initiation, though statistical significance was not reached; the rates of moderate exacerbations were similar between patient subgroups. These findings align with previous similar studies conducted in the US (prompt vs delayed MITT initiation), which found no significance in moderate exacerbations,6 and the UK (prompt vs delayed MITT initiation), which found that frequencies of exacerbations after treatment initiation were comparable.21 Due to the timing of the study, the majority of patients were initiated on MITT as opposed to SITT, due to the recent introduction of SITT in Japan at the time of the study.19,20 A previous study in the US with prompt vs delayed SITT initiation showed that prompt initiators had significantly reduced overall, moderate, and severe exacerbations compared to delayed initiators. Although the results of the current study may indicate a lack of differentiation between prompt and delayed initiators for this particular endpoint, it is also important to consider factors such as low sample size, or any unknown and/or unmeasured variables not included in the models. For example, almost half of prompt initiators initiated triple therapy on the same date as the index exacerbation (ie, day 0), whilst timing of delayed therapy was more evenly distributed across the 31–180 days. Further studies would be required to (a) determine the impact of immediate (day 0) initiation of triple therapy vs prompt or delayed initiation on exacerbations and (b) determine the optimal timing of SITT given the current lack of available data.
For the sensitivity analysis, for which the alternative definition of ≤14 days was used for prompt initiation, no significant differences in the rate of subsequent exacerbations between prompt and delayed initiators were observed; prompt initiators experienced a numerically lower rate of severe exacerbations, but a numerically higher rate of moderate-to-severe and moderate exacerbations compared with delayed initiators. This contrast in findings may be because the number of moderate exacerbations ranged from 0–41 and 0–10 in patients initiating triple therapy within 0–14 and 15–30 days of index, respectively, possibly skewing the analysis.
Prompt initiation of triple therapy following a first exacerbation was associated with significantly reduced all-cause (total, medication, and outpatient) and COPD-related (medication and total) costs; all-cause hospitalization costs, and COPD-related outpatient and hospitalization costs, were numerically (if not statistically significantly) lower. There were also significantly fewer days spent in hospital per month (all-cause and COPD-related) for prompt initiators compared with delayed. These findings, despite the differences in global healthcare systems, align with similar studies conducted in the US and UK, which found all-cause and COPD-related medical costs to be lower in those who promptly initiated MITT treatment compared with those who had a delayed initiation to MITT,6,21 and highlight the potential cost benefit to the healthcare system in Japan of initiating triple therapy promptly. This evidence would also be of value to healthcare decision makers; GOLD guidelines cite “there is no high-quality evidence to support initial pharmacological treatment strategies in newly-diagnosed patients with COPD”.11
It should be noted that the study period of the current analysis spans the COVID-19 pandemic period; however, feasibility assessments for both the current study and other ongoing GSK studies involving the MDV database found that the number of patients with COPD in Japan with disease codes for COVID-19 between January and October 2020 was small. The results of the current study are therefore not expected to have been impacted by COVID-19.
The following limitations may impact the generalizability of the study results to the wider Japanese population: the MDV database is composed of information from only contracted diagnosis procedures combination hospitals, thus information from other medical sites is unknown; the prevalence of comorbidities in this study was also higher than what has been presented in previous studies. For example, almost 90% of patients in this study also had an asthma diagnosis in addition to their COPD diagnosis, which is much higher compared to previous studies.21 Additionally, in this study, asthma was defined based on ICD-10 codes only, regardless of treatment use. Patients who had only one asthma diagnosis code during the 24 months prior to index date were also included in this study. This could have potentially overestimated the number of patients with comorbid asthma. However, patients with comorbid asthma were not excluded from this study in order to reflect the real-world population and to keep a sufficient sample size of patients. The MDV database includes a large number of cancer therapeutic facilities, which may account for the high prevalence of cancer observed.28 Additionally, the inclusion of suspected cases as well as confirmed diagnoses for comorbidities may have led to the over-estimation of comorbidities such as cancer. In this study, exacerbations were partly based on claims of antibiotics and systemic corticosteroids within the same calendar month as the patient’s outpatient COPD diagnosis. Although the patient population had multiple comorbidities, and these antibiotics and systemic corticosteroids may have been claimed for other comorbidities rather than COPD, this is unlikely to have had a major impact on misclassification as the algorithm also required an ICD-10 code for COPD exacerbation in addition to the prescription of OCS or antibiotics. Furthermore, overlapping prescriptions forming open combinations were considered for the baseline treatment strategies; subsequently, patients who received LABA/LAMA at baseline as well as an overlapping ICS-containing therapy would have been presumed to be receiving triple therapy. This likely explains why no patients were reported to be receiving LABA/LAMA at baseline, whilst 149 (14.7%) patients received LABA/LAMA immediately prior to index, where overlapping therapies were not considered. Due to data privacy laws in Japan, if a patient is transferred to another hospital within the MDV database, their data can no longer be tracked continuously as they will have a unique, un-linkable patient record for each hospital. This may cause calculations for readmission-based study objectives and patients’ follow-up to be underestimated due to this limitation, impacting the power of the study (the extent to which patients attend multiple hospitals in Japan is unknown); information on clinical measures (eg, lung function) and some patient characteristics (eg, geographic region) are unavailable from the MDV database, and some patient characteristics (eg, BMI) are only partially available among patients who were hospitalized; the health insurance reimbursement system in Japan has challenges with linking disease diagnoses to specific events or treatment, and this may result in some events being mis-classified as COPD-related (these mis-classifications should be non-differential and we anticipate that this would bias results towards the null (ie, underestimate any benefit of prompt initiation).
Conclusion
In this real-world study from Japan, rates of moderate-to-severe and severe exacerbations following a first exacerbation, were numerically lower among prompt initiators of triple therapy compared with delayed initiators of triple therapy. Additionally, all-cause (total, medication, and outpatient) and COPD-related (medication and total) costs were significantly lower among patients who initiated triple therapy treatment promptly, compared with those who experienced a delay. There were also significantly fewer days in hospital (all-case and COPD-related) for patients initiated promptly. These data suggest that earlier initiation of triple therapy may reduce the overall costs of COPD management in Japan. Further studies focusing on the benefits of prompt vs delayed initiation of SITT in this population are warranted.
Abbreviations
¥, Japanese Yen; ADL, Activities of Daily Living; BMI, body mass index; BUD/GLY/FOR, budesonide/glycopyrronium/formoterol; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DPC, Diagnosis Procedures Combination; FF/UMEC/VI, fluticasone furoate/umeclidinium/vilanterol; FU, follow up; GERD, gastro-esophageal reflux disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HCRU, healthcare resource utilization; HR, hazard ratio; ICD-10, International statistical Classification of Diseases, 10th revision; ICS, inhaled corticosteroid; IPTW, inverse probability of treatment weighting; KM, Kaplan–Meier; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor agonist; MDV, Medical Data Vision Co., Ltd.; MITT, multiple-inhaler triple therapy; RR, rate ratio; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic antagonist; SCS, systemic corticosteroids; SD, standard deviation; SITT, single-inhaler triple therapy; SMD, standardized mean difference; UK, United Kingdom; US, United States.
Data Sharing Statement
The data analyzed in this publication are derived from the MDV hospital database (https://www.mdv.co.jp/). Authors had access to the study data for the purposes of this work only. Data were accessed through an existing GSK license to address the pre-specified research questions only. Therefore, the data cannot be broadly disclosed or made publicly available at this time. Access to the MDV database can be requested via the website.
Ethics Approval and Informed Consent
This study used existing, fully de-identified data and the subject(s) cannot be identified, directly or through identifiers. Study results were in tabular form and aggregate analyses that omits subject identification. This study was therefore exempt from ethical approval and informed consent based on the Japanese national Ethical Guidelines for Medical and Health Research Involving Human Subjects, Ministry of Health, Labour and Welfare. There are no other national legislations or regulations which require informed consent or Institutional Review Board approval for retrospective anonymized data. This study also complied with all applicable laws regarding subject privacy, Declaration of Helsinki. No direct subject contact or primary collection of individual human subject data has occurred in this study.
Acknowledgments
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Lindsay Uglow of Apollo, OPEN Health Communications, and was funded by GSK. The current affiliation of Takeo Ishii is Moderna, Tokyo, Japan. A summary of the results of this study was presented as a mini symposium presentation (Presentation number MS265) at the 63rd Annual Meeting of the Japanese Respiratory Society in Tokyo, Japan (April 28–30, 2023).
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the manuscript; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by GSK (study number 217636). GSK-affiliated authors were involved in study conception and design, data analysis, data interpretation, and the decision to submit the article for publication. GSK funded the article processing charges and open access fee.
Disclosure
Shoko Akiyama, Masao Yarita, Kenichi Hashimoto, Kieran J Rothnie, and Afisi S Ismaila are employees of, and/or hold stocks/shares in, GSK. Alexandrosz Czira and Takeo Ishii were employees of GSK at the time of the study. Robert P Wood, Lucinda J Camidge, Hannah Wallis, Thomas Jennison, and Rosie AC Wild are employees of Adelphi Real World. Adelphi Real World received funding from GSK to conduct the study only. The authors report no other conflicts of interest in this work.
References
1. Forum of International Respiratory Societies (FIRS). The global impact of respiratory disease; 2021. Available from: https://www.firsnet.org/images/publications/FIRS_Master_09202021.pdf.
2. Takemura H, Hida W, Sasaki T, Sugawara T, Sen T. Prevalence of chronic obstructive pulmonary disease in Japanese people on medical check-up. Tohoku J Exp Med. 2005;207(1):41–50. doi:10.1620/tjem.207.41
3. Igarashi A, Fukuchi Y, Hirata K, et al. COPD uncovered: a cross-sectional study to assess the socioeconomic burden of COPD in Japan. Int J Chron Obstruct Pulmon Dis. 2018;13:2629–2641. doi:10.2147/COPD.S167476
4. Minakata Y, Ichinose M. [Epidemiology of COPD in Japan]. Nihon Rinsho. 2011;69(10):1721–1726. Japanese.
5. Blasi F, Cesana G, Conti S, et al. The clinical and economic impact of exacerbations of chronic obstructive pulmonary disease: a cohort of hospitalized patients. PLoS One. 2014;9(6):e101228. doi:10.1371/journal.pone.0101228
6. Bogart M, Stanford RH, Reinsch T, Hull M, Buikema A, Hulbert E. Clinical characteristics and medication patterns in patients with COPD prior to initiation of triple therapy with ICS/LAMA/LABA: a retrospective study. Respir Med. 2018;142:73–80. doi:10.1016/j.rmed.2018.07.009
7. Dalal AA, Shah M, D’Souza AO, Rane P. Costs of COPD exacerbations in the emergency department and inpatient setting. Respir Med. 2011;105(3):454–460. doi:10.1016/j.rmed.2010.09.003
8. Mannino D, Bogart M, Germain G, et al. Benefit of prompt versus delayed use of single-inhaler fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) following a COPD exacerbation. Int J Chron Obstruct Pulmon Dis. 2022;17:491–504. doi:10.2147/COPD.S337668
9. Sicras Mainar A, Huerta A, Navarro Artieda R, Monso E, Landis SH, Ismaila AS. Economic impact of delaying initiation with multiple-inhaler maintenance triple therapy in Spanish patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:2121–2129. doi:10.2147/COPD.S211854
10. Decramer M, Cooper CB. Treatment of COPD: the sooner the better? Thorax. 2010;65(9):837–841. doi:10.1136/thx.2009.133355
11. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Diagnosis, Management and Prevention 2023 Update; 2023. Available from: https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf.
12. Japanese Respiratory Society. Guidelines for COPD (chronic obstructive pulmonary disease) diagnosis and treatment [6th edition]; 2022.
13. Gaduzo S, McGovern V, Roberts J, Scullion JE, Singh D. When to use single-inhaler triple therapy in COPD: a practical approach for primary care health care professionals. Int J Chron Obstruct Pulmon Dis. 2019;14:391–401. doi:10.2147/COPD.S173901
14. Halpin DMG, Worsley S, Ismaila AS, et al. INTREPID: single- versus multiple-inhaler triple therapy for COPD in usual clinical practice. ERJ Open Res. 2021;7(2):00950–02020. doi:10.1183/23120541.00950-2020
15. Pharmaceuticals and Medical Devices Agency (PMDA). List of approved products - new drugs approved in FY 2013; 2013. Available from: https://www.pmda.go.jp/files/000232771.pdf.
16. Pharmaceuticals and Medical Devices Agency (PMDA). List of approved products - new drugs approved in FY 2008; 2008. Available from: https://www.pmda.go.jp/files/000232775.pdf.
17. Pharmaceuticals and Medical Devices Agency (PMDA). List of approved products - new drugs approved in FY 2004; 2004. Available from: https://www.pmda.go.jp/files/000232776.pdf.
18. GlaxoSmithKline KK Serevent Diskus® [package insert]; 2022. (in Japanese). Available from: https://www.info.pmda.go.jp/go/pack/2259708G1022_1_18/?view=frame&style=XML&lang=ja.
19. Pharmaceuticals and Medical Devices Agency (PMDA). List of approved products - new drugs approved in FY 2018; 2018. Available from: https://www.pmda.go.jp/files/000235288.pdf.
20. Pharmaceuticals and Medical Devices Agency (PMDA). List of approved products - new drugs approved in FY 2019; 2019. Available from: https://www.pmda.go.jp/files/000235289.pdf.
21. Sansbury LB, Wood RP, Anley GA, Nam Y, Ismaila AS. Quantifying the economic impact of delayed multiple-inhaler triple therapy initiation in patients with COPD: a retrospective cohort study of linked electronic medical record and hospital administrative data in England. Int J Chron Obstruct Pulmon Dis. 2021;16:2795–2808. doi:10.2147/COPD.S312853
22. Ismaila A, Jakubanis R, Holbrook T, Punekar Y, Wood R, Meeraus W. Cost impact of time to initiation of triple therapy in COPD patients in Japan: a retrospective analysis of healthcare claims data. Eur Resp J. 2017;50(suppl 61):
23. Medical Data Vision (MDV). About MDV database. Available from: https://en.mdv.co.jp/about-mdv-database/.
24. Laurent T, Simeone J, Kuwatsuru R, et al. Context and considerations for use of two Japanese real-world databases in Japan: Medical Data Vision and Japanese Medical Data Center. Drugs Real World Outcomes. 2022;9(2):175–187. doi:10.1007/s40801-022-00296-5
25. Statistics of Japan. e-Stat Consumer Price Index Monthly Reports; 2021. Available from: https://www.e-stat.go.jp/en/stat-search/files?page=1&layout=datalist&toukei=00200573&tstat=000001084976&cycle=1&tclass1=000001085955&tclass2val=0.
26. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi:10.1097/01.mlr.0000182534.19832.83
27. Flattet Y, Garin N, Serratrice J, Perrier A, Stirnemann J, Carballo S. Determining prognosis in acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:467–475. doi:10.2147/COPD.S122382
28. Tsutsue S, Tobinai K, Yi J, Crawford B. Nationwide claims database analysis of treatment patterns, costs and survival of Japanese patients with diffuse large B-cell lymphoma. PLoS One. 2020;15(8):e0237509. doi:10.1371/journal.pone.0237509
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





