Back to Journals » Infection and Drug Resistance » Volume 16
Benefit of Nasal Douche in COVID-19 Patients with Recurrence of Positive SARS-CoV-2 Viral RNA
Authors Liao X, Guan Y, Asakawa T , Lin Z, Tang Q, Ma Z, Wu S, Wang X, Dong J, Zhang L, Deng J, Liao Z, Yang S, Wang C, Song S, Yi H, Wu S , Lu H
Received 8 June 2023
Accepted for publication 7 September 2023
Published 21 September 2023 Volume 2023:16 Pages 6269—6276
DOI https://doi.org/10.2147/IDR.S421380
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Xuejiao Liao,1,* Yuan Guan,1,2,* Tetsuya Asakawa,1,* Zixun Lin,1 Qingrong Tang,3 Zhenghua Ma,1 Shuting Wu,1 Xiaobin Wang,1 Jingke Dong,1 Liping Zhang,1 Jiayu Deng,1 Zhonghui Liao,1,4 Sumei Yang,1,4 Cheng Wang,1,4 Shuo Song,1 Hongyang Yi,1 Song Wu,5 Hongzhou Lu1
1Institute for Hepatology, National Clinical Research Center for Infectious Disease, The Third People’s Hospital of Shenzhen, The Second Affiliated Hospital, School of Medicine, Southern University of Science and Technology, Shenzhen, People’s Republic of China; 2Department of Epidemiology, School of Public Health Fudan University, Shanghai, People’s Republic of China; 3The First Hospital of Changsha, Infectious Diseases Department, Changsha, People’s Republic of China; 4School of Public Health, Bengbu Medical College, Bengbu, People’s Republic of China; 5Department of Central Laboratory, South China Hospital, Medical School, Shenzhen University, Shenzhen, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongzhou Lu, Institute for Hepatology, National Clinical Research Center for Infectious Disease, The Third People’s Hospital of Shenzhen, The Second Hospital Affiliated with the School of Medicine, Southern University of Science and Technology, No. 29 Bulan Road, Long Gang District, Shenzhen, Guangdong, 518112, People’s Republic of China, Email [email protected]; Song Wu, Department of Central Laboratory, South China Hospital, Medical School, Shenzhen University, 3688 Nanhai Dadao, Nanshan District, Shenzhen, 518060, People’s Republic of China, Email [email protected]
Purpose: The purpose was to review relevant clinical data and formulate recommendations supporting the use of saline as a simple rinse for an early reassuring intervention to reduce the occurrence of re-positive COVID-19 patients.
Methods: We conducted a single-centre retrospective cohort study, which enrolled patients with confirmed re-testing positive COVID-19 during 7– 60 days after discharge from Third People’s Hospital of Shenzhen. By one-to-two propensity score matching for age and sex, the control group of those not re-testing positive during the same period served as matched control.
Results: A total of 223 patients were included in our study, 94 in re-positive group and 129 in non-re-positive group. The result shows that the rates of nasal douche treatment in the non-re-positive group were considerably higher than that of the re-positive group. And the Ct value of nasal douche group increased faster than that of non-nasal douche group after the Ct value reaching ≥ 35. Further analysis revealed that the higher the Ct value at the time of readmission, the shorter the time of average Ct values to reach ≥ 35.
Conclusion: These findings suggest that nasal douche is beneficial to shorten the time of virus nucleic acid turning negative, thereby reducing the incidence of re-positive. The prevention and control of epidemics focuses on re-positive patients with Ct values < 35.
Keywords: COVID-19, nasal douche, re-positive, RT-PCR
Introduction
After the first confirmed case in 2019, coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2), has caused the current worldwide pandemic.1 Infections with SARS-CoV-2 with the Omicron variant have surged in recent years. Globally, as of 6 April 2023, more than 762 million confirmed cases suffered from this disease, and more than 6.8 million patients have died following infection with SARS-CoV-2, which pose severe challenges for medical institutions and public health.2 The cornerstone to optimal COVID-19 management is the rapid identification of SARS-CoV-2 cases. Currently, reverse-transcription polymerase chain reaction (RT-PCR) for the detection of SARS-CoV-2 RNA in nasopharyngeal and oropharyngeal swab specimens is a highly reliable test for detecting infectivity and severity of COVID-19.3,4
The outbreak has been controlled in different ways by many countries and regions. In China, all the SARS-CoV-2 cases needed to strict quarantine. In accordance with the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonias (Trial Version 9) published by the National Health Commission of China, before COVID-19 patients are discharged from the hospital, two consecutive nucleic acid tests of respiratory pathogens (sampling interval of at least 24 hours) must be negative.5 During the initial recovery stage, a considerable number of discharged COVID-19 patients were found to re-test positive for SARS-CoV-2 by using nucleic acid RT-PCR.6–8 About 7–10% of COVID-19 patients who were discharged re-tested positive for SARS-CoV-2 RNA by RT-PCR, depending on the region, sample size, patient age, and specimen type.9 As a result of the potential for virus transmission among recovered patients with re-positive virus detection, it was required that recovered patients quarantine locations for another 7 days following discharge, during which they were tested for coronavirus nucleic acids (COVNAT).5 Additionally, long-term COVNAT needs to be closely monitored in COVID-19 patients released from quarantine.
Coronavirus can colonize nasal mucosa due to its abundance of blood vessels, mucinous glands, and serous glands, which create humid conditions.10 Due to this, the upper respiratory tract and mucosa need to be protected. In addition to pharmacological preventive measures, saline nasal irrigations (SNIs) remove antigens, inflammatory mediators, and microorganisms from the nose cavity. It is particularly effective when SNIs are used in the nasal cavity to reduce viral loads.11 Despite the fact that chloride ions in saline may help cells mount an antiviral defense by producing hypochlorous acid, nasal irrigation has not been found to have therapeutic effect on Omicron.
As of now, there are few studies on SNI’s effectiveness in reducing the occurrence of re-positives among COVID-19 patients after discharge. Consequently, we reviewed relevant clinical data and formulated recommendations in support of simple saline rinses as an early intervention for reducing COVID-19 re-positives patients caused by the Omicron variant of SARS-CoV-2.
Materials and Methods
Study Design and Participants
We conducted a single-centre retrospective cohort study, which enrolled patients with confirmed re-testing positive (re-positive) COVID-19 discharge from Third People’s Hospital of Shenzhen between March 15 and September 30, 2022. During the study period, 3507 COVID-19 patients recovered and were discharged from the hospital. Of the two hundred and fifty-three potential re-positive cases, one hundred and forty were ineligible for the study due to infection abroad, and nine were excluded as the baseline information is incomplete. Of the remaining, ten patients were excluded as the data during health management were incomplete. In the end, 94 re-positive patients were included in the study. By one-to-two propensity score matching for age and sex, the control group of those not re-testing positive (non-re-positive) during the same period in our study served as matched controls. A total of 188 non-re-positive patients were selected as the control group, forty-seven were ineligible for the study due to infection abroad, twelve patients were excluded as the information is incomplete. Therefore, 129 non-re-positive patients were included in the control group, and a total of 223 subjects were included in the final analysis (Figure 1).
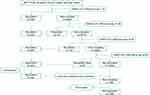 |
Figure 1 Study flow diagram. |
Patient informed consents were exempted on account of the patient had signed informed consent at the time of initial hospitalization. We abided by all ethical standards established by the Helsinki Declaration of 1975. The study protocol was approved by the Ethics Committee of Third People’s Hospital of Shenzhen (IRB 2022-074-02).
Clinical Definition
In this study, re-positives are defined as confirmed COVID-19 patients who have detected SARS-CoV-2 RNA during 7–60 days after discharge. The non-re-positive group were confirmed COVID-19 patient without detected SARS-CoV-2 RNA-positive 7 days after post-discharge.
For COVID-19 patients, the date of onset of the disease was determined by the first RT-PCR positive result for the virus or the date of onset of symptoms.
The solution of nasal douche consisted of 30mL 10% sodium chloride added to 250mL 0.09% sodium chloride, and then 225mL of mixed solution was taken out and added to 75mL PVP-I gargle. Nasal douche was administered twice a day and continued for 5–7 days.
According to the Treatment Protocol for COVID-19 (Trial Version 9), patients were discharged once they met all the criteria:1 More than 3 days of normal body temperature;2 Recovery of respiratory symptoms to a significant degree;3 Lung imaging demonstrating apparent absorption and recovery of acute exudative lesions; and4 A two-sample interval of at least 24 hours was used for the nucleic acid tests of respiratory pathogens for the N gene and ORF gene. The health management of these discharged patients continued for two months after discharge.
Data Collection
The demographic and epidemiological data [ie, age, sex, basic diseases, COVID-19 vaccines], treatment data, and clinical endpoints, especially the RNA test of SARS-CoV-2 were obtained using a standardized data collection form from the electronic medical records database of Affiliated Third People’s Hospital of Shenzhen by two physicians. For COVID-19 patients, there is no definitive treatment, and the specific medications chosen depend on the patient’s condition and the most recent guidelines at the time.12,13
Strategy for Nucleic Acid Amplification Tests
The RNA of SARS-CoV-2 was detected using real-time RT-PCR using nasal swabs. Within the two months after discharge from the hospital, all patients received at least one additional test for the presence of SARS-Cov-2 RNA at day 7–14, 15–28, 29–45 and 46–60. In the event that patients were re-admitted to the hospital after being discharged from the hospital but tested positive for SARS-CoV-2 RNA during follow-up, they would be re-admitted until the virus was cleared from their system.
Open reading frame 1ab (ORF1ab) and nucleocapsid protein (N) were two of the target genes of SARS-CoV-2 as detected by a kit (Bio-germ, Shanghai, China) recommended by the Chinese Center for Disease Control and Prevention (CCDC). This study detected both ORF1b and N genes of SARS-CoV-2 in specimens with a cycle threshold (Ct) value of 40 for both tests, which was considered positive. It was determined that single-gene-positive specimens would be considered positive if the Ct values were 40 or greater from the repeat tests. The specimens were collected and tested again when the results were indeterminate.
Statistical Analysis
In this study, all statistical analyses were conducted using SPSS 23.0 (IBM Corp., Armonk, NY, USA). The median and interquartile range (IQR) are used for continuous variables when the data are not normally distributed, and n (%) is used for categorical variables. The Mann–Whitney U-test (continuous variables) and the chi-square test or Fisher’s exact test (categorical variables) were used to compare baseline characteristics and clinical data between groups. The statistical significance level was set at 0.05 using a two-sided test.
Results
Demographic and Clinical Characteristics of Study Participants
A total of 3507 COVID-19 patients were selected as the initial study population from Third People’s Hospital of Shenzhen between March 15 and September 30, 2022. There were no deaths during hospitalization, and all patients were discharged. Finally, 223 patients were included in our study. The rate of re-positive test after hospital discharge was 7.2% (253/3507) in this study. The mean age of the 223 patients was 36.3 ± 17.6 years, with a range of 10 months to 82 years; twenty-eight patients were younger than 14 years among them, and the 10 months old boy was negative with retested RT-PCR. The re-positive patients were younger than that of the non-re-positive patients, but the difference was not significant (P = 0.631). The re-positive patients had a higher percentage of basic diseases than did the non-re-positive patients (P = 0.066). Twenty-six patients have not been vaccinated against COVID-19 at enrollment. The mean lowest CoV ORF or N gene levels during positive period were 20.3 ± 6.7. There were no significant differences in the cycle threshold and COVID-19 vaccines between the two groups. The mean duration of hospital stay was 16.8 ± 5.0 days for all the patients, 17.4 ± 4.9 days for the non-re-positive retest group, and 15.9 ± 5.0 days for the re-positive group (P < 0.05). In total, thirteen (5.8%) participants reported at least one sequelae of COVID-19. The rate of sequelae in re-positive was 7.4% and non-re-positive group was 4.7%, in which the difference was not significant (P = 0.379). The demographic and clinical characteristics of the patients are shown in Table 1.
 |
Table 1 Demographics and Characteristics of Patients with COVID-19 |
Comparison of Treatment Between the Two Groups
Of the 223 patients, 68.6% were treated with nasal douche. The rates of nasal douche treatment were 85.3% for the non-re-positive group and 45.7% for the re-positive group (P < 0.05). Sixty (26.7%) patients received Traditional Chinese Medicine (TCM). There was no significant difference in TCM between the re-positive and non-re-positive patients. The rate of Thymosin was 10.9 for the non-re-positive group and 14.9% for the re-positive group (P > 0.05). The treatment on the first admission is shown in Table 2.
 |
Table 2 Treatment of Patients with COVID-19 |
Demographic and Clinical Characteristics of Re-Positive Group
The re-positive patients were divided into three subgroups according to Ct values on readmission. And the re-positive group comprised 19 patients in Ct values <35, 37 patients in Ct values 35–40 group, 38 patients in Ct values ≥40 group. The mean age of Ct values ≥40 group was 32.7 ± 13.4 years, which was younger than that of the Ct values <35 group and Ct values 35–40 group. The difference was not significant (P = 0.403). The mean lowest CoV ORF or N gene levels during positive period of Ct values 35–40 group were 18.9 ± 5.9 which was lower than that of the Ct values <35 group and Ct values ≥40 group (P > 0.05). There were no significant differences in the sex, basic diseases, lowest CoV ORF or N gene levels, COVID-19 vaccines, hospitalization time and sequelae of COVID-19 between the three subgroups. The median readmission time of Ct values <35 group was 6.6 ± 2.7 days, which was more than that of the Ct values 35–40 group and Ct values ≥40 group. The difference was significant (P = 0.001). The characteristics of re-positive groups in the stratification analysis are shown in Table 3.
 |
Table 3 Characteristics of Re-Positive Groups in the Stratification Analysis |
Comparison of Treatment Between the Re-Positive Groups
The nasal douche proportion of Ct values ≥40 group on the first admission was 57.9% which was more than that of the Ct values 35–40 group and Ct values <35 group. There were no significant differences in the nasal douche, Thymosin and Traditional Chinese Medicine on the first admission (P > 0.05). There were no significant differences in the nasal douche and Traditional Chinese Medicine on readmission. The treatment of re-positive groups in the stratification analysis is shown in Table 4.
 |
Table 4 Treatment of Re-Positive Groups in the Stratification Analysis |
Dynamic Change of Cycle Threshold (Ct) for ORF1ab and N RNA Amplification
When amplification of N RNA and ORF1ab was conducted during the first hospitalization, Ct values increased gradually over time (Figure 2). This indicated a gradual decrease in viral load. The time for the average Ct value to reach ≥35 in re-positive group was longer than that of non-re-positive group (Figure 2A). The Ct value of nasal douche group increased faster than that of non-nasal douche group after the Ct value reaching ≥35 (Figure 2B). According to the nucleic acid value at the time of readmission, the re-positive COVID-19 patients was subsequently divided into two groups (Ct values <35 and Ct ≥35) (Figure 2C). The time for the average Ct value to reach ≥35 in Ct values ≥35 group was shorter than that of Ct values <35 group.
Discussion
The re-positive results of nucleic acid amplification tests in recovered COVID-19 patients present challenges to eradicating the disease.14,15 This retrospective cohort study identified the prevalence and characteristics of positive retest COVID-19 patients 7–60 days after discharge from hospital. The rate of re-positive test 7–60 days after hospital discharge was 7.2% in this study which was similar to the 7–10% of COVID-19 patients re-test positive for SARS-CoV-2 RNA by RT-PCR.9 There was no significant difference in gender between the two groups, which was consistent with the findings of Wan et al.16 The two groups of patients had a similar cycle threshold of ORF1ab and N RNA amplification, defined as the number of cycles required for the fluorescent signal to cross the threshold. These results suggested that the re-positive and non-re-positive patients had the same viral load when they were initially diagnosed. The sequelae of COVID-19 are an emerging problem in the post-epidemic era. And we observed 13 (5.8%) of participants had at least one sequelae of COVID-19, findings similar to our previous research.17 There was no significant difference sequelae of COVID-19 between the re-positive and non-re-positive patients. This indicated that the prevalence of sequelae is not high in the COVID-19 patients with SARS-CoV-2 Omicron variant, and the re-positive patients seem to have no significantly persistent symptoms of long COVID.
In patients with viral upper respiratory tract infections (URTIs), nasal irrigation with saline solution relieves symptoms, loosens secretions, and prevents secondary bacterial complications.18–20 As an easy, safe and low-cost intervention measure, 68.6% participants were treated with nasal douche in our study. The utilization rate of nasal douche in non-re-positive group was higher than that in re-positive group. And the Ct value of nasal douche group increased faster than that of non-nasal douche group after the Ct value reaching ≥35. These findings suggest that nasal douche is beneficial to shorten the time of virus nucleic acid turning negative in patients meeting discharge criteria but with positive nucleic-acid results, thereby reducing the incidence of re-positive. However, due to the differences in other treatment measures, further randomized controlled trials (RCT) and prospective studies are needed to explore the effect of nasal douche on re-positive.
By depicting the dynamic change of cycle threshold (Ct) for ORF1ab and N RNA amplification, we found that the time of virus nucleic acid turning negative in re-positive group was longer than that of non-re-positive group. Therefore, future research must focus on COVID patients whose nucleic acid results are difficult to turn negative. Adjuvant therapy includes nasal douche that should be used to shorten time of viral nucleic acid turning negative later in the disease course. Previous studies have shown that COVID-19 patients with Ct value is ≥35 are not infectious in convalesce, because live virus can no longer be isolated.21 For these re-positive population, if they have no symptoms or signs of COVID-19, there is no need for further treatment and community control. By instructing patients with saline nasal irrigation at home, the time of virus nucleic acid turning negative can be shortened. However, our study found that 20.2% of patients in the re-positive group had a CT value less than 35 on readmission. And the median readmission time of Ct values <35 was 6 days, which was more than that of the Ct values ≥35. Attention should be paid to this population, and the transmission risk should be rapidly assessed by combining the disease course and dynamic changes of Ct values. If there is a risk of transmission, the patient should be managed as an infected person. Once fever, cough and other related clinical manifestations appear, they should be immediately transferred to designated medical institutions. Scientific classified management is conducive to saving medical resources and reducing the pressure of prevention and control.
This study has several limitations. Firstly, this was a retrospective study with some missing data, which may have led to bias. It is possible that different methods and parts of specimen collection can result in false negatives, so our observation may not be a realistic estimate of the number of patients who will re-test positive. Additionally, this study involved a single centre and a small sample size, which may have led to biases. The need for large-scale, multicenter studies must be addressed.
Conclusion
In conclusion, there are 7.2% patients retested positive by RT-PCR for SARS-CoV-2 7–60 days after hospital discharge. Nasal douche is beneficial to shorten the time of virus nucleic acid turning negative and reduce the incidence of re-positive. Attention should be paid to the re-positive patients with low Ct values to reduce the potential for disease transmission.
Acknowledgments
The authors wish to thank all of the subjects who participated in this study.
Funding
This study is supported by the Shenzhen Science and Technological Foundation (no. JCYJ20210324115611032, JSGG20220226090002003 and JSGG20220301090005007), the Shenzhen High-level Hospital Construction Fund (no. G2021027 and G2022062), and CAMS Innovation Fund for Medical Sciences (no. 2022-I2M-C&T-B-113).
Disclosure
The authors have no conflicts of interest to disclose for this work.
References
1. Stein C, Nassereldine H, Sorensen RJD. COVID-19 Forecasting Team. Past SARS-CoV-2 infection protection against re-infection: a systematic review and meta-analysis. Lancet. 2023;401(10379):833–842. doi:10.1016/S0140-6736(22)02465-5
2. World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int/.
3. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi:10.1056/NEJMc2001468
4. Hoehl S, Rabenau H, Berger A, et al. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N Engl J Med. 2020;382(13):1278–1280. doi:10.1056/NEJMc2001899
5. China NHCotPsRo. Diagnosis and treatment plan for COVID-19 (trial version 9). Chin J Clin Infect Dis. 2022;15:81–89.
6. Hoang VT, Dao TL, Gautret P. Recurrence of positive SARS-CoV-2 in patients recovered from COVID-19. J Med Virol. 2020;92(11):2366–2367. doi:10.1002/jmv.26056
7. Jiang M, Li Y, Han M, Wang Z, Zhang Y, Du X. Recurrent PCR positivity after hospital discharge of people with coronavirus disease 2019 (COVID-19). J Infect. 2020;81(1):147–178. doi:10.1016/j.jinf.2020.03.024
8. Yuan J, Kou S, Liang Y, Zeng J, Pan Y, Liu L. Polymerase chain reaction assays reverted to positive in 25 discharged patients with COVID-19. Clin Infect Dis. 2020;71(16):2230–2232. doi:10.1093/cid/ciaa398
9. Cao H, Ruan L, Liu J, Liao W. The clinical characteristic of eight patients of COVID-19 with positive RT-PCR test after discharge. J Med Virol. 2020;92(10):2159–2164. doi:10.1002/jmv.26017
10. Casale M, Rinaldi V, Sabatino L, Moffa A, Ciccozzi M. Could nasal irrigation and oral rinse reduce the risk for COVID-19 infection? Int J Immunopathol Pharmacol. 2020;34:2058738420941757. doi:10.1177/2058738420941757
11. Huijghebaert S, Hoste L, Vanham G. Essentials in saline pharmacology for nasal or respiratory hygiene in times of COVID-19. Eur J Clin Pharmacol. 2021;77(9):1275–1293. doi:10.1007/s00228-021-03102-3
12. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi:10.1001/jama.2020.12839
13. Xiao AT, Tong YX, Zhang S. False negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: rather than recurrence. J Med Virol. 2020;92(10):1755–1756. doi:10.1002/jmv.25855
14. Dao TL, Hoang VT, Gautret P. Recurrence of SARS-CoV-2 viral RNA in recovered COVID-19 patients: a narrative review. Eur J Clin Microbiol Infect Dis. 2021;40(1):13–25. doi:10.1007/s10096-020-04088-z
15. Chen D, Xu W, Lei Z, et al. Recurrence of positive SARSCoV-2 RNA in COVID-19: a case report. Int J Infect Dis. 2020;93:297–299. doi:10.1016/j.ijid.2020.03.003
16. Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92(7):797–806. doi:10.1002/jmv.25783
17. Liao X, Guan Y, Liao Q, et al. Long-term sequelae of different COVID-19 variants: the original strain versus the Omicron variant. Glob Health Med. 2022;4(6):322–326. doi:10.35772/ghm.2022.01069
18. Radulesco T, Lechien JR, Michel J. Nasal saline irrigations in the COVID-19 pandemic. JAMA Otolaryngol Head Neck Surg. 2021;147(2):218. doi:10.1001/jamaoto.2020.4326
19. Burton MJ, Clarkson JE, Goulao B, et al. Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them. Cochrane Database Syst Rev. 2020;9(9):CD013627. doi:10.1002/14651858.CD013627.pub2
20. Farrell NF, Klatt-Cromwell C, Schneider JS. Benefits and safety of nasal saline irrigations in a pandemic-washing COVID-19 away. JAMA Otolaryngol Head Neck Surg. 2020;146(9):787–788. doi:10.1001/jamaoto.2020.1622
21. Liang L, Guo Q, Zhang H, et al. Low infectious risk of re-positive COVID-19 patients: a single-center study. Int J Infect Dis. 2021;111:5–9. doi:10.1016/j.ijid.2021.08.019
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

