Back to Journals » Cancer Management and Research » Volume 10
Behavior change techniques for increasing physical activity in cancer survivors: a systematic review and meta-analysis of randomized controlled trials
Authors Finne E, Glausch M, Exner AK, Sauzet O , Stölzel F, Seidel N
Received 6 April 2018
Accepted for publication 11 June 2018
Published 30 October 2018 Volume 2018:10 Pages 5125—5143
DOI https://doi.org/10.2147/CMAR.S170064
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Emily Finne,1,* Melanie Glausch,2,* Anne-Kathrin Exner,1 Odile Sauzet,1,3 Friederike Stölzel,2 Nadja Seidel2
1School of Public Health, Bielefeld University, Bielefeld, Germany; 2University Cancer Center, University Hospital Carl Gustav Carus, Dresden University of Technology, Dresden, Germany; 3Center for Statistics (ZeSt), Bielefeld University, Bielefeld, Germany
*These authors contributed equally to the paper
Purpose: The purpose of this systematic review and meta-analysis is to investigate how physical activity (PA) can be effectively promoted in cancer survivors. The effect of PA-promoting interventions in general, behavior change techniques (BCTs), and further variables as moderators in particular are evaluated.
Methods: This study included randomized controlled trials of lifestyle interventions aiming at an increase in PA that can be carried out independently at home, published by December 2016, for adults diagnosed with cancer after completion of the main treatment. Primary outcomes were subjective and objective measures of PA prior to and immediately after the intervention. Meta-analysis and meta-regression were used to estimate effect sizes (ES) in terms of standardized mean differences, variation between ES in terms of heterogeneity indices (I2), and moderator effects in terms of regression coefficients.
Results: This study included 30 studies containing 45 ES with an overall significant small positive effect size of 0.28 (95% confidence interval=0.18–0.37) on PA, and I2=54.29%. The BCTs Prompts, Reduce prompts, Graded tasks, Non-specific reward, and Social reward were significantly related to larger effects, while Information about health consequences and Information about emotional consequences, as well as Social comparison were related to smaller ES. The number of BCTs per intervention did not predict PA effects. Interventions based on the Theory of Planned Behavior were associated with smaller ES, and interventions with a home-based setting component were associated with larger ES. Neither the duration of the intervention nor the methodological quality explained differences in ES.
Conclusion: Certain BCTs were associated with an increase of PA in cancer survivors. Interventions relying on BCTs congruent with (social) learning theory such as using prompts and rewards could be especially successful in this target group. However, large parts of between-study heterogeneity in ES remained unexplained. Further primary studies should directly compare specific BCTs and their combinations.
Keywords: exercise, lifestyle, intervention methods, behavior change, moderator effects, tumor
Background
About 14.1 million new cancer cases and 8.2 million cancer-related deaths were recorded worldwide in 2012.1 A person is defined as a cancer survivor from the moment of cancer diagnosis throughout life.2 Early detection, improved diagnostics, and treatment have resulted in increased survival rates,3,4 leading to almost 32.6 million cancer survivors worldwide with a diagnosis in the previous 5 years.1 Numerous disease- or treatment-related adverse effects, such as secondary cancers, fatigue, or depression, can decrease the length and quality of life.5
In the last two decades it has been demonstrated that physical activity (PA) plays an important role not only in cancer prevention but also during and after cancer treatment.6,7 PA increases physical functioning among cancer survivors and provides physiological and psychological benefits.8–11 It is recommended that cancer survivors should become or stay physically active as soon as possible after diagnosis. They should engage in at least 150 minutes per week of moderately intense or 75 minutes per week of vigorous intense aerobic PA and should perform muscle-strengthening activities at least twice per week.12 Despite this evidence, cancer survivors show low levels of PA13–15 and a decline during cancer treatment without returning to PA levels prior to diagnosis.16,17
Changing behavior from a mainly sedentary to a physically active lifestyle poses a challenge to most people but particularly to those with chronic diseases such as cancer.18,19 PA that is easily performed at home (for example aerobics, walking, biking) is more convenient and accessible for patients and can play an important role in developing an active lifestyle.20
Various interventions have been developed in recent years, aiming at promoting PA in cancer survivors.21–24 Although reviews show beneficial effects in terms of PA increases23,25,26 and exercise tolerance,24 substantial variance in effect sizes indicates a moderating effect of intervention characteristics such as study design, theoretical foundation, and content.
Regarding study design as a moderator, a recent meta-analysis on PA promoting interventions in different target groups found a strong methodological quality to be related to smaller intervention effects.27 This finding suggests that, by including methodologically weak studies with larger effects in previous meta-analyses, overall effects may potentially have been overestimated.
Theories most commonly used as a basis of behavior change interventions are the Transtheoretical Model (TTM), Social Cognitive Theory (SCT), Health Belief Model, and Theory of Planned Behavior (TPB).28 It is expected that interventions are more effective when built on a theoretical foundation.29 However, studies show ambiguous results.30,31
Examining intervention content as a potential moderator is challenging, since behavior change interventions are usually built out of multiple components. To facilitate consistent classification of intervention content by researchers and clinicians, Michie et al32 developed a taxonomy of behavior change techniques (BCTs) by employing a systematic expert consensus approach.32 A BCT is defined as “an observable, replicable and irreducible component of an intervention designed to alter or redirect causal processes that regulate behavior”.33 The taxonomy of Michie et al33 (BCTT v1) consists of standardized definitions of 93 different BCTs. No consistent matching of BCT definitions from BCTT v1 with theories or theory-based determinants of PA behavior is available yet. Studies that analyzed effective BCTs in interventions to increase PA30,31,34–38 showed equivocal results. Regarding cancer survivors, so far neither the amount of PA nor BCTs were compared between more or less successful trials.24
The purpose of the present review and meta-analysis is to summarize the efficacy of interventions that aim at increasing overall PA that can be carried out independently at home in cancer survivors after completion of main cancer treatment and, particularly, to analyze which BCTs are most effective in this target group. Additional intervention features such as intervention duration, number of applied BCTs, and theoretical foundation as well as patient characteristics are analyzed as possible moderators of treatment effects.
Methods
To ensure correct proceedings along the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), a PRISMA checklist was created and PRISMA review guidelines were followed (Figure S1).39 Every study and intervention feature was extracted by two reviewers independently and ambiguity in this process was resolved by consulting with a third reviewer. Since only published results were analyzed and no individual data gathered, no ethical approval was obtained.
Search strategy
The electronic database MEDLINE was searched for articles from the earliest possible year to December 2016. The search strategy included medical subheadings and text word terms in different combinations, for example: neoplasms, cancer survivor, exercise, PA, physical fitness, muscle strength, health promotion, health education, behavior therapy, and randomized controlled trial (RCT). The complete search strategy is shown in Figure S2. Additionally, reference lists of retrieved articles and published reviews and meta-analyses on PA interventions in cancer survivors were screened.
Study inclusion criteria
For eligibility, studies had to meet the following conditions:
- Type of study: RCT. Exclusion: RCT-pilot study subsequently evaluated in a main study.
- Type of participants: Adult patients (18 years of age or older) diagnosed with cancer. Exclusion: Current active treatment (except hormonal treatment) or end-of-life-care patients.
- Type of intervention: Lifestyle intervention aiming at an increase in PA behavior including exercise intervention and multicomponent program focusing on PA and further lifestyle factors; interventions that aim at increasing PA that can be easily carried out independently at home. Exclusion: Interventions aiming at PA that requires professional guidance, specific equipment or facilities (eg, fitness machines).
- Type of control group: Usual care or wait-list control group.
- Type of outcome measure: PA (self-reported or objectively measured) prior to and immediately after the intervention. PA is defined as any bodily movement produced by skeletal muscles resulting in energy expenditure.
Follow-up measurements were not taken into account, since these were reported only for a subset of interventions and were of varying duration. Only full-text articles written in English and German were included. To identify studies meeting the inclusion criteria, titles and/or abstracts of studies retrieved with the search strategy were screened. The full texts of these studies were retrieved and assessed for eligibility.
Data extraction
Extracted information of study details included author, year, research question, the country where the study was carried out, recruitment source, inclusion and exclusion criteria, study design, and description of the control group (CG) vs intervention group (IG). Regarding participant characteristics, cancer type, age, gender, and time since diagnosis or treatment were extracted. Furthermore, the following intervention details were recorded: name, frequency and total duration of the intervention, setting, type of delivery, theoretical basis, and BCTs used. Regarding PA outcome, the method of measuring PA, the number of participants randomly assigned and assessed, as well as the PA level were extracted.
Coding of methodological quality
Methodological quality was assessed according to the Effective Public Health Practice Project Quality Assessment Tool for Quantitative Studies (EPHPP-Tool).40 The tool consists of questions to help with assessing the quality concerning the six criteria selection bias, study design, confounders, blinding, data collection methods, as well as withdrawals and dropouts, which are combined to a global rating of methodological quality. Additionally, intervention integrity and statistical analyses (including intention-to-treat approach) are evaluated.
Coding of behavior change techniques
The BCT taxonomy v132 was used to identify and code the BCTs reported in each IG. The most comprehensive published description of the intervention content (eg, from study protocols) was used. Coding was carried out by MG and EF independently after completing the BCT taxonomy v1 Online Training41 using the given BCT definitions and coding rules. BCTs were coded as present or absent, and only the BCTs exclusively applied in the IG were extracted. After coding the first interventions, definitions and coding rules were discussed and additional coding rules established to interpret ambiguities. To quantify intercoder agreement, Cohen’s kappa42 was calculated for BCTs and studies (Table S1) based on the semi-final coding after this discussion. Prevalence-adjusted bias-adjusted kappa (PABAK)42 values are additionally reported. These are kappa values adjusted for a potential bias by the overall proportion of “yes”-responses as well as by differences in this proportion between the coders. Remaining disagreements in coding were then solved by discussion and consulting with a third reviewer (AE).
Data synthesis (meta-analysis and meta-regression)
Since the majority of studies reported means and standard deviations (SD or equivalents) as outcome and only a few studies reported change scores, Hedge’s g was computed as an effect size from the PA scores immediately after the intervention. Since only RCTs were included, possible baseline differences between groups should be random. For calculating SDs from other measures, formulae from the Cochrane Handbook were used.43
Effect sizes and variances were calculated within the package “metafor”44 for the Software R45 and, where effect sizes had to be calculated from F-values, P-values or proportions, these calculations were performed with the package “compute.es”,46 using conversion formulas from Cooper et al.47 If required, SDs of PA outcomes following the intervention were estimated by regressing the log SDs on the log means following the Cochrane Handbook.43
Available objective and self-reported measures were included, since both measure slightly different aspects of PA behavior and are only moderately correlated.48,49 Within-study dependencies of effect sizes were accounted for.
For studies that provided multiple self-report measures, only one effect size regarding these measures was included. Scores of total moderate to vigorous physical activity were prioritized over scores solely including low-intensity activities or those limited to only moderate or vigorous intensity activities. Overall PA was preferred over measures of sport or exercise, and measures related to total volume (ie, combining intensity, frequency, and duration) were favored over the duration of PA. Validated self-report instruments were given priority. One study did not report any measure of PA volume or frequency, but instead gave a “relative treatment effect” and the corresponding SD for both groups.50 This study was included in the meta-analysis, since this outcome measure seemed directly related to overall PA, and sensitivity analysis excluding this study did not change our results meaningfully.
For studies which included different treatment conditions compared to the same CG, dependencies between effect sizes were also taken into account in the models.
Within-study covariances were estimated following Gleser and Olkin51 and Pustejovsky52 for multiple outcomes, multiple treatment conditions, or both. In cases without a reported correlation, the estimation of covariances was based on a correlation between self-reported and objective outcomes of r=0.51, as reported for cancer survivors.49 Significance tests and CIs were based on robust estimation methods to adjust for a potentially misspecified variance–covariance matrix, since all but one covariance could only be estimated.
First, a multivariate mixed effects meta-analysis (a random effects meta-analysis that allows for effect sizes of different outcomes to be correlated within a study) with the function “rma.mv” within the “metafor” package was conducted to estimate the overall effect size and between-study heterogeneity for self-reported and objective PA outcomes using the restricted maximum likelihood estimation method. Heterogeneity due to differences between the true effects was estimated by calculating a variant of I2 for multivariate meta-analysis based on a multivariate generalization of H2, as suggested by Jackson et al.53 Publication bias was examined by visual inspection of the funnel plot and testing the association of study sampling variances with effect sizes within the multivariate mixed model (similar to Egger’s regression test).
To determine effects of individual BCTs, separate meta-regression models were then calculated with each BCT (coded as absent or present) as moderator. Only BCTs that were coded as being present for at least five comparisons were included. Additional models with further intervention characteristics as moderators followed the same approach.
Results
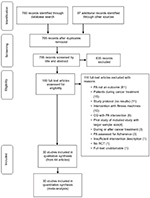
From a database search and reference checking of recent systematic reviews, 795 records were identified (Figure 1). After screening, 44 articles reporting on 30 trials met the inclusion criteria.54–96 Of these articles, all 30 trials provided sufficient data for inclusion into the meta-analysis.
Description of included trials
Three of the 30 RCTs included compared more than one IG to an untreated CG, resulting in the investigation of a total of 34 comparisons for self-reported PA outcomes and 11 comparisons for objectively measured PA outcomes. All studies were published between 2006 and 2016.
In total, 4,507 cancer survivors were included (M=150, range=22–641) with a mean age of 57.1 years (median (Md)=56.7, SD=7.71, range=33.6–73.1), and an overall percentage of females of M=74.14% (Md=90.93, SD=35.25, range=0%–100%). Most samples were survivors of breast cancer (k=13 trials) or mixed types of cancer (k=11). The majority of trials were from the US (k=19) and mostly used standard care as control comparison (k=21, eight wait-list control, and one not stated).
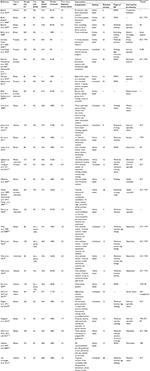
The duration of interventions ranged from one-time recommendation (zero months) to 12 months (M=4.26, Md=3, SD=2.87), and treatment took place at home (k=16), at different treatment facilities (k=4) or both combined (k=10). Study characteristics are depicted in Table 1.
Methodological quality
Results of the methodological quality assessment are presented in Figure 2 (for details see also Table S2). Overall, nine of the 30 studies were rated as methodologically strong, 16 as moderate, and five as weak. Regarding patient selection bias, many of the studies were rated weak (k=18), while the remaining were rated moderate. Confounders were controlled for in most of the studies (k=26). For blinding, only two of the studies were ranked strong. In the remaining studies, the blinding process was either not explained, the outcome assessors were aware of the exposure status of the participant, or the participants were aware of the research question. Reliable and valid outcome measures were used in most of the studies (k=26). Regarding drop-outs, 21 studies had a rate of less than 20%, and were, therefore, rated as strong. Nine studies had 60%–79% drop-outs, and one study did not report on drop-outs. Half of the studies (k=15) reported a sample-size calculation. Many of the studies had small sample sizes, suggesting difficulties in providing adequate statistical power to detect between-group differences, even if they were present. Intention-to-treat analyses were used in 20 studies. The remaining studies used either per-protocol analysis (k=7) or analyses were not explained (k=3).
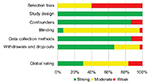  | Figure 2 Quality assessment results presented as percentages across all included studies (k=30). |
Regarding intervention integrity (ie, the degree to which an intervention is implemented as intended), three studies reported that more than 80% of participants of the IG received the intervention, in two studies 60%–79% of participants received the intervention, while the remaining 25 studies did not communicate the information. Four studies measured the consistency of the intervention (ie, if all individuals receive the same intervention). Unfortunately, 26 studies did not report on the consistency of the intervention.
Behavior change technique coding
Overall, 41 of the 93 BCTs were coded at least once in the semi-final and 37 in the final coding. For the individual BCTs, based on the semi-final coding, Cohen’s kappa ranged from 0.30 (BCT Reduce prompts) to 1.0, with the exception of four BCTs that were coded only once or twice by one reviewer but not the other, resulting in κ=0. Including these values, mean κ was 0.76 and reflects substantial agreement.97 PABAK values ranged from 0.65–1.0 (M=0.93) for the different BCTs. Regarding the different trials, Cohen’s κ ranged from 0.67–1.0 (M=0.91) and PABAK from 0.74–1.0 (M=0.94) (see Table S1). Overall, a substantial agreement could be reached.
The included interventions used an average of 10.44 BCTs (Md=11, SD=4.44), ranging from 1–17. The BCTs most commonly used were Goal setting (behavior) and Social support (unspecified) (in k=27 IGs), followed by Problem solving and Self-monitoring of behavior (k=26), Instructions on how to perform the behavior (k=24), Behavioral practice/rehearsal (k=23), and Adding objects to the environment (k=21, often related to the use of pedometers).
Overall meta-analysis
The meta-analysis included k=45 effect sizes (34 for self-reported, 11 for objective PA outcomes) within 30 trials. All trials used self-reported PA as an outcome and eight trials (effect sizes from 10 IGs) additionally used objectively measured PA. Raw means and SDs for the PA outcomes immediately after the intervention were extracted for 33 effects. The SD had to be estimated in eight studies (10 comparisons overall). For the prediction of SDs from log means the values R2=0.946 for the IG and R2=0.918 for the CG were detected. The estimated SDs were controlled for plausibility. In two cases the effect size was estimated based on available data, comparing group proportions by converting effect sizes to the standardized mean difference.
The funnel plot (Figure S3) and regression of effect size on sampling variance (β=3.65, t(df = 28)=2.273, P=0.031) indicated a significant asymmetry in the distribution of standard errors related to observed study outcomes mainly caused by small studies with particularly large effect sizes. The estimated pooled effect size may, therefore, be slightly overestimated. However, with a fail-safe N (number of unpublished studies with nonsignificant findings that would have to exist for the overall effect to become insignificant) of 1,859 for a probability of error of alpha=5%, an overall positive effect seems likely.
The model resulted in an overall estimated effect size in terms of standardized mean difference of g=0.276 (95% CI=0.183–0.369 based on robust variance estimation), indicating a significant effect in favor of the IG (P<0.001; Figure 3). There was significant heterogeneity of effect sizes (Q(df=44)=94.081, P<0.001), with τ2=0.048 (95% CI=0.013–0.132) for subjective outcomes and τ2=0.007 (95% CI=0.000–0.097) for objective outcomes, respectively. About 54.29% of the total variation in effect sizes was estimated to be caused by heterogeneity of true effects (I2). On average, effect sizes for self-reported PA were higher than for objective outcomes (g=0.316 as compared to 0.182, F(1,28)=4.642, P=0.040).
Meta-regression of PA outcomes on behavior change techniques and other potential moderators
Results of multivariate mixed effects models on BCTs
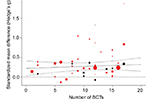
Number of BCTs
Figure 4 shows predicted and observed effect sizes of the included studies in relation to the number of BCTs used in the analyzed interventions. Effect sizes did not increase meaningfully with the number of BCTs per intervention (estimated increase per additional BCT, β=0.005, 95% CI=–0.007–0.017, P=0.408).
Moderator effects of specific BCTs
Of the final 37 BCTs exclusively applied in an IG, 27 were coded as being present for at least five effect sizes and were, therefore, analyzed as possible moderators in the meta-regression models for self-reported and objectively measured PA outcomes (see Table 2). The BCTs Prompts/cues, Reduce prompts/cues, Graded tasks, Nonspecific reward, and Social reward were significantly related to larger effect sizes, while Information about health consequences and Information about emotional consequences as well as Social comparison were used in interventions with smaller treatment effects (P<0.05). The largest differences in effect sizes associated with the use of specific BCTs were comparable in size to the magnitude of the overall effect. With a borderline significant trend, the potential moderator Self-monitoring of behavior was associated with larger effect sizes and the moderators Discrepancy between current behavior and goal and Information about social and environmental consequences with smaller effect sizes (P<0.10).
None of these individually tested BCTs reduced the unexplained heterogeneity in effects to a nonsignificant level. Overall, the amount of heterogeneity explained by the mentioned BCTs was higher for objective than for self-reported outcomes, although the heterogeneity estimates were rather imprecise due to the small number of effect sizes for objective outcomes.
Of the seven mentioned BCTs coded most often (see “BCT coding” above), only Self-monitoring of behavior had a trend to significance while the other six BCTs were clearly not significant.
Further study characteristics analyzed as potential moderators
Intervention features
Theories that were mentioned as the foundation of interventions were analyzed as further potential moderators concerning an increase in PA (see Table 3). Some interventions referred to more than one theory so that frequently mentioned theories were coded binary as absent or present for each study. The most frequently used theories were Social Cognitive Theory (SCT, in 25 IGs), the Transtheoretical Model (TTM, in 11), and Theory of Planned Behavior (TPB, in 7), whereas seven interventions used other theories and for eight interventions no theoretical foundation was mentioned. Interventions based on the SCT reported slightly but not significantly larger effect sizes. No difference was found for interventions based on the TTM. Interventions based on the TPB revealed significantly smaller effects (P<0.001). No significant moderating effects were found for interventions that did not mention a theoretical foundation or those that were based on other theories when compared to the remaining interventions.
Effect sizes varied with the setting of the intervention (P<0.001). Interventions that included a home-based training component seemed more effective than those that only took place at facilities like clinics or gyms, and those that combined both settings showed the largest effect sizes.
The duration of the intervention did not explain differences in effects sizes. However, effect sizes for longer interventions had a tendency to be smaller (mean effect size=0.30 for duration <3 months, 0.36 for 3 months, and 0.19 for >3 months; F(2, 27)=1.452, P=0.252).
Study characteristics
Methodological quality had no significant effect on effect size (see Table 3). Particularly, studies with lower quality did not result in larger effect sizes. Studies published more currently reported slightly larger effect sizes with an estimated increase per year of β=0.022 (P<0.10). Effects for trials with a wait-list CG did not differ meaningfully from those with a standard care comparison.
Patient characteristics
Effect sizes had a tendency to be smaller in studies with older participants, with an estimated decrease in effect size of β=0.016 per year of mean age of the study population (P<0.10). No differential effects were found for cancer survivors with different types of cancer, neither did the proportion of females vs males have a significant effect on outcomes.
Discussion
Within this meta-analysis, treatment effects from 30 RCTs on interventions for cancer survivors following acute treatment and aiming at an increase in PA which can be carried out independently at home were analyzed. The results show an overall significant positive effect (Hedge’s g=0.28; 95% CI=0.18–0.37) after the end of treatment, which is in line with the magnitude of effect sizes of other meta-analyses for PA interventions in cancer patients and survivors.23,26,98 Therefore, the effect is of small magnitude in terms of established rules and typical for studies in this field.
Behavior change techniques
Our results show that some specific BCTs were associated with larger effects on PA increases. Namely, including prompts or cues to perform PA behavior (eg, adding a pedometer to a workbook as a behavioral cue) or employing intermittent telephone calls as prompts79 and gradually decreasing such prompts (or the frequency/intensity of interventions) over time were significantly associated with larger PA increases. The same was true for setting graded tasks that increase in difficulty (eg, increasing the frequency and/or duration of exercise sessions from week to week was a common strategy or progressing to more demanding exercises) and for delivering different kinds of rewards for effort or progress toward PA behavior (eg, immediate reinforcement via positive automated messaging for participants who attained their personal exercise goal,99 praise for achievement of goals, or progress toward goal61). In general, interventions using a larger number of these specific BCTs showed larger PA increases.
In contrast, BCTs including information about the consequences of PA behavior in terms of health benefits or positive emotional effects were used in less successful interventions. Likewise, social comparison, ie, to draw attention to the performance of others compared to the patient her/himself, or emphasizing discrepancies between PA goals and actual behavior was associated with smaller intervention effects in our analysis. Combinations of more than two of these BCTs were associated with smaller PA increases.
In contrast, the overall number of BCTs used in an intervention had no significant impact on the achieved PA increase immediately after the intervention compared to standard care or wait-list controls in our study. This result is in line with meta-regressions that included BCTs to increase PA behavior in adults with obesity37 or diabetes.36 In contrast, Samdal et al100 found more BCTs associated with larger intervention effects in overweight or obese adults.
Overall, results on the effectiveness of specific BCTs for increasing PA do not show a consistent pattern, and our analysis is only partly consistent with previous research. Rewards were associated with greater success, in line with other studies on healthy adults of the general population as well as older adults.34,35 However, a negative effect was found for Graded tasks in one study,34 while others found a positive effect of graded tasks only on long-term but not on immediate success.100 On the other hand, BCTs similar to Social comparison, which was associated with lower effect sizes in our study, showed a positive PA effect in healthy adults,34 but a negative effect in a meta-analysis on older adults.35 In terms of Information on consequences of the behavior, we obtained a negative association with effect size, whereas others found no effect.36,100 One meta-analysis showed a positive effect.34 None of the compared studies30,34–37,100,101 endorse the positive effects we found in relation to Prompts and cues.
Several authors came to the conclusion that a combination of BCTs fostering self-regulation, ie, Self-monitoring, Goal setting, Feedback on performance, and Reviewing goals are especially promising in increasing PA in different populations.31,34,38 Of these only Self-monitoring showed a marginally significant effect in our study, while no effects were found for other self-regulatory BCTs. One reason could be that cancer survivors as a specific target group react differentially to certain BCTs than other groups. This conclusion is backed by the results of French et al,35 who found these techniques were not successful in older adults in contrast to younger populations. A similar mechanism could apply to Social comparison.35 Social comparison might be more important for younger people than for cancer survivors or the elderly, and, thus, might not be as effective when applied as BCT in these target groups.
In addition to potential target group effects,102 reasons for the differences observed may lie in different taxonomies used for coding of BCTs (ie, CALO-RE taxonomy vs BCTT v1) or in the challenge of adequate translation of intervention methods into practical application,103,104 as well as the varying numbers of included studies.
In our analysis, the most frequently used BCTs, including some of the self-regulatory techniques mentioned above, were not associated with a more successful PA increase. In other reviews and meta-analyses on different target groups, these or similarly defined BCTs were shown to be associated with larger PA effects (ie, Instructions on how to perform behavior,34 Problem solving,30,35 Goal setting,31,100 Behavioral practice31).
One possible explanation for this finding may be that some of these BCTs were used in too many interventions to enable meaningful comparisons in our analysis. Further studies should specifically test intervention effects of such frequently employed BCTs in different target groups alone and in combination with other techniques.103
Further moderators
Overall, results on the use of specific theories in PA interventions are equivocal. Most studies did not find differences between specific theories reported as the basis of PA interventions101,105 or the use of theory in general.27,30,106
Interventions that were described as based on the TPB showed lower effect sizes. Husebø et al,107 on the other hand, reported that constructs of the TPB (ie, intention, perceived behavioral control) were weakly but significantly associated with better exercise adherence in eight studies with cancer patients and survivors. The most frequently stated theory in our analysis was SCT and interventions based on SCT reported slightly but not significantly larger effect sizes. While some of the identified BCTs directly match SCT constructs (like Setting graded tasks to increase self-efficacy), other techniques fitting into the SCT framework (like Role modeling) were not associated with better success.
While the authors of the present study could not identify a cluster of BCTs that completely matches one specific theory of behavior change, BCTs that proved advantageous in our study seem mostly congruent with principles of (social) learning theory, ie, rewards, including sense of achievement and situational cues which are faded out gradually and may promote habit building. In contrast, those BCTs relying on knowledge and rational decision making (ie, setting goals, providing information, problem solving) seemed less successful in increasing PA in cancer survivors. The latter is consistent with smaller effect sizes for TPB-based interventions which often rely on information and rationality.
However, in many cases it remains unclear how exactly theory is implemented in interventions. An explicit methodology for linking BCTs to theories is currently being developed and will help to clarify relations between BCTs, mechanisms of action, and other variables such as modes of delivery, populations, settings, and types of behavior.33 Further research should establish whether the results of the present study can be replicated for cancer survivors compared to other groups, since this would have theoretical implications for planning interventions for this target group.
In terms of intervention duration, we did not find a meaningful effect on PA increase. A study by Bernard et al27 even reports a shorter duration of theory-based interventions designed to promote PA, ie, less than 14 weeks, to be associated with larger treatment effects in different target groups. Although not significant, our results point in the same direction with the lowest effect sizes for those interventions longer than 3 months. These findings underline that duration alone may not be the best measure of overall intervention intensity and that, in fact, more complex or longer interventions do not necessarily lead to greater success.
Methodological quality of included studies was also not associated with the magnitude of intervention effects indicating that the results are not contorted by differences in study quality. In contrast, two recent studies27,100 reported significant moderator effects suggesting overestimation of the efficacy of PA interventions due to methodological weaknesses. Both of these studies employed the Cochrane tool for assessing risk of bias,108 while the present study used the EPHPP-Tool.40 As Armijo-Olivo et al109 demonstrated, ratings using the EPHPP-Tool may differ from those resulting from the Cochrane tool. This may explain the diverging results. However, it was also found that the EPHPP tool seems superior in terms of interrater reliability.109
Strengths and limitations
This is the first study that systematically analyzed the associations of BCTs with PA increases in cancer survivors after treatment employing a current BCT taxonomy, where two coders evaluated studies independently, arriving at good interrater reliabilities. Furthermore, self-reported as well as objectively measured PA outcomes were included while adjusting the analysis for intratrial correlations.
Some limitations must also be taken into account. As our search was limited to MEDLINE, missing studies may lead to publication bias. However, we also accounted for reference lists of other current reviews and meta-analyses.23,98
Although the overall methodological quality of trials was not related to effect sizes, only a subset implemented an intention-to-treat analysis or reported on intervention integrity. As these criteria are not included in the overall quality rating, they may have biased the results.
Our meta-analysis is limited to outcomes measured directly after completion of interventions. Effects of specific BCTs might differ between immediate and long-term outcomes, as recently shown by Samdal et al.100 Further research on long-term effects is, therefore, desirable.
Eight trials added an objectively measured PA outcome to a self-report measure. Since these trials contained more effect size information than those relying only on self-report measures, this may have influenced our results. However, including all available information by using a multivariate model and adjusting for dependencies between outcomes seemed more appropriate than including only information on self-reported measures. The overall effect size was similar to other studies on cancer survivors. Due to the limited amount of data on objective outcomes, it was not suitable to distinguish the results of meta-regression models by subjective vs objective outcome.
Peters et al103 described limitations of analyzing effectiveness of BCTs by meta-analytical techniques. Within the included studies, BCTs may not have been transferred to intervention strategies effectively or may not have been adapted to target groups and contexts adequately. A prerequisite for finding an effect of BCTs in a meta-regression is the exclusive use of a BCT in the IG, but not in the CG. Since most of the included interventions only provided a very short description of the “standard care,” it was impossible to code and, therefore, detect BCTs for the CG accurately.
Effects of different BCTs may have confounded each other or may have been confounded by other study characteristics such as the overall number of BCTs applied in an intervention. Due to a large number of analyzed potential moderators compared to the limited number of included studies, calculation of more complex models allowing analyses of confounding effects were not possible.
Since some BCTs were often used in combination in the same studies (see Table S3) we adjusted for within-study dependencies of effect sizes and show “dose-response” relations for the use of those BCTs associated with effect sizes, but we cannot rule out that trial characteristics other than these BCTs were responsible for differences in PA effects. Furthermore, many BCTs were tested as moderators in separate models without adjustment for multiple testing.
Furthermore, some BCTs were used in very many or very few interventions, reducing the power of tests for moderator effects of these BCTs. A nonsignificant effect may not be interpreted as a proof of lack of effectiveness. The results are, therefore, exploratory and do not allow definite or causal conclusions.
We found some BCT definitions and coding rules not being clearly outlined and, therefore, added more specific coding rules, attaining good intercoder reliabilities afterwards. Others report similar issues.100 Cradock et al36 also developed extensive additional coding rules, which we included in our discussion process.
Notwithstanding these limitations, meta-analyzing the effectiveness of BCTs for increasing PA in cancer survivors and comparing the results to other target groups can be seen as one constituent of further developing theory-based interventions aimed at health behavior change in this target group.103
Conclusions
A growing body of evidence shows the positive effects of PA in cancer survivors. Thus, identifying the relevant characteristics of interventions is of great importance. The present meta-analysis shows significant effects for interventions aiming at an increase in PA that can be carried out independently by cancer survivors. The magnitude of PA increase seems neither to depend on the duration of the intervention nor on the number of BCTs used, but certain techniques were associated with significantly larger or smaller PA-increasing effects. Interventions relying on BCTs congruent with (social) learning theory, such as using prompts and rewards and setting graded tasks, could be especially successful in this target group. However, large parts of between-study heterogeneity in effect sizes remained unexplained by single moderator variables. Other factors than those studied here may impact on the success of PA interventions in cancer survivors, or synergistic effects of moderators may exist that can only be revealed in more complex analyses which require larger meta-studies. To strengthen validity, the results should be replicated and in addition be complemented by the analysis of long-term effects and direct comparisons to other target groups. Further primary studies should directly test and compare specific BCTs and their combinations. Coding instruments should be more precise with an extension of definitions and anchor examples for different interventions goals.
Acknowledgments
This research was partially supported by Stiftung Hochschulmedizin Dresden and by Deutsche Krebshilfe (German Cancer Aid) in the Program for the “Development of Interdisciplinary Oncology Centers of Excellence in Germany”, reference number: 107,759. We acknowledge support by the German Research Foundation and the Open Access Publication Funds of the Sächsische Landesbibliothek – Staats- und Universitätsbibliothek Dresden/Technische Universität Dresden. FS and NS contributed equally to this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
Stewart BW, Wild CP. World Cancer Report 2014. online ed. Lyon: International Agency for Research on Cancer/World Health Organization; 2014. | ||
Centers for Disease Control and Prevention. Cancer survivors—United States, 2007. MMWR Morb Mortal Wkly Rep. 2011;60(9):269–272. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: A Cancer Journal for Clinicians. 2017;67(1):7–30. | ||
Cancer Research UK. Cancer survival statistics. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/survival. Accessed September 15, 2017. | ||
Hewitt M, Greenfield S, Stovall E. editors. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academy Press; 2006. | ||
Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. | ||
World Cancer Research Fund InternationalAmerican Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. 1. publ. Washington, DC: AICR; 2007. | ||
Speck RM, Gross CR, Hormes JM, et al. Changes in the Body Image and Relationship Scale following a one-year strength training trial for breast cancer survivors with or at risk for lymphedema. Breast Cancer Res Treat. 2010;121(2):421–430. | ||
Mishra SI, Scherer RW, Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8(29):CD007566. | ||
Bouillet T, Bigard X, Brami C, et al. Role of physical activity and sport in oncology: scientific commission of the National Federation Sport and Cancer CAMI. Crit Rev Oncol Hematol. 2015;94(1):74–86. | ||
Fong DY, Ho JW, Hui BP, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012; 344(Jan 30 5):e70. | ||
Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA: A Cancer J Clin. 2012;62(4):242–274. | ||
Littman AJ, Tang M-T, Rossing MA. Longitudinal study of recreational physical activity in breast cancer survivors. Journal of Cancer Surviv. 2010;4(2):119–127. | ||
Harrison S, Hayes SC, Newman B. Level of physical activity and characteristics associated with change following breast cancer diagnosis and treatment. Psychooncology. 2009;18(4):387–394. | ||
Irwin ML, Crumley D, McTiernan A, et al. Physical activity levels before and after a diagnosis of breast carcinoma: The Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97(7):1746–1757. | ||
Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. The Journal of Alternative and Complementary Medicine. 1997;3(3):215–226. | ||
Devoogdt N, van Kampen M, Geraerts I, et al. Physical activity levels after treatment for breast cancer: one-year follow-up. Breast Cancer Res Treat. 2010;123(2):417–425. | ||
Pinto BM, Ciccolo JT. Physical activity motivation and cancer survivorship. Recent Results Cancer Res. 2011;186:367–387. | ||
Courneya KS, Karvinen KH, Vallance JKH. Exercise motivation and behavior change. In: Feuerstein M, editor. Handbook of Cancer Survivorship. Boston: Springer US; 2007:113–132. | ||
Lee MK, Kim NK, Jeon JY. Effect of the 6-week home-based exercise program on physical activity level and physical fitness in colorectal cancer survivors: a randomized controlled pilot study. PLoS One. 2018;13(4):e0196220. | ||
Demark-Wahnefried W, Jones LW. Promoting a healthy lifestyle among cancer survivors. Hematol Oncol Clin North Am. 2008;22(2):319–342. | ||
Pekmezi DW, Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011;50(2):167–178. | ||
Bluethmann SM, Vernon SW, Gabriel KP, Murphy CC, Bartholomew LK. Taking the next step: a systematic review and meta-analysis of physical activity and behavior change interventions in recent post-treatment breast cancer survivors. Breast Cancer Res Treat. 2015;149(2):331–342. | ||
Bourke L, Homer KE, Thaha MA, et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev. 2013;9:CD010192. | ||
Short CE, James EL, Stacey F, Plotnikoff RC. A qualitative synthesis of trials promoting physical activity behaviour change among post-treatment breast cancer survivors. Journal of Cancer Survivorship. 2013;7(4):570–581. | ||
Stacey FG, James EL, Chapman K, Courneya KS, Lubans DR. A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors. Journal of Cancer Survivorship. 2015;9(2):305–338. | ||
Bernard P, Carayol M, Gourlan M, et al. Moderators of theory-based interventions to promote physical activity in 77 randomized controlled trials. Health Education & Behavior. 2017;44(2):227–235. | ||
Painter JE, Borba CPC, Hynes M, Mays D, Glanz K. The use of theory in health behavior research from 2000 to 2005: a systematic review. Annals of Behavioral Medicine. 2008;35(3):358–362. | ||
Glanz K, Bishop DB. The role of behavioral science theory in development and implementation of public health interventions. Annu Rev Public Health. 2010;31(1):399–418. | ||
Avery L, Flynn D, Dombrowski SU, van Wersch A, Sniehotta FF, Trenell MI. Successful behavioural strategies to increase physical activity and improve glucose control in adults with type 2 diabetes. Diabetic Medicine. 2015;32(8):1058–1062. | ||
Greaves CJ, Sheppard KE, Abraham C, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health. 2011;11(1):119. | ||
Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Annals of Behavioral Medicine. 2013;46(1):81–95. | ||
Michie S, Carey RN, Johnston M, et al. From theory-inspired to theory-based interventions: a protocol for developing and testing a methodology for linking behaviour change techniques to theoretical mechanisms of action. Annals of Behavioral Medicine. 2016;369(10). | ||
Williams SL, French DP. What are the most effective intervention techniques for changing physical activity self-efficacy and physical activity behaviour--and are they the same? Health Educ Res. 2011;26(2):308–322. | ||
French DP, Olander EK, Chisholm A, Mc Sharry J. Which behaviour change techniques are most effective at increasing older adults’ self-efficacy and physical activity behaviour? A systematic review. Annals of Behavioral Medicine. 2014;48(2):225–234. | ||
Cradock KA, Ólaighin G, Finucane FM, Gainforth HL, Quinlan LR, Ginis KAM. Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: a systematic review and meta-analysis. Int J Behav Nutr Phys Act. 2017;14(1):18. | ||
Dombrowski SU, Sniehotta FF, Avenell A, Johnston M, MacLennan G, Araújo-Soares V. Identifying active ingredients in complex behavioural interventions for obese adults with obesity-related co-morbidities or additional risk factors for co-morbidities: a systematic review. Health Psychol Rev. 2012;6(1):7–32. | ||
Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychology. 2009;28(6):690–701. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(Jul 21 1):b2535. | ||
Effective Public Health Practice Project. Quality Assessment tool for Quantitative Studies. Available from: http://www.ephpp.ca/tools.html. Accessed August 7, 2017. | ||
BCTTv1 Online Training. Available from: http://www.ucl.ac.uk/health-psychology/. Accessed August 7, 2017. | ||
Byrt T, Bishop J, Carlin JB. Bias, prevalence and kappa. J Clin Epidemiol. 1993;46(5):423–429. | ||
Higgins JPT, Green S. editors. Cochrane Handbook for Systematic Reviews of Interventions. Repr. Chichester: Wiley-Blackwell; 2012. | ||
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3). | ||
R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. | ||
Del Re A.C. compute.es: Compute Effect Sizes: R Package version 0.2-2; 2013. Available from: http://cran.r-project.org/web/packages/compute.es. Accessed June 30, 2017. | ||
Cooper H, Hedges LV, Valentine JC, editors. The Handbook of Research Synthesis and Meta-Analysis. New York: Russell Sage Foundation; 2009. | ||
Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5(1):56. | ||
Boyle T, Lynch BM, Courneya KS, Vallance JK. Agreement between accelerometer-assessed and self-reported physical activity and sedentary time in colon cancer survivors. Supportive Care in Cancer. 2015;23(4):1121–1126. | ||
Rau J, Teichmann J, Petermann F. Motivation zu sportlicher Aktivität bei onkologischen Patienten nach der Rehabilitationsmaßnahme – Ergebnisse einer randomisiert-kontrollierten Wirksamkeitsstudie. PPmP - Psychotherapie · Psychosomatik · Medizinische Psychologie. 2009;59(08):300–306. | ||
Gleser LJ, Olkin I. Stochastically dependent effect sizes. In: Cooper H, Hedges LV, Valentine JC, editors. The Handbook of Research Synthesis and Meta-Aeta-analysis. New York: Russell Sage Foundation; 2009:357–376. | ||
Pustejovsky J.E. Correlations between standardized mean differences. Available from: http://jepusto.github.io/Correlations-between-SMDs. Accessed August 2, 2017. | ||
Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 2012;31(29):3805–3820. | ||
Bantum EO, Albright CL, White KK, et al. Surviving and thriving with cancer using a web-based health behavior change intervention: randomized controlled trial. J Med Internet Res. 2014;16(2):e54. | ||
Basen-Engquist K, Taylor CLC, Rosenblum C, et al. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Educ Couns. 2006;64(1-3):225–234. | ||
Bennett JA, Lyons KS, Winters-Stone K, Nail LM, Scherer J. Motivational interviewing to increase physical activity in long-term cancer survivors: a randomized controlled trial. Nurs Res. 2007;56(1):18–27. | ||
Bloom JR, Stewart SL, D’Onofrio CN, Luce J, Banks PJ. Addressing the needs of young breast cancer survivors at the 5 year milestone: can a short-term low intensity intervention produce change? J Cancer Surviv. 2008;2(3):190–204. | ||
Carmack Taylor CL, Smith MA, de Moor C, et al. Quality of life intervention for prostate cancer patients: design and baseline characteristics of the active for life after cancer trial. Control Clin Trials. 2004;25(3):265–285. | ||
Carmack Taylor CL, Demoor C, Smith MA, et al. Active for Life After Cancer: a randomized trial examining a lifestyle physical activity program for prostate cancer patients. Psychooncology. 2006;15(10):847–862. | ||
Culos-Reed SN, Robinson JW, Lau H, et al. Physical activity for men receiving androgen deprivation therapy for prostate cancer: benefits from a 16-week intervention. Supportive Care in Cancer. 2010;18(5):591–599. | ||
Demark-Wahnefried W, Clipp EC, McBride C, et al. Design of FRESH START: a randomized trial of exercise and diet among cancer survivors. Medicine & Science in Sports & Exercise. 2003;35(3):415–424. | ||
Demark-Wahnefried W, Clipp EC, Lipkus IM, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. Journal of Clinical Oncology. 2007;25(19):2709–2718. | ||
Ottenbacher AJ, Day RS, Taylor WC, et al. Long-term physical activity outcomes of home-based lifestyle interventions among breast and prostate cancer survivors. Supportive Care in Cancer. 2012;20(10):2483–2489. | ||
Hatchett A, Hallam JS, Ford MA. Evaluation of a social cognitive theory-based email intervention designed to influence the physical activity of survivors of breast cancer. Psychooncology. 2013;22(4):829–836. | ||
Hébert JR, Hurley TG, Harmon BE, Heiney S, Hebert CJ, Steck SE. A diet, physical activity, and stress reduction intervention in men with rising prostate-specific antigen after treatment for prostate cancer. Cancer Epidemiol. 2012;36(2):e128–e136. | ||
Ibfelt E, Rottmann N, Kjaer T, et al. No change in health behavior, BMI or self-rated health after a psychosocial cancer rehabilitation: results of a randomized trial. Acta Oncol. 2011;50(2):289–298. | ||
Irwin ML, Cadmus L, Alvarez-Reeves M, et al. Recruiting and retaining breast cancer survivors into a randomized controlled exercise trial: the Yale Exercise and Survivorship Study. Cancer. 2008;112(11 Suppl):2593–2606. | ||
Høybye MT, Dalton SO, Christensen J, et al. Research in Danish cancer rehabilitation: social characteristics and late effects of cancer among participants in the FOCARE research project. Acta Oncol. 2008;47(1):47–55. | ||
James EL, Stacey F, Chapman K, et al. Exercise and nutrition routine improving cancer health (ENRICH): the protocol for a randomized efficacy trial of a nutrition and physical activity program for adult cancer survivors and carers. BMC Public Health. 2011;11(1):236. | ||
James EL, Stacey FG, Chapman K, et al. Impact of a nutrition and physical activity intervention (ENRICH: Exercise and Nutrition Routine Improving Cancer Health) on health behaviors of cancer survivors and carers: a pragmatic randomized controlled trial. BMC Cancer. 2015;15(1):710. | ||
Kim SH, Shin MS, Lee HS, et al. Randomized pilot test of a simultaneous stage-matched exercise and diet intervention for breast cancer survivors. Oncol Nurs Forum. 2011;38(2):106–E106. | ||
Lahart IM, Metsios GS, Nevill AM, Kitas GD, Carmichael AR. Randomised controlled trial of a home-based physical activity intervention in breast cancer survivors. BMC Cancer. 2016;16(1):234. | ||
Ligibel JA, Meyerhardt J, Pierce JP, et al. Impact of a telephone-based physical activity intervention upon exercise behaviors and fitness in cancer survivors enrolled in a cooperative group setting. Breast Cancer Res Treat. 2012;132(1):205–213. | ||
Livingston PM, Salmon J, Courneya KS, et al. Efficacy of a referral and physical activity program for survivors of prostate cancer [ENGAGE]: rationale and design for a cluster randomised controlled trial. BMC Cancer. 2011;11(1):237. | ||
Livingston PM, Craike MJ, Salmon J, et al. Effects of a clinician referral and exercise program for men who have completed active treatment for prostate cancer: a multicenter cluster randomized controlled trial (ENGAGE). Cancer. 2015;121(15):2646–2654. | ||
Matthews CE, Wilcox S, Hanby CL, et al. Evaluation of a 12-week home-based walking intervention for breast cancer survivors. Supportive Care in Cancer. 2007;15(2):203–211. | ||
Morey MC, Snyder DC, Sloane R, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301(18):1883–1891. | ||
Demark-Wahnefried W, Morey MC, Sloane R, et al. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. Journal of Clinical Oncology. 2012;30(19):2354–2361. | ||
Snyder DC, Morey MC, Sloane R, et al. Reach out to ENhancE Wellness in Older Cancer Survivors (RENEW): design, methods and recruitment challenges of a home-based exercise and diet intervention to improve physical function among long-term survivors of breast, prostate, and colorectal cancer. Psychooncology. 2009;18(4):429–439. | ||
Park JH, Lee J, Oh M, et al. The effect of oncologists’ exercise recommendations on the level of exercise and quality of life in survivors of breast and colorectal cancer: a randomized controlled trial. Cancer. 2015;121(16):2740–2748. | ||
Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005;23(15):3577–3587. | ||
Pinto BM, Rabin C, Papandonatos GD, Frierson GM, Trunzo JJ, Marcus BH. Maintenance of effects of a home-based physical activity program among breast cancer survivors. Supportive Care in Cancer. 2008;16(11):1279–1289. | ||
Pinto BM, Papandonatos GD, Goldstein MG. A randomized trial to promote physical activity among breast cancer patients. Health Psychology. 2013;32(6):616–626. | ||
Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N. Home-based physical activity intervention for colorectal cancer survivors. Psychooncology. 2013;22(1):54–64. | ||
Rabin C, Pinto B, Fava J. Randomized trial of a physical activity and meditation intervention for young adult cancer survivors. J Adolesc Young Adult Oncol. 2016;5(1):41–47. | ||
Reif K, de Vries U, Petermann F, Görres S. Chronische Fatigue bei Krebspatienten. Med Klin. 2010;105(11):779–786. | ||
Reif K, de Vries U, Petermann F, Görres S. A patient education program is effective in reducing cancer-related fatigue: a multi-centre randomised two-group waiting-list controlled intervention trial. European Journal of Oncology Nursing. 2013;17(2):204–213. | ||
Rogers LQ, McAuley E, Anton PM, et al. Better exercise adherence after treatment for cancer (BEAT Cancer) study: rationale, design, and methods. Contemp Clin Trials. 2012;33(1):124–137. | ||
Rogers LQ, Courneya KS, Anton PM, et al. Effects of the BEAT cancer physical activity behavior change intervention on physical activity, aerobic fitness, and quality of life in breast cancer survivors: a multicenter randomized controlled trial. Breast Cancer Res Treat. 2015;149(1):109–119. | ||
Sheppard VB, Hicks J, Makambi K, Hurtado-de-Mendoza A, Demark-Wahnefried W, Adams-Campbell L. The feasibility and acceptability of a diet and exercise trial in overweight and obese black breast cancer survivors: The Stepping STONE study. Contemp Clin Trials. 2016;46:106–113. | ||
Short CE, James EL, Girgis A, McElduff P, Plotnikoff RC. Move more for life: the protocol for a randomised efficacy trial of a tailored-print physical activity intervention for post-treatment breast cancer survivors. BMC Cancer. 2012;12(1):172. | ||
Short CE, James EL, Plotnikoff RC. Theory-and evidence-based development and process evaluation of the Move More for Life program: a tailored-print intervention designed to promote physical activity among post-treatment breast cancer survivors. Int J Behav Nutr Phys Act. 2013;10(1):124. | ||
Short CE, James EL, Girgis A, D’Souza MI, Plotnikoff RC. Main outcomes of the Move More for Life Trial: a randomised controlled trial examining the effects of tailored-print and targeted-print materials for promoting physical activity among post-treatment breast cancer survivors. Psychooncology. 2015;24(7):771–778. | ||
Vallance JKH, Courneya KS, Plotnikoff RC, Yasui Y, Mackey JR. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. Journal of Clinical Oncology. 2007;25(17):2352–2359. | ||
Vallance JK, Courneya KS, Taylor LM, Plotnikoff RC, Mackey JR. Development and evaluation of a theory-based physical activity guidebook for breast cancer survivors. Health Education & Behavior. 2008;35(2):174–189. | ||
von Gruenigen V, Frasure H, Kavanagh MB, et al. Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): a randomized controlled trial. Gynecol Oncol. 2012;125(3):699–704. | ||
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. | ||
Bourke L, Homer KE, Thaha MA, et al. Interventions to improve exercise behaviour in sedentary people living with and beyond cancer: a systematic review. Br J Cancer. 2014;110(4):831–841. | ||
Lee MK, Park H-A, Yun YH, Chang YJ. Development and Formative evaluation of a web-based self-management exercise and diet intervention program with tailored motivation and action planning for cancer survivors. JMIR Res Protoc. 2013;2(1):e11. | ||
Samdal GB, Eide GE, Barth T, Williams G, Meland E. Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults; systematic review and meta-regression analyses. Int J Behav Nutr Phys Act. 2017;14(1):42. | ||
McDermott MS, Oliver M, Iverson D, Sharma R. Effective techniques for changing physical activity and healthy eating intentions and behaviour: asystematic review and meta-analysis. Br J Health Psychol. 2016;21(4):827–841. | ||
Michie S, West R. Behaviour change theory and evidence: a presentation to Government. Health Psychol Rev. 2013;7(1):1–22. | ||
Peters GY, de Bruin M, Crutzen R. Everything should be as simple as possible, but no simpler: towards a protocol for accumulating evidence regarding the active content of health behaviour change interventions. Health Psychol Rev. 2015;9(1):1–14. | ||
Kok G, Gottlieb NH, Peters GY, et al. A taxonomy of behaviour change methods: an Intervention Mapping approach. Health Psychol Rev. 2016;10(3):297–312. | ||
Gourlan M, Bernard P, Bortolon C, et al. Efficacy of theory-based interventions to promote physical activity. A meta-analysis of randomised controlled trials. Health Psychol Rev. 2016;10(1):50–66. | ||
Prestwich A, Sniehotta FF, Whittington C, Dombrowski SU, Rogers L, Michie S. Does theory influence the effectiveness of health behavior interventions? Meta-analysis. Health Psychology. 2014;33(5):465–474. | ||
Husebø AML, Dyrstad SM, Søreide JA, Bru E. Predicting exercise adherence in cancer patients and survivors: a systematic review and meta-analysis of motivational and behavioural factors. J Clin Nurs. 2013;22(1-2):4–21. | ||
Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(Oct 2):d5928. | ||
Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract. 2012;18(1):12–18. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.