Back to Journals » Infection and Drug Resistance » Volume 16
Bedaquiline Resistance and Molecular Characterization of Rifampicin-Resistant Mycobacterium Tuberculosis Isolates in Zhejiang, China
Authors Tong E, Zhou Y, Liu Z, Zhu Y, Zhang M, Wu K, Pan J, Jiang J
Received 14 August 2023
Accepted for publication 10 October 2023
Published 31 October 2023 Volume 2023:16 Pages 6951—6963
DOI https://doi.org/10.2147/IDR.S429003
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Enyu Tong,1,* Ying Zhou,1,* Zhengwei Liu,2 Yelei Zhu,2 Mingwu Zhang,2 Kunyang Wu,2 Junhang Pan,2 Jianmin Jiang1– 3
1School of Public Health, Hangzhou Normal University, Hangzhou, 311100, People’s Republic of China; 2Tuberculosis Control Department, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, 310051, People’s Republic of China; 3Key Laboratory of Vaccine, Prevention and Control of Infectious Disease of Zhejiang Province, Hangzhou, 310051, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Junhang Pan; Jianmin Jiang, Email [email protected]; [email protected]
Purpose: This study aimed to determine the prevalence and molecular characterization of bedaquiline (BDQ) resistance among rifampicin-resistant tuberculosis (RR-TB) isolates collected from Zhejiang, China.
Patients and Methods: A total of 245 RR-TB isolates were collected from 19 municipal TB hospitals in Zhejiang province, China between January and December 2021. Microplate assays were used to determine the minimum inhibitory concentrations (MIC) of BDQ. Whole-genome sequencing (WGS) was performed on isolates with MIC values for BDQ ≥ 0.25 μg/mL.
Results: Five (2.04%) BDQ-resistant strains were isolated from 245 tuberculosis patients. The resistance rate of BDQ was not correlated to the sex, age, treatment history, or occupation of patients. Four BDQ-resistant isolates and three BDQ-sensitive isolates were found to carry Rv0678 mutations, and one BDQ-resistant strain carried both Rv0678 and pepQ mutations. No mutations within the atpE and Rv1979c genes were observed.
Conclusion: BDQ demonstrated strong in vitro antibacterial activity against RR-TB isolates, and the Rv0678 gene was identified as the primary mechanism contributing to BDQ resistance among RR-TB isolates from Zhejiang, China. Furthermore, in addition to the four currently known resistance-associated genes (atpE, Rv0678, Rv1979c, and pepQ), other mechanisms of resistance to BDQ may exist that need further study.
Plain Language Summary: This study looked at a bacterial species called Mycobacterium tuberculosis, which causes the highly problematic disease, tuberculosis. Certain strains of this bacterium have developed resistance to conventional antibiotics used in tuberculosis treatment, necessitating an investigation into the efficacy of the newer antibiotic, bedaquiline. We collected 245 rifampicin-resistant tuberculosis samples from patients in Zhejiang, China, subjecting them to bedaquiline susceptibility testing. Concurrently, we conducted a genetic analysis of the bacteria to pinpoint mutations linked to bedaquiline resistance. Out of the 245 samples, 5 were found to be resistant to bedaquiline. We found that mutations in a gene called Rv0678 were the main reason for this resistance. This gene had mutations in four of the bedaquiline-resistant samples and three of the bedaquiline-susceptible samples. One of the bedaquiline-resistant samples had mutations in both Rv0678 and another gene called pepQ. We also found that bedaquiline was effective at killing drug-resistant tuberculosis bacteria in the lab. However, there may be other genes or mechanisms that make other bacteria resistant to the drug, which will need further study. Overall, this study helps us understand how bedaquiline works against drug-resistant tuberculosis bacteria and identifies a genetic mechanism that can cause resistance to the drug.
Keywords: Mycobacterium tuberculosis, rifampicin-resistant tuberculosis, bedaquiline, whole-genome sequencing
Introduction
Mycobacterium tuberculosis (MTB) has been the leading cause of human death caused by a single infectious agent, preceding the COVID-19 pandemic, and was responsible for approximately 10.6 million new cases and 1.6 million deaths in 2021.1 The rise of drug-resistant tuberculosis (DR-TB) strains, particularly multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) strains, have presented significant challenges to the global goal of controlling TB. The goal of TB control is to reduce the burden of TB by implementing various strategies, such as early detection and treatment of active TB cases, infection control measures to prevent transmission, and improving access to TB care and prevention services.2,3 According to the World Health Organization (WHO), an estimated 450,000 new rifampicin-resistant TB (RR-TB) cases occurred globally in 2021, with 78% being MDR-TB.1 Treating RR/MDR-TB is challenging due to the extended duration of treatment, limited options for effective therapies, lower cure rates, increased risk of side effects, and higher associated costs.4 Therefore, there is an urgent need to develop new and effective drugs to enhance the chemotherapy of RR/MDR-TB.5
Bedaquiline (BDQ), an innovative oral diarylquinoline drug, received FDA approval in 2012 for the treatment of MDR-TB and XDR-TB.6 Initial clinical trials have demonstrated the efficacy of BDQ in MDR-TB patients, resulting in improved sputum-negative rates with no cross-resistance observed with current first-line anti-TB drugs.7–10 However, the emergence of BDQ resistance has been observed following its introduction into treatment regimes, with several mechanisms of resistance having been identified. Non-synonymous mutations in four genes are known to cause BDQ resistance. Mutations in the atpE gene, which encodes subunit C of the ATP synthase and can prevent BDQ from binding to the C subunit, resulting in resistance;11–13 mutations in Rv0678, which codes the repressor of the MmpS5-MmpL5 efflux system, was associated with resistance to BDQ;14 and mutations in genes encoding the uncharacterized transporter, Rv1979c, and the cytoplasmic peptidase, PepQ, have also been reported to confer BDQ resistance.15–17
Zhejiang province is situated in southeast China and has a total population of 65.77 million. The annual incidence rate of TB in Zhejiang is estimated to be 41.92 cases per 100,000 population, which is lower than the national average rate of 59.93 cases per 100,000 population.18 In addition, among all incident TB cases, approximately 2.5% were RR-TB, with 4.3% of previously treated TB cases being RR-TB, of which 81.8% were MDR-TB.19
BDQ, a novel drug for the treatment of MDR-TB, was introduced into routine care in China in 2018.20 Several studies have suggested that patients receiving BDQ-containing regimens exhibit higher rates of culture conversion and treatment success compared to those receiving BDQ-free regimens.21,22 However, since limited information is available on the prevalence and molecular characteristics of BDQ resistance in Zhejiang, it is crucial to investigate these features of BDQ resistance in RR-TB isolates using whole-genome sequencing (WGS). This investigation will improve the diagnosis and treatment of RR-TB patients.
Materials and Methods
Bacterial Strains
In our study, Löwenstein–Jensen (L-J) medium was used for isolating and culturing mycobacteria, including MTB. The growth of tubercle bacilli on L-J medium often taking 2–6 weeks to observe distinct colonies.23 The samples for this study were collected from 19 municipal TB hospitals located across 10 municipalities of Zhejiang province, China. Between January 2021 and December 2021, RR-TB isolates were collected from consecutive patients receiving treatment for smear-positive and Xpert MTB/RIF rapid test rifampicin-resistant tuberculosis. Subsequently, all isolates were subcultured on Löwenstein–Jensen (L-J) medium for 4 weeks at 37 °C, and serial samples from the same patients were excluded from this study.
Data Collection
The basic information on RR-TB patients involved in this study is obtained from the National Tuberculosis Information Management System (TBIMS). This system records registration details of tuberculosis patients, such as sex, age, treatment history, occupation, and other relevant information.
Conventional Drug Susceptibility Testing
Conventional drug susceptibility testing (DST) was performed using the 1% proportion method on the L-J medium, according to the Clinical and Laboratory Standards Institute guidelines.24 The Mycobacterium tuberculosis (MTB) suspension was inoculated into an L-J medium which contained anti-TB drugs; an L-J medium without drugs was utilized as a control. The concentrations of the antituberculosis drugs for susceptibility testing were as follows: 0.2 μg/mL for isoniazid (INH), 40μg/mL for rifampin (RIF), 4.0 μg/mL for streptomycin (SM), 2.0 μg/mL for ethambutol (EMB), 2 μg/mL for ofloxacin (OFX), 30 μg/mL for kanamycin (KAN), 2 μg/mL for moxifloxacin (MXF), and 2 μg/mL for levofloxacin (LFX).25 MDR-TB was defined as any strain with resistance to at least INH and RIF. Pre-extensively drug-resistant tuberculosis (Pre-XDR-TB) was defined as any MDR-TB isolates with additional resistance to either OFX or KAN. XDR-TB was defined as resistance to RIF, any fluoroquinolone, and at least one of BDQ or Linezolid (LZD).1
Minimum Inhibitory Concentrations
The susceptibility of RR-TB isolates to BDQ was assessed using microplate assay (Wuhan Baso Medical Device Co., China). The testing procedures were conducted according to the manufacturer’s instructions.
To prepare the MTB suspension, fresh colonies from the L-J medium (Wuhan Baso Medical Device Co., China) were ultrasonically milled into a 1.0 McFarland suspension. The suspension was diluted 100-fold with culture medium containing 10% oleic acid-albumin-dextrose-catalase (OADC). An inoculum of 1.0×105 CFU/mL was added to each well using an Automatic Sample Adding Device (Wuhan Baso Medical Device Co., China). The plates were incubated at 37 °C for 14–21 days and DST results were read by two trained operators using the Microbial Drug Sensitivity Analysis System (Wuhan Baso Medical Device Co., China). A third experimenter reviewed inconsistent results. The MIC was defined as the lowest antibiotic concentration that inhibited visible growth. To ensure valid results, no wells were skipped, there was no contamination, and positive-control growth was observed.26 Each batch of drug susceptibility testing included H37Rv (ATCC 27294) as a pan-susceptible control. The breakpoint concentrations for BDQ were defined as 0.25 μg/mL according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines.27 Duplicate DSTs were performed for each isolate, and the MIC50, MIC90, and MIC95 values were defined as the concentration required to inhibit the growth of 50%, 90%, and 95% of the strains, respectively.
DNA Extraction and Sequencing
In this study, isolates with bedaquiline MIC values ≥ 0.25 μg/mL were scraped from the L-J medium slant, and their genomic DNA were extracted using the cetyltrimethylammonium bromide (CTAB) method.28 Sequencing libraries were prepared following the manufacturer’s protocol using the Illumina Nextera kit and sequenced on Illumina Hisseq 2500 system (Illumina, Inc., San Diego, CA, USA) with 2×150 paired-end (PE) strategies. All whole-genome sequencing procedures were conducted by Lianchuan Biotechnology Co., Ltd (Hangzhou, China).
Quality Control and Genome Alignment
To ensure sample purity and minimize contamination, we employed the Kraken software (http://ccb.jhu.edu/software/kraken/) for species identification. Specifically, we excluded reads identified as different species or with MTB ratios below 80%. This step helped to enhance the reliability of our analysis by focusing on reads that predominantly represented MTB.29
(Burrows-Wheeler Alignment Maximal exact matches) BWA-MEM was used to align the remaining sequencing reads to the reference genome H37Rv (NC_000962.2).30 Only clean data with an average sequencing depth greater than 200× and genome coverage >95% were retained for subsequent WGS data analysis.
Genotypic Mutations and Lineage Analysis
The drug susceptibility of the RR-TB was predicted using WGS-based analysis with TB-profiler software (https://github.com/jodyphelan/TB-profiler).31 This software can identify genetic polymorphisms associated with drug resistance and provides information on the lineage and sublineage of each isolate.31
Statistical Analysis
Statistical analysis was conducted using the chi-square test or Fisher’s exact test to compare proportions or resistant rates, and statistical significance was defined as P < 0.05. The statistical analyses were conducted using PASW Statistics v.26.0 software (IBM SPSS Statistics, Armonk, NY, USA).
Ethics Statement
The study was approved by the ethics review committee of Zhejiang Center for Disease Control and Prevention (Ref. No AF/SC-06/01.0). The patient’s informed consent was waived as the samples were collected from existing specimens obtained during routine examinations. Data retrieval was facilitated through a secure interface to prevent unauthorized access.
Results
Demographic Information
A total of 297 patients were initially enrolled, however, 37 patients (12.46%, 37/297) failed to produce specimen cultures and 9 specimens (3.03%, 9/297) were contaminated. Out of the 251 strains (84.51%, 251/297) that tested positive for Mycobacterium culture, three were identified as susceptible MTB (1.20%, 3/251) and three (1.20%, 3/251) were non-tuberculous Mycobacterium (NTM). The remaining 245 RR-TB strains (97.61%, 245/251) were used for subsequent analysis, with 171 (69.80%, 171/245) strains from patients with new cases and 74 (30.20%, 74/245) from retreated cases. The majority of patients were male, accounting for 75.51% (185/245), with 24.49% (60/245) female patients. No statistically significant difference was observed between the genders (P = 0.057). Patients’ age ranged from 14 to 87 years, with a mean age (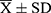 ) of 45.34±16.80 years, and no significant difference was found between age groups (P=0.693) or occupation groups (P=0.070). Detailed demographic information is presented in Table 1.
) of 45.34±16.80 years, and no significant difference was found between age groups (P=0.693) or occupation groups (P=0.070). Detailed demographic information is presented in Table 1.
 |
Table 1 Analysis of Basic Demographic Characteristics of Patients with RR-TB |
Drug Susceptibility Patterns
DST results for the nine anti-TB drugs revealed that among the 245 RR-TB isolates, the most prevalent resistance was to rifampin (100%, 245/245) and isoniazid (64.08%, 157/245), followed by ethambutol (24.08%, 59/245), ofloxacin (9.39%, 23/245), kanamycin (2.04%, 5/245), bedaquiline (2.04%, 5/245), streptomycin (0.82%, 2/245), levofloxacin (0.82%, 2/245), and moxifloxacin (0.41%, 1/245). The proportions of MDR, Pre-XDR, and XDR isolates were 64.08% (157/245), 11.02% (27/245), and 0.41% (1/245), respectively (Figure 1).
Bedaquiline Resistance Across Various Drug Resistance Patterns
Drug susceptibility testing was performed on 245 RR-TB isolates to evaluate the MIC values of BDQ. The MIC values ranged from 0.016 μg/mL to 0.031 μg/mL; the quality control strain, H37Rv, was used to verify the reproducibility of the results. For all RR-TB isolates, the overall MIC range of BDQ was 0.016–1.0 μg/mL, with MIC50, MIC90, and MIC95 values of 0.031μg/mL, 0.125 μg/mL, and 0.25 μg/mL, respectively. Of the 245 RR-TB isolates, 240 strains (97.96%) were susceptible and 5 (2.04%) were resistant to BDQ based on the susceptible breakpoint of ≤0.25 μg/mL. For MDR-TB isolates, two isolates (1.27%) showed bedaquiline MICs ≥ 0.25 μg/mL and 243 isolates (98.73%) showed MICs ≤0.25 μg/mL. bedaquiline MICs ranged from 0.016 μg/mL to 1 μg/mL, with MIC50, MIC90, and MIC95 values of 0.031 μg/mL, 0.125 μg/mL, and 0.125 μg/mL, respectively. For Pre-XDR isolates, one (3.70%) showed a bedaquiline MIC ≥ 0.25 μg/mL and 26 (96.30%) showed MICs ≤0.25 μg/mL; bedaquiline MICs ranged from 0.016 μg/mL to 0.5 μg/mL, with MIC50, MIC90, and MIC95 values of 0.063 μg/mL, 0.25 μg/mL, and 0.25 μg/mL, respectively. The MIC values of bedaquiline were compared among RR-TB isolates resistant to various TB therapeutic agents. The bedaquiline MIC50 values for RR-TB isolates resistant to isoniazid, ethambutol, streptomycin, and kanamycin were all 0.031μg/mL, while the MIC50 values for ofloxacin, levofloxacin, and moxifloxacin were 0.063 μg/mL. Furthermore, the MIC90 values of bedaquiline were 0.125 μg/mL for isoniazid, ethambutol, and streptomycin, 0.25 μg/mL for ofloxacin, and 0.063 μg/mL for levofloxacin, moxifloxacin, and kanamycin, respectively. The MIC95 values of bedaquiline for isoniazid and streptomycin-resistant strains were found to be consistent at 0.125 μg/mL, while ethambutol and ofloxacin-resistant strains showed consistency at 0.25 μg/mL. levofloxacin, moxifloxacin, and kanamycin-resistant strains also exhibited uniformity in MIC95 values at 0.063 μg/mL. These bedaquiline MIC results are summarized in Table 2.
 |
Table 2 MIC Distribution of Mycobacterium Tuberculosis isolates Against Bedaquiline According to Different Resistance Patterns |
Patient Demographics and Bedaquiline Resistance in RR-TB Patients
Table 3 shows the results of the statistical analysis of patient demographics and their association with BDQ resistance in RR-TB patients. There was no significant difference in the rate of BDQ resistance based on sex, age, treatment history, or occupation (Table 3). Out of the five patients identified with BDQ-resistant MTB, two were diagnosed with MDR-TB, one was diagnosed with pre-XDR-TB, one was diagnosed with XDR-TB, and one had RIF-resistant MTB. The MIC values for BDQ were 0.5 μg/mL for three of the strains, and the remaining two strains had MIC values of 1 μg/mL. Among the five patients, three were new cases and two had a history of prior treatment with anti-TB drugs.
 |
Table 3 Comparison of Bedaquiline Resistance Rates According to RR-TB Patient Demographics |
Genetic Mutations Associated with Bedaquiline Resistance in RR-TB Isolates
Thirteen RR-TB isolates with MIC values for BDQ ≥ 0.25 μg/mL were subjected to WGS to identify BDQ-resistant mutants (Table 4). No mutation within the atpE gene was detected in these isolates. However, five BDQ-resistant and three BDQ-susceptible isolates carried mutations in Rv0678 and PepQ. A total of five mutation types were identified in BDQ-resistant isolates, including G192 insertion, A275 duplication, U273G mutation leading to an amino acid change of Phe79Cys, A413G mutation causing Glu138Gly amino acid change, A187C mutation causing an amino acid change of Ser63Arg, and PepQ c.131G>A. The identified mutations in BDQ-sensitive isolates are detailed as follows: a GCC305GUC mutation, a missense mutation, causing an amino acid substitution from Alanine to Valine at position 102 in the Rv0678 gene; an AGC5AUC mutation, also a missense mutation, resulting in an amino acid substitution of Serine to Isoleucine at position 2; and a G198 duplication, representing a duplication mutation, within the Rv0678 gene (Table 4). Among these five BDQ-resistant isolates with mutations in Rv0678 and pepQ, four belonged to the Beijing genotype and one to the non-Beijing genotype. Furthermore, of the three BDQ-susceptible isolates with mutations in the Rv0678 gene, one was the non-Beijing genotype and two were the Beijing genotype. Moreover, of the eight patients with MIC values of BDQ ≥ 0.25 μg/mL with mutations in Rv0678 and pepQ, four were retreated cases and the remaining four were new cases based on treatment history (Table 5).
 |
Table 4 Mutation Analysis of BDQ-Resistant Genes Among 13 RR-MTB Isolates |
 |
Table 5 Drug Resistance Data Among 8 RR-MTB Isolates with Gene Mutation |
Discussion
BDQ has demonstrated high efficacy in the treatment of RR/MDR-TB,32 however, insufficient or incomplete use may lead to the emergence of drug-resistant strains. Despite this, there are limited studies that have investigated the resistance status of BDQ in patients with RR/MDR-TB in Zhejiang province, China.33 In this study, we evaluated the distribution of MIC values and resistance among 245 RR-TB isolates to BDQ, focusing on identifying any strains with a resistance to the drug. Five of the isolates were found to be resistant to BDQ, based on the breakpoint of 0.25 μg/mL. Our findings indicate that BDQ exhibited comparable in-vitro efficacy against clinical isolates resistant to various RR-TB agents, such as isoniazid, streptomycin, ethambutol, ofloxacin, levofloxacin, moxifloxacin, and kanamycin. The MIC values for most BDQ-susceptible isolates ranged from 0.016–0.25 μg/mL, while those for BDQ-resistant isolates ranged from 0.5–1.0 μg/mL. Notably, the resistance rate of RR-TB to BDQ in Zhejiang province, China, was found to be lower (2.04%) than that of commonly used first- and second-line drugs, indicating that BDQ has strong in-vitro antibacterial activity against RR-TB strains. However, the resistance rates of BDQ vary significantly in different countries and regions, ranging from approximately 1.0% in France,34 1.3% in Russia,35 2.2–3.9% in China,36–38 and 2.3% in multinational populations.8,39,40 Such inconsistencies may be attributed to factors such as differences in epidemic strains, medication history, and the local genotype of epidemic strains. Furthermore, previous studies have shown that BDQ and clofazimine (CFZ) exhibit cross-resistance, and prior exposure to CFZ could lead to the development of resistance to BDQ.15 Therefore, it is crucial to closely monitor the mechanisms, changes in MIC values, and clinical outcomes during drug therapy to prevent the increase in resistance and the spread of drug-resistant strains.
Based on the 2022 Global Tuberculosis Report, a notable sex disparity in TB incidence exists between men and women. Globally, the prevalence ratio stands at approximately 1.7:1, rising to about 2.0:1 in the WHO Western Pacific region.1 Some studies revealed that the risk of both TB and DR-TB is notably higher in men compared to women.41–44 Several factors contribute to this gender-based disparity. Firstly, young and middle-aged men are often significant contributors as primary earners in families, particularly among migrant workers, leading to increased population mobility and higher occupational exposure to TB. Secondly, economic pressures, substandard living conditions, and detrimental habits like smoking and excessive drinking prevalent among men can compromise their immune systems, heightening the risk of drug-resistant tuberculosis.45,46 Furthermore, research has demonstrated lower treatment compliance among men, contributing to a higher occurrence of drug-resistant tuberculosis.47 Given these findings, future TB prevention and control efforts should prioritize men as a key demographic. Public awareness campaigns targeting TB prevention and control must be intensified within this group, and relevant policies need to be implemented to address this gender-specific health challenge.
Studies from Taiwan have reported that over 95% of patients with RR-TB are also resistant to INH, suggesting that rifampicin resistance detection can serve as a surrogate marker for multidrug-resistant tuberculosis.48 However, a meta-analysis study revealed a wide variation in the overall detection rate of INH resistance among RR-TB patients; Xinjiang had the lowest detection rate at 57%, whereas Hong Kong and Macao had the highest rates of INH resistance, with an overall detection rate of over 90%.49 In contrast, Shen et al found that 83% of rifampicin-resistant strains were also classified as MDR-TB.50 As a result, the use of INH resistance as a screening marker for MDR-TB remains controversial and requires further study.
In this study, we found that all five BDQ-resistant isolates had mutations in known BDQ-resistance-associated genes. The Rv0678 gene, which encodes a transcriptional repressor of the efflux pump system, had the highest mutation rate of 100%. The genes MmpS5 (Rv0677c) and MmpL5 (Rv0676c) encode for a drug efflux pump. Mutations in these genes result in the overexpression of the efflux pump MmpS5/MmpL5 and reduced intracellular drug concentration.14 The association between mutations in the Rv0678 gene and BDQ resistance has been previously reported. In a study by Omar et al51 130 isolates of MTB were sequenced and all isolates were found to carry the Rv0678 mutation. The most common mutations associated with resistance in the Rv0678 gene were shifts in the guanylate homodimer at nt192–198 and in the low sequence complexity regions at nt138–144 and nt212–216.51 In a Ugandan study, frameshift mutations in Rv0678 Glu49fs were detected in two XDR-TB isolates resistant to bedaquiline. Notably, patient non-adherence, evident in the clinical history, played a significant role in the development of this acquired resistance.52 Another study by Guo, focusing on bedaquiline-resistant mutants in TB, thoroughly analyzed 168 mutants. The results notably revealed that a significant majority (157/168) of these mutants had mutations in the Rv0678 gene, specifically identified at nucleotide positions (286–287 and 198–199). Additionally, this study identified a newly recognized gene, glpK, showcasing a prevalent mutation (85.7%) and displaying a strong correlation with bedaquiline resistance.53 In our study, no mutations in the atpE gene (Rv1305), which encodes the target of BDQ, were identified. Mutations in the atpE gene result in reduced binding of BDQ to the c subunit of the F0 structural domain of ATP synthase, which leads to decreased H+ transfer and ATP production. Some studies have reported that the atpE gene is mostly mutated at positions 28 and 63.11–13 The pepQ (Rv2535c) gene encodes Xaa-Pro cytoplasmic peptidase, and its mutation is also associated with reduced sensitivity to BDQ.15 However, the exact mechanism by which mutations in the pepQ and Rv1979c genes lead to reduced sensitivity to BDQ remains unclear. Monitoring the development of these mutations during treatment is crucial in preventing the spread of drug-resistant strains.
Previous studies have reported that differences in gene expression profiles can lead to differences in drug resistance between strains of MTB, and strains that are susceptible to drug resistance may have higher transmission rates and relapse rates.54,55 Currently, MTB is globally classified into seven major lineages (Lineage 1 to Lineage 7), with Lineage 2 (mainly composed of the Beijing sublineage family, Lineage 2.2) and Lineage 4 being the most prevalent in China, while Lineage 1 and Lineage 3 are less common. The Beijing family, which is a subtype of lineage is highly prevalent, with wider transmission and a higher proportion of drug-resistant strains than non-Beijing families. However, the prevalence of the Beijing family varies across regions and countries.56–58 Our data also suggested that the most prevalent MTB subtype in Zhejiang province, China, was lineage 2.2.1. Therefore, future studies should focus on monitoring drug resistance in lineage 2 strains, particularly in the Beijing family.
Several limitations in this study should be acknowledged. First, WGS was only performed on isolates with BDQ MIC ≥ 0.25 μg/mL, and it remains unclear whether isolates with lower MIC values have mutations that confer resistance to BDQ. This highlights the need for further investigation to determine the genetic basis and underlying mechanisms of BDQ resistance in these strains. Second, the analysis of genetic diversity was based on the TB-profiler platform, which may not have identified all resistance genes due to limited coverage of gene loci. Future studies should consider a more comprehensive approach to analyze and mine the WGS data. Third, the resistance rate of BDQ is currently low and the number of strains that were analyzed for resistance in this study was relatively small, which result in a limited representation of the molecular characteristics of resistance. Future studies should include larger sample sizes to improve the understanding of BDQ resistance. Fourth, the lack of relevant treatment information, especially for patients who failed treatment, is another limitation of this study. Comprehensive genotype data and treatment information should be reported in subsequent studies to facilitate a better understanding of the correlation between genotype and phenotype in BDQ resistance.
Conclusion
The results of this study suggest that BDQ exhibits remarkable efficacy against RR-TB in Zhejiang province, China, with no significant correlation observed between the resistance rate of BDQ and demographic or clinical characteristics. Furthermore, the analysis of the genetic basis of BDQ resistance revealed that mutations in the Rv0678 gene are the predominant mechanism underlying BDQ resistance. These findings provide valuable insights into the use of BDQ in the treatment of RR-TB and emphasize the importance of continued monitoring and surveillance of BDQ resistance in clinical settings.
Abbreviations
BDQ, Bedaquiline; RR-TB, rifampicin-resistant Mycobacterium tuberculosis; MIC, minimum inhibitory concentrations; WGS, Whole-genome sequencing; TB, Tuberculosis; DR-TB, drug-resistant tuberculosis; MDR-TB, multidrug-resistant TB; XDR-TB, extensively drug-resistant TB; DST, drug susceptibility testing; L-J, Löwenstein–Jensen medium; MTB, Mycobacterium tuberculosis; INH, isoniazid; RIF, rifampin; SM, streptomycin; EMB, ethambutol; OFX, ofloxacin; KAN, kanamycin; MXF, moxifloxacin; LFX, levofloxacin; Pre-XDR-TB, Pre-extensively drug-resistant tuberculosis; LZDLinezolid; OADC, oleic acid-albumin-dextrose-catalase; EUCAST, European Committee on Antimicrobial Susceptibility Testing; CTAB, cetyltrimethylammonium bromide; NTM, non-tuberculous Mycobacterium.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
All the protocol were approved by the ethics review committee of Zhejiang Center for Disease Control and Prevention (Ref. No AF/SC-06/01.0). The patient’s informed consent was waived as the samples were collected from existing specimens obtained during routine examinations. This study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the Natural Science Foundation of China (Grant no. 82173581).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. World Health Organization. Global tuberculosis report 2022[EB/OL]. Available from:https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022.
2. El Hamdouni M, Bourkadi JE, Benamor J, Hassar M, Cherrah Y, Ahid S. Treatment outcomes of drug resistant tuberculosis patients in Morocco: multi-centric prospective study. BMC Infect Dis. 2019;19(1):1–7. doi:10.1186/s12879-019-3931-5
3. Getahun B, Ameni G, Medhin G, Biadgilign S. Treatment outcome of tuberculosis patients under directly observed treatment in Addis Ababa, Ethiopia. Braz J Infect Dis. 2013;17(5):521–528. doi:10.1016/j.bjid.2012.12.010
4. World Health Organization. World health statistics; 2021. Available from: https://www.who.int/data/gho/publications/world-health-statistics.
5. Yao C, Guo H, Li Q, et al. Prevalence of extensively drug-resistant tuberculosis in a Chinese multidrug-resistant TB cohort after redefinition. Antimicrob Resist Infect Control. 2021;10(1):126. doi:10.1186/s13756-021-00995-8
6. Angula KT, Legoabe LJ, Beteck RM. Chemical classes presenting novel antituberculosis agents currently in different phases of drug development: a 2010–2020 review. Pharmaceuticals. 2021;14(5):461. doi:10.3390/ph14050461
7. Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360(23):2397–2405. doi:10.1056/NEJMoa0808427
8. Diacon AH, Pym A, Grobusch MP, et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med. 2014;371(8):723–732. doi:10.1056/NEJMoa1313865
9. Padhi A, Naik SK, Sengupta S, Ganguli G, Sonawane A. Expression of Mycobacterium tuberculosis NLPC/p60 family protein Rv0024 induce biofilm formation and resistance against cell wall acting anti-tuberculosis drugs in Mycobacterium smegmatis. Microbes Infect. 2016;18(4):224–236. doi:10.1016/j.micinf.2015.11.007
10. Diacon A, Donald P, Pym A, et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob Agents Chemother. 2012;56(6):3271–3276. doi:10.1128/AAC.06126-11
11. Huitric E, Verhasselt P, Koul A, Andries K, Hoffner S, Andersson DI. Rates and mechanisms of resistance development in Mycobacterium tuberculosis to a novel diarylquinoline ATP synthase inhibitor. Antimicrob Agents Chemother. 2010;54(3):1022–1028. doi:10.1128/aac.01611-09
12. Segala E, Sougakoff W, Nevejans-Chauffour A, Jarlier V, Petrella S. New mutations in the mycobacterial ATP synthase: new insights into the binding of the diarylquinoline TMC207 to the ATP synthase C-ring structure. Antimicrob Agents Chemother. 2012;56(5):2326–2334. doi:10.1128/aac.06154-11
13. Zimenkov DV, Nosova EY, Kulagina EV, et al. Examination of bedaquiline- and linezolid-resistant Mycobacterium tuberculosis isolates from the Moscow region. J Antimicrob Chemother. 2017;72(7):1901–1906. doi:10.1093/jac/dkx094
14. Andries K, Villellas C, Coeck N, et al. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One. 2014;9(7):e102135. doi:10.1371/journal.pone.0102135
15. Almeida D, Ioerger T, Tyagi S, et al. Mutations in pepQ confer low-level resistance to bedaquiline and clofazimine in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2016;60(8):4590–4599. doi:10.1128/aac.00753-16
16. Nieto Ramirez LM, Quintero Vargas K, Diaz G. Whole genome sequencing for the analysis of drug resistant strains of Mycobacterium tuberculosis: a systematic review for bedaquiline and delamanid. Antibiotics. 2020;9(3):133. doi:10.3390/antibiotics9030133
17. Degiacomi G, Sammartino JC, Sinigiani V, Marra P, Urbani A, Pasca MR. In vitro study of bedaquiline resistance in Mycobacterium tuberculosis multi-drug resistant clinical isolates. Front Microbiol. 2020;11:559469. doi:10.3389/fmicb.2020.559469
18. Qian W, Yu Z, Kui L, Wei W, Bin C, Songhua C. Epidemiological characteristics of pulmonary tuberculosis in Zhejiang Province from 2016 to 2020. Prev Med. 2022;34(05):487–491. doi:10.19485/j.cnki.issn2096-5087.2022.05.013
19. Zhou L, Wu B, Huang F, et al. Drug resistance patterns and dynamics of tuberculosis in Zhejiang Province, China: results from five periodic longitudinal surveys. Front Public Health. 2022;10:1047659. doi:10.3389/fpubh.2022.1047659
20. Agnarson AM, Wang XC, Potluri R, et al. Long-term impact of the adoption of bedaquiline-containing regimens on the burden of drug-resistant tuberculosis in China. BMC Infect Dis. 2020;20(1):113. doi:10.1186/s12879-020-4795-4
21. Gao M, Gao J, Xie L, et al. Early outcome and safety of bedaquiline-containing regimens for treatment of MDR- and XDR-TB in China: a multicentre study. Clin Microbiol Infect. 2021;27(4):597–602. doi:10.1016/j.cmi.2020.06.004
22. Zhang SJ, Yang Y, Sun WW, et al. Effectiveness and safety of bedaquiline-containing regimens for treatment on patients with refractory RR/MDR/XDR-tuberculosis: a retrospective cohort study in East China. BMC Infect Dis. 2022;22(1):715. doi:10.1186/s12879-022-07693-9
23. Naveen G, Peerapur BV. Comparison of the Lowenstein-Jensen Medium, the Middlebrook 7H10 Medium and MB/BacT for the Isolation of Mycobacterium Tuberculosis (MTB) from Clinical Specimens. J Clin Diagn Res. 2012;6(10):1704–1709. doi:10.7860/JCDR/2012/4603.2635
24. Lewis I, James S Performance standards for antimicrobial susceptibility testing. 2022.
25. World Health Organization. Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. World Health Organization; 2014.
26. He W, Liu C, Liu D, et al. Prevalence of Mycobacterium tuberculosis resistant to bedaquiline and delamanid in China. J Glob Antimicrob Resist. 2021;26:241–248. doi:10.1016/j.jgar.2021.06.007
27. Kaniga K, Aono A, Borroni E, et al. Validation of bedaquiline phenotypic drug susceptibility testing methods and breakpoints: a multilaboratory, multicountry study. J Clin Microbiol. 2020;58(4). doi:10.1128/jcm.01677-19
28. Kigozi E, Kasule GW, Musisi K, et al. Prevalence and patterns of rifampicin and isoniazid resistance conferring mutations in Mycobacterium tuberculosis isolates from Uganda. PLoS One. 2018;13(5):e0198091. doi:10.1371/journal.pone.0198091
29. Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;15(3):R46. doi:10.1186/gb-2014-15-3-r46
30. Freschi L, Vargas JR, Husain A, et al. Population structure, biogeography and transmissibility of Mycobacterium tuberculosis. Nat Commun. 2021;12(1):6099. doi:10.1038/s41467-021-26248-1
31. Coll F, McNerney R, Preston MD, et al. Rapid determination of anti-tuberculosis drug resistance from whole-genome sequences. Genome Med. 2015;7(1):51. doi:10.1186/s13073-015-0164-0
32. Ahmad N, Ahuja SD, Akkerman OW, et al. Collaborative Group for the Meta-Analysis of Individual Patient Data in MDRTBt. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018;392(10150):821–834. doi:10.1016/S0140-6736(18)31644-1
33. Khoshnood S, Goudarzi M, Taki E, et al. Bedaquiline: current status and future perspectives. J Glob Antimicrob Resist. 2021;25:48–59. doi:10.1016/j.jgar.2021.02.017
34. Veziris N, Bernard C, Guglielmetti L, et al. Rapid emergence of Mycobacterium tuberculosis bedaquiline resistance: lessons to avoid repeating past errors. Eur Respir J. 2017;49(3):1601719. doi:10.1183/13993003.01719-2016
35. Peretokina IV, Krylova LY, Antonova OV, et al. Reduced susceptibility and resistance to bedaquiline in clinical M. tuberculosis isolates. J Infect. 2020;80(5):527–535. doi:10.1016/j.jinf.2020.01.007
36. Pang Y, Zong Z, Huo F, et al. In vitro drug susceptibility of bedaquiline, delamanid, linezolid, clofazimine, moxifloxacin, and gatifloxacin against extensively drug-resistant tuberculosis in Beijing, China. Antimicrob Agents Chemother. 2017;61(10):128. doi:10.1128/AAC.00900-17
37. Liu Y, Gao M, Du J, et al. Reduced susceptibility of mycobacterium tuberculosis to bedaquiline during antituberculosis treatment and its correlation with clinical outcomes in China. Clin Infect Dis. 2021;73(9):e3391–e7. doi:10.1093/cid/ciaa1002
38. Yang J, Pang Y, Zhang T, et al. Molecular characteristics and in vitro susceptibility to bedaquiline of Mycobacterium tuberculosis isolates circulating in Shaanxi, China. Int J Infect Dis. 2020;99:163–170. doi:10.1016/j.ijid.2020.07.044
39. Pym AS, Diacon AH, Tang S-J, et al. Bedaquiline in the treatment of multidrug- and extensively drug-resistant tuberculosis. Eur Respir J. 2016;47(2):564–574. doi:10.1183/13993003.00724-2015
40. Villellas C, Coeck N, Meehan CJ, et al. Unexpected high prevalence of resistance-associated Rv0678 variants in MDR-TB patients without documented prior use of clofazimine or bedaquiline. J Antimicrob Chemother. 2017;72(3):684–690. doi:10.1093/jac/dkw502
41. Zhao Y, Xu S, Wang L, et al. National survey of drug-resistant tuberculosis in China. N Engl J Med. 2012;366(23):2161–2170. doi:10.1056/NEJMoa1108789
42. Dookie N, Rambaran S, Padayatchi N, Mahomed S, Naidoo K. Evolution of drug resistance in Mycobacterium tuberculosis: a review on the molecular determinants of resistance and implications for personalized care. J Antimicrob Chemother. 2018;73(5):1138–1151. doi:10.1093/jac/dkx506
43. El Mahalli AA, Al-Qahtani MF. Predictors of drug resistance in tuberculosis patients in the Eastern Province, Saudi Arabia. J Egypt Public Health Assoc. 2015;90(1):24–28. doi:10.1097/01.EPX.0000461677.83722.78
44. Jacobs MG, Pelissari DM, Pinto VL. Factors associated with the drug-resistant tuberculosis incidence rate in Brazil. Int J Tuberc Lung Dis. 2018;22(6):675–680. doi:10.5588/ijtld.17.0667
45. Faustini A, Hall AJ, Perucci CA. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax. 2006;61(2):158–163. doi:10.1136/thx.2005.045963
46. Nava-Aguilera E, Andersson N, Harris E, et al. Risk factors associated with recent transmission of tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2009;13(1):17–26.
47. Baluku JB, Mukasa D, Bongomin F, et al. Gender differences among patients with drug resistant tuberculosis and HIV co-infection in Uganda: a countrywide retrospective cohort study. BMC Infect Dis. 2021;21(1):1093. doi:10.1186/s12879-021-06801-5
48. Jou R, Chuang PC, Wu YS, Yan JJ, Luh KT. Drug-resistant Mycobacterium tuberculosis, Taiwan. Emerg Infect Dis. 2006;12(5):871–872. doi:10.3201/eid1205.051688
49. Liu Z, Dong H, Wu B, et al. Is rifampin resistance a reliable predictive marker of multidrug-resistant tuberculosis in China: a meta-analysis of findings. J Infect. 2019;79(4):349–356. doi:10.1016/j.jinf.2019.08.004
50. Xiuli S, Mingxia J, Zhaofen W, et al. Current situation of drug resistance of 236 mycobacterium tuberculosis from Qinghai. Chin J Dis Cont Prev. 2017;21(04):353–356. doi:10.16462/j.cnki.zhjbkz.2017.04.008
51. Omar SV, Ismail F, Ndjeka N, Kaniga K, Ismail NA. Bedaquiline-Resistant Tuberculosis Associated with Rv0678 Mutations. N Engl J Med. 2022;386(1):93–94. doi:10.1056/NEJMc2103049
52. Kabahita JM, Kabugo J, Kakooza F, et al. First report of whole-genome analysis of an extensively drug-resistant Mycobacterium tuberculosis clinical isolate with bedaquiline, linezolid and clofazimine resistance from Uganda. Antimicrob Resist Infect Control. 2022;11(1):68. doi:10.1186/s13756-022-01101-2
53. Guo Q, Bi J, Lin Q, et al. Whole genome sequencing identifies novel mutations associated with bedaquiline resistance in Mycobacterium tuberculosis. Front Cell Infect Microbiol. 2022;12:807095. doi:10.3389/fcimb.2022.807095
54. Gao Q, Kripke KE, Saldanha AJ, Yan W, Holmes S, Small PM. Gene expression diversity among Mycobacterium tuberculosis clinical isolates. Microbiology. 2005;151(1):5–14. doi:10.1099/mic.0.27539-0
55. Mudliar SKR, Kulsum U, Rufai SB, Umpo M, Nyori M, Singh S. Snapshot of Mycobacterium tuberculosis phylogenetics from an Indian State of Arunachal Pradesh Bordering China. Genes. 2022;13(2):263. doi:10.3390/genes13020263
56. Karmakar M, Trauer JM, Ascher DB, Denholm JT. Hyper transmission of Beijing lineage Mycobacterium tuberculosis: systematic review and meta-analysis. J Infect. 2019;79(6):572–581. doi:10.1016/j.jinf.2019.09.016
57. van Soolingen D, Qian L, de Haas PE, et al. Predominance of a single genotype of Mycobacterium tuberculosis in countries of east Asia. J Clin Microbiol. 1995;33(12):3234–3238. doi:10.1128/jcm.33.12.3234-3238.1995
58. Ramazanzadeh R, Sayhemiri K. Prevalence of Beijing family in Mycobacterium tuberculosis in world population: systematic review and meta-analysis. Int J Mycobacteriol. 2014;3(1):41–45. doi:10.1016/j.ijmyco.2014.01.001
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

