Back to Journals » Breast Cancer: Targets and Therapy » Volume 13
Based on BATMAN-TCM to Explore the Molecular Mechanism of Xihuang Pill Regulating Immune Function to Treat Breast Precancerous Lesions
Authors Li D , Fan H, Dong J, Sun C, Su Y, Liu J , Gu Y
Received 16 September 2021
Accepted for publication 19 November 2021
Published 23 December 2021 Volume 2021:13 Pages 725—742
DOI https://doi.org/10.2147/BCTT.S339607
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Dehui Li, Huanfang Fan, Jingfei Dong, Chunxia Sun, Yifan Su, Jiao Liu, Yiting Gu
Hebei Province Hospital of Chinese Medicine;The First Affiliated Hospital of Hebei University of Chinese Medicine, Shi Jiazhuang, 050011, People’s Republic of China
Correspondence: Dehui Li Email [email protected]
Objective: The specific mechanism of Xihuang Pill in the treatment of breast precancerous lesions and breast cancer has not yet been elucidated.
Methods: In our study, BATMAN-TCM (a Bioinformatics Analysis Tool for Molecular mechanisms of Traditional Chinese Medicine) was used to forecast the relationship among chemical components, immune targets, and diseases of each herb in Xihuang Pill and constructed a component-target-disease network. Taking breast precancerous lesion model rats as the research object, the molecular mechanism of Xihuang Pill regulating immunity was analyzed.
Results: BATMAN-TCM prediction showed that 309 genes were enriched in the biological process of “immune system response”, which was the target of Xihuang Pill to regulate the immune system. The target of breast cancer disease and the genes related to Xihuang Pill’s immune system response were crossed, and 88 cross genes were obtained. According to the enrichment results of GO/KEGG pathway, T cell activation was found to be the most relevant. We select Th1 cells (IL-2, IFN-γ) and Th2 cells (IL-4, IL-10) among them for animal experiment verification. The results show that Xihuang Pill can upregulate the serum IFN-γ and IL-2 levels, reduce the IL-4 and IL-10 levels, and regulate the balance of Th1/Th2 cells in the peripheral blood of rats with breast precancerous lesions.
Conclusion: Xihuang Pill targets a variety of immune-related molecules related to breast precancerous lesions and is a traditional Chinese medicine formula that effectively regulates immune function.
Keywords: BATMAN-TCM, Xihuang Pill, breast precancerous lesions, Th1/Th2
Introduction
Breast cancer is the highest morbidity and mortality in women with malignant tumors, which seriously threatens women’s health and life safety.1 Modern studies have proved that the development of breast cancer can experience a continuous pathological process of “normal cells→simple hyperplasia→atypical hyperplasia→carcinoma in situ→invasive carcinoma”.2 Precancerous lesion (PL) refers to a lesion with a certain degree of atypical hyperplasia in histomorphology and the potential for canceration.3,4 According to WHO, all kinds of lesions with more than 20% possibility of canceration belong to PL.5 Breast precancerous lesions refer to a certain degree of morphological atypical hyperplasia of breast duct epithelial cells. After follow-up, some breast hyperplasia lesions can develop into carcinoma.6 The histological classification of pre-mammary lesions in the “WHO Histological Classification of Breast Tumors” in the fourth edition of 2012 includes:7 (1) Ductal carcinoma in situ (DCIS): divided into low nuclear grade DCIS, medium nuclear grade DCIS, high nuclear grade DCIS; (2) Lobular tumors: divided into lobular carcinoma in situ (LCIS) and atypical lobular hyperplasia (ALH); (3) Atypical ductal hyperplasia (ADH). Precancerous lesions are the necessary stage of the progression of breast cancer, and active intervention measures at this stage can block or even reverse the carcinogenesis process.2 The intervention of breast precancerous lesions and blocking its development to breast cancer is the key to the prevention and treatment of breast cancer.8
Xihuang Pill (XHP) is a traditional Chinese herbal formula, which is published in “waike zhengzhi quansheng ji”. It consists of NIU HUANG, SHE XIANG, RU XIANG (vinegar), and MO YAO (vinegar). It has the effects of promoting blood circulation and removing blood stasis, clearing heat and detoxicating, reducing phlegm and resolving mass, decreasing swelling, and relieving pain. It is a classic prescription for breast precancerous lesions and prophylaxis and treatment of breast cancer, with definite clinical effects.9,10 Our previous studies have shown that,11–13 XHP can inhibit the activity of MCF-10AT cells in precancerous breast lesions and induce apoptosis by inhibiting the expression of mTOR and VEGF in PI3K/AKT/mTOR signaling pathway, and improve the microcirculation and hemorheology of breast precancerous lesions induced by 7,12-dimethylbenzanthracene (DMBA) combined with estrogen and progesterone. Modern studies have shown that immune function status corresponds to the occurrence and progression of breast cancer, and cellular immunity plays an essential part of anti-breast cancer immunity. The immune microenvironment of breast cancer transformation, that is, the local internal environment formed by the immune cells that infiltrate into the breast cancer tissue and the cytokines they secrete together with the cancerous cells, plays an important role in regulating the growth, proliferation and metastasis of cancerous cells.14 The immune cells in the immune microenvironment are mainly T lymphocytes, accounting for about 70%-80%, and the rest are composed of B lymphocytes, macrophages, natural killer cells, and antigen presenting cells.15 Th1 and Th2 cells are important immune cells in the human body, which can more sensitively reflect the immune status of the body. An increase in the Th1/Th2 value indicates a better immune function and a better prognosis.16 In breast cancer, the ratio of Th1 and Th2 cells is found to be unbalanced, accompanied by abnormal expression of related cytokines.17 In breast cancer patients, Th2 release of cytokines is increased, while patients with Th1 dominating the response have a higher survival rate and a lower rate of cancer recurrence.18 The main mechanism of immunotherapy for breast cancer is to change the tumor microenvironment, so that the tumor immune microenvironment that is in a failed state can play a role again, so that the original immune function of the body is improved, and the purpose of anti-tumor is achieved.19 However, whether XHP plays an immunomodulatory role in the occurrence and development of breast cancer remains to be fully elucidated. Therefore, it is necessary to research the immunomodulatory mechanism of XHP and provide an experimental basis for the development of new drugs.
Due to the variety of components of traditional Chinese medicine and the complicacy of the interaction between traditional Chinese medicine and the human body, it is still very hard to reveal its internal molecular mechanism. Elucidating the molecular mechanism of traditional Chinese medicine has become the bottleneck of modernization and internationalization of traditional Chinese medicine. In recent years, with the fast development of bioinformatics, systems biology, and multi-pharmacy, network pharmacology based on network goals is a promising method for the new generation of traditional Chinese medicine or traditional Chinese medicine prescription drug research and development mode.20,21 BATMAN-TCM (http://bionet.ncpsb.org.cn/batman-tcm/) (a Bioinformatics Analysis Tool for Molecular mechanism of Traditional Chinese Medicine) is an online bioinformatics analysis tool specially designed for the research of traditional Chinese medicine molecular mechanisms. It can provide the prediction of potential targets of traditional Chinese medicine, prescriptions and components as well as KEGG and GO analysis, systematically reveal the prediction of multi-components and targets of traditional Chinese medicine, and provide the correlation analysis of component target pathway and component target disease correlation, which has a good prediction effect on the mechanism research of traditional Chinese medicine.22–24 In this research, BATMAN-TCM online database was used to gather the components of XHP from multiple databases to predict its potential targets, pathways, and diseases. We constructed a component-targeted disease network to predict the immunomodulatory effect of XHP on breast cancer. Finally, combined with animal experiments, the mechanism of XHP in the prophylaxis and treatment of breast cancer through regulating immunity was preliminarily elucidated. See Figure 1.
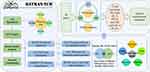 |
Figure 1 Network pharmacology and validation roadmap of XHP regulating immune function in the treatment of breast precancerous lesions. |
Materials and Methods
Prediction of Active Components and Their Targets
BATMAN-TCM (Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine) was carried out in this study. XHP is composed of NIU HUANG, SHE XIANG, RU XIANG (vinegar), and MO YAO (vinegar). We input XHP prescription drugs “NIU HUANG, SHE XIANG, RU XIANG, MO YAO” to the BATMAN-TCM platform and set the “drug-target” similarity model threshold: Score cutoff ≥ 20, P ≤ 0.05, search and screen the active components and corresponding potential targets of XHP.
Analysis of KEGG Pathway in XHP
Based on the abundance analysis of BATMAN-TCM online platform (KEGG pathway and Gene Ontology), the signal pathway of XHP target enrichment was predicted.
Construction and Analysis of Component-Target-Pathway-Disease Network of XHP
According to the predicted results, the association analysis between the significantly enriched KEGG signaling pathway and OMIM/TTD disease phenotype was carried out, and the component-target-pathway-disease network was structured to comprehensively and intuitively clarify the pharmacological mechanism of XHP.
Mining the Compounds with the Strongest Immunomodulatory Activity of XHP in the Treatment of Breast Cancer
Use GeneCards, OMIM, PharmGkb, TTD, DrugBank databases, and use “breast cancer” as a keyword to screen and merge breast cancer-related genes. Construct a component-immune gene network to comprehensively and intuitively explore the compounds with the strongest immunomodulatory activity of XHP.
Experimental Verification
Laboratory Animals
Sixty 6-week-old SPF healthy female SD rats, weighing (180±20) g, were provided by Hebei experimental animal center with animal Certificate No. 1705351. Animal experiments were approved by the Medical Ethics Committee of Hebei University of Chinese Medicine and were carried out in accordance with the Animal Welfare Guidelines of the Medical Ethics Committee of Hebei University of Chinese Medicine, in line with the guidelines for the care and use of laboratory animals by the Chinese Institute of Health, and in compliance with all regulatory guidelines. They ate and drank freely. The experiment was conducted after one week of conventional feeding.
Medicines and Reagents
XHP (Zhejiang Tianyitang Pharmaceutical Co., Ltd Division, lot No.: 1703011). Estradiol benzoate injection (Ningbo No.2, hormone factory, lot No.: 110252511). Progesterone injection (Ningbo No.2 hormone factory, lot No.: 110251670). Tamoxifen citrate tablets (Yangzijiang Pharmaceutical Group Co., Ltd., lot No.: 17041311). DMBA (7, 12-Dimethylbenz [a]anthracen, TCI, CAS57-97-6). Rat IFN-γ, IL-10, IL-2, IL-4 quantitative ELISA Kit (Shanghai senxiong Technology Industry Co., Ltd.).
Drug Preparation
DMBA preparation: precisely weigh 7, 12-dimethylbenz [a] anthracene (DMBA), soluble it in sesame oil at a proportion of 7 mg·mL−1, and put it in a waterbath box at 60°C, then dissolved by ultrasonic vibration for use.
XHP aqueous solution preparation: soak the XHP in a little distilled water the day before the experiment, whichever is just enough to soak the drug particles. The soaked XHP was crushed on the day of the experiment. Distilled water was used to prepare XHP aqueous solutions with concentrations of 27 mg·mL−1, 55 mg·mL−1, and 137 mg·mL−1 for use.
Tamoxifen aqueous solution preparation: 0.4 mg·mL−1 tamoxifen solution was prepared with distilled water.
Animal Model Replication
On the first day of the experiment, the rats were intragastrically administered with DMBA-dissolved sesame oil at a rate of 1 mL·100 g−1. From the second day, 5 days as a cycle (On days 1–3, rats were injected with 0.5 mg·kg−1·d−1 estradiol benzoate on the inside of the hind legs. On the 4th day, the rats were injected with progesterone 4 mg·kg−1·d−1 on the inside of the hind legs. Observation on day 5th). For 12 consecutive cycles, a rat model of a breast precancerous lesion was established.25 Model success judgment standard reference Ma et al.26
Grouping and Administration Method
According to weight stratification and random grouping method, 60 rats were divided into normal control group (n = 10) and disease model group (n = 50). The methods of administration were as follows: Normal control group: After one-time gavage of 1 mL·100 g−1 of sesame oil without DMBA, regular feeding; disease model group: Copy the model according to Animal Model Replication method and fed routinely. After 10 weeks of successful modeling, rats in the disease model group were stochastically divided into 5 groups, 10 rats in each group: disease model group, tamoxifen group, and XHP low, middle and high dose groups. The administration methods were as follows: Disease model group: fed routinely. Tamoxifen group: tamoxifen (4 mg·kg−1) 1 mL·100g−1 was given by gavage one time a day for 28 days, fed routinely. XHP low, middle, and high dose groups: Xihuang pills were given low (270 mg·kg−1), middle (550 mg·kg−1) and high-dose (1370 mg·kg−1) 1 mL·100g−1 by gavage, once a day for 28 days, fed routinely.25
Specimen Collection and Testing
The 14th weekend of an animal experiment, the materials were collected. The rats in each group fasted but drank freely for 24 hours before taking the material. After anesthesia, blood was collected from the inferior vena cava. The levels of IFN-γ, IL-10, IL-2, and IL-4 were detected by enzyme-linked immunosorbent assay (ELISA) according to the instructions of the kit. Take 6 pairs of mammary glands and surrounding skin and subcutaneous tissue of rat chest and abdomen under aseptic conditions, about 1.0 cm × 1.0 cm. The tissues were fixed in neutral formalin for HE staining. Refer to literature for histological observing the degree of hyperplasia under a light microscope.27
Statistical Methods
SPSS 20.0 statistical software was used for analysis. The experimental data was expressed as mean ± standard deviation (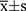 ). One way ANOVA was used for comparison between groups. The Wilcoxon rank-sum test was used for grade data. P < 0.05 was considered statistically significant.
). One way ANOVA was used for comparison between groups. The Wilcoxon rank-sum test was used for grade data. P < 0.05 was considered statistically significant.
Results
Active Components and Targets of XHP
XHP includes NIU HUANG, SHE XIANG, RU XIANG (vinegar), and MO YAO (vinegar). The active components and potential targets of XHP were retrieved and sieved by searching BATMAN-TCM. A total of 139 major chemical components were obtained, 74 of which had potential targets. Among the main chemical components obtained from SHE XIANG, 30 correspond to 1076 potential targets; among the main chemical components obtained from RU XIANG, 19 correspond to 573 potential targets; among the main chemical components obtained from MO YAO, 15 correspond to 411 potential targets; among the main chemical components obtained from NIU HUANG, 13 correspond to 386 potential targets. There are 91 cross targets of four drugs. The compound information and its corresponding targets see Figure 2 and Table 1 for details.
 |
Table 1 The Chemical Components of Drugs in XHP Prescriptions |
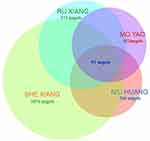 |
Figure 2 Venn diagram of cluster target set comparison of four drugs in XHP. |
KEGG Pathway Analysis of XHP Targets
The abundance analysis of BATMAN-TCM online platform (KEGG pathway and Gene Ontology) was used to predict the signal pathway of XHP target enrichment. GO functional enrichment analysis turns out that the action pathway of XHP was primarily related to 141 biological processes such as immune system reaction, signal transduction activity, kinase activity, cell differentiation, cell proliferation and death, among which 309 genes were enriched in the biological process “immune system reaction”. They include Th1 cells (IL-2, IFN-γ) and Th2 cells (IL-4, IL-10). (Figure 3). It is suggested that XHP may regulate the immune system and regulate the balance of Th1/Th2 cells through IL-2, IFN-γ, IL-4, and IL-10. KEGG pathway analysis showed that XHP acted on 30 signaling pathways including the immune system, endocrine system, digestive system, and circulatory system. The immune system includes 19 signaling pathways including chemokine signaling pathway, complement coagulation cascade, and platelet activation, etc. (Table 2). This hinted that the regulation of immune function is an important molecular mechanism of XHP.
 |
Table 2 KEGG Signaling Pathway of XHP Immune System |
 |
Figure 3 XHP with immune system response-related genes. The red box in the figure represents IL-2, IFN-γ, IL-4, IL-10. |
Component-Target-Pathway-Disease Network Construction and Analysis of XHP
According to the predicted results, the association analysis between the significantly enriched KEGG signaling pathway and OMIM/TTD disease phenotype was carried out, and the component-target-pathway-disease network was structured to comprehensively and intuitively clarify the pharmacological mechanism of XHP. The heptagonal node represents the main active component, the pentagram node represents the main target, the circular node represents the main KEGG signaling pathway, and the square node represents OMIM/TTD disease. The node size of the target, pathways and diseases is proportional to their weight in the network, including the number of active components, the number of targets involved in the pathway, and the number of targets of known disease-related genes. From the figure, we can see that XHP can treat breast cancer, pain and other diseases, and its component-target-pathway-disease network is shown in Figure 4.
 |
Figure 4 Component-target-pathway-disease network of XHP. |
Mining the Compounds with the Strongest Immunomodulatory Activity of XHP in the Treatment of Breast Cancer
Use GeneCards, OMIM, PharmGkb, TTD, DrugBank databases, and use “breast cancer” as a keyword to screen and merge breast cancer-related genes. A total of 1105 related genes were screened. 309 genes involved in the “immune system response” of the biological process are enriched with XHP. The target of breast cancer disease and the genes related to XHP’s immune system response were crossed, and 88 cross genes were obtained. As shown in Figure 5. The active ingredient compounds of XHP’s four-flavor medicines are correlated with 88 immune genes to construct a component-immune gene network, which comprehensively and intuitively presents the strongest immunomodulatory activity of the compounds in XHP. The results are shown in Figure 6. The hexagonal nodes represent the main active ingredient compounds, and the diamond nodes represent immune genes. The size of compound nodes and immune gene nodes is proportional to their weight in the network, including the target number of compound-related immune genes. Among them, immune genes include IL-2, IFN-γ, IL-4, and IL-10. The six compounds 5-Cis-Cyclopentadecen-1-One, 17-Beta-Estradiol, Androst-4-Ene-3,17-Dione, P-Menth-4-En-3-One, Testosterone, 5-Cis-Cyclotetradecen-1-One have the strongest immunomodulatory activity, and their corresponding immune gene degrees are 41, 34, 27, 26, 26 and 26, respectively. See Figures 5 and 6.
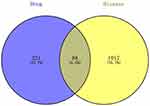 |
Figure 5 Veen diagram of the intersection of XHP and breast cancer related genes. |
 |
Figure 6 XHP compounds and immune genes network diagram. |
Study the Enrichment Analysis of GO and KEGG Signaling Pathways of the Target of XHP in the Treatment of Breast Cancer
With the aid of R 4.0.2 software, carry out enrichment analysis of the target GO biological process of XHP in the treatment of breast cancer and the metabolic pathways in the KEGG. According to the enrichment results of GO/KEGG pathway, T cell activation was discovered to be the most relevant. Th1/Th2 cell balance is closely related to T cell activation, so we choose Th1 cells (IL-2, IFN-γ), Th2 cells (IL-4, IL-10) for verification. See Figures 7 and 8.
 |
Figure 7 The GO enrichment analysis bar graph of the target of XHP in the treatment of breast cancer. |
 |
Figure 8 Analysis of the KEGG pathway of the target of XHP in the treatment of breast cancer. |
XHP Can Block and Reverse the Pathomorphology Changes of Breast Tissue in Precancerous Lesion Rats
According to Kruskal Wallis method (H-test) of multi sample comparison, χ2 = 358.58, P ≤ 0.001, according to the level of α = 0.05, it was considered that the degree of hyperplasia of mammary gland tissue in each group is different, and the degree of hyperplasia in the disease model group was the heaviest (average rank = 501.93). The results of the Mean-Whitney U-test showed that the degree of breast tissue hyperplasia in the disease model group was higher than that in the normal control group (Z = 14.529, P ≤ 0.001); the degree of breast tissue hyperplasia in the tamoxifen group and XHP low, middle and high dose groups was lower than that in the disease model group (P < 0.05 or P < 0.01) (Table 3). It is suggested that XHP can prevent and reverse the pathomorphology changes of breast tissue in rats with precancerous lesions induced by DMBA combined estrogen and progesterone.
 |
Table 3 The Effect of XHP on the Pathological Degree of Breast Tissue in Rat Model of Precancerous Lesions of Breast Cancer (n=10) |
Effect of XHP on Serum Cytokines in Breast Precancerous Lesion Rats
ELISA method was used to detect the changes in serum cytokine content of rats in each group, and the results showed (see Figures 9 and 10): Compared with rats in the disease model group, each dose group of XHP can significantly increase DMBA combined with estrogen and progesterone sequential induction serum IFN-γ and IL-2 levels (P < 0.01 or P < 0.05) in breast precancerous lesion rats, lower IL-4 and IL-10 levels (P < 0.01 or P < 0.05), and regulate peripheral blood Th1/Th2 cells balance.
Discussion
Chinese herbal medicine has been used to cure malignant tumors for thousands of years. In recent decades, studies have confirmed that Chinese herbal medicine (natural products) possesses the features of “multi-component, multi-target, and multi-pathway”.28 Because of this feature, it is tough to conduct a comprehensive and extensive study of Chinese herbal compounds.23 After Hopkins put forward the idea of “network Pharmacology” in 2008,29 network pharmacology is an effective tool to systematically analyze the mechanism of multi-compound traditional Chinese medicine formulas, providing a new strategy for studying the mechanism of complex Chinese herb formulas. BATMAN-TCM has been used in the pharmacological research of a variety of traditional Chinese medicines and compound prescriptions.5,30 In this project, BATMAN-TCM online analysis tool was used to forecast the mechanism of XHP in the treatment of breast precancerous lesions through immune regulation, and preliminary verification was carried out through animal experiments.
XHP is a classic prescription for the prevention and treatment of breast cancer. Clinical studies have shown that9 XHP combined with chemotherapy has a better curative effect, and the neoplasm response and survival condition of breast cancer patients have been significantly improved, which can significantly reduce the adverse reactions caused by chemotherapy, including nausea and vomiting, leucopenia, thrombocytopenia and hemoglobin reduction. Whereas, it is still limited the acceptance and application of XHP due to lack of sufficient molecular evidence in foreign countries. Consequently, the antitumor mechanism of XHP needs further study. In recent years, tumor immune treatment has become an important method of cancer treatment and one of the hot topics in the field of cancer study. Breast cancer is also an immunogenic disease, and some patients do benefit from immunotherapy. Whether XHP plays an immunomodulatory role in the occurrence and development of breast cancer remains to be fully elucidated. Thus, it becomes necessary to research the immunomodulatory action of XHP and provide the experimental basis for the new drug exploitation.
XHP include four traditional Chinese medicines: NIU HUANG, SHE XIANG, RU XIANG (vinegar), and MO YAO (vinegar). A total of 139 main chemical components were retrieved by BATMAN-TCM, of which 74 chemical components have potential targets, resulting in 2446 targets, 91 cooperative targets of four Chinese medicines. Afterward, enrichment analysis was carried out. We discovered that biological pathway enrichment analysis revealed many potential targeting pathways to XHP. Immune system response, signal transduction activity, kinase activity, cell differentiation, cell proliferation, and death play a major role in the progression of breast cancer. There are 309 genes enriched in the biological process of “immune system response”, including Th1 cells (IL-2, IFN-γ) and Th2 cells (IL-4, IL-10). It is pointed out that the regulation of immune function is an important molecular mechanism of XHP. XHP may regulate the immune system through IL-2, IFN-γ, IL-4, and IL-10. Significantly rich disease phenotypes include breast cancer, pain, and other diseases. The target of breast cancer disease and the genes related to XHP’s immune system response were crossed, and 88 cross genes were obtained. Associate the active ingredient compounds of XHP four herbs with 88 immune genes to construct a component-immune gene network. And found that six compounds 5-Cis-Cyclopentadecen-1-One, 17-Beta-Estradiol, Androst-4-Ene-3, 17-Dione, P-Menth-4-En-3-One, Testosterone, 5-Cis-Cyclotetradecen-1-One have the strongest immunomodulatory activity. With the aid of R 4.0.2 software, carry out enrichment analysis of the target GO biological process of XHP in the treatment of breast cancer and the metabolic pathways in the KEGG. According to the enrichment results of GO/KEGG pathway, T cell activation was found to be the most relevant. Th1/Th2 cell balance is closely related to T cell activation, so we choose Th1 cells (IL-2, IFN-γ), Th2 cells (IL-4, IL-10) for verification. It is suggested that IL-2, IFN-γ, IL-4, and IL-10 may be potential targets of XHP in the treatment of breast cancer. We carried out further experimental verification.
The role of 5-Cis-Cyclopentadecen-1-One, Androst-4-Ene-3,17-Dione, P-Menth-4-En-3-One, Testosterone, 5-Cis-Cyclotetradecen-1-One on immune regulation has not yet been reported. It is reported that 17-Beta-Estradiol has dual functions of immune enhancement and immunosuppression,31 which can promote the vitality, activation and maturation of B cells and enhance immunity.32 It can also inhibit the proliferation of IM-9 cells (peripheral blood B lymphocytes), and down-regulate the expression of IgG,33 inhibit T lymphocytes, and induce their apoptosis.34 17-Beta-Estradiol has two sides, which can stimulate the growth of breast cancer cells at some time, and the appropriate time can also inhibit the growth of breast cancer cells.35,36 Studies have found that monitoring the expression of FAS in CTCs in patients with advanced third-line endocrine therapy-resistant breast cancer, giving estrogen therapy can achieve a certain effect, and it is possible to restore the sensitivity of some patients to aromatase inhibitors without obvious side effects.37 It is clinically proven that XHP combined with endocrine therapy has a certain attenuation and synergistic effect on estrogen-dependent breast cancer.38 We speculate that this may be related to the 17-Beta-Estradiol contained in XHP. Testosterone, there are accumulated data to support the protective effect of androgens in breast tissue.39 Androgens have been successfully used to treat breast cancer.40,41 Androst-4-Ene-3,17-Dione is an important steroid drug intermediate. Androstenedione can be used as a hormone for the biosynthesis of estrogen and more effective androgens, such as testosterone.42 There is no report on its treatment of breast cancer. We speculate that its mechanism of treatment of breast cancer is similar to 17-Beta-Estradiol and Testosterone. There is no report on the treatment of breast cancer with active ingredients such as 5-Cis-Cyclotetradecen-1-One, 5-Cis-Cyclopentadecen-1-One, P-Menth-4-En-3-One, etc. Its efficacy and mechanism in the treatment of breast cancer need to be further studied. 17-Beta-Estradiol has two sides, can promote the occurrence and development of breast cancer, and can also treat breast cancer. In terms of immune regulation, it has the dual effects of immune enhancement and immunosuppression. The role of this two-way regulation is very interesting, and it is worthwhile for us to further study its mechanism.
At present, the main mechanisms of flavonoids against breast cancer are: inhibit aerobic glycolysis, promote apoptosis, block cell cycle, inhibit invasion and migration, cause DNA damage, inhibit aromatase, inhibit microtubule production, etc.43 In terms of immunity, flavonoids can affect the immune phenotype of breast cancer cells.44 Since flavonoids are similar in chemical structure to estrogen, they can exert estrogen-like effects to regulate estrogen receptor activity and downstream signals. Some flavonoids can also inhibit the synthesis of estrogen by inhibiting aromatase.45 XHP contains flavonoids such as quercetin, luteolin and hesperetin. Studies have found that quercetin promotes breast cancer cell apoptosis and inhibits the proliferation of cancer cells by regulating the GAS5/Notch1, EGFR/AKT/mTOR signaling pathways.46,47 Luteolin can inhibit the P13K/Akt, MAPK/Erk1/2 signaling pathway and inhibit the proliferation of human breast cancer cell MCF-7.48–50 Hesperetin can inhibit the PI3K-AKT-Paxillin-FAK-Src signaling pathway and inhibit the migration and adhesion of breast cancer MDA-MB-231 cells.51 In summary, flavonoids such as quercetin, luteolin and hesperetin have the effect of inhibiting the occurrence and development of breast cancer. In our research, we found that these flavonoids have little relationship with immunity.
The immune microenvironment of breast cancer is the local internal environment formed by the immune cells that infiltrate into the breast cancer tissue and the cytokines secreted by them together with the cancerous cells. It plays a major part in regulating the growth, proliferation, and metastasis of breast cancer cells. The main components of infiltrating immune cells contain T cells, B cells, macrophage cells, natural killer cells, and dendritic cells, of which T cells account for about 80%, playing a leading role. According to the function of T cells in the immune response and different surface markers, they can be divided into CD8 + cytotoxic T lymphocytes (CTL) and CD4 + helper T lymphocytes (including 4 cell subgroups of Th1, Th2, Th17 and Treg cells). CD4 + T cell subgroups Th1, Th2, Th17, Treg and their related cytokines play a significant part in the tumor immune microenvironment and are crucial to the occurrence, growth and prognosis of breast cancer. CD4 + T cells differentiate into Th1 and Th2 cells under the stimulation of tumor antigens presented by MHCII molecules on the surface of tumor antigen-presenting cells (APCs). Th1 cells activate CD8 + T cells by excreting IL-2 and IFN-γ cytokines to promote cellular immunity. Th2 cells promote B cell proliferation, maturation, and antibody production by secreting IL-4, IL-10, and other cytokines, promote humoral immunity and inhibit cellular immunity. Tumor immunity is mainly cellular immunity. The immune response induced by Th1 cells inhibits the proliferation of malignant tumors. If Th2 cells are dominant in tumor patients, the cellular immune function will be inhibited, thus promoting the occurrence and progress of the tumor. Studies have shown that in animal models of breast cancer or human breast cancer, the number of Th2 cells infiltrated into the tumor stroma is significantly higher than that of Th1 cells, Th1/Th2 immune balance is broken, Th1 floats to Th2, and cellular immunity of the body is.52
The 7.12-dimethylbenz[a]anthracene (DMBA) revealed breast cancer model is a widely used experimental model in chemistry research. DMBA has extremely high selectivity and specificity for breast tissue (70 – 80%),53 which can completely reappear the whole process of breast carcinogenesis (i e “normal cells→simple hyperplasia→atypical hyperplasia→carcinoma in situ→invasive carcinoma”).54 The success rate of breast precancerous lesion modeling was 92.5%.55 In addition, studies have also shown that exogenous or endogenous estrogen and progesterone are important factors leading to breast cancer, DMBA combined with estrogen and progesterone can significantly improve the occurrence rate of breast precancerous lesions.12,13,26,56 In this study, the model of breast precancerous lesions was established by one-time intragastric administration of DMBA combined with estrogen and progesterone. The outcomes of the normal control group breast tissue were normal glands and a small amount of general hyperplasia; the disease model group, breast tissue was primarily precancerous lesions and a small amount of invasive carcinoma; the number of precancerous lesions and invasive carcinoma in each dose group of XHP was significantly reduced, suggesting that XHP can reverse and block the pathological changes of breast tissue caused by DMBA combined with estrogen and progesterone. The results of ELISA showed that contrasted with the normal control group rats, the serum IFN-γ and IL-2 levels of the disease model group rats were decreased. At the same time, the IL-4 and IL-10 levels were increased, indicating that the Th1/Th2 immune balance of the animal model of breast precancerous lesions was broken, and the cellular immunity of the body was inhibited. XHP in each dose group can significantly increase the serum levels of IL-2 and IFN-γ, reduce the levels of IL-10 and IL-4, regulate the equilibrium of Th1/Th2 cells in peripheral blood, and effectively inhibit the progress of breast precancerous lesions. It is suggested that the results of network pharmacological analysis of XHP are consistent with the results of animal experiments, which have a certain reference value.
One of the advantages of this study is to forecast the immunotherapeutic targets, therapeutic pathways, and therapeutic diseases of XHP components, and to establish the component-target-disease network of XHP. We also identified IL-2, IFN-γ, IL-4, and IL-10 targets in the rat model, an up-and-coming therapeutic target, probably. However, the mechanism of the beneficial effect of XHP on breast precancerous lesions is complicated. However, note that our research has some limitations. For example, we only forecasted potential immune-related targets, but the signaling pathways and concrete mechanisms of their were not research. In the next step of the research, we will touch on the biological processes and signaling pathways targeting these targets and identify which compounds in this traditional Chinese medicine formula are more likely to play a therapeutic role by regulating immunity, to further explore the micro mechanism of XHP for breast precancerous lesions.
Conclusion
Based on network pharmacology, the components and potential targets of XHP were discussed and analyzed through BATMAN-TCM, and the immune genes of Th1 cells (IL-2, IFN-γ), Th2 cells (IL-4, IL-10) were found. It is suggested that the regulation of immune function is an important molecular mechanism of XHP. XHP may regulate the immune system of many diseases through IL-2, IFN-γ, IL-4, and IL-10. We further found that XHP acts as immune genes and related immune active compounds in breast cancer, which provides evidence for XHP in the treatment of breast precancerous lesions by immunomodulation. Animal experiments have verified that XHP can significantly inhibit the progression of breast precancerous lesions. This may be achieved by increasing serum IFN-γ and IL-2 levels, reducing IL-4 and IL-10 levels, and regulating Th1/Th2 cell balance. This may be one of the important functions of XHP to regulate immunity. It is suggested that XHP is a promising immunomodulatory drug, and it has a good development trend in the future immunotherapy of breast precancerous lesions. In the future, we hope to further verify the role of compounds with immunomodulatory activity in XHP and explore the mechanism of treatment of breast precancerous lesions in molecular biology.
Data Sharing Statement
Data will be obtained from the author upon reasonable request.
Acknowledgments
This research was financially supported by grants from the Youth Program of National Natural Science Foundation of China (Grant No. 81603412), Key r&d Projects of Hebei Province (Grant No. 18277731D), Research Project of Administration of Traditional Chinese Medicine of Hebei Province (Grant No. 2017163, 2019008, 2020014), General Projects for Improving Scientific Research Capacity of Hebei University of TCM (Grants No. KTY2019009), Hebei Key Laboratory of Chinese Medicine Research on Cardio-Cerebrovascular Disease, Hebei Key Laboratory of Integrative Medicine on Liver-kidney Patterns (Grants No. A201902), Hebei Province “three three three talent project” funded project (Grants No. A202002008).
Disclosure
The authors declare no conflicts of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Han B, Du Y, Fu T, et al. Differences and relationships between normal and atypical ductal hyperplasia, ductal carcinoma in situ, and invasive ductal carcinoma tissues in the breast based on raman spectroscopy. Appl Spectrosc. 2017;71(2):300–307. doi:10.1177/0003702816681009
3. Hodorowicz-Zaniewska D, Brzuszkiewicz K, Szpor J, et al. Clinical predictors of malignancy in patients diagnosed with atypical ductal hyperplasia on vacuum-assisted core needle biopsy. Wideochir Inne Tech Maloinwazyjne. 2018;13(2):184–191. doi:10.5114/wiitm.2018.73528
4. Zhang LY, Hemminki O, Zheng GQ, et al. Comparison of familial clustering of anogenital and skin cancers between in situ and invasive types. Sci Rep. 2019;9(1):16151. doi:10.1038/s41598-019-51651-6
5. Zhang GJ, Jiang XF, Liu YS, et al. Therapeutic efficiency of an external chinese herbal formula of Mammary precancerous lesions by BATMAN-TCM online bioinformatics analysis tool and experimental validation. Evid Based Complement Alternat Med. 2019;2019:2795010. doi:10.1155/2019/2795010
6. Myers DJ, Walls AL, Myers DJ, Walls AL. Breast, atypical hyperplasia. [Updated 2018 Oct 27]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2018. Available from https://www.ncbi.nlm.nih.gov/books/NBK470258/.
7. Sinn HP, Kreipe H. A brief overview of the WHO classification of breast tumors, 4th edition, focusing on issues and updates from the 3rd edition. Breast Care. 2013;8(2):149–154. doi:10.1159/000350774
8. Dettogni RS, Stur E, Laus AC, et al. Potential biomarkers of ductal carcinoma in situ progression. BMC Cancer. 2020;20(1):119. doi:10.1186/s12885-020-6608-y
9. Mao D, Feng L, Huang SQ, et al. Meta-analysis of xihuang pill efficacy when combined with chemotherapy for treatment of breast cancer. Evid Based Complement Alternat Med. 2019;2019:3502460. doi:10.1155/2019/3502460
10. Zheng WX, Han SY, Jiang ST, et al. Multiple effects of Xihuang pill aqueous extract on the Hs578T triple-negative breast cancer cell line. Biomed Rep. 2016;5(5):559–566. doi:10.3892/br.2016.769
11. Li DH, Fan HF, Sun CX, et al. Effects of liquid extract of Xihuang pills on mTOR and VEGF expression in precancerous cells of human breast cancer. Hunan J Tradit Chin Med. 2017;33(06):145–148. doi:10.16808/j.cnki.issn1003-7705.2017.06.068
12. Li DH, Su YF, Fan HF, et al. Effect of Xihuang Pill on microcirculation in DMBA combined estrogen and progesterone induced breast precancerous lesions rats. IOP Conf. 2020;474(5):52053–52055. doi:10.1088/1755-1315/474/5/052053
13. Li DH, Su YF, Fan HF, et al. Effect of Xihuang Pill on hemorheological properties in DMBA combined estrogen and progesterone induced breast precancerous lesions rats. Basic Clin Pharmacol Toxicol. 2020;127:14–15. doi:10.1111/bcpt.13461
14. Wagner J, Rapsomaniki MA, Chevrier S, et al. A single-cell atlas of the tumor and Immune ecosystem of human breast cancer. Cell. 2019;177(5):1330–1345. doi:10.1016/j.cell.2019.03.005
15. Burugu S, Asleh-Aburaya K, Nielsen TO. Immune infiltrates in the breast cancer microenvironment: detection, characterization and clinical implication. Breast Cancer. 2017;24(1):3–15. doi:10.1007/s12282-016-0698-z
16. Chen LJ, Wang J, Zhang WJ. Relationship between Th1/Th2 cell imbalance and disease severity, lung injury in patients with sepsis. J Mol Diag Ther. 2020;12(10):1315–1318. doi:10.3969/j.issn.1674-6929.2020.10.010
17. Liu C, Liu HY, Fan H, et al. Cellullar immune state and the change of Th1/Th2 cytokines in the triple negative breast cancer. J Mod Oncol. 2016;24(2):234–236. doi:10.3969/j.issn.1672-4992.2016.02.019
18. Guo QR, Liu Y, Su CY, et al. Non-coding RNA and tumor immune regulation. Acta Pharm Sin. 2019;54(10):1783–1791. doi:10.16438/j.0513-4870.2019-0570
19. Zeng TL, Li JX, Yin ZP, et al. Research progress of PD-1/PD-L1 inhibitors in immunotherapy of triple-negative breast cancer. Chin J Immunol. 2019;35(19):2423–2429. doi:CNKI:SUN:ZMXZ.0.2019-19-024
20. Sakle NS, More SA, Mokale SN. A network pharmacology-based approach to explore potential targets of Caesalpinia pulcherima: an updated prototype in drug discovery. Sci Rep. 2020;10(1):17217. doi:10.1038/s41598-020-74251-1
21. Huang SJ, Mu F, Li F, et al. Systematic elucidation of the potential mechanism of Erzhi Pill against drug-induced liver injury via network pharmacology approach. Evid Based Complement Alternat Med. 2020;2020:1–15. doi:10.1155/2020/6219432
22. Long SR, Yuan CH, Wang Y, et al. Network pharmacology analysis of damnacanthus indicus C.F.Gaertn in gene-phenotype. Evid Based Complement Alternat Med. 2019;2019:1368371. doi:10.1155/2019/1368371
23. Tang YJ, Zhang Y, Li L, et al. Kunxian capsule for rheumatoid arthritis: inhibition of inflammatory network and reducing adverse reactions through drug matching. Front Pharmacol. 2020;11:485. doi:10.3389/fphar.2020.00485
24. Liu ZY, Guo FF, Wang Y, et al. BATMAN-TCM: a bioinformatics analysis tool for molecular mechANism of traditional chinese medicine. Sci Rep. 2016;6(1):21146. doi:10.1038/srep21146
25. Fan HF, Li DH, Guo N, et al. Xihuang Pill inhibits the development of DMBA combined estrogen and progesterone induced breast precancerous lesions rats by PI3K/AKT/mTOR signaling pathway; 2021. doi:10.21203/rs.3.rs-151758/v1
26. Ma M, Li DH, Zhang GJ, et al. Study of Rats’ Mammary Precancer models-induced by DMBA combined estrogen and progesterone.
27. Frank GA, Danilova NV, Andreeva II, et al. WHO classification of tumors of the breast, 2012. Arkh Patol. 2013;75(2):53–63.
28. Song YN, Wang HY, Pan YJ, et al. Investigating the multi-target pharmacological mechanism of Hedyotis diffusa Willd acting on prostate cancer: a network pharmacology approach. Biomolecules. 2019;9(10):591. doi:10.3390/biom9100591
29. Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4(11):682–690. doi:10.1038/nchembio.118
30. Zhu JQ, Li B, Ji YS, et al. βelemene inhibits the generation of peritoneum effusion in pancreatic cancer via suppression of the HIF1AVEGFA pathway based on network pharmacology. Oncol Rep. 2019;42(6):2561–2571. doi:10.3892/or.2019.7360
31. Yuan WD. Effects of 17β-estradiol on immunocompetence of peritoneal macrophages of rat in Vitro. J Jining Med Univ. 2007;11(4):296–298. doi:10.3969/j.issn.1000-9760.2007.04.010
32. Li XX. 17β-estradiol regulates DC to influence the immune response of B cells. Nanjing University; 2010.
33. Song XF, Niu ZG, Guo JQ, et al. Regulatory effect of 17-β-estradiol on IM-9 cells of human B lymphocyte line. Chin J Cell Mol Immunol. 2009;25(03):
34. Song XF, Sun X, Wang H. Regulating effect of estrogen and progesterone on the growth of T lymphocytes. J Cell Mol Immunol. 2005;126(02):249–253. doi:CNKI:SUN:XBFM.0.2005-02-00Y
35. Ellis MJ, Dehdahti F, Kommareddy A, et al. A randomized Phase 2 trial of low dose (6 mg daily) versus high dose (30 mg daily) estradiol for patients with estrogen receptor positive aromatase inhibitor resistant advanced breast cancer. Cancer Res. 2009:69. doi:10.1158/0008-5472
36. Lonning PE, Taylor PD, Anker G, et al. High-dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine therapy. Breast Cancer Res Treat. 2001;67(2):111–116. doi:10.1023/A:1010619225209
37. Tang DB, Zhang QY, Wang JX, et al. A clinical study of estradiol therapy in endocrine-resistant advanced breast cancer. J Pract Oncol. 2012;26(04):289–292. doi:10.3969/j.issn.1002-3070.2012.04.001
38. Pan GF. Clinical research and experimental study on Xihuang Pill treating breast cancer based on estrogen receptor. Chin J Exper Tradit Med Form. 2012;18(23):330–333. doi:10.13422/j.cnki.syfjx.2012.23.099
39. Glaser R, Dimitrakakis C. Testosterone and breast cancer prevention. Maturitas. 2015;82(3):291–295. doi:10.1016/j.maturitas.2015.06.002
40. Boni C, Pagano M, Panebianco M, et al. Therapeutic activity of testosterone in metastatic breast cancer. Anticancer Res. 2014;34(3):1287–1290.
41. Glaser RL, Dimitrakakis C. Rapid response of breast cancer to neoadjuvant intramammary testosterone-anastrozole therapy: neoadjuvant hormone therapy in breast cancer. Menopause. 2014;21(6):673–678. doi:10.1097/GME.0000000000000096
42. Kent LN, Konno T, Soares MJ. Phosphatidylinositol 3 kinase modulation of trophoblast cell differentiation. BMC Dev Biol. 2010;10(1):97. doi:10.1186/1471-213X-10-97
43. Pang BB, Chu YK, Yang H. Anti-breast cancer mechanism of flavonoids. China J Chin Mater Med. 2018;43(05):913–920. doi:10.19540/j.cnki.cjcmm.20171211.005
44. Coombs MR, Harrison ME, Hoskin DW. Apigenin inhibits the inducible expression of programmed death ligand 1 by human and mouse mam mary carcinoma cells. Cancer Lett. 2016;380(2):424. doi:10.1016/j.canlet.2016.06.023
45. Pan ZY, Zhou LP, Qi B, et al. Research progress on antitumor mechanism of phytoestrogen. Chin J Biochem Pharmaceut. 2014;34(9):174–176. doi:CNKI:SUN:SHYW.0.2014-09-052
46. Jiang DC, Jin L, Chen HX, et al. Quercetin promotes apoptosis of breast cancer cells by targeting GAS5/Notch1 signaling pathway. Chin Pharmacol Bull. 2021;37(5):637–644.
47. Mu CJ, Pan W, Wang J. Quercetin modulates the proliferation and apoptosis of human breast cancer cells T47D by regulating EGFR/AKT/mTOR signaling pathway. J Clin Exper Med. 2019;18(14):1460–1464. doi:10.3969/j.issn.1671-4695.2019.14.002
48. Li WF, Ou Q, Zhang H, et al. Inhibition effect of luteolin on SDF-1α/CXCR4 signal pathway in breast cancer cells MDA-MB-231. J Basic Clin Oncol. 2014;3:199–202. doi:10.3969/j.issn.1673-5412.2014.03.005
49. Wang LM, Xie KP, Huo HN, et al. Luteolin inhibits proliferation induced by IGF-1 pathway dependent ERα in human breast cancer MCF-7 cells. Asia Pac J Cancer Prev. 2012;13(4):1431–1437. doi:10.7314/APJCP.2012.13.4.1431
50. Sui JQ, Xie KP, Xie MJ. Inhibitory effect of luteolin on the proliferation of human breast cancer cell lines induced by epidermal growth factor. Acta Physiol Sin. 2016;68(1):27–34.
51. Mao CM, Yu DH, Gui H. Effect and mechanism of hesperetin on P-selectin mediated breast cancer MDA-MB-231 metastasis. Chin Tradit Herb Drugs. 2017;591(4):714–721. doi:10.7501/j.issn.0253-2670.2017.04.017
52. Olkhanud PB, Rochman Y, Bodogai M, et al. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J Immunol. 2011;186(10):5656–5662. doi:10.4049/jimmunol.1100463
53. Sharma D, Smits BM, Eichelberg MR, et al. Quantification of epithelial cell differentiation in mammary glands and carcinomas from DMBA- and MNU-exposed rats. PLoS One. 2011;6(10):e26145. doi:10.1371/journal.pone.0026145
54. Thompson HJ, Singh M. Rat models of premalignant breast disease. J Mammary Gland Biol Neoplasia. 2000;5(4):409–420. doi:10.1023/A:1009582012493
55. Song AL, Ye L, Li JW, et al. Effect of Rufu Decoction on the microcirculation of model rats with atypical hyperplasia of mammary glands. Shandong J Tradit Chin Med. 2003;22(10):622–625. doi:CNKI:SUN:SDZY.0.2003-10-030
56. Wang F, Ma ZB, Wang F, et al. Establishment of novel rat models for premalignant breast disease. Chin Med J. 2014;127(11):2147–2152. doi:10.3760/cma.j.issn.0366-6999.20130276
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


