Back to Journals » Journal of Inflammation Research » Volume 16
Bariatric Surgery and Gut-Brain-Axis Driven Alterations in Cognition and Inflammation
Authors Custers E , Franco A, Kiliaan AJ
Received 25 August 2023
Accepted for publication 31 October 2023
Published 22 November 2023 Volume 2023:16 Pages 5495—5514
DOI https://doi.org/10.2147/JIR.S437156
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Emma Custers,* Ayla Franco,* Amanda Johanne Kiliaan
Department of Medical Imaging, Anatomy, Radboud University Medical Center, Donders Institute for Brain Cognition and Behaviour, Nijmegen, the Netherlands
*These authors contributed equally to this work
Correspondence: Amanda Johanne Kiliaan, Department of Medical Imaging, Anatomy, Preclinical Imaging Centre, Radboud Alzheimer Center, Radboud University Medical Center, Donders Institute for Brain, Cognition and Behaviour, Geert Grooteplein 21N, Nijmegen, 6525 EZ, the Netherlands, Tel +31 24 3614378, Email [email protected]
Abstract: Obesity is associated with systemic inflammation, comorbidities like diabetes, cardiovascular disease and several cancers, cognitive decline and structural and functional brain changes. To treat, or potentially prevent these related comorbidities, individuals with obesity must achieve long-term sustainable weight loss. Often life style interventions, such as dieting and increased physical activity are not successful in achieving long-term weight loss. Meanwhile bariatric surgery has emerged as a safe and effective procedure to treat obesity. Bariatric surgery causes changes in physiological processes, but it is still not fully understood which exact mechanisms are involved. The successful weight loss after bariatric surgery might depend on changes in various energy regulating hormones, such as ghrelin, glucagon-like peptide-1 and peptide YY. Moreover, changes in microbiota composition and white adipose tissue functionality might play a role. Here, we review the effect of obesity on neuroendocrine effects, microbiota composition and adipose tissue and how these may affect inflammation, brain structure and cognition. Finally, we will discuss how these obesity-related changes may improve after bariatric surgery.
Keywords: obesity, cognitive impairment, inflammation, gut hormones, adipose tissue, bariatric surgery
Introduction
Obesity is a major risk factor for the development of several comorbidities, including type 2 diabetes (T2DM), cardiovascular disease and several cancers.1 Thirty-nine percent of the adults are overweight (25–29.9 kg/m2) and 13% are obese (BMI≥30kg/m2).2 Recently, it has been found that obesity may also affect brain function and structure, as it is associated with impaired cognition and alterations in gray matter (GM) and white matter (WM).3 Obesity is negatively associated with GM integrity in many brain regions such as thalamus, caudate nucleus, putamen, globus pallidus, hippocampus and nucleus accumbens.4 Moreover, a higher BMI and waist-to-hip ratio (WHR) are associated with lower fractional anisotropy (FA) values, indicating a global distortion of WM integrity in multiple WM tracts, including the corpus callosum, periventricular WM and the brainstem.5,6 Moreover, it is proposed that obesity increases the risk of developing dementia later in life by 60–90%, versus healthy weight individuals.7 The underlying mechanisms responsible for these obesity-related brain changes are still poorly understood. However, adipose dysfunction, increased inflammation and mood disorders are suggested to play a role.8–10
Increasing evidence shows that obesity driven cognitive impairment may be reversible by weight loss,11 with the largest improvements in working memory12 and executive function.13,14 However, substantial long-term weight loss is often hard to achieve with dietary interventions.15 Though, bariatric surgery (BS) can provide a good solution. BS is an effective treatment for obesity leading to rapid and sustainable weight loss. Common procedures are the vertical sleeve gastrectomy and the Roux-en-Y gastric bypass,16 which are restrictive and malabsorptive surgical procedures that induce approximately 25% total body weight loss.17 Moreover, BS leads to the remission of several comorbidities, improving glycaemic and lipid metabolism and reducing all-cause mortality.16,18–22 Still little is known about the physiological changes that occur after BS. However, it is thought that hormonal alterations in the gastrointestinal tract, pancreas and adipose tissue are partially responsible for the effectiveness of BS.23
The gut-brain axis consists of a bidirectional communication system, connected through the vagus nerve, spinal fibers and sympathetic and parasympathetic fibers which are directly innervating the gastrointestinal tract.24 These elements communicate through endocrine messengers, neuroimmune mediators and neuroactive metabolites. This review will focus on the gut-brain axis in obesity. In particular, we will focus on the neuroendocrine effects of ghrelin, insulin, glucagon-like-peptide-1 (GLP-1) and Peptide YY (PYY), the microbiota and its metabolites, and finally we will focus on adipokine secretion by adipose tissue on inflammation, cognition and brain structure before, and after BS. We searched the PubMed database for original articles published in English from 1996 to 2023, using appropriate search terms related to obesity (eg “obesity”, “body mass index”, “BMI”, “adiposity”, “white adipose tissue”), cognition (eg “cognition”, “memory”, “learning”), inflammation (eg “inflammation”, “neuroinflammation”, “microglia”, “immune system”), hormones (eg “gut hormones”, “GLP-1”, “insulin”, “insulin resistance”, “diabetes”, “adipokines”, “ghrelin”, “microbiome”, “microbiota”, “short chain fatty acids”, “SCFA”) and bariatric surgery (eg “bariatric surgery”, “gastric sleeve”, “gastric bypass”). Both human and animal studies investigating the mechanisms of action were included. Relevant reviews and references lists of selected articles were also examined for suitable articles. Our selection criteria for human participants were: (1) medically considered obese with a BMI ≥ 30 kg/m², (2) measurement or associations between adiposity and cognition/inflammation were established and/or the effect of BS was determined, (3) control group of non-obese participants or using placebo for intervention studies. Human studies were excluded when eating disorders were documented (Table 1). Inclusion criteria for animal models were (1) induced obesity, either through diet (eg high fat diet, western diet, etc) or genetic manipulation (eg ob/ob model), (2) measurement of cognition or inflammation was established and/or the effect of BS was determined, (3) control group present (Table 2).
 |
Table 1 Summary of Included Human Studies Based on Our Inclusion Criteria |
 |
Table 2 Summary of Included Animal Studies Based on Our Inclusion Criteria |
The Role of Hormones
Ghrelin
Ghrelin is an amino-acid hormone that increases appetite due to its action on the type 1a growth hormone secretagogue receptor (GHSR1a) in the hippocampus, hypothalamus and pituitary gland.94–97 Ghrelin is produced mainly in the stomach, and affects the brain directly via the vagus nerve to inhibit food intake.98 Obese mice show a decreased response to ghrelin,81 which can be caused by lower ghrelin levels passing the blood–brain barrier (BBB)99 and/or a decreased ghrelin receptor expression in the brain.70,83 After consumption of a high-fat diet (HFD) in mice, the peripheral and central effect of ghrelin on food intake is reduced, indicating that ghrelin’s satiety effect is negated when following a HFD.70,83,100,101 In humans, obesity is associated with decreased secretion and lower circulating levels of ghrelin.95 Moreover, ghrelin levels do not increase after food intake in persons with obesity, while they do in healthy individuals.102 Thus, reduced ghrelin levels are involved in increased food intake and could thereby contribute to the development of obesity.
Ghrelin can improve memory, learning and behaviour by activating the GHSR1a receptor, which is highly expressed in the hippocampus.103 In C57BL/6J mice harboring thy1-green fluorescent protein, ghrelin was shown to modulate synaptic plasticity by increasing the dendritic spine density and promoting the expression of BDNF-mRNA species.104 Furthermore, a ghrelin receptor antagonist (GRA) restored passive avoidance behaviours in male rats and improved spatial learning and increased activity in mice.105 While animal studies tend to show a positive effect of ghrelin on cognition, one human study revealed the opposite effect. Here, it was demonstrated that increased serum ghrelin is associated with reduced performance on several cognitive domains.106 Although, in humans very little research has been conducted to examine the effect of ghrelin on cognitive or behavioural functions and therefore more research should be performed. For now, it is suggested that reduced ghrelin levels might be involved in obesity-related cognitive impairment.
Ghrelin also has anti-inflammatory effects. HFD-induced inflammation in lean C57BL/6J mice was characterized by increased expression levels of toll-like receptor 4 (TLR4) in globlet cells of the intestine and interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), ionized calcium-binding adaptor molecule 1 (IBA1) and eosinophil surface receptor 1(EMR-1) in the nodose ganglion and hypothalamus.84 Ghrelin administration reduced cytokine expression and thereby ameliorated HFD-induced inflammation.84 This anti-inflammatory effect of ghrelin also blocks the NF-kB pathway and reduces IL-6, IL-1β and TNF-α expression in endothelial cells.107 An in vitro model showed decreased levels of leptin induced inflammatory cytokines after ghrelin treatment, suggesting that ghrelin can control immune cell activation and inflammation.108 Finally, there seems to be a relation between ghrelin resistance and metabolic inflammation, which both are related to obesity and can be improved by calorie restriction.109
Ghrelin affects meal initiation and as a consequence increases calorie intake,110 which might be circumvented through bariatric surgeries. The vertical sleeve gastrectomy involves the removal of the gastric fundus, the primary source of ghrelin, leading to a reduction of fasting and postprandial ghrelin on short and long term.32,38,45 In comparison, the effect of biliopancreatic diversion on ghrelin is much lower as the gastric fundus is maintained which induces no change30,42 or increased ghrelin levels.28,111 Results on Roux-en-Y gastric bypass remain inconclusive, as some studies reveal decreased ghrelin levels, even 5 years post-surgery,28,29,37 while others showed either no change32,48 or higher post-operative ghrelin levels.40,61 Increased ghrelin levels may arise from a compensatory mechanism after weight loss, whereas the difference in ghrelin levels after surgery may be explained by different surgeons performing the surgeries. The remaining gastric pouch and alimentary limb can differ in size and length as these are determined by the surgeon, causing differences in ghrelin levels. In conclusion, altered ghrelin levels in obesity might be involved in the development of obesity-related cognitive impairment and inflammation, but its levels seem to be restored after BS.
Insulin
Insulin is secreted by β-cells in the islet of Langerhans in the pancreas and regulates glucose homeostasis, via its hypoglycaemic effect.112 Insulin is initially released after food intake through the readily releasable pool within the plasma membrane of pancreatic B-cells, while the second release is more sustained and derives from granules stored in the reserve pool which resides deeper within the cell.113 Insulin can peripherally activate glucose transporter type 4 (GLUT4), which subsequently transports glucose to the liver, muscles and adipose tissue.114 Individuals with obesity have increased levels of insulin and therefore have an increased risk of insulin resistance, compared to lean controls.68 Insulin resistance is described as the failure of tissues to respond to the constant release of insulin, increasing insulin levels independent of blood glucose, a phenomenon known as hyperinsulinemia.115 Obesity is also a known risk factor for the development of T2DM as it is associated with abnormal fasting glucose levels and impaired glucose tolerance.116
Disrupted glycaemic control not only affects peripheral organs but also the brain, determined by impaired brain insulin sensitivity.72,86 Moreover, deficits in memory,46 attention and learning117 have been implicated in T2DM. Disrupted glycaemic control is associated with high glucose levels, which induce vasoconstriction and therewith also reduce the blood flow to the brain,118 which may explain the deceased GM and WM volumes observed in diabetes type I and II.119,120 Nonetheless, insulin driven cognitive impairment already arises in pre-diabetes, as pre-diabetic individuals perform poorer on memory and cognitive tasks, show a smaller total brain volume and have reduced WM integrity compared to non-diabetic participants.58,121,122 These memory impairments in pre-diabetics are directly attributed to insulin resistance and not to increased glucose levels,123 suggesting that obesity-related insulin resistance might induce changes in cognition and the brain.
Insulin resistance is associated with increased production of reactive oxygen species (ROS), leading to swelling and conformational changes in the mitochondria.80,86 ROS can trigger mitochondrial membrane permeability and consequently mitochondrial dysfunction. Proteins from the mitochondrial intermembrane space are expulsed and cause apoptosis via various pathways.124 Oxidative stress driven by ROS notably affects the brain as it impairs regeneration and limits the degree of antioxidant products.125 Finally, ROS is a modulator of NF-kB and mitogen-activated protein kinase pathways,126 and can thereby activate the brain’s resident immune cells, the microglia, and increase levels of pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α.125 All these changes alter adenosine triphosphate (ATP) production, which is highly important to maintain neuronal functions.127 In addition, activation of these pathways induces apoptosis and creates a domino effect; ATP is released extracellularly by injured and apoptotic cells,128 which in turn activates microglia and the release of cyclooxygenase-2, TNF-α and IL6, ultimately leading to more neuronal death.129
Roux-en-Y gastric bypass restores fasting insulin levels and improves insulin sensitivity.130 Increased secretion of GLP-1 and gastric inhibitory polypeptide (GIP) are driving forces for insulin release due to their insulinotropic effects.35 It is suggested that bypassing a portion of the small intestine inhibits the production and secretion of GLP-1 and GIP and might reduce insulin resistance. Moreover, it is believed that a longer bypass of the proximal intestine leads to a higher reduction of insulin resistance.131 Therefore, it is assumed that the biliopancreatic diversion is more successful in terms of restoring insulin sensitivity compared to Roux-en-Y gastric bypass. This is independent from weight loss, as the difference in insulin resistance between surgeries was observed in the early post-operative stages.131 Diabetic and non-diabetic patients with obesity confirmed previous results, as they showed increased insulin clearance after bypassing both the duodenum and proximal jejunum versus bypassing solely the duodenum.41 In summary, obesity-induced insulin resistance is associated with (neuro)inflammation, cognitive impairment, albeit BS seems to reverse these negative effects.
Glucagon-Like-Peptide-1
GLP-1 is an incretin hormone, which upon food ingestion, is secreted by L cells in the ileum and by neurons in the nucleus tractus solitarius. GLP-1 has an anorectic effect. It increases insulin synthesis and secretion, and inhibits glucagon, and therewith decreases blood sugar levels and slows down digestion.132 In obesity, the anorectic effect of GLP-1 seems to be reduced, since ob/ob mice show a reduction of GLP-1 receptors in the cortex and hippocampus.75 Additionally, clinical studies also indicated that weight gain promotes functional deficits in GLP-1 signalling, which maintain the obese phenotype.133
Previous research showed that GLP-1 can enhance associative and spatial learning via the GLP-1 receptor (GLP-1R). It was demonstrated that GLP-1R deficient mice show deficits in contextual fear conditioning which restored after hippocampal Glp1r gene transfer.134 Additionally, in rats, intranasal administration of synthetic GLP-1 analogues increased spatial learning capacities as shown in the Morris water maze (MWM) and passive avoidance paradigm.134 Long-term potentiation, a mechanism which strengthens synaptic connections and important for learning and memory135 is increased in obese ob/ob mice after GLP-1 agonist administration compared to controls.78 Moreover, these mice showed improved glycaemic control.78 These findings were supported by HFD induced ob/ob mice which showed decreased body weight, improved performance on the object recognition test and improved non-fasting insulin levels after daily injections of Liraglutide, a GLP-1 agonist.71 Memory improvements were observed in an Alzheimer mouse model after administration of insulin together with exenatide (a GLP-1 agonist), compared to insulin alone, suggesting that the neuroprotective effect of GLP-1 does not depend on insulin.136 In addition, GLP-1 analogue consumption in HFD induced obese mice also increased neurogenesis and proliferation in the hippocampus, a region involved in memory consolidation and storage.79 Thus, GLP-1 plays an important role in cognitive function, suggesting that functional deficits in GLP-1 might commit to obesity related cognitive impairment.
GLP-1 has anti-inflammatory effects as it inhibits the production of cytokines after lipopolysaccharides (LPS) induction.137 In vitro and in vivo studies demonstrate that GLP-1 agonists activate GLP-1R, which promotes pre-adipocytes proliferation and inhibits apoptosis and therewith increase adipocyte formation and improve lipid homeostasis.138 Animal studies also provided evidence for the anti-inflammatory effect of GLP-1. Recombinant adenovirus producing GLP-1 administration in obese ob/ob mice resulted in decreased expression of proinflammatory cytokines such as IL-6, TNF-α and monocyte chemoattractant protein-1 in adipose tissue. Moreover, LPS-induced inflammation and expression of M1 macrophage-specific genes was decreased in these GLP-1 treated ob/ob mice.74 Recently, GLP-1 agonists are being prescribed as medication to lose weight in for example diabetic patients. GLP-1 agonists may exert a positive effect on glucose homeostasis, which help to maintain weight, and directly affect adipocytes.74 Besides, human trials also highlighted the potential of GLP-1 agonists to significantly reduce inflammation.139 These anti-inflammatory effects might also contribute to the neuroprotective effect of GLP-1. It was revealed in an animal study that the proglucagon gene and TNF-α expression levels were reduced in obese ob/ob mice, compared to control mice.75 Moreover, a positive correlation between TNF-α and proglucagon mRNA expression levels was found in control and ob/ob mice. Furthermore, it was stated that activated microglia, induced by LPS, reduced the secretion of GLP-1, while TNF-α and proglucagon levels acutely and transiently increased. This indicates that the acute effect of LPS treatment initially inhibits the GLP-1 secretory mechanism. It was speculated that the correlation of pro-inflammatory cytokines to mRNA expression of a neuroprotective element confers increased protection to neurons whilst fighting off pathogens.75
BS has also been shown to increase GLP-1 levels.59,60 For Roux-en-Y gastric bypass, this elevation in GLP-1 levels remained 10 years after surgery.36 Moreover, GLP-1 was positively correlated with weight loss post-operatively.140 Sleeve gastrectomy also increased GLP-1, albeit levels were markedly lower one year after surgery compared to the Roux-en-Y gastric bypass group.37 In summary, deficits in GLP-1 signalling are linked to obesity, cognitive changes and increased inflammation. Nonetheless, BS can restore GLP-1 levels and thereby improve obesity-related alterations.
Peptide YY
Peptide YY1-36 (PYY) is secreted postprandially from L cells in ileum and colon. It is an anorexigenic hormone and is converted to PYY3-36 in the circulation when cleaved by dipeptidyl-peptidase IV (DPP-IV). Circulating levels of PYY3-36 are more abundant than PYY1-36. PYY3-36 has a high affinity for the Y2 receptor, and this inhibitory G-coupled receptor decreases levels of cyclic-AMP and intracellular calcium.141 It is thought that the anorexigenic effect of PYY3-36 is induced by Y2 receptor stimulation in the hypothalamus, which then increases neuropeptide Y effects and consequently reduces hunger by increasing satiety.44 The ileum and colon have the highest levels of PYY1-36. In subjects with normal weight or obesity, plasma concentrations of PYY were significantly increased after a high-fat meal, suggesting that PYY secretion is primarily determined by the fat content of the meal.34 In obesity, the secretory profile of PYY has been altered. Fasting PYY levels are negatively correlating with BMI and are diminished in participants with obesity.25,27,54 Individuals with obesity showed not only decreased levels of circulating PYY but also lower postprandial secretion of PYY in comparison to healthy individuals.31 Nonetheless, results are contradictory as a large population-based study (2094 participants – 75%female) showed no differences in PYY levels between participants with obesity, overweight and a healthy weight.44 PYY gene expression and subsequent PYY secretion are increased by the histone deacetylase inhibitory activity of short-chain fatty acids (SCFA; eg butyrate and propionate). SCFA is a product of carbohydrate fermentation by the gut microbiota and is thus linked to fiber intake. Therefore, previous results might not depend on obesity per se but relay more the current diet of participants.142 Moreover, it is known that the microbiota composition in obesity is significantly altered compared to lean individuals, which can also impair SCFA expression levels.143 Accordingly, it is suggested that altered SCFA expression levels, as a consequence of lower diet quality and a dysbalanced microbiota, could contribute to differences in PYY levels in obesity and normal weight.
The Y2 receptor is widely expressed, making it possible for PYY to activate neural circuits throughout the brain. Notably, the mesolimbic, nigrostriatal dopaminergic pathways, brainstem and orbitofrontal cortex were activated after peripheral injection of PYY3-36 in healthy participants. Moreover, it was shown that PYY could switch activity in areas predicting calorie intake from a homeostatic (eg hypothalamus) to a more hedonic area (eg orbitofrontal cortex).144 Subjects showed similar BOLD patterns after feeding and after PYY3-36 infusions during the fasted state in brain reward regions such as insula, left nucleus accumbens and left orbitofrontal cortex.145 This widespread activation was also seen in Long Evans rats.146 After peripheral PYY administration, brain activity was detected in the nucleus tractus solitarii, hypothalamic arcuate nucleus, paraventricular nucleus but also in hedonic centres such as the amygdala and the nucleus accumbens.146 PYY also increased novelty seeking behaviours in a dose-dependent manner, determined by the novel object exploration test,147 in animals administered with PYY compared to controls. On the contrary, it has been found that PYY can impair selective associative learning, spatial working memory and goal-directed behaviour in mice, determined by the latent inhibition paradigm and water maze test.148 Another study revealed that increased PYY levels were associated with decreased nesting behaviour and increased food intake, which could be restored by a T2 receptor antagonist.149
PYY is also strongly involved in the immune system, yet its role in inflammation is still uncertain. The PYY promoter gene contains two potential NF-kB binding domains. In vitro stimulation of the NF-kB pathway by TLR agonists lead to an 80 to 100% increased expression of PYY. This was seen after activation of TLR 2 and 6, which have a strong effect on NF-kB.150 Although, TLR 7 and 8 agonists did not elicit an increase in NF-kB activation they still increased PYY expression. PYY derived from PYY3-36, inhibits gut motility by decreasing neuronal activity, while PYY1-36 enhances gut motility by increasing muscle contractions.150 Therefore, increased PYY induced by TLR activity might increase colonic motility, a physical mechanism to eliminate pathogens and restrict nutrient availability in the infected area. Additionally, colitis is negatively associated with PYY plasma levels and deregulated intestinal motility. Moreover, PYY increases the production and degranulation of invariant natural killer T cells, is associated with increased CD4+ T cell activation, and it increases immune activity.63 These results suggest that PYY levels increase as a host response to colonic infection.
Both sleeve gastrectomy and gastric bypass have been shown to increase PYY levels 6 month post-surgery.54 PYY fasting levels were significantly higher after sleeve gastrectomy, whereas such effect was not seen after Roux-en-Y gastric bypass. Both the sleeve gastrectomy and Roux-en-Y gastric bypass improved postprandial levels of PYY.62,67 However, diet-induced weight loss showed no significant changes in PYY levels. Therefore, these improvements may be attributed to a mechanistical effect of the surgery and not the weight loss itself.67
Microbiota and Short Chain Fatty Acids
The gastrointestinal tract is home to a multitude of bacteria, and dysbiosis in the gut microbiota has been associated with obesity.73,88 Notably, HFD induced obese mice showed decreased microbiota diversity, determined by increased Firmicutes and Oscillibacter abundance, decreased levels of Bacteroidetes and Lactobacillus and increased Firmicutes/Bacteroidetes ratio.73,82,151 Similarly in humans, obesity is accompanied by reduced bacterial diversity, increased abundance of potential proinflammatory proteobacteria and decreased Bacteroidetes/Firmicutes ratio.43 Other important features for microbiota diversity are the SCFA, which are the primary metabolites produced in the colon after fermentation of fibers and non-digestible starch by the gut bacteria.152 In particular, acetate and propionate are produced by Bacteroidetes, while butyrate is produced by Firmicutes.152 Previous research shows contradictory results, including positive and negative relationships between SCFA and obesity.153 Martínez-Cuesta et al compared the metabolite production of the microbiota from obese and normal weight participants cultured in high and normal energy medium.66 Here, it was found that the microbiota of obese participants cultured in high energy medium produced more SCFA compared to microbiota from normal weight participants cultured in both mediums. This finding suggests that energy harvesting is optimized in the microbiota of participants with obesity. Moreover, obese ob/ob mice showed an enrichment in pathways encoding polysaccharide digesting enzymes compared to lean mice, supporting the enhanced energy harvest potential of the obese microbiota.69 In comparison, SCFA concentration in the stool of children with obesity, but not overweight, was lower compared to normal weight children.50 Obesity is induced by a low fiber and high fat diet, potentially explaining the observed negative relation between SCFA and obesity. As results are inconsistent, more studies with a larger cohort are needed to define the direction and causality of this relationship, but also to identify other factors involved in SCFA production, excretion and absorption in humans.
Alterations in the microbiota also seem to be associated with mood, cognition and behaviour. In mice, Bacteroidetes were negatively associated with performance in the Y-maze and object recognition test, indicating that Bacteroidetes play a role in reference and working memory.88 It is known that the gut microbiota can directly influence the central nervous system via the kynurenine pathway and as a consequence of tryptophan metabolism. The gut may influence emotional regulation and has been implicated in mood disorders such as anxiety via the connection between the gut and brain.154 A high-fiber diet reduced anxiety in mice, reflected by increased time spent in the open arm and elevated plus‐maze test.90 These mice also showed significantly improved performance on the MWM task. In humans, improved mood was demonstrated after six weeks of prebiotic consumption.155 Additionally, the Scale of Positive and Negative Experience revealed a decrease in negative emotions and enhanced flexibility in subjects with obesity after intake of prebiotics.65 Moreover, improvements in the MWM were linked to a healthier gut microbiota profile and their metabolites, including serotonin, tauroursodeoxycholic acid (TUDCA) and 3-indolepropionic acid (IPA).91 Intermittent fasting in obese diabetic mice (db/db model) was also shown to improve cognition, mitochondrial function, post-synaptic density and insulin sensitivity. Individual metabolite administration revealed that the observed improvements were induced by SCFA, TUDCA, IPA and serotonin.91 Moreover, after antibiotic administration, these positive effects diminished, highlighting the impact of the gut microbiota.
As mentioned before, SCFA are the metabolic products of fiber fermentation by anaerobic bacteria in the gut. They are important fuel for intestinal epithelial cells, but also have to ability to act on different inflammatory cells, including macrophages and neutrophils.156 Butyrate, for example, is able to increase peripheral regulatory T-cells and induces secretion of GLP-1 from intestinal endocrine cells, and thereby decreases inflammation.157 Butyrate can regulate inflammation in the epithelium by increased production of anti-inflammatory cytokines and activation of dendritic cells.158 Butyrate administration in mice can decrease microglial inflammation after HFD-induced obesity, reflected by decreased ionized calcium-binding adapter molecule 1 expression in the thalamus and hippocampus.8 Supplementation of a fiber-rich diet improved cognitive impairment, gut microbiota dysbiosis, endotoxemia and systemic inflammation.92 Moreover, SCFAs might inhibit inflammation in de central nervous system as valproic acid, butyrate, and trichostatin A induced anti-neuroinflammatory and neuroprotective effects in rats with brain ischemia.156 These results indicate the importance of a healthy diet, microbiota and metabolite production in order to regulate inflammatory processes.
The gut microbiota is important to protect the gut mucosal barrier and maintaining a proper barrier function via the production of butyrate159 which is transported into epithelial cytoplasm and used as cellular fuel after β-oxidation inducing epithelial proliferation.158 When this mechanism fails, the gut may become permeable and increase LPS-induced translocation of bacterial metabolites into the circulation.23 Increased LPS activates TLR which further triggers the release of inflammatory cytokines, alteration of white adipose tissue and impairment of insulin sensitivity.160,161 Probiotic administration has shown to reduce LPS inflammation and improve gut barrier function in vitro,162 probably as a consequence of SCFA production, as they are derived from fiber fermentation.142 Participants with overweight and obesity showed decreased plasma LPS, proinflammatory chemokines and white blood cells after three months pasteurised Akkermansia mucinphila supplementation.53 However, supplementation with Bifidobacterium adolescentis and Bifidobacterium lactis did not change lipopolysaccharide-binding protein (LBP) or plasma LPS levels in participants with obesity.51 Synbiotic supplementation of Lacticaseibacillus paracasei, Bifidobacterium breve and galacto-oligosaccharides also did not improve inflammatory markers nor glycaemic control compared to controls.64 In conclusion, the gut microbiota is involved in the maintenance of a healthy gut barrier by the regulation of inflammation, in which every bacteria has its own effect.
BS leads to anatomical changes and thereby alters the passage of nutrients and increases the pH of the digestive tract. This creates a swift from an anaerobic environment towards a more aerobic environment. Therefore, BS will also create a shift in microbial composition.23,163 Every BS procedure will induce different anatomical changes in the digestive tract, and therewith alter the gut microbiota in distinct ways.164 Studies have observed increased levels of Bacteroidetes, Proteobacteria and increased variety of Fusobacteria and the Verrucomibia phyla, with decreasing levels of Firmicutes and Actinobacteria in mice and men after gastric bypass.33,49,77 Akkermansia muciniphila is of particular interest as it is inversely associated with obesity.52 pH changes accompanied by Roux-en-Y gastric bypass also increase levels of Akkermansia muciniphila.33 Moreover, Akkermansia muciniphila is associated with mucin degradation,165 increased GLP-1 levels,85 reversed adipose driven inflammation, insulin sensitivity and fat reduction,76 all contributing to a healthier intestinal barrier function. Nevertheless, the changes in gut microbiota post-BS display large variability between patients, which could also contribute to differences in weight loss.166 It is unclear whether the change in gut microbiota composition after BS is induced by a significant change in diet or reduction in gastric volume.167 In summary, BS induces changes in the gut microbiota composition which vary between patients and surgical procedures. However, increased microbiota diversity after BS might improve adipose tissue, gut barrier integrity, insulin sensitivity and many more.
Adipose Tissue
Adipose tissue is an endocrine organ which recently gained more attention.168 Adipose depots are an intricate mesh, involving adipocytes, preadipocytes, stem cells and immune cells.169 Adipocytes are most abundant in the white adipose tissue (WAT), which constitutes 95% of the humans body fat. Brown adipose tissue (BAT) constitutes 2% and beige adipose tissue constitutes roughly 3% of the humans body fat.169 The main function of WAT is the storage of energy as it maintains high levels of triglycerides. BAT, on the other hand, produces mainly heat, and therewith induces thermogenesis when needed.168 Beige fat can act as WAT or BAT, and depending on the stimulus it can store energy or increase mitochondrial activity to produce heat.170 WAT is an endocrine organ secreting important hormones involved in food regulation, such as leptin and adiponectin. In obesity, secretion of these hormones is dysregulated, with leptin being increased and adiponectin decreased.68 Adipose tissue also secretes serum amyloids A (SAA) and plasminogen activator inhibitor 1 (PAI-1) which inhibits anticlotting factors and is highly associated with thrombosis.171 PAI-1 is associated with visceral fat depots but not with subcutaneous fat in humans.39 In women, WHR is associated with carotid intima-media thickness (CIMT) decrease, indicating that visceral fat is related to atherosclerosis.26 The correlation between CIMT and visceral fat remained significant after BMI and total fat correction. Additionally, a correlation was found between flow mediated dilation (FMD) and abdominal fat size.172 Other pro-inflammatory cytokines, such as SAA and angiotensinogen have been associated with an impaired cardiovascular system, and are thought to be involved in the development of hypertension, atherosclerosis and thrombosis.3,171,173 Altogether, it is clear that increased adiposity, depending on the fat depot, has many consequences for human health.
Evidence revealed that impaired visual recognition memory and memory flexibility were associated with visceral adiposity and HFD-induced weight gain in Black 6 mice.87 In humans, similar results have been found. Elevated adiposity was associated with impaired working memory and cognitive flexibility in young adults.55 Moreover, total fat percentage was negatively associated with visual memory and visuospatial skills in young healthy obese men,47 suggesting that adipose tissue can influence cognitive function.
In obesity, adipocytes become hypertrophic, which changes their secretory profile and induces low-grade inflammation. Inflamed adipocytes secrete various inflammatory cytokines and chemokines such as IL-6 and TNF-α.174 These cytokines can activate and mobilise macrophages, but also recruit dendritic cells and B cells.175 Leptin is also able to increase expression levels of IL-6, IL-1β, and TNF-α in vitro.108 Altogether, this contributes to low-grade systemic inflammation, which is a hallmark of excess fat depots. Cytokines and free fatty acids can translocate through the BBB, stimulating immune cells in the brain, such as microglia, and thereby induce neuroinflammation. Moreover, systemic inflammation alters the BBB integrity, which increases peripheral immune cell infiltration and further exacerbates neuroinflammation.176
As shown by magnetic resonance imaging, reduction in abdominal WAT is mainly responsible for BS-induced weight loss, followed by liver and pancreatic fat, whereas no changes in BAT have been observed.56,57 The significant reduction in visceral adipose depots after BS also partially reversed the prothrombotic state seen in obesity.39 Laparoscopic adjustable gastric banding or gastric bypass improved both functional and structural markers of atherosclerosis, in terms of CIMT and FMD measures. It is speculated that the cardiovascular pathologies are a multifactorial problem, which can be improved by lower levels of visceral fat and subsequent improvements in adipokine and cytokine secretion, instead of weight loss alone.172 After BS, obese sprague Dawley rats showed tissue weight loss and increased adiponectin expression levels, which upregulated the expression of sirtuin 1 and therewith increased WAT browning.89 Serglycin (SRGN) is a dominant proteoglycan in inflammatory cell types that infiltrate WAT in the context of obesity.93 In both mice and humans, SRGN is accompanied by higher expression levels of several inflammatory markers, albeit it is demonstrated that fat loss after BS can reduce mRNA expression levels of SRGN and inflammatory genes (including macrophage markers).93 Furthermore, Srgn-/- mice demonstrated decreased levels of proinflammatory M1 macrophages and crown-like-structures, a hallmark of adipocyte driven inflammation, compared to Srgn +/+ mice after sugar and fat-induced obesity.93 These findings suggest that SRGN is associated with adipose tissue accumulating immune cell populations under obese conditions, which can be restored after BS. Finally, lower inflammatory makers (eg CRP, SAA, TNF-α, IL-1β), lower leptin and increased adiponectin levels were observed in humans 6 months after Roux-en-Y gastric bypass.11 In summary, these results indicate that BS can restore adipose tissue functionality and thereby might improve obesity-related comorbidities.
Despite all positive effects of BS discussed in this review, one should also be aware of the negative consequences. BS can induce post-operative gastrointestinal complications and thereby reduce the quality of life, but also vitamin and nutritional deficiencies have been observed in post-BS individuals.177,178
Conclusion
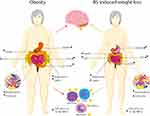
A growing body of evidence reveals that obesity is related with alterations in neuroendocrine production and secretion, including ghrelin, insulin, GLP-1 and PYY (Figure 1). These alterations increase food intake and reduce insulin sensitivity, leading to increased adiposity and the development of T2DM. Obesity is also associated with a dysbalanced gut microbiota and consequently with an impaired metabolite profile, which can increase gut barrier permeability and low-grade systemic inflammation. Finally, obesity is linked to dysfunctional WAT, leading to changes in adipokine and cytokine secretion profiles. The aforementioned effects can alter human health in distinct ways, and through direct or indirect pathways can promote the development of obesity-related comorbidities as well as cognitive impairment. Luckily, BS is an effective treatment for obesity. It decreases body weight not only due to physical effects such as reduced food intake and malabsorption but also due to the various neuroendocrine changes which affect energy homeostasis and hunger/satiety. Moreover, BS might improve the gut microbiota diversity and restore WAT function, which can improve obesity-related immunological and cognitive impairments. However, future research should focus on the long-term effects of BS, to be able to investigate the neuroendocrine, microbiota and WAT changes and to potentially determine the new “normal” after homeostatic adjustments. Various studies have focused on neuroendocrine alterations already after six months. Six months post-surgery patients lose weight rapidly and generally still follow their post-operative diet. Therefore, the observed effects 6 months post-surgery might differ at longer follow-ups, when patients achieve a stable weight, or regain weight. To summarize, BS is a good procedure to treat obesity and its related pathologies, however long-term effects remain unsolved. Future studies should focus on long-term effects after BS and try to determine potential factors (eg gut microbiota and hormones) that are involved in successful weight loss after surgery.
Funding
No funding was received to assist with the preparation of this manuscript.
Disclosure
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
1. Fruh SM. Obesity: risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract. 2017;29(S1):S3–S14. doi:10.1002/2327-6924.12510
2. World Health Organization. Fact sheet: obesity and overweight; 2021.
3. Nota MHC, Vreeken D, Wiesmann M, Aarts EO, Hazebroek EJ, Kiliaan AJ. Obesity affects brain structure and function- rescue by bariatric surgery? Neurosci Biobehav Rev. 2020;108:646–657. doi:10.1016/j.neubiorev.2019.11.025
4. Dekkers IA, Jansen PR, Lamb HJ. Obesity, brain volume, and white matter microstructure at MRI: a cross-sectional UK Biobank Study. Radiology. 2019;291(3):763–771. doi:10.1148/radiol.2019181012
5. Verstynen TD, Weinstein AM, Schneider WW, Jakicic JM, Rofey DL, Erickson KI. Increased body mass index is associated with a global and distributed decrease in white matter microstructural integrity. Psychosom Med. 2012;74(7):682–690. doi:10.1097/PSY.0b013e318261909c
6. Lampe L, Zhang R, Beyer F, et al. Visceral obesity relates to deep white matter hyperintensities via inflammation. Ann Neurol. 2019;85(2):194–203. doi:10.1002/ana.25396
7. Shaw ME, Sachdev PS, Abhayaratna W, Anstey KJ, Cherbuin N. Body mass index is associated with cortical thinning with different patterns in mid- and late-life. Int J Obesity. 2018;42(3):455–461. doi:10.1038/ijo.2017.254
8. Arnoldussen IAC, Wiesmann M, Pelgrim CE, et al. Butyrate restores HFD-induced adaptations in brain function and metabolism in mid-adult obese mice. Int J Obes. 2017;41(6):935–944. doi:10.1038/ijo.2017.52
9. Forny-Germano L, De Felice FG, Vieira M. The role of leptin and adiponectin in obesity-associated cognitive decline and Alzheimer’s disease. Front Neurosci. 2018;12:1027. doi:10.3389/fnins.2018.01027
10. Dionysopoulou S, Charmandari E, Bargiota A, Vlahos N, Mastorakos G, Valsamakis G. The role of hypothalamic inflammation in diet-induced obesity and its association with cognitive and mood disorders. Nutrients. 2021;13(2):498. doi:10.3390/nu13020498
11. Vreeken D, Seidel F, Custers EM, et al. Factors associated with cognitive improvement after bariatric surgery among patients with severe obesity in the Netherlands. JAMA Netw Open. 2023;6(5):e2315936. doi:10.1001/jamanetworkopen.2023.15936
12. Brinkworth GD, Buckley JD, Noakes M, Clifton PM, Wilson CJ. Long-term effects of a very low-carbohydrate diet and a low-fat diet on mood and cognitive function. Arch Intern Med. 2009;169(20):1873–1880. doi:10.1001/archinternmed.2009.329
13. Horie NC, Serrao VT, Simon SS, et al. Cognitive effects of intentional weight loss in elderly obese individuals with mild cognitive impairment. J Clin Endocrinol Metab. 2016;101(3):1104–1112. doi:10.1210/jc.2015-2315
14. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. doi:10.1016/S0140-6736(15)60461-5
15. Varkevisser RDM, van Stralen MM, Kroeze W, Ket JCF, Steenhuis IHM. Determinants of weight loss maintenance: a systematic review. Obes Rev. 2019;20(2):171–211. doi:10.1111/obr.12772
16. Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324(9):879–887. doi:10.1001/jama.2020.12567
17. Nuzzo A, Czernichow S, Hertig A, et al. Prevention and treatment of nutritional complications after bariatric surgery. Lancet Gastroenterol Hepatol. 2021;6(3):238–251. doi:10.1016/S2468-1253(20)30331-9
18. Courcoulas AP, Yanovski SZ, Bonds D, et al. Long-term outcomes of bariatric surgery: a National Institutes of Health symposium. JAMA Surg. 2014;149(12):1323–1329. doi:10.1001/jamasurg.2014.2440
19. Cummings DE, Cohen RV. Bariatric/metabolic surgery to treat type 2 diabetes in patients with a BMI <35 kg/m2. Diabetes Care. 2016;39(6):924–933. doi:10.2337/dc16-0350
20. Schauer DP, Feigelson HS, Koebnick C, et al. Association between weight loss and the risk of cancer after bariatric surgery. Obesity. 2017;25(Suppl 2):S52–S57. doi:10.1002/oby.22002
21. Morris A. Life expectancy: benefits of bariatric surgery clarified. Nat Rev Endocrinol. 2021;17(1):4–5. doi:10.1038/s41574-020-00440-7
22. Syn NL, Cummings DE, Wang LZ, et al. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet. 2021;397(10287):1830–1841. doi:10.1016/S0140-6736(21)00591-2
23. Martinou E, Stefanova I, Iosif E, Angelidi AM. Neurohormonal changes in the gut-brain axis and underlying neuroendocrine mechanisms following bariatric surgery. Int J Mol Sci. 2022;23(6):3339. doi:10.3390/ijms23063339
24. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. doi:10.1152/physrev.00018.2018
25. Alvarez Bartolomé M, Borque M, Martinez-Sarmiento J, et al. Peptide YY secretion in morbidly obese patients before and after vertical banded gastroplasty. Obes Surg. 2002;12(3):324–327. doi:10.1381/096089202321088084
26. De Michele M, Panico S, Iannuzzi A, et al. Association of obesity and central fat distribution with carotid artery wall thickening in middle-aged women. Stroke. 2002;33(12):2923–2928. doi:10.1161/01.STR.0000038989.90931.BE
27. Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349(10):941–948. doi:10.1056/NEJMoa030204
28. Frühbeck G, Diez-Caballero A, Gil MJ, et al. The decrease in plasma ghrelin concentrations following bariatric surgery depends on the functional integrity of the fundus. Obes Surg. 2004;14(5):606–612. doi:10.1381/096089204323093363
29. Stoeckli R, Chanda R, Langer I, Keller U. Changes of body weight and plasma ghrelin levels after gastric banding and gastric bypass. Obes Res. 2004;12(2):346–350. doi:10.1038/oby.2004.43
30. Garcia-Fuentes E, Garrido-Sanchez L, Garcia-Almeida JM, et al. Different effect of laparoscopic Roux-en-Y gastric bypass and open biliopancreatic diversion of Scopinaro on serum PYY and ghrelin levels. Obes Surg. 2008;18(11):1424–1429. doi:10.1007/s11695-008-9560-5
31. Batterham RL, Heffron H, Kapoor S, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4(3):223–233. doi:10.1016/j.cmet.2006.08.001
32. Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247(3):401–407. doi:10.1097/SLA.0b013e318156f012
33. Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365–2370. doi:10.1073/pnas.0812600106
34. Rigamonti AE, Resnik M, Compri E, et al. The cholestyramine-induced decrease of PYY postprandial response is negatively correlated with fat mass in obese women. Horm Metab Res. 2011;43(8):569–573. doi:10.1055/s-0031-1280783
35. Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes. 2011;60(9):2308–2314. doi:10.2337/db11-0203
36. Dar MS, Chapman WH, Pender JR, et al. GLP-1 response to a mixed meal: what happens 10 years after Roux-en-Y gastric bypass (RYGB)? Obes Surg. 2012;22(7):1077–1083. doi:10.1007/s11695-012-0624-1
37. Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22(5):740–748. doi:10.1007/s11695-012-0622-3
38. Ramón JM, Salvans S, Crous X, et al. Effect of Roux-en-Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J Gastrointest Surg. 2012;16(6):1116–1122. doi:10.1007/s11605-012-1855-0
39. Tschoner A, Sturm W, Engl J, et al. Plasminogen activator inhibitor 1 and visceral obesity during pronounced weight loss after bariatric surgery. Nutr, Metab Cardiovasc Dis. 2012;22(4):340–346. doi:10.1016/j.numecd.2010.07.009
40. Barazzoni R, Zanetti M, Nagliati C, et al. Gastric bypass does not normalize obesity-related changes in ghrelin profile and leads to higher acylated ghrelin fraction. Obesity. 2013;21(4):718–722. doi:10.1002/oby.20272
41. Salinari S, Bertuzzi A, Guidone C, Previti E, Rubino F, Mingrone G. Insulin sensitivity and secretion changes after gastric bypass in normotolerant and diabetic obese subjects. Ann Surg. 2013;257(3):462–468. doi:10.1097/SLA.0b013e318269cf5c
42. Tsoli M, Chronaiou A, Kehagias I, Kalfarentzos F, Alexandrides TK. Hormone changes and diabetes resolution after biliopancreatic diversion and laparoscopic sleeve gastrectomy: a comparative prospective study. Surg Obes Relat Dis. 2013;9(5):667–677. doi:10.1016/j.soard.2012.12.006
43. Verdam FJ, Fuentes S, de Jonge C, et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity. 2013;21(12):E607–E15. doi:10.1002/oby.20466
44. Cahill F, Ji Y, Wadden D, et al. The Association of Serum Total Peptide YY (PYY) with obesity and body fat measures in the CODING Study. PLoS One. 2014;9(4):e95235. doi:10.1371/journal.pone.0095235
45. Yousseif A, Emmanuel J, Karra E, et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obes Surg. 2014;24(2):241–252. doi:10.1007/s11695-013-1066-0
46. Cheke LG, Bonnici HM, Clayton NS, Simons JS. Obesity and insulin resistance are associated with reduced activity in core memory regions of the brain. Neuropsychologia. 2017;96:137–149. doi:10.1016/j.neuropsychologia.2017.01.013
47. Bove RM, Gerweck AV, Mancuso SM, Bredella MA, Sherman JC, Miller KK. Association between adiposity and cognitive function in young men: hormonal mechanisms. Obesity. 2016;24(4):954–961. doi:10.1002/oby.21415
48. Kruljac I, Mirošević G, Kirigin LS, et al. Changes in metabolic hormones after bariatric surgery and their predictive impact on weight loss. Clin Endocrinol. 2016;85(6):852–860. doi:10.1111/cen.13160
49. Palleja A, Kashani A, Allin KH, et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016;8(1):67. doi:10.1186/s13073-016-0312-1
50. Barczynska R, Litwin M, Slizewska K, et al. Bacterial microbiota and fatty acids in the faeces of overweight and obese children. Pol J Microbiol. 2018;67(3):339–345. doi:10.21307/pjm-2018-041
51. Krumbeck JA, Rasmussen HE, Hutkins RW, et al. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome. 2018;6(1):121. doi:10.1186/s40168-018-0494-4
52. Dao MC, Belda E, Prifti E, et al. Akkermansia muciniphila abundance is lower in severe obesity, but its increased level after bariatric surgery is not associated with metabolic health improvement. Am J Physiol Endocrinol Metab. 2019;317(3):E446–E459. doi:10.1152/ajpendo.00140.2019
53. Depommier C, Everard A, Druart C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096–1103. doi:10.1038/s41591-019-0495-2
54. Guida C, Stephen SD, Watson M, et al. PYY plays a key role in the resolution of diabetes following bariatric surgery in humans. EBioMedicine. 2019;40:67–76. doi:10.1016/j.ebiom.2018.12.040
55. Huang T, Chen Z, Shen L, Fan X, Wang K. Associations of cognitive function with BMI, body fat mass and visceral fat in young adulthood. Medicina. 2019;55(6):221. doi:10.3390/medicina55060221
56. Hui SCN, Wong SKH, Ai Q, Yeung DKW, Ng EK, Chu WCW. Observed changes in brown, white, hepatic and pancreatic fat after bariatric surgery: evaluation with MRI. Eur Radiol. 2019;29(2):849–856. doi:10.1007/s00330-018-5611-z
57. Maïmoun L, Lefebvre P, Aouinti S, Picot MC, Mariano-Goulart D, Nocca D. Acute and longer-term body composition changes after bariatric surgery. Surg Obes Relat Dis. 2019;15(11):1965–1973.
58. Marseglia A, Fratiglioni L, Kalpouzos G, Wang R, Bäckman L, Xu W. Prediabetes and diabetes accelerate cognitive decline and predict microvascular lesions: a population-based cohort study. Alzheimers Dement. 2019;15(1):25–33. doi:10.1016/j.jalz.2018.06.3060
59. Perakakis N, Kokkinos A, Peradze N, et al. Circulating levels of gastrointestinal hormones in response to the most common types of bariatric surgery and predictive value for weight loss over one year: evidence from two independent trials. Metabolism. 2019;101:153997. doi:10.1016/j.metabol.2019.153997
60. Svane MS, Bojsen-Møller KN, Martinussen C, et al. Postprandial nutrient handling and gastrointestinal hormone secretion after Roux-en-Y gastric bypass vs sleeve gastrectomy. Gastroenterology. 2019;156(6):1627–41.e1. doi:10.1053/j.gastro.2019.01.262
61. Tsouristakis AI, Febres G, McMahon DJ, et al. Long-term modulation of appetitive hormones and sweet cravings after adjustable gastric banding and Roux-en-Y gastric bypass. Obes Surg. 2019;29(11):3698–3705. doi:10.1007/s11695-019-04111-z
62. Lopez-Nava G, Negi A, Bautista-Castaño I, Rubio MA, Asokkumar R. Gut and metabolic hormones changes after Endoscopic Sleeve Gastroplasty (ESG) vs Laparoscopic Sleeve Gastrectomy (LSG). Obes Surg. 2020;30(7):2642–2651. doi:10.1007/s11695-020-04541-0
63. Han K, Singh K, Rodman MJ, et al. Identification and validation of nutrient state-dependent serum protein mediators of human CD4(+) T cell responsiveness. Nutrients. 2021;13(5):1492. doi:10.3390/nu13051492
64. Kanazawa A, Aida M, Yoshida Y, et al. Effects of synbiotic supplementation on chronic inflammation and the gut microbiota in obese patients with type 2 diabetes mellitus: a randomized controlled study. Nutrients. 2021;13(2):558. doi:10.3390/nu13020558
65. Leyrolle Q, Cserjesi R, Dghm M, et al. Prebiotic effect on mood in obese patients is determined by the initial gut microbiota composition: a randomized, controlled trial. Brain Behav Immun. 2021;94:289–298. doi:10.1016/j.bbi.2021.01.014
66. Martínez-Cuesta MC, Del Campo R, Garriga-García M, Peláez C, Requena T. Taxonomic characterization and short-chain fatty acids production of the obese microbiota. Front Cell Infect Microbiol. 2021;11:598093. doi:10.3389/fcimb.2021.598093
67. Agarwal K, Maki KA, Vizioli C, et al. The neuro-endo-microbio-ome study: a pilot study of neurobiological alterations pre- versus post-bariatric surgery. Biol. Res. Nurs. 2022;24(3):362–378. doi:10.1177/10998004221085976
68. Lejawa M, Osadnik K, Czuba Z, Osadnik T, Pawlas N. Association of metabolically healthy and unhealthy obesity phenotype with markers related to obesity, diabetes among young, healthy adult men. Analysis of MAGNETIC Study. Life. 2021;11(12):1350. doi:10.3390/life11121350
69. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi:10.1038/nature05414
70. Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 2010;151(10):4745–4755. doi:10.1210/en.2010-0556
71. Porter DW, Kerr BD, Flatt PR, Holscher C, Gault VA. Four weeks administration of Liraglutide improves memory and learning as well as glycaemic control in mice with high fat dietary-induced obesity and insulin resistance. Diabetes Obes Metab. 2010;12(10):891–899. doi:10.1111/j.1463-1326.2010.01259.x
72. Pratchayasakul W, Kerdphoo S, Petsophonsakul P, Pongchaidecha A, Chattipakorn N, Chattipakorn SC. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci. 2011;88(13–14):619–627. doi:10.1016/j.lfs.2011.02.003
73. Lam YY, Ha CW, Campbell CR, et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7(3):e34233. doi:10.1371/journal.pone.0034233
74. Lee YS, Park MS, Choung JS, et al. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia. 2012;55(9):2456–2468. doi:10.1007/s00125-012-2592-3
75. Kappe C, Tracy LM, Patrone C, Iverfeldt K, Sjöholm Å. GLP-1 secretion by microglial cells and decreased CNS expression in obesity. J Neuroinflammation. 2012;9(1):276. doi:10.1186/1742-2094-9-276
76. Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–9071. doi:10.1073/pnas.1219451110
77. Liou AP, Paziuk M, Luevano JM, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5(178):178ra41. doi:10.1126/scitranslmed.3005687
78. Porter WD, Flatt PR, Hölscher C, Gault VA. Liraglutide improves hippocampal synaptic plasticity associated with increased expression of Mash1 in ob/ob mice. Int J Obes. 2013;37(5):678–684. doi:10.1038/ijo.2012.91
79. Lennox R, Porter DW, Flatt PR, Holscher C, Irwin N, Gault VA. Comparison of the independent and combined effects of sub-chronic therapy with metformin and a stable GLP-1 receptor agonist on cognitive function, hippocampal synaptic plasticity and metabolic control in high-fat fed mice. Neuropharmacology. 2014;86:22–30. doi:10.1016/j.neuropharm.2014.06.026
80. Pintana H, Sripetchwandee J, Supakul L, Apaijai N, Chattipakorn N, Chattipakorn S. Garlic extract attenuates brain mitochondrial dysfunction and cognitive deficit in obese-insulin resistant rats. Appl Physiol Nutr Metab. 2014;39(12):1373–1379. doi:10.1139/apnm-2014-0255
81. Uchida A, Zechner JF, Mani BK, Park WM, Aguirre V, Zigman JM. Altered ghrelin secretion in mice in response to diet-induced obesity and Roux-en-Y gastric bypass. Mol Metab. 2014;3(7):717–730. doi:10.1016/j.molmet.2014.07.009
82. Hamilton MK, Boudry G, Lemay DG, Raybould HE. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am J Physiol Gastrointest Liver Physiol. 2015;308(10):G840–G851. doi:10.1152/ajpgi.00029.2015
83. Naznin F, Toshinai K, Waise TM, et al. Diet-induced obesity causes peripheral and central ghrelin resistance by promoting inflammation. J Endocrinol. 2015;226(1):81–92. doi:10.1530/JOE-15-0139
84. Waise TMZ, Toshinai K, Naznin F, et al. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem Biophys Res Commun. 2015;464(4):1157–1162. doi:10.1016/j.bbrc.2015.07.097
85. Yan M, Song MM, Bai RX, Cheng S, Yan WM. Effect of Roux-en-Y gastric bypass surgery on intestinal Akkermansia muciniphila. World J Gastrointest Surg. 2016;8(4):301–307. doi:10.4240/wjgs.v8.i4.301
86. Sa-Nguanmoo P, Tanajak P, Kerdphoo S, et al. SGLT2-inhibitor and DPP-4 inhibitor improve brain function via attenuating mitochondrial dysfunction, insulin resistance, inflammation, and apoptosis in HFD-induced obese rats. Toxicol Appl Pharmacol. 2017;333:43–50. doi:10.1016/j.taap.2017.08.005
87. Pétrault O, Pétrault M, Ouk T, Bordet R, Bérézowski V, Bastide M. Visceral adiposity links cerebrovascular dysfunction to cognitive impairment in middle-aged mice. Neurobiol Dis. 2019;130:104536. doi:10.1016/j.nbd.2019.104536
88. Zhang P, Yu Y, Qin Y, et al. Alterations to the microbiota-colon-brain axis in high-fat-diet-induced obese mice compared to diet-resistant mice. J Nutr Biochem. 2019;65:54–65. doi:10.1016/j.jnutbio.2018.08.016
89. Liu L, Zhang T, Hu J, et al. Adiponectin/SIRT1 axis induces white adipose browning after vertical sleeve gastrectomy of obese rats with type 2 diabetes. Obes Surg. 2020;30(4):1392–1403. doi:10.1007/s11695-019-04295-4
90. Liu Z, Li L, Ma S, et al. High-dietary fiber intake alleviates antenatal obesity-induced postpartum depression: roles of gut microbiota and microbial metabolite short-chain fatty acid involved. J Agric Food Chem. 2020;68(47):13697–13710. doi:10.1021/acs.jafc.0c04290
91. Liu Z, Dai X, Zhang H, et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun. 2020;11(1):855. doi:10.1038/s41467-020-14676-4
92. Shi H, Wang Q, Zheng M, et al. Supplement of microbiota-accessible carbohydrates prevents neuroinflammation and cognitive decline by improving the gut microbiota-brain axis in diet-induced obese mice. J Neuroinflammation. 2020;17(1):77. doi:10.1186/s12974-020-01760-1
93. Doncheva AI, Norheim FA, Hjorth M, et al. Serglycin is involved in adipose tissue inflammation in obesity. J Immunol. 2022;208(1):121–132. doi:10.4049/jimmunol.2100231
94. Howard AD, Feighner SD, Cully DF, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273(5277):974–977. doi:10.1126/science.273.5277.974
95. Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707–709. doi:10.2337/diabetes.50.4.707
96. Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci U S A. 2004;101(13):4679–4684. doi:10.1073/pnas.0305930101
97. Mani BK, Walker AK, Lopez Soto EJ, et al. Neuroanatomical characterization of a growth hormone secretagogue receptor-green fluorescent protein reporter mouse. J Comp Neurol. 2014;522(16):3644–3666. doi:10.1002/cne.23627
98. Date Y. Ghrelin and the vagus nerve. Methods Enzymol. 2012;514:261–269.
99. Banks WA, Burney BO, Robinson SM. Effects of triglycerides, obesity, and starvation on ghrelin transport across the blood-brain barrier. Peptides. 2008;29(11):2061–2065. doi:10.1016/j.peptides.2008.07.001
100. Gardiner JV, Campbell D, Patterson M, et al. The hyperphagic effect of ghrelin is inhibited in mice by a diet high in fat. Gastroenterology. 2010;138(7):2468–76, 76.e1.
101. Perreault M, Istrate N, Wang L, Nichols AJ, Tozzo E, Stricker-Krongrad A. Resistance to the orexigenic effect of ghrelin in dietary-induced obesity in mice: reversal upon weight loss. Int J Obes Relat Metab Disord. 2004;28(7):879–885. doi:10.1038/sj.ijo.0802640
102. English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JP. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab. 2002;87(6):2984. doi:10.1210/jcem.87.6.8738
103. Seminara RS, Jeet C, Biswas S, et al. The neurocognitive effects of ghrelin-induced signaling on the hippocampus: a promising approach to Alzheimer’s disease. Cureus. 2018;10(9):e3285.
104. Perea Vega ML, Sanchez MS, Fernández G, Paglini MG, Martin M, de Barioglio SR. Ghrelin treatment leads to dendritic spine remodeling in hippocampal neurons and increases the expression of specific BDNF-mRNA species. Neurobiol Learn Mem. 2021;179:107409. doi:10.1016/j.nlm.2021.107409
105. Edwards CM, Dolezel T, Rinaman L. Ghrelin receptor signaling contributes to fasting-induced suppression of conditioned avoidance behavior and neural circuit activation in male rats. bioRxiv. 2022. doi:10.1101/2022.02.11.480168
106. Spitznagel MB, Benitez A, Updegraff J, et al. Serum ghrelin is inversely associated with cognitive function in a sample of non-demented elderly. Psychiat Clin Neuros. 2010;64(6):608–611. doi:10.1111/j.1440-1819.2010.02145.x
107. Tesauro M, Schinzari F, Iantorno M, et al. Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation. 2005;112(19):2986–2992. doi:10.1161/CIRCULATIONAHA.105.553883
108. Dixit VD, Schaffer EM, Pyle RS, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114(1):57–66. doi:10.1172/JCI200421134
109. Naznin F, Toshinai K, Waise TMZ, Okada T, Sakoda H, Nakazato M. Restoration of metabolic inflammation-related ghrelin resistance by weight loss. J Mol Endocrinol. 2018;60(2):109–118. doi:10.1530/JME-17-0192
110. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–1719. doi:10.2337/diabetes.50.8.1714
111. García-Unzueta MT, Fernández-Santiago R, Domínguez-Díez A, Vazquez-Salví L, Fernández-Escalante JC, Amado JA. Fasting plasma ghrelin levels increase progressively after biliopancreatic diversion: one-year follow-up. Obes Surg. 2005;15(2):187–190. doi:10.1381/0960892053268453
112. Ojha A, Ojha U, Mohammed R, Chandrashekar A, Ojha H. Current perspective on the role of insulin and glucagon in the pathogenesis and treatment of type 2 diabetes mellitus. Clin Pharmacol. 2019;11:57–65. doi:10.2147/CPAA.S202614
113. Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2013;9(1):25–53. doi:10.2174/157339913804143225
114. Erichsen JM, Fadel JR, Reagan LP. Peripheral versus central insulin and leptin resistance: role in metabolic disorders, cognition, and neuropsychiatric diseases. Neuropharmacology. 2022;203:108877. doi:10.1016/j.neuropharm.2021.108877
115. Sripetchwandee J, Chattipakorn N, Chattipakorn SC. Links between obesity-induced brain insulin resistance, brain mitochondrial dysfunction, and dementia. Front Endocrinol. 2018;9:496. doi:10.3389/fendo.2018.00496
116. Garber AJ. Incretin effects on β-cell function, replication, and mass: the human perspective. Diabetes Care. 2011;34(Suppl 2):S258–S263. doi:10.2337/dc11-s230
117. Palta P, Schneider AL, Biessels GJ, Touradji P, Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J Int Neuropsychol Soc. 2014;20(3):278–291. doi:10.1017/S1355617713001483
118. Sivitz WI, Wayson SM, Bayless ML, Sinkey CA, Haynes WG. Obesity impairs vascular relaxation in human subjects: hyperglycemia exaggerates adrenergic vasoconstriction arterial dysfunction in obesity and diabetes. J Diabetes Complicat. 2007;21(3):149–157. doi:10.1016/j.jdiacomp.2005.12.003
119. Hughes TM, Ryan CM, Aizenstein HJ, et al. Frontal gray matter atrophy in middle aged adults with type 1 diabetes is independent of cardiovascular risk factors and diabetes complications. J Diabetes Complicat. 2013;27(6):558–564. doi:10.1016/j.jdiacomp.2013.07.001
120. Hsu JL, Chen YL, Leu JG, et al. Microstructural white matter abnormalities in type 2 diabetes mellitus: a diffusion tensor imaging study. Neuroimage. 2012;59(2):1098–1105. doi:10.1016/j.neuroimage.2011.09.041
121. Ennis GE, Saelzler U, Umpierrez GE, Moffat SD. Prediabetes and working memory in older adults. Brain Neurosci Adv. 2020;4:2398212820961725. doi:10.1177/2398212820961725
122. Liang M, Cai X, Tang Y, et al. Diffusion tensor imaging of white matter in patients with prediabetes by trace-based spatial statistics. J Magn Reson Imaging. 2019;49(4):1105–1112. doi:10.1002/jmri.26290
123. Willmann C, Brockmann K, Wagner R, et al. Insulin sensitivity predicts cognitive decline in individuals with prediabetes. BMJ Open Diabetes Res Care. 2020;8(2):e001741. doi:10.1136/bmjdrc-2020-001741
124. Galluzzi L, Blomgren K, Kroemer G. Mitochondrial membrane permeabilization in neuronal injury. Nat Rev Neurosci. 2009;10(7):481–494. doi:10.1038/nrn2665
125. Simpson DSA, Oliver PL. ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants. 2020;9(8):743. doi:10.3390/antiox9080743
126. Zhang J, Wang X, Vikash V, et al. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 2016;2016:4350965. doi:10.1155/2016/4350965
127. van Horssen J, van Schaik P, Witte M. Inflammation and mitochondrial dysfunction: a vicious circle in neurodegenerative disorders? Neurosci Lett. 2019;710:132931. doi:10.1016/j.neulet.2017.06.050
128. Volonté C, Amadio S, Cavaliere F, D’Ambrosi N, Vacca F, Bernardi G. Extracellular ATP and neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2003;2(6):403–412. doi:10.2174/1568007033482643
129. Xu P, Xu Y, Hu B, et al. Extracellular ATP enhances radiation-induced brain injury through microglial activation and paracrine signaling via P2X7 receptor. Brain Behav Immun. 2015;50:87–100. doi:10.1016/j.bbi.2015.06.020
130. Stenberg E, Thorell A. Insulin resistance in bariatric surgery. Curr Opin Clin Nutr Metab Care. 2020;23(4):255–261. doi:10.1097/MCO.0000000000000657
131. Mingrone G, Cummings DE. Changes of insulin sensitivity and secretion after bariatric/metabolic surgery. Surg Obes Relat Dis. 2016;12(6):1199–1205. doi:10.1016/j.soard.2016.05.013
132. Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today. 2016;21(5):802–818. doi:10.1016/j.drudis.2016.01.013
133. Anandhakrishnan A, Korbonits M. Glucagon-like peptide 1 in the pathophysiology and pharmacotherapy of clinical obesity. World J Diabetes. 2016;7(20):572–598. doi:10.4239/wjd.v7.i20.572
134. During MJ, Cao L, Zuzga DS, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9(9):1173–1179. doi:10.1038/nm919
135. Martinez JL, Derrick BE. Long-term potentiation and learning. Annu Rev Psychol. 1996;47(1):173–203. doi:10.1146/annurev.psych.47.1.173
136. Robinson A, Lubitz I, Atrakchi-Baranes D, et al. Combination of insulin with a GLP1 agonist is associated with better memory and normal expression of insulin receptor pathway genes in a mouse model of Alzheimer’s disease. J Mol Neurosci. 2019;67(4):504–510. doi:10.1007/s12031-019-1257-9
137. Iwai T, Ito S, Tanimitsu K, Udagawa S, Oka J. Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci Res. 2006;55(4):352–360. doi:10.1016/j.neures.2006.04.008
138. Challa TD, Beaton N, Arnold M, Rudofsky G, Langhans W, Wolfrum C. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J Biol Chem. 2012;287(9):6421–6430. doi:10.1074/jbc.M111.310342
139. Bendotti G, Montefusco L, Lunati ME, et al. The anti-inflammatory and immunological properties of GLP-1 receptor agonists. Pharmacol Res. 2022;182:106320. doi:10.1016/j.phrs.2022.106320
140. Dirksen C, Jørgensen NB, Bojsen-Møller KN, et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int J Obes. 2013;37(11):1452–1459. doi:10.1038/ijo.2013.15
141. Stadlbauer U, Woods SC, Langhans W, Meyer U. PYY3-36: beyond food intake. Front Neuroendocrinol. 2015;38:1–11. doi:10.1016/j.yfrne.2014.12.003
142. Larraufie P, Martin-Gallausiaux C, Lapaque N, et al. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. 2018;8(1):74. doi:10.1038/s41598-017-18259-0
143. Liu BN, Liu XT, Liang ZH, Wang JH. Gut microbiota in obesity. World J Gastroenterol. 2021;27(25):3837–3850. doi:10.3748/wjg.v27.i25.3837
144. Batterham RL, Ffytche DH, Rosenthal JM, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450(7166):106–109. doi:10.1038/nature06212
145. De Silva A, Salem V, Long CJ, et al. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011;14(5):700–706. doi:10.1016/j.cmet.2011.09.010
146. Stadlbauer U, Arnold M, Weber E, Langhans W. Possible mechanisms of circulating PYY-induced satiation in male rats. Endocrinology. 2013;154(1):193–204. doi:10.1210/en.2012-1956
147. Stadlbauer U, Weber E, Langhans W, Meyer U. The Y2 receptor agonist PYY(3-36) increases the behavioural response to novelty and acute dopaminergic drug challenge in mice. Int J Neuropsychopharmacol. 2014;17(3):407–419. doi:10.1017/S1461145713001223
148. Stadlbauer U, Langhans W, Meyer U. Administration of the Y2 receptor agonist PYY3-36 in mice induces multiple behavioral changes relevant to schizophrenia. Neuropsychopharmacology. 2013;38(12):2446–2455. doi:10.1038/npp.2013.146
149. Yamada C, Mogami S, Kanno H, Hattori T. Peptide YY causes apathy-like behavior via the dopamine D2 receptor in repeated water-immersed mice. Mol Neurobiol. 2018;55(9):7555–7566. doi:10.1007/s12035-018-0931-1
150. Larraufie P, Doré J, Lapaque N, Blottière HM. TLR ligands and butyrate increase Pyy expression through two distinct but inter-regulated pathways. Cell Microbiol. 2017;19(2):e12648. doi:10.1111/cmi.12648
151. Olsthoorn L, Vreeken D, Kiliaan AJ. Gut microbiome, inflammation, and cerebrovascular function: link between obesity and cognition. Front Neurosci Switz. 2021;15. doi:10.3389/fnins.2021.761456
152. Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol. 2020;11:25. doi:10.3389/fendo.2020.00025
153. Kim KN, Yao Y, Ju SY. Short Chain fatty acids and fecal microbiota abundance in humans with obesity: a systematic review and meta-analysis. Nutrients. 2019;11(10):2512. doi:10.3390/nu11102512
154. Evrensel A, Ünsalver B, Ceylan ME. Neuroinflammation, gut-brain axis and depression. Psychiatry Investig. 2020;17(1):2–8. doi:10.30773/pi.2019.08.09
155. Marotta A, Sarno E, Del Casale A, et al. Effects of Probiotics on cognitive reactivity, mood, and sleep quality. Front Psychiatry. 2019;10:10. doi:10.3389/fpsyt.2019.00010
156. Vinolo MAR, Rodrigues HG, Nachbar RT, Curi R. Regulation of Inflammation by short chain fatty acids. Nutrients. 2011;3(10):858–876. doi:10.3390/nu3100858
157. Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi:10.1038/nature12726
158. Salvi PS, Cowles RA. Butyrate and the intestinal epithelium: modulation of proliferation and inflammation in homeostasis and disease. Cells. 2021;10(7):1775. doi:10.3390/cells10071775
159. Yang R, Hu X, Xie X, et al. Propionic acid targets the TLR4/NF-κB signaling pathway and inhibits LPS-induced intestinal barrier dysfunction: in vitro and in vivo studies. Front Pharmacol. 2020;11:573475. doi:10.3389/fphar.2020.573475
160. Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Bäckhed F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. 2015;22(4):658–668. doi:10.1016/j.cmet.2015.07.026
161. Vaure C, Liu Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol. 2014;5:316. doi:10.3389/fimmu.2014.00316
162. Han C, Ding Z, Shi H, Qian W, Hou X, Lin R. The role of probiotics in lipopolysaccharide-induced autophagy in intestinal epithelial cells. Cell Physiol Biochem. 2016;38(6):2464–2478. doi:10.1159/000445597
163. Sánchez-Alcoholado L, Gutiérrez-Repiso C, Gómez-Pérez AM, García-Fuentes E, Tinahones FJ, Moreno-Indias I. Gut microbiota adaptation after weight loss by Roux-en-Y gastric bypass or sleeve gastrectomy bariatric surgeries. Surg Obes Relat Dis. 2019;15(11):1888–1895. doi:10.1016/j.soard.2019.08.551
164. Magouliotis DE, Tasiopoulou VS, Sioka E, Chatedaki C, Zacharoulis D. Impact of bariatric surgery on metabolic and gut microbiota profile: a systematic review and meta-analysis. Obes Surg. 2017;27(5):1345–1357. doi:10.1007/s11695-017-2595-8
165. de Vos WM. Microbe Profile: akkermansia muciniphila: a conserved intestinal symbiont that acts as the gatekeeper of our mucosa. Microbiology. 2017;163(5):646–648. doi:10.1099/mic.0.000444
166. Debedat J, Clement K, Aron-Wisnewsky J. Gut microbiota dysbiosis in human obesity: impact of bariatric surgery. Curr Obes Rep. 2019;8(3):229–242. doi:10.1007/s13679-019-00351-3
167. Farup PG, Valeur J. Changes in faecal short-chain fatty acids after weight-loss interventions in subjects with morbid obesity. Nutrients. 2020;12(3):802. doi:10.3390/nu12030802
168. Cypess AM, Ingelfinger JR. Reassessing human adipose tissue. N Engl J Med. 2022;386(8):768–779. doi:10.1056/NEJMra2032804
169. Kahn CR, Wang G, Lee KY. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J Clin Invest. 2019;129(10):3990–4000. doi:10.1172/JCI129187
170. Rabiee A. Beige fat maintenance; toward a sustained metabolic health. Front Endocrinol. 2020;11:634. doi:10.3389/fendo.2020.00634
171. Kiliaan AJ, Arnoldussen IA, Gustafson DR. Adipokines: a link between obesity and dementia? Lancet Neurol. 2014;13(9):913–923. doi:10.1016/S1474-4422(14)70085-7
172. Sturm W, Sandhofer A, Engl J, et al. Influence of visceral obesity and liver fat on vascular structure and function in obese subjects. Obesity. 2009;17(9):1783–1788. doi:10.1038/oby.2009.81
173. Arnoldussen IAC, Kiliaan AJ, Gustafson DR. Obesity and dementia: adipokines interact with the brain. Eur. Neuropsychopharmacol. 2014;24(12):1982–1999. doi:10.1016/j.euroneuro.2014.03.002
174. Balasubramanian B, Kim HJ, Mothana RA, Kim YO, Siddiqui NA. Role of LXR alpha in regulating expression of glucose transporter 4 in adipocytes - Investigation on improvement of health of diabetic patients. J Infect Public Health. 2020;13(2):244–252. doi:10.1016/j.jiph.2019.09.008
175. Perry RJ, Camporez JG, Kursawe R, et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160(4):745–758. doi:10.1016/j.cell.2015.01.012
176. Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21.
177. Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8(9):544–556. doi:10.1038/nrendo.2012.48
178. Ma IT, Madura JA. Gastrointestinal complications after bariatric surgery. Gastroenterol Hepatol. 2015;11(8):526–535.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.