Back to Journals » International Journal of Nanomedicine » Volume 19
Bacteria-Based Nanoprobes for Cancer Therapy
Authors Lu Y, Mei N, Ying Y, Wang D, Li X, Zhao Y, Zhu Y, Shen S, Yin B
Received 5 September 2023
Accepted for publication 4 January 2024
Published 23 January 2024 Volume 2024:19 Pages 759—785
DOI https://doi.org/10.2147/IJN.S438164
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor R.D.K. Misra
Yiping Lu,1,* Nan Mei,1,* Yinwei Ying,1,* Dongdong Wang,1 Xuanxuan Li,1 Yajing Zhao,1 Yuqi Zhu,1 Shun Shen,2,3 Bo Yin1
1Department of Radiology, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; 2Pharmacy Department, Shanghai Pudong Hospital, Fudan University Pudong Medical Center, Shanghai, People’s Republic of China; 3Center for Medical Research and Innovation, Shanghai Pudong Hospital, Fudan University Pudong Medical Center, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bo Yin; Shun Shen, Email [email protected]; [email protected]
Abstract: Surgical removal together with chemotherapy and radiotherapy has used to be the pillars of cancer treatment. Although these traditional methods are still considered as the first-line or standard treatments, non-operative situation, systemic toxicity or resistance severely weakened the therapeutic effect. More recently, synthetic biological nanocarriers elicited substantial interest and exhibited promising potential for combating cancer. In particular, bacteria and their derivatives are omnipotent to realize intrinsic tumor targeting and inhibit tumor growth with anti-cancer agents secreted and immune response. They are frequently employed in synergistic bacteria-mediated anticancer treatments to strengthen the effectiveness of anti-cancer treatment. In this review, we elaborate on the development, mechanism and advantage of bacterial therapy against cancer and then systematically introduce the bacteria-based nanoprobes against cancer and the recent achievements in synergistic treatment strategies and clinical trials. We also discuss the advantages as well as the limitations of these bacteria-based nanoprobes, especially the questions that hinder their application in human, exhibiting this novel anti-cancer endeavor comprehensively.
Keywords: bacteria-based nanoprobe, bacteria-mediated synergistic cancer therapy, nanomedicine, tumor targeting, tumor immune microenvironment, clinical trial
Graphical Abstract:
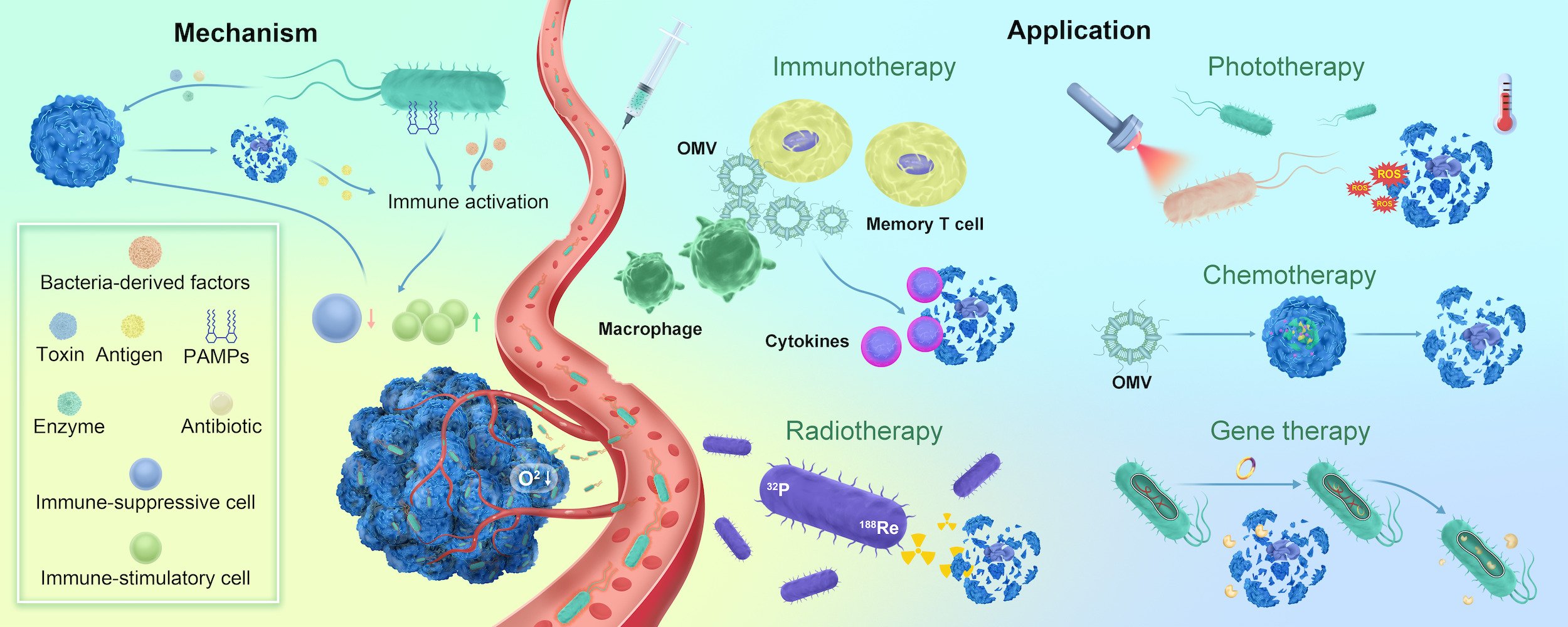
Introduction
Nowadays, cancer is considered to rank as the leading cause of death and the single most important barrier to increasing life expectancy in every country of the world in the 21st century.1 Numerous efforts have been made to conquer cancers. Surgical resection with tumor removal is known as the first-line treatment of many kinds of tumors in their early stage, but for unresectable ones, additional tools are indispensable. Since the late 20th century, new methods, especially chemotherapy and radiotherapy, which utilizes different types of chemotherapeutic drugs or high doses of radiation, are applied to kill tumor cells and shrink tumors. When compared to chemotherapy, the utility of radiotherapy is rather limited since it is only suitable for a tumor confined to a discrete anatomical area.2 As a systemic therapy, chemotherapy with anti-tumor drugs is capable to treat cancer throughout the whole body even with metastasis.3 However, the therapeutic effect of chemotherapy is often modest. It was reported that cancer treatment based on a broad use of cytotoxic chemotherapy in unselected cancer patients had reached its therapeutic plateau.4 Furthermore, the systemic toxicity caused by the lack of selectivity and resistance to chemotherapy and radiotherapy had also severely hampered the effectiveness of these traditional treatments.5 Therefore, the need to find better treatment alternatives was underscored.
The phenomenon of the selective accumulation of nanometric devices into tumoral tissues discovered first by Matsumura and Maeda in 1986, known as enhanced permeation and retention (EPR) effect, has given nanomedicine an advantage over conventional medicine and started a new chapter of nanomedicine against cancer.6,7 Since then, hundreds of different nanoparticle (NP)-based drug delivery systems (DDSs) have been developed, from inorganic systems, or organic ones, to hybrid NPs. Theoretically, when administered systemically, NPs leak from the tumor blood vessels into the tumor interstitium through the hyperpermeable tumor vasculatures and are then retained in the tumor due to reduced lymphatic drainage which could potentially increase the effectiveness of anti-tumor drugs.8 The characteristics including passive accumulation within tumor via EPR effect, decoration of various targeting ligands and multiple types of triggers of payload release enable NPs to achieve excellent performance in vitro and in vivo models.9–13 However, the nano-delivery system mainly based on EPR effect could not always achieve satisfactory therapeutic response in real clinical situations. So far, only a few nano-systems have reached the market and most of them are still associated with adverse effects14,15 Also, the increase in overall survival of patients with nanomedicine treatment is modest for many cases.16
Several reasons might reduce the therapeutic effect of NPs. First, NPs are passively targeting the cancers which would sometimes result in insufficient drug loading-NPs to reach the target sites.3 Second, the dense extracellular matrix of tumor hampers the particle penetration and limits the distribution of the nanocarrier only on the tumor periphery.17 Furthermore, the high interstitial pressure presenting in solid tumors makes NP diffusion rather difficult.18 Despite the less toxicity than conventional therapeutics, the stability, biocompatibility and long-term potential safety of the synthetic nanocarriers are far from ideal for in vivo application.19 In these cases, biological carriers, especially bacterial-based carriers, exhibit promising properties and gradually attract people’s attention.
In comparison to synthetic NPs, bacteria-based NPs exert many benefits in cancer therapy. On the one hand, bacteria and their derivatives are endowed with tumor targeting intrinsically rather than based on EPR effect. In particular, it is the tumor hypoxic and immunosuppressive microenvironment and its special structure like flagellum and chemotaxis that enable bacteria to target, colonize and proliferate.20 On the other hand, owing to the intrinsic targeting ability of bacteria, the loaded toxic drugs of bacterial NPs play a therapeutic role with spatial and temporal precision and without systemic effects as much as possible.21,22 Moreover, it is promising to help ensure biocompatibility of NPs by means of choosing a commensal or probiotic bacterial strain, or live attenuated bacteria or derivatives.23
This review focuses on bacterial therapy against cancer from the following three aspects: development, mechanism and advantages. Subsequently, the bacteria-based nanoprobes against cancer and the recent achievements in synergistic treatment strategies and clinical trials are introduced. Ultimately, the limitations and future prospects of bacteria-based nanoprobes are anticipated. These will provide scientific guidance and strategic reference for expanding the construction of bacteria-based nanosystems and their significance in cancer therapy.
Bacteria vs Cancer—History, Mechanism and Advantages
The History of Bacterial Therapy
The history of bacteria-based anticancer treatment dates back to 1813, when Vautier reported his observation of tumor regression in patients who concurrently suffered from gas gangrene caused by a Clostridium infection.24 Inspired by this, several attempts were made to shrink cancers upon infection of erysipelas. In 1891, W.B. Coley, an American surgeon, reported his experience in treating sarcoma by inoculation of streptococcus erysipelatous.25 He dedicated his whole career to exploring the anti-cancer effect after the inoculation of the mixed toxin of the streptococcus erysipelas and the Bacillus Prodigiosus, known as ‘Coley’s toxin’, whose active agent was later reported to be interleukin-12.26,27 At that time, with stable replicability in clinical use, Coley’s work was controversial, and gradually the pioneering bacterial therapy gave way to arising chemotherapy and radiotherapy. But science will never stop its pace.
In 1929, Pearl found that cancer survivors had a higher incidence of active or healed tuberculosis (TB) than individuals dying of cancer, implying TB’s protection against cancer.28 In the second part of 20th century, the anticancer effects of bacilli Calmette-Guérin (BCG), a live attenuated obligate anaerobic strain of Mycobacterium bovis, were intensively investigated, during which the underlying theories and substances in bacterial-based immunotherapy were subsequently discovered.29,30 Efforts were made to treat cancers by BCG vaccine and in 1990, the general use of intravesical BCG for the treatment of noninvasive bladder cancer was officially approved by FDA.31,32 The breakthrough in synthetic biology, nanotechnology and genetic engineering makes the use of live attenuated bacteria and their derivatives as anti-cancer therapeutics a tangible reality.
Main Mechanism of Bacterial Therapy
Intrinsic Tumor Targeting
It is well documented that when administered systemically, therapeutic bacteria disseminate to both tumor and healthy tissues with similar bacterial amount mainly in a passive way. However, several hours or days later, live bacteria could only be detected in solid tumor rather than normal, inflammatory or non-tumoral hypoxic regions. This tumor-targeting phenomenon could be explained by two intrinsic characteristics of the tumor: hypoxia and immunosuppression.
The unlimited proliferation of malignant cells and chaotic neovascularity leads to tumor metabolism environment (TME), which is well summarized as (a) hypoxia, oxygen concentrations ≤ 10mmHg; (b) anaerobic metabolism-induced acidity status; and (c) necrosis, contributing to bacterial proliferation in the tumor tissue.33,34 The hypoxia status of a tumor leads to resistance to cytotoxic agents and radiation, and finally brings poor prognosis. Furthermore, the function of immune cells in vivo was affected by the over-expression of HIF-1α and VEGF, contributing to the immune privilege of solid tumors.35,36 It is the hypoxic status and lack of immune clearance in tumor region that provide a suitable environment for live bacteria to colonize and proliferate.
On the bacteria side, live bacteria can move by acquiring energy from the surrounding environment. The flagellum and chemotaxis enable these live creatures penetrate deep into the tumor against the high interstitial fluid pressure (IFP), differentiating these bacteria from other anti-cancer systems that could only diffuse passively.37 Chemotaxis and tumor necrosis factor (TNF-α) are also proved to contribute to the initial phase of the penetration of bacteria into tumor tissue from vessels. For example, the chemotactic compounds produced by the dead cancer cells within the tumor attract Salmonella to adhere to the vessel walls of tumor. As early as 30 minutes after injection, the concentration of TNF-α increased significantly in parallel to the start of the intra-tumoral colonization of bacteria, and peaked between 30–120 min.38 The cytokine, TNF-α, leads to vessel disruption, hemorrhage and necrosis, thereafter, creating ideal environment for bacterial germination. The pre-treatment of anti-TNF-α could not inhibit but retard the intra-tumoral colonization of Salmonella, which convinced the effect of TNF-α in tumor invasion behavior of bacteria.
Other factors including EPR effect in nano-scale bacteria and possible entrapment of bacteria by occluded/dying vessels also contribute to the intrinsic tumor-targeting ability of bacteria.39 Some bacteria-derived nanovesicles like minicells can be equipped with the specific antibodies or ligands in order to achieve the precise tumor-targeting.40
Anticancer Agents Secreting and Immune Response Activating
Bacteria have evolved sophisticated mechanisms to inhibit the growth of competitors, and in cancer, the competitors are challengeable cancer cells.41 Many successful experiments have proved the ability of live bacteria to cause tumor regression. The native therapeutic mechanism of the attenuated live bacteria could be roughly devoted to 2 parts: the secretion of anti-cancer agents and activation of immune response.
The anticancer agents of bacteria mainly include 3 categories: antibiotics, toxins and enzymes.42,43 Several antibiotics of bacteria origin against tumor were intensively investigated in the past and now are available in market, for instance, Bleomycin and Doxorubicin. Toxins are groups of ribosomal-synthesized bacterial proteins to disrupt the physiological behavior of target cells, and some of them exhibit anticancer activities. For example, colicins, provided by different strains of Escherichia coli, represent cytotoxicity towards cancer cells by a series of mechanism including depolarization of the cytoplasmic membrane, DNase and RNase activity, etc, exhibiting anticancer properties toward a variety of human ovarian cell lines.44,45 Another common group of toxins, including Cytolysin A and Listeriolysin O etc., belongs to pore-forming toxin family, which can damage cell membrane, ablate the chemical gradients needed to keep a cell functioning or allow entrance of bacteria into cytosol, often leading to cell death.46,47 Some enzymes of bacterial origin display anticancer properties as well, and the representatives of which were the nitrate and nitrite reductases provided by Salmonella strains.48 Under hypoxia circumstances, nitrate and nitrite reductases are capable to convert nitrate and nitrite to the nitric oxide free radical molecule (NO), which has anticancer activity via arresting tumor proliferation.49
Besides the anticancer agents mentioned above, bacteria’s ability to arouse immune activation is another key to kill tumor. Tumors are known to maintain immune tolerance by resembling normal tissue, but bacteria act as a strong immune stimulator. By secreting bacteria-derived factors, pathogen-associated molecular patterns (PAMPs), for instance, and releasing tumor antigens after disruption of tumor cells, bacteria successfully activate both the innate and adaptive immune systems in the tumor environment, exhaust immune-suppressive cells and increase pro-immunostimulatory lymphocyte and CD8+ T cell infiltrates within the tumor50–53 Finally, with the immune effects of multiple immune cells, cytokines and chemokines, the immune system is modulated and activated, and cancer cells are recognized and attacked as the consequences.
Due to the inherited cargoes from the donor bacteria, bacteria-derived nanovesicles possess potent immunogenicity in immune response activation. For example, with the same antigens or PAMPs like lipopolysaccharide (LPS), peptidoglycan, and flagellin as the original bacteria, outer membrane vesicles (OMVs) are highly immunogenic nanovesicles that can robustly activate a variety of toll-like receptor (TLR) signaling pathways.54,55 It was found that intravenous administration of OMVs alone could elicit interferon (IFN)-γ production and nonspecific antitumor immune response.56 Bacterial ghosts (BGs) can not only directly activate immune cells including dendritic cells (DCs), macrophages, and B or T cells, but also activate epithelial cells, fibroblasts, and keratinocytes, all of which can indirectly attract immune cells.57 Also, BGs can be able to induce higher quality immune responses with the preservation of the epitopes of virulent bacteria strains.
The Advantages of Bacteria-Based Tumor Treatment
With the tumor-targeting and specific anti-cancer therapeutic effects, bacteria-based tumor treatment has comparative advantages over traditional treatments. Bacteria 1) have the capability of preferentially colonizing the core area of the tumor to achieve accurate drug delivery and therapeutic effects; 2) can effectively activate immune system in the tumor immunosuppressive microenvironment and eliminate tumor cells both directly and indirectly; 3) can be developed as carriers to exert multifunctional anti-tumor efficacy constructed with various nanomaterials; 4) like probiotic strains, live attenuated bacteria or engineered derivatives, can enhance its biocompatibility and biosafety; 5) can be decorated with specific luminescent materials or imaging agents to realize therapeutic visualization in vitro and in vivo.
More and more attempts have been made to synthesize bacteria and its components to digger the bacterial ability as carriages to deliver medicine, antibodies, cytokines, etc. to trigger more intensive and profound anti-cancer results.58,59 In the next part, we aim to introduce the most frequently used live attenuated bacteria and their engineered derivatives which have joined the fight against cancers as NPs.
The Bacteria-Based NPs to Fight Cancer
These bacteria-based nanoprobes against cancer can be further categorized by their structure and the preloaded treating materials.
Live Attenuated Bacteria
The qualified anti-cancer bacteria need to be attenuated, which means they can infect a host and induce a strong immune response against tumor, but do not cause disease. Historically, the attenuated bacterial vectors were once achieved by chemical mutagenesis or serial passages under laboratory conditions, whose successful examples were illustrated as Salmonella enterica serovar Typhi Ty21a and Mycobacterium bovis BCG.60 The modern biotechnology has revolutionized the mechanism of attenuation by deleting or mutating the essential genes involved in virulence regulatory system through recombinant DNA techniques. These mutations attenuate the strain whilst preserving its immunogenicity.61 Next, we will introduce the most frequently used live attenuated bacteria as NPs to fight cancers.
Salmonella
Salmonella is a genus of rod-shaped Gram-negative bacteria of the family Enterobacteriaceae. They are facultative anaerobic, intracellular pathogens with cell diameters between about 0.7 and 1.5 μm, lengths from 2 to 5 μm, equipped with peritrichous flagella (all around the cell body). The serotypes of Salmonella can be divided into two main groups—typhoidal and nontyphoidal. A member in typhoidal group, Salmonella enterica serovar Typhimurium (hereafter S. Typhimurium), has been extensively studied as an antitumoral tool. Many well-known engineered S. Typhimurium strains are used in anti-tumoral research, including VNP20009 (pur I;-/msbB−), A1-R (leu−/arg−), SL7207 (aroA-) and the ΔppGpp strain SHJ2037 (relA−/spoT−), etc.62–65 For example, in S. Typhimurium strain VNP20009, purI and msbB are deleted chromosomally, resulting in the deficiency of adenine synthesis and modifying the Lipid-A structure. Thus, the virulence of VNP20009 is greatly attenuated. The maximum tolerated dose (MTD) of VNP20009 is significantly reduced by 50,000 times compared to that of the wild-type strain, while the LD50 of it was increased more than 10,000-fold compared with the wild-type strain.66 S. Typhimurium strain A1-R, auxotrophic for leucine and arginine, can suppress the growth of different types of tumors, including prostate, breast and pancreatic cancers and spinal cord glioma, and eradicate cancer metastases.67–72 Besides virulence attenuation, by placing the essential gene asd under a hypoxia-induced promoter, an obligate anaerobic strain called YB1was created from SL7207 strain. With the characteristics of anaerobia, YB1 can only colonize in the hypoxic and necrotic regions of tumor, which enhances the tumor specification.65,73,74
S. Typhimurium can seek the openings between the disorganized endothelial cells and entered the tumor in both active and passive ways. In the passive aspect, the hemodynamics played an important role in the attachment of Salmonella to the tumor vessel wall. In vessels with low flow rates (defined as <~1.5mm/sec), S. Typhimurium were observed to have interaction with vessel wall, but in vessel with higher blood velocity, no bacteria adherence was detected.35 Except the hemodynamic factors, the motility of S. Typhimurium also facilitated its intra-tumoral accumulation. The necessary components for the mobility of S. Typhimurium include the proper flagella constructs, signal transduction proteins, active motor function and chemotaxis. Forbes et al proved the chemotaxis, directed by different chemoreceptors, guided S. Typhimurium into tumor cylindroids. These chemoreceptors, including the aspartate receptor, the serine receptor and the ribose/galactose receptor played vital roles in the process of chemotaxis initiation, tumor penetration and necrosis colonization.75,76 In addition, the nutrients and protection provided by hypoxic and necrotic environments further explained the accumulation of S. Typhimurium in tumor rather than in normal tissues.77 After colonization, S. Typhimurium was reported to kill cancer cells directly by inducing apoptosis and autophagy through a variety of mechanisms.68,78 Moreover, Salmonella can inhibit angiogenesis and activate the host immune response against tumor tissue to suppress tumor growth.79–82
Listeria
Listeria monocytogenes (hereafter Lm) are Gram-positive, facultatively anaerobic, rod-shaped bacteria with the size about 0.5*2-3um.83 The motility mechanism of Lm largely depends on environmental temperature. At 20–25 °C, the flagella are mainly responsible for its motility, but at 37 °C, Lm can only move within eukaryotic cells by explosive polymerization of actin filaments.83 It is famous for its ability to infect humans (especially the immunodeficient ones) and produce a variety of symptoms including gastroenteritis, meningitis, and encephalitis.84 The entrance of Lm into the mammalian cells started from their binding to receptors on host cells and endocytosis thereafter via the guidance of internalin A (InlA) and internalin B (InlB) proteins.85 Then, Lm are free to move throughout the host cell cytosol via expression of the virulence factor actin assembly-inducing protein (ActA) and finally form protrusions which can be internalized by neighboring cells, leading to dissemination of the infection.86
Since wild-type Lm is pathogenic, many efforts have been made to create the attenuated, safe strains for clinical usage. Basically, the attenuation strategies of Lm can be divided into three types: deletion of virulence genes, episomal replacement of virulence or metabolic genes and heat-killing but with active metabolism.87 In detail, the representative of gene-deleted Lm is Lm ∆actA/∆inlB strain, which is also known as Live Attenuated Double-Deleted (LADD) (Aduro BioTech Inc., Berkeley, CA, USA). The deletion of both inlB and actA makes it possible for the strain to selectively infect antigen-presenting cells and reduce toxicity.88 The deletion of a gene followed by reintroduction of that gene via plasmid can also result in attenuation, whose example is LM ∆dal/∆dat strain (LMDD). The dal and dat genes are necessary for Lm to synthesize D-alanine to build peptidoglycan and lipoteichoic acid.89 After deleting dal and dat genes, the strain is transformed with a plasmid encoding the dal gene under a constitutively active promoter, thus achieving the restoration of virulence.90–93
After photochemical treatment, the DNA of Lm was unreversibly destroyed. Nevertheless, the strain is still able to invade host cells and secrete TAA products to elicit antitumor CD8+ T-cell responses. The DNA-destroyed strain is called killed but metabolically active (KBMA) Lm strain, which is not available in clinical trials yet.94,95
The common strategy to enhance the anti-tumoral response in Lm is to fuse TAA with native antigens of the strain.96 The two most common fusion partners with TAAs are modified versions of Listeriolysin O (LLO) and ActA. LLO is a famous virulence factor, which helps Lm perforate the phagosome and enter the cytosol. Since LLO is not phagosome-specific, a truncated version of LLO (tLLO) has been engineered and widely used in anti-tumoral vaccines.97,98 In the explorative study designed by Gunn et al, two recombinant Lm strains, Lm-E7 and Lm-LLO-E7 were injected into mice with tumors secreting the human papilloma virus-16 (HPV-16) E7 protein, which is expressed in HPV-16-associated cervical cancer cells and Lm-LLO-E7, but not Lm-E7, was found to induce the complete regression in 75% E7-expressing tumors. Since then, numerous studies have been carried out to explain the antitumor effects of tLLO and several hypotheses have been testified. First, tLLO contains PEST-like sequences, which can improve vaccine responses towards TAAs.99,100 Second, tLLO and other pore-forming toxins can signal through pattern recognition receptors.101,102
Moreover, tLLO may enhance antitumor responses through changes in Tregs.103,104 Although not completely explained, tLLO still makes Lm a promising candidate as an anti-tumor vaccine. Another different strategy for Lm to evoke anti-tumor response involves fusing TAAs to ActA. The truncated ActA sequences vary in lengths.95,105 Similar to tLLO, fusion of E7 with tActA induces superior antitumor immunity compared to E7 alone.106 Like tLLO, tActA contains PEST domains, but it may possess PAMP-like attributes as well, leading to the production of pro-inflammatory cytokines.106,107 However, the precise mechanisms for its adjuvant-like properties are not fully understood yet. After comparison, ActA-E7 or tLLO-E7 Lm stains resulted in similar levels of circulating E7-specific CD8+ T cells and yielded similar antitumor effects, while tLLO-E7 seemed to induce higher percentages of tumor-infiltrating E7-specific CD8+ T cells.99,105 Similar tumor-response level of both strains indicates the anti-tumor potency of Lm.
Clostridium
As one of the largest prokaryotic genera, Clostridium comprises a phylogenetically heterogeneous group of strictly anaerobic, Gram-positive bacteria. One of their distinct features is the production of bottle-shaped endospores. Cells are in rod shape and motile with flagellum. Well-studied species linked to human diseases are Clostridium tetani, the causative agent of tetanus, and Clostridium botulinum, which is related to food poisoning and causes botulism.24
Although Vautier reported the phenomenon of cancer regression in patients suffered from gas gangrene in 1813, it was in 1947 that the first research on the anti-tumor potential of clostridia spores was reported.108 They found that the subsequent vegetative growth of the organism resulted in significant tumour lysis and extended their survival compared with tumour-bearing mice that were not treated with clostridial spores. Since then, multiple studies have been carried out to investigate the therapeutic effects of Clostridium against cancer, which involved multiple stains, including C. histolyticum, C. tetani, C. oncolyticum (sporogenes), C. beijenrickii (acetobutylicum), etc109,110 The most frequently investigated Clostridium strain is Clostridium novyi-NT (hereafter C. novyi-NT), which was the α-toxin-free strain derived from Clostridium novyi. After intravenous injection of 108 spores, C. novyi-N strain colonized and proliferated in the necrotic regions of tumor, causing cytolysis and tumor growth retardation, but unfortunately, without tumor control.111
Although unable to eliminate tumor thoroughly, the anti-tumoral mechanism of C. novyi-NT is still deeply investigated. A variety of secreted bacterial toxins (eg phospholipases, haemolysins) and enzymes (eg proteases and lipases) destroy the neighboring hypoxic cancer cells and activate the immune response in order to eliminate pathogens.112,113 Once C. novyi-NT spores reach cancer cells, they induce expression of specific antibodies against hypoxia inducing factor-1 (HIF-1) as well as different cytokines such as TNF-α, IL-2 and IL-12 to inhibit the growth of the cancer cells and angiogenesis.114
Escherichia coli
Escherichia coli, also known as E. coli, is a member of the bacterial family of Enterobacteriaceae. It is a Gram-negative, facultative anaerobe, nonsporulating bacterium with rod shape.115 The size of it is around 2.0 μm in length and 0.25–1.0 μm in diameter.116,117 Different from S. Typhimurium and Lm strains mentioned above, the frequently used tumor-targeting probiotic E. coli strains were nonvirulent. Among them, Escherichia coli Nissle 1917 (EcN) is the most famous one, which is an intestinal probiotic isolated from a soldier who survived from a severe outbreak of diarrhea during World War I.118 Since EcN strain does not produce any toxins with great tolerance in various populations, it is safe to administer it orally or through injection.119 It was found that the tumor/liver ratio of EcN colonization at day 2 after intravenous injection was at least 10,000:1 in 4T1 tumor-bearing BALB/c mice, indicating the characteristics of high tumor-selective replication of EcN.120 After genetical modification, EcN strain can express therapeutic proteins, aurin and tumstatin for instance, which was proved to trigger the apoptosis of melanoma cells and inhibit angiogenesis.121,122 Symbioflor-2, another probiotic E. coli strain, is also investigated for its antitumoral potency.123,124 It was proved to be systemically administered and induced an early, albeit lower TNFα response compared with the attenuated S. Typhimurium strain SL7207.124 Altogether, the safety of probiotic E. coli strains is guaranteed; however, the therapeutic response is not that prominent.
Other strains of bacteria include Lactococcus Lactis,125,126 Lactobacillus acidophilus,127 etc. have also shown promising anti-cancer efficiency in pre-clinical trials which could be found in other papers.
Bacteria-Derived Nanovesicles
Given the potential proliferation of bacteria, the nanovesicles synthesized from bacteria components were equivalently useful and much safer in cancer treatment.
Bacterial Ghost
BG is the envelope of gram-negative bacteria emptied of its cytoplasmic contents through cloned lysis/tunnel formation. Since the cytoplasmic components are removed, BG cannot proliferate but the cell morphology and cell surface structure, including outer membrane proteins, adhesins, LPS and peptidoglycan layer are preserved.128 BGs derived from different bacteria (eg E. coli, Helicobacter pylori, Mannheimia haemolytica) have been proved to drive efficient maturation and activation of DCs and have the potential to act as adjuvants and/or candidate vaccines through improving maturation and functioning of DCs.129,130 Besides these, the surface of BGs can be modified to present antigens to elicit specific humoral/cellular responses to target cancer cells while the empty space of BGs can be used to contain different types of payloads like drugs, contrast agents, etc.131
Bacterial Minicells
Bacterial minicells are nano-sized (100–300 nm diameter), non-living, anucleated, non-dividing cells that attributed to the mutation in minC or minD gene.132 These mutations usually lead to polar septation instead of the mid-cell one, thus resulting in the subsequent formation of the small spherical minicells lacking chromosomal DNA.133 Bacterial minicells contain all the components similar to their parent bacterial cells, except DNA. Although cannot proliferate, minicells are still metabolically active, like ATP synthesis, translation of mRNA, transcription and translation of plasmid DNA.134 The basic bioprocess of minicells in target cells are endocytosis, intracellular degradation and payloads release.135 Bacterial minicells are proved to minimize the side effects and increase the biocompatibility of cancer therapy and can reduce phagocytosis.136,137 They are transported across the macrophage membranes and maintained stable for around 6 hours inside the cells.137 The capability of encapsulating drug molecules of bacterial minicells is prominent as well. One minicell can encapsulate one million molecules of doxorubicin (DOX), whereas only around approximately 10,000 drug molecules are loaded into each liposome.133,138 Moreover, with the combined characteristics of NPs and bacteria, minicells are more likely to target in the hypoxic regions of cancer and avoid clearance for a long period. But that is not enough. In order to achieve the precise targeting, the minicells are equipped with the bispecific antibodies (bsAbs), in which one arm interacts with the O-polysaccharide component present in LPS on the minicell surface and other arm interacts with the cell surface receptor specific to the target cancer cells.138 EGFR and Her2/neu are the major targets of minicells modified with a bsAb, and several studies have testified the ability of bacterial minicells as an ideal delivery system for cancer treatment.139–141
Outer Membrane Vesicles
OMVs are nanosized (20–250 nm diameter), spherical proteo-liposomes. They were first discovered by S. Chatterjee as the secretion products from Vibrio Cholerae, but currently OMVs are known as originated nanoscale vesicles from the surface of various Gram-negative bacteria by the route of blebbing or endolysin-triggered cell lysis.142,143 OMVs consist of an outer leaflet of LPS and an inner leaflet of phospholipid. Multiple studies have illustrated that OMVs contain periplasmatic and cytosolic proteins, DNA and RNA.144–147 Phospholipids and LPS, as the main lipid component of OMVs, derivate from the outer membrane and the cell wall of gram-negative bacteria, respectively, representing the same antigens and PAMPs as the original bacteria.55 These antigen proteins and PAMPs are transmitted to antigen presenting cells (APCs), thereby eliciting a pro-inflammatory host immune response.148 Equipped with NP-specific EPR effect and a strong immune response induced by a variety of proteins and PAMPs, OMVs exhibit an enormous potential in anti-tumoral therapy.
Magnetosomes
Magnetotactic bacteria (MTB) are iron-rich particles within intracytoplasmic membrane vesicles, observed and reported by Bellini and Blakemore, respectively, in the 20th century.149 They are composed of an iron crystal, either magnetite (an iron oxide) or greigite (an iron sulfide), with the size of 20–60 nm surrounded by a proteolipidic membrane.150 Magnetosomes are produced by MTB in a complex genetically controlled process that leads to the formation of colloidally stable particles naturally covered by lipids, with high magnetism and a narrow size distribution. The size, morphology and alignment of magnetosomes varied depending on the MTB species and the minerals inside basically belong to 2 types: an iron oxide (Fe2+Fe3+2O4), also called magnetite crystals, and greigite, an iron sulfide (Fe2+Fe3+2S4), also called greigite crystals.151,152 These ferrimagnetic materials consist of one or several magnetic domains, but the mature magnetosomes are stable single domain with the magnetic moments of all their constituting atoms pointing to the same direction.153 Magnetosomes do not display toxic properties for eukaryotic models, therefore they are low-risk for systemic administration.154 Due to their magnetic behavior, magnetosomes are currently used as efficient contrast agents for MRI. Compared with chemical contrast agents, magnetosomes do offer better contrast images.155,156 After surface decoration, the imaging of magnetosomes can be specific to cancer regions.157 They can also be used for curative purposes. The magnetism of magnetosomes is used to enhance the treatment efficiency in magnetic hyperthermia or photothermia against cancer.158 Furthermore, after genetically modified, the magnetosomes can load chemotherapeutic drugs or gene therapy agents, target certain cancer cells and exhibit long-term release.159–161
S-Layer of Bacteria
Many strains of archaea and bacteria (both Gram-positive and Gram-negative) contain regular protein arrays as the outermost component of their cell envelopes, which is referred as surface layer, or S-layers.162 They are highly porous protein/glycoprotein meshworks with unit cell sizes of 3–30 nm. The key features of S-layer proteins include self-assembly, structural regularity, mechanical and osmotic stability, predictable physicochemical properties, making it the ideal vehicles for drug delivery.163,164 Moreover, the uniform patterning, unique morphology and the presence of functional groups make them useful biopolymers in biotechnology applications, such as designer matrices, porous ultrafiltration membranes, and substrates for the immobilization of active compounds.165,166
The Treatment Strategy of Bacteria-Based NPs
Since the anti-tumor efficiency of bacteria monotherapy is not prominent, bacteria and their derivatives are frequently employed in hybrid therapies. In this section, we elaborated in detail on the current knowledge of the bacteria-mediated synergistic cancer therapy (BMSCT) and the recent achievements.
Synergistic Bacteria-Mediated Chemotherapy
Chemotherapy has long been the main treatment for various types of primary cancer and metastasis. However, the delivery efficiency is still impeded by the off-target side effects and the limited intratumoral delivery. Although NPs or other target delivery system have gained some improvements, the chaotic vascular structure and high interstitial fluid pressure can still diminish the drug supply to the tumor center, which severely affect the therapeutic effects of the drugs. Given the specific characteristics of bacteria like active mobility, tumor-targeting and hypoxic-region penetration, bacteria-mediated chemotherapy may overcome the obstacles chemotherapy encountered currently.
In the bacteria-mediated hybrid chemotherapy, bacteria and their derivatives usually participate as the vectors. Fu S et al successfully developed a series of feasible strategies for the targeted delivery of chemotherapeutic drugs to the interior hypoxic areas of solid tumor tissue in virtue of the inherent-targeting ability of the anaerobic Bifidobacterium infantis (B. infantis, Bif), thus potentially improving efficacy and alleviate toxic side effects.167–170 For instance, a Bif-based biohybrid (Bif@PDA-PTX-NPs) was designed to deliver polydopamine (PDA)-coated paclitaxel NPs (PTX-NPs) to tumor tissues, which realized the growth inhibition of A549 xenografts in nude mice, and the survival prolongation of the tumor-bearing mice without any systemic or localized toxicity, providing a new therapeutic strategy for targeted chemotherapy to solid tumors167 (Figure 1).
 |
Figure 1 Schematic diagram shows the construction of the Bif@PDA-PTX-NPs biohybrid and its intelligent responsibility to reductive tumor microenvironment through self-driven targeting to hypoxic regions of tumor. Notes: Adapted with permission from Shi H, Chen L, Liu Y, et al. Bacteria-Driven Tumor Microenvironment-Sensitive Nanoparticles Targeting Hypoxic Regions Enhances the Chemotherapy Outcome of Lung Cancer. International Journal of Nanomedicine. 2023;18:1299–1315.167 |
With much smaller size (20–250 nm) compared with their parent bacteria, bacterial OMVs and minicells were more frequently used to carry chemotherapeutic drugs to treat colorectal carcinoma, lung cancer, glioma, breast cancer, etc. For example, the OMV-coated, tegafur-loaded (a prodrug of 5-FU) polymeric micelles demonstrated substantial improvement in terms of tumor inhibitory rate and survival extension in B16F10 metastatic mouse models.171 Kuerban et al developed another doxorubicin-loaded Klebsiella pneumonia-derived OMV system which presented a substantial tumor growth inhibition with favorable tolerability with extensive apoptotic cells and necrosis in A549 tumor tissues in vivo.172
For non-pathogenetic bacteria, two concerns should be noted. One is the toxicity at the dose required for therapeutic efficacy. A lower dose provides safety but treatment efficacy, which has resulted in the termination of some clinical trials.173 The other concern is the deterioration of motility of bacteria after loading drugs. Intestinal probiotics EcN, with the loading of DOX via acid-labile linkers of cis-aconitic anhydride, have overcome both challenges. The number of conjugated DOX molecules per one bacterium was around 4.7× 105, and the average velocities of the conjugated and free EcN were 9.0 and 9.8 um/s, respectively. The constructed EcN-Dox treatment realized the bacteria-directed accumulation and acid-responsive release of anticancer drugs in tumors, inhibited tumor growth, prolonged animal survivals and induced the apoptosis of tumor cells141,174 (Figure 2).
 |
Figure 2 Schematic illustration depicting the construction of the minicellspHLIP for targeted delivery of chemotherapeutic drugs into the hypoxic regions of solid tumors to kill cancer cells. Note: Adapted with permission from Zhang Y, Ji W, He L, et al. E. coli Nissle 1917-Derived Minicells for Targeted Delivery of Chemotherapeutic Drug to Hypoxic Regions for Cancer Therapy. Theranostics. 2018;8(6):1690–1705.141 |
Synergistic Bacteria-Mediated Radiotherapy
Different from external beam radiotherapy, the bacteria-mediated radiotherapy usually refers to targeted radionuclide therapy, in which the bacteria system carries radionuclides to the targeted tumor region and releases radioactive particles, finally destroying the cancerous cells. Claudia G. et al have made some attempts in the treatment of early and advanced pancreatic ductal adenoma by establishing attenuated Listeria-based radionuclide delivery platform. They demonstrated in the metastatic pancreatic mouse tumor model that live attenuated Listeria coupled with radionuclide 188Rhenium, could deliver radioactivity to the metastases and less abundantly to primary tumors without affecting normal cells, and finally killed tumor cells.175 Four years later, their team developed another Listeria-based radionuclide delivery system by incorporating attenuated Listeria with 32P. They found 32P-Listeria predominantly accumulated in tumors and metastases and exhibited a great anti-tumor effect on primary and metastatic tumors in both pancreatic cancer models Panc-02 and KPC at early and advanced stages of pancreatic cancer, while Listeria alone did not have significant effect on advanced pancreatic cancer.176 However, no matter the way they used to connect radionuclide with bacteria, the radio-toxicity, especially the harm induced by free radionuclide shedding from the delivery system should be given enough attention.
Synergistic Bacteria‑Mediated Phototherapy
Phototherapy has long been a method to eradicate tumor via the exposure of patients to light after the administration of photosensitizing agents. Basically, it can be further divided into 2 main approaches: photothermal therapy (PTT) and photodynamic therapy (PDT). In brief, in PTT, photothermal agents are irradiated by light of a specific wavelength, absorbed energy from photons and transformed the energy into heat, resulting in localized thermal damage. Different from PTT, PDT is largely a photochemical process that relies on light interaction with a photosensitizer, generating reactive oxygen species (ROS) and finally inducing cytotoxic effects.177 However, there are still some challenges, for example, the limited depth of light penetration, the tumoral resistance to PTT and the most important one, the relatively low delivery efficiency of photothermal transduction agents (PTAs). Therefore, the tumor-targeting characteristics of bacteria have inspired new thoughts to the precise target in the field of phototherapy.
In the work conducted by Shuang Q. et.al, the authors establish a bacteria-related NP platform by employing calcium phosphate (CaP) to cover the surface of E.coli-derived OMVs (Figure 3). They selected an FDA-approved photosensitizer, ICG, to integrate with OMV@CaPs. For mice treated with OMV@CaP-ICGs. The tumor temperature rises up to ≈45°C after a mild NIR irradiation. Flow cytometry analysis indicated that OMV@CaP-ICGs treatment promoted the DC activation from 2.19% to 40.18% and increased the percentage of CD8+ T cells in vitro.
 |
Figure 3 OMV@CaPs for enabling efficient tumor accumulation and overcoming the severe inflammatory effect. Notes: (a) Experimental design for testing tumor accumulation of OMV@CaPs in CT26-tumor-bearing mice with different injection modes (×1, ×3, and ×5). Each injection dose of OMV@CaPs contained 5 µg total protein of OMVs. Red dots mean administration of Cy7-labeled OMVs, while black dots mean unlabeled OMVs. (b) Half-life of OMVs with different injection modes (n = 3). (c) Representative fluorescence images of CT26- tumor-bearing mice 24 h after intravenous injection with Cy7-labeled OMV@CaPs (left) and their relative tumor accumulation efficiencies (n = 3) (right). (d) Experimental design for in vivo toxicity evaluation. (e) SR of each group at the endpoint (n = 8). (f) Systemic cytokines (IL-6, TNF-α, and IFN-γ) release profiles at different time points (n = 3). (g) Weight of spleens in each group and the corresponding representative photographs at the endpoint (n = 3). (h) H&E staining of liver (scale bar: 200 µm) and the serum level of ALT at different time points (n = 3). (i) Representative bioluminescence images of IFN-γ-IRES-Venus-AkaLuc mice after intravenous injection with OMVs and OMV@CaPs (equivalent protein amount) at different time points (left) and their corresponding total flux of luciferase signals (n = 3) (right). Data in (b, c), and (f–i) are presented as the mean ± SEM. Statistical significance was calculated via one-way ANOVA with a Tukey post hoc test. ns, not significant. Reprinted with permission from Qing S, Lyu C, Zhu L, et al. Biomineralized Bacterial Outer Membrane Vesicles Potentiate Safe and Efficient Tumor Microenvironment Reprogramming for Anticancer Therapy. Adv Mater. 2020;32:1–14.178 © 2020 Wiley-VCH GmbH. |
Then OMV@CaP- ICGs were intravenously injected into mice with luc-4T1 tumors every other day for 5 times. Twenty-four hours after the first injection, the tumors were irradiated with an 808 nm laser at a low power density (0.5 W/cm2) for 15 min and the temperature of tumor reached around 45°C. It was recorded that after this PTT treatment, the tumor growth was completely suppressed and all mice in OMV@CaP- ICG group stayed alive until the endpoint at day 80.178
In terms of good biodegradability and biocompatibility, organic PTAs bring negligible health risk and are favorable in recent bacterial-mediated phototherapy studies. WF. Chen et al developed a polydopamine-mediated photothermal system by coating VNP20009 with polydopamine molecules. Three days after injected via the tail vein with 0.1 mL pDA-VNP, C57BL/6 mice bearing B16F10 tumor (tumor volumes were close to 80 mm3) were irradiated for 5 min with a near-infrared laser (808 nm, 1.18 W/cm2). Then tumor temperature reached up to 55.0 °C and all mice treated with pDA-VNP PPT showed a complete tumor regression without apparent tumor relapse or metastasis during the 90-day experiment.179 Similarly, Vipul Gujrati et al produced OMVs encapsulating biopolymer-melanin (OMVMel) using a E. coli K12 strain expressing a tyrosinase transgene, which can be further detected by optoacoustic imaging. After intra-tumoral injection of OMVMel in mice bearing 4T1 subcutaneous tumors, the tumor surface temperature in the laser-exposed area (800 nm, 1.5 W/cm2, 6 min) reached as high as 56 °C and the tumor mass nearly disappeared. Therefore, the combination of bacterial components and organic PTAs could noninvasively be monitored the spatio-temporally, achieved therapeutic effect and resulted in very limited toxicity.180
Bacteria-mediated phototherapy system does help in the tumoral accumulation of PTAs, but other challenges still exist, for example, the heat resistance caused by heat shock protein (HSP). In the research performed by QW. Chen et al, they developed a tumor-targeting a photothermal bacterium (PTB) based on Shewanella oneidensis MR-1 and hybridize Zeolitic imidazole frameworks-90 (ZIF-90) encapsulating photosensitizer methylene blue (MB) on the surface of the living PTB. Under light illumination, MB could be released at mitochondria and cause mitochondrial dysfunction, which further contributed to adenosine triphosphate (ATP) synthesis inhibition and HSP down-regulated expression, overcoming the obstacle of heat tolerance and achieving the treatment enhancement of PTT181 (Figure 4). Although the history of bacteria-mediated phototherapy is short, the promising results in relative studies encourage more brave attempts in this field.182–184
 |
Figure 4 Schematic illustration of (A) synthetic procedure of ZIF-90/MB, and (B) synthetic procedure of PTB and PTB@ZIF-90/MB, and (C) mechanism of tumor targeting and photothermal tumor ablation of PTB@ZIF-90/MB upon laser irradiation. Notes: Reprinted with permission from Chen QW, Liu XH, Fan JX, et al. Self-Mineralized Photothermal Bacteria Hybridizing with Mitochondria-Targeted Metal–Organic Frameworks for Augmenting Photothermal Tumor Therapy. Adv Funct Mater. 2020;30.181 © 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. |
Synergistic Bacteria‑Mediated Gene Therapy
Beside these traditional treatments, gene therapy provides an alternative to cancer patients, especially those with metastasis. Basically, gene therapy involves the delivery of genetic information to patients to facilitate the production of therapeutic proteins. The main problem of gene therapy is how to deliver these genetic products into target cells precisely. The characteristics of hypoxia region targeting of bacteria makes them ideal carriers for gene delivery.
Cancer cell is the main target of gene therapy, and the induction of cell apoptosis is a direct method to eradicate cancer. The VP3 protein of Chicken anemia virus, named apoptin, is capable of inducing G2/M arrest and apoptosis in several tumor cells. Cecropin B is a small molecule which could potentiate apoptin-induced apoptosis. Therefore, Xi Wang et al combined these two genes and used bacterial magnetic particles (Magnetospirillum Gryphiswaldense MRS-1) as carriers to deliver these genes to cancer cells. The constructed delivery system, BMP-pVAX1-VA, was proved to promote apoptosis of HepG2 by enhancing membrane deficiency and caspases upregulation in vitro. Via injection subcutaneously adjacent to tumor, BMP-pVAX1-VA showed ability to inhibit tumor growth in HepG2-bearing mice, which demonstrated that bacterium was an efficient and attractive gene delivery system.160
Besides gene transfection, another strategy to induce apoptosis in tumor cells is to take advantages of gene modification of bacteria themselves. Recently, the expression of apoptosis-related proteins by modifying genes in bacteria is popular in anti-cancer treatment. In the work conducted by Huan Wang et al, a eukaryotic expressing VNP20009-AbVec-Igκ-AIF (V-A-AIF) strain was established. It was noted that the AIF expressed by the V-A-AIF strain significantly enhanced the apoptosis of B16F10 cells in vitro and decreased the tumor volume, and prolonged the lifespan in a melanoma-bearing mouse model after intravenous injection.185 Another example is the construction of Bax BH3 peptide-released Salmonella Typhimurium SL3261. Inspired by the previous findings from Armando M. et al, they developed a peptide delivery system by applying the live attenuated Salmonella enterica serovar Typhimurium strain SL3261 expressing and releasing cell-permeable Bax BH3 peptide through the MisL autotransporter system. In vitro experiments demonstrated that the cell-permeable Bax BH3 peptide released from S. enterica promotes apoptosis of Romos cells whereas the in vivo study also proved the existence of antitumor activity of this bacterial-based system in a murine xenograft model of human B NHL.186 Many molecules could activate apoptosis of cancer cells, which could be further investigated.187
Aside from apoptosis induction, disruption of tumor angiogenesis is also promising in retarding tumor growth. Basic fibroblast growth factor (BFGF), as a compensative molecule of VEGF, can cause resistance to anti-VEGF therapy and is regarded as another potential antitumor target. Based on these knowledgements, Huang et al constructed a BFGF-expressing E. coli DH5α competent strain and collected the BFGF-OMVs. After 3 times of BFGF-OMVs immunization, TC-1 tumor cells were subcutaneously inoculated into mice. These studies indicated that the growth of both the primary tumor and metastasis was completely suppressed by strong anti-BFGF autoantibody expression, angiogenesis inhibition and the reversion of tumor immune suppression barriers.188 Similarly, an engineered strain of EcN was established to express Tum-5, the anti-angiogenic active fragment encoding amino acids of Tumstatin. It was proved that EcN (Tum-5) could specifically colonize the tumor and B16 melanoma tumor growth was remarkably restrained compared with pure EcN or Tum-5 deliveries121 (Figure 5).
 |
Figure 5 Tum-5 protein (yellow) could be solubly expressed in EcN and secreted to the medium. The engineered bacteria (blue) were rapidly specially colonized in mouse tumors. Tum-5 bound to integrin receptors on the surface of vascular endothelial cells to induce endothelial cell apoptosis. This process would cause blood vessels to shrink, then the tumor growth was suppressed. Notes: Adapted with permission from He L, Yang H, Liu F, et al. Escherichia coli Nissle 1917 engineered to express Tum-5 can restrain murine melanoma growth. Oncotarget. 2017;8(49):85772–85782.121 |
Synergistic Bacteria‑Mediated Immunotherapy
Immunotherapy, as an emerging and promising strategy in cancer treatment, brings hopes for precision cancer treatment. However, hypoxia is considered as one of the key characteristics of TME, which facilitates the immune suppressive tumor environment.189,190 Bacteria‑mediated therapy combined with immunology can enhance the efficacy of immunology through potentiating precise tumor targeting and immune response modulation.191,192
Bacteria-based NPs can reduce tumor burden synergistically with other treatment by eliciting an antitumor host immune response through producing immune-stimulating molecules and metabolites. Bifidobacterium, an anaerobic commensal bacterium residing in the GI tract with low toxicity, enhanced the antitumor efficacy of CD47-based immunotherapy. Live bacteria could produce secondary metabolites to trigger the STING signaling pathway inside DCs and modulate the immune response elicited by CD47 blockade through the capacity to actively target and colonize in the tumor microenvironment193 (Figure 6). Bifidobacterium bifidum modulated the immunological background and enhanced PD-1 blockade or oxaliplatin treatment efficacy by facilitating the production of interferon-γ, probably through the enhanced biosynthesis of immune-stimulating molecules and metabolites.194
 |
Figure 6 Schematic illustration depicts the mechanism by which gut microbiota (Bifidobacterium) preferentially colonize in tumor sites and facilitate immunotherapy via STING signaling. Notes: Adapted with permission of Rockefeller University Press, from Shi Y, Zheng W, Yang K. Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. J Exp Med. 2020;217(5):e20192282; permission conveyed through Copyright Clearance Center, Inc.193 |
In addition to the aforementioned utilization of bacteria products as immunostimulants, bacteria can be developed as drug carriers to elicit immunology modulation, such as polarization of M2 macrophages to M1 macrophages. It was proposed that EcNZ/F@Au loaded with macrophage phenotype regulator zoledronic acid (ZOL) enhanced valid polarization of tumor-associated macrophages toward the M1 phenotype and an effective production of proinflammatory cytokines, leading to a synergistic efficacy on tumor growth inhibition.195
All these new synthesized therapies are all benefiting from the precise tumor-targeting characteristics of bacteria. With editable gene materials and bacteria-induced immune responses, the bacteria-based delivery system is destined to have bright future in human’s fight against cancer.
The Clinical Trials of Bacteria-Based NPs
With great success of various bacteria-based anti-cancer treatments in pre-clinical studies, bacteria and their derivatives are expected to be alternatives of treatments for primary and metastatic tumors (Supplementary Table 1). However, the very first trial of VNP20009 was claimed to fail with some tumor localization and no tumor retardation effects in the Phase I study, although no safety concerns were detected.196 In 2020, another live attenuated strain of S. Typhimurium called Salmonella-IL2, was used to treat metastatic gastrointestinal cancers in 22 patients. Although no significant toxicities were detected by the doses of up to 1010 colony forming unit, but still, no survival advantage was observed. Nevertheless, the demonstration of significant increase in circulating NK and NK-T cell suggested the immunological impact of Salmonella-IL2 on human body, which is a progression forward.197
Another strain which has been investigated intensely is CRS-207, a live, attenuated LM strain carrying double deletions (LADD) and secreting mesothelin. Mesothelin is a tumor-associated antigen present on normal mesothelial cells and highly expressed by many human tumor types, including mesotheliomas, pancreatic adenocarcinomas, non-small cell lung cancers (NSCLC), and ovarian cancers. Therefore, CRS-207 was investigated in the clinical trials to explore the usefulness and safety in cancer treatment. A phase I study in patients with advanced mesothelioma, non–small-cell lung cancer, ovarian cancer, and pancreatic adenocarcinoma first revealed that CRS-207 was well tolerated at a dose of 109 CFU and could induce a robust innate and adaptive mesothelin-specific T-cell response (NCT00327652)198 Then, an open-label, randomized, controlled Phase II study of 90 patients with previously treated metastatic pancreatic cancer found that the overall survival did improve by sequential administration of low-dose cyclophosphamide (Cy), GVAX (granulocyte-macrophage colony-stimulating factor-secreting allogeneic pancreatic tumor cells) followed by CRS-207 compared with Cy/GVAX alone.199 In light of the promising results, a phase II b randomized, multicenter study was further conducted to compare Cy/GVAX+CRS-207 with chemotherapy in patients with metastatic pancreatic cancer (NCT02004262). But it turned out that in a larger cohort (213 patients) the combination of Cy/GVAX+CRS-207 did not improve survival over chemotherapy.200 Except metastatic pancreatic cancer, another CRS-207 clinical trial aimed to investigate the treatment efficiency of malignant pleural mesothelioma (MPM). A total of 60 patients with unresectable MPM were enrolled in this multicenter, open-label phase Ib study. The results showed that administration of CRS-207 was safe and the combination treatment with CRS-207 immunotherapy and chemotherapy led to tumor size reduction, disease control and improved survival.201 Further clinical trials concerning the treating efficiency of CRS-207 are anticipated.
Bacterial derivatives, like minicells, packaged with chemotherapeutic drugs are also translating from laboratory to clinics. Preclinical studies showed that epidermal growth factor receptor (EGFR)-targeted, paclitaxel-loaded Salmonella Typhimurium-derived minicells (EGFRminicellsPac) have antitumor effects in xenograft models. Then, an open-label, multi-center, first-time-in-human Phase I trial was launched. Twenty-eight patients with advanced solid tumors were enrolled and received infusion of EGFRminicellsPac with different concentrations. This study confirmed that the maximum tolerated dose was defined as 1010 EGFRminicellsPac and nearly 50% patients achieved stable disease as their best response (ACTRN12609000672257).139
Recent clinical trials are exploring the treatment safety and possibility of various live attenuated bacteria. For instance, the administration of SGN1, a genetically modified strain of Salmonella typhimurium (VNP20009-M) that expresses L-Methioninase, is ready to recruit patients with advanced solid tumors to assess the safety and tolerability (NCT05038150, NCT05103345). Other trials involve genetically modified Bifidobacterium longum, Clostridium novyi-NT spores, Yersinia enterocolitica et al against different advanced solid tumors. Although more and more Phase I/II trials are launched, it still needs a period of time to reveal the real treating efficacy of these mini-robots in human body. Typical clinical trials are summarized in Table 1.
 |
Table 1 Examples of Anti-Cancer Bacterial Clinical Studies |
Challenges and Future Perspectives
In all the published works, the promising anti-cancer bacteria and their derivatives have the common features including 1) targeting only tumor cells; 2) having tolerable toxicities and side effects and 3) being generically stable, which are the foundation of the optimal microorganism to be applied in human trials. Although many bacteria-based nanoprobes did perform well in animal experiments, we still need to consider the following questions which may hinder their application in human.
The first question is how to guarantee the high local concentration of the bacteria-derived carriers. It is known that bacteria are relatively small compared to mammalian cells, in order to avoid severe side effects, the concentration of injected bacteria or their derivatives should not be very high, which means that the functional proteins they delivered must be effective at a low concentration. Therefore, the tumoral concentration of bacteria-based materials after administration should be investigated carefully which can help to estimate the treatment efficacy of the subsequent molecules.
Second is the how to control the tumor localization and delivery toward efficient bacterial therapy. Bacteria, given their intrinsic mobility, are proved to have tumor targeting capability in both active and passive methods. With deepening insight into bacteria-based treatments, the live attenuated bacteria and their derivatives are modified in their structures and some are designed to carry various functional materials, which may deprive them of their original mobility which in turn may prolong the time they used and reduce the number of bacteria for localization or drug delivery. Moreover, diverse small-sized nanomaterials can accumulate intracellularly rather than outside the cell or on the membrane so as to exert potentially specific mechanisms of antitumor effects toward activation of precise cancer cell death pathways.208 Hence, it is a novel field in anti-tumor bacterial therapy to investigate more about how to target specific organelles intracellularly.
The third question is how to guarantee the safety of the bacterial therapy. In almost all phase I studies of anti-tumor bacterial treatments, few side effects were reported. However, off-target effects and possible bacterial infection remain to be unavoidable concerns. Since robust intratumoral colonization of bacteria is essential for optimal therapeutic effect, such aggressive infection will inevitably cause infectious symptoms and laboratory changes. More researches are required to investigate how to keep or shift the balance towards an effective therapy with minimal safety concerns.209 For example, multifunctional synergetic therapy combined with novel cancer therapy with engineered bacteria or extracted individual component, with high therapeutic effects and low toxic and side effects, is developed as one of the novel directions in the realm of anti-tumor bacterial therapy.
Translation from pre-clinical studies to clinical usage is a huge step. Differing from non-viable clinical agents, live therapeutic bacteria cannot be sterilized by the conventional standard. It is an uphill struggle to guarantee the safety, consistency and stability of the products. The mature and standard manufacturing processes of the bacterial-based products, like good manufacturing practices (GMP), need to be well-established in each case from producing, purifying to harvesting live bacteria following strict aseptic protocols with real-time supervision. Furthermore, more regulated issues including unintended spreading among public, the potential infection etc. should also be considered as well.
Conclusion
Bacteria and bacteria-derived NPs have demonstrated promising potential in anti-cancer treatment. These bacterial-derived organisms are smart to realize intrinsic tumor targeting in both active and passive ways and retard tumor growth with anti-cancer agents secreted and immune response. Based on these advantages, bacteria and their derivatives are further developed into various platforms by delivering additional molecules and drugs in synergistic bacteria-mediated anticancer treatments. These platforms achieved excellent anti-tumor performances in vivo and in vitro studies and some of them have started clinical trials which brings hope to advanced tumor patients.
Nevertheless, the challenges concerning bacterial therapy still exist. How to guarantee the efficiency and reduce the possible infection after bacteria-derived agents injected? How to successfully translate the bacterial-related NPs from animal experiments to application in human? Current clinical trials are still in the exploration stage of bacteria-inspired medicine. We still have much to learn about the bacteria-based anti-cancer treatment. But with the deepening insights and ever-growing knowledge, these tiny robots may join us as strong weapons in our war against cancer in the near future.
Abbreviations
EPR, Enhanced permeation and retention; NP, Nanoparticle; DDS, Drug delivery systems; TB, Tuberculosis; BCG, Bacilli Calmette-Guérin; TME, Tumor metabolism environment; IFP, Interstitial fluid pressure; TNF-α, Tumor necrosis factor; NO, nitric oxide; PAMP, Pathogen-associated molecular patterns; LPS, lipopolysaccharide; OMV, Outer membrane vesicles; TLR, toll-like receptor; IFN, interferon; BG, Bacterial ghost; DC, dendritic cells; S. Typhimurium, Salmonella enterica serovar Typhimurium; MTD, Maximum tolerated dose; LM, Listeria monocytogenes; InlA, internalin A; InlB, internalin B; ActA, actin assembly-inducing protein; LADD, Live attenuated double-deleted; LMDD, LM ∆dal/∆dat strain; KBMA, Killed but metabolically active; LLO, Listeriolysin O; tLLO, truncated version of LLO; HPV-16, Human papilloma virus-16; C. novyi-NT, Clostridium novyi-NT; HIF-1, hypoxia inducing factor-1; EcN, Escherichia coli Nissle 1917; DOX, Doxorubicin; bsAb, bispecific antibodies; APC, antigen presenting cell; MTB, Magnetotactic bacteria; BMSCT, Bacteria-mediated synergistic cancer therapy; Bif, Bifidobacterium infantis; PDA, polydopamine; PTT, Photothermal therapy; PDT, Photodynamic therapy; ROS, Reactive oxygen species; PTA, Photothermal transduction agent; CaP, Calcium phosphate; OMVMel, OMVs encapsulating biopolymer-melanin; HSP, Heat shock protein; PTB, Photothermal bacterium; ZIF-90, Zeolitic imidazole frameworks-90; MB, Methylene blue; ATP, Adenosine triphosphate; V-A-AIF, VNP20009-AbVec-Igκ-AIF; BFGF, Basic fibroblast growth factor; ZOL, zoledronic acid; NSCLC, non-small cell lung cancers; Cy, cyclophosphamide; MPM, Malignant pleural mesothelioma; EGFR, Epidermal growth factor receptor; GMP, Good Manufacturing Practices.
Acknowledgments
Yiping Lu, Nan Mei, Yinwei Ying are co-first authors and Bo Yin and Shun Shen are co-corresponding authors for this review.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work has been supported by the National Natural Science Foundation of China (Grant No. 82271966), Shanghai Sailing Program (Grant No. 21YF1404800), Science and Technology Innovation Action Plan of Shanghai Science and Technology Commission (Grant No. 22S31905900), Youth Program of Special Project for Clinical Research of Shanghai Municipal Health Commission Health industry (Grant No. 20204Y0423), Youth Medical Talents—Medical Imaging Practitioner Program (No.3030256001), Shanghai Zhou Liangfu Medical Development Foundation “Brain Science and Brain Diseases Youth Innovation Program” and Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01), ZJ Lab, and Shanghai Center for Brain-Inspired Technology. However, these funding sources had no involvement in any processes of the research completion.
Disclosure
The authors declare that they have no competing interests.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a Phase 2 single-arm study. Lancet Oncol. 2016;17(3):287–298. doi:10.1016/S1470-2045(15)00167-9
3. Li H, Jin H, Wan W, Wu C, Wei L. Cancer nanomedicine: mechanisms, obstacles and strategies. Nanomedicine. 2018;13(13):1639–1656. doi:10.2217/nnm-2018-0007
4. Schiller JH, Harrington D, Belani CP, et al. Comparison of Four Chemotherapy Regimens for Advanced Non–Small-Cell Lung Cancer. N Engl J Med. 2002;346(2):92–98. doi:10.1056/NEJMoa011954
5. Sharma R, Tobin P, Clarke SJ. Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhoea. Lancet Oncol. 2005;6(2):93–102. doi:10.1016/S1470-2045(05)01735-3
6. Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392.
7. Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjug Chem. 2010;21(5):797–802. doi:10.1021/bc100070g
8. Kobayashi H, Turkbey B, Watanabe R, Choyke PL. Cancer drug delivery: considerations in the rational design of nanosized bioconjugates. Bioconjug Chem. 2014;25(12):2093–2100. doi:10.1021/bc500481x
9. Wu C, Li H, Zhao H, et al. Potentiating antilymphoma efficacy of chemotherapy using a liposome for integration of CD20 targeting, ultra-violet irradiation polymerizing, and controlled drug delivery. Nanoscale Res Lett. 2014;9(1):9–12. doi:10.1186/1556-276X-9-447
10. Bar-Zeev M, Livney YD, Assaraf YG. Targeted nanomedicine for cancer therapeutics: towards precision medicine overcoming drug resistance. Drug Resist Updat. 2017;31:15–30. doi:10.1016/j.drup.2017.05.002
11. Choi JH, Ha T, Shin M, Lee SN, Choi JW. Nanomaterial-based fluorescence resonance energy transfer (Fret) and metal-enhanced fluorescence (mef) to detect nucleic acid in cancer diagnosis. Biomedicines. 2021;9(8):928. doi:10.3390/biomedicines9080928
12. Li M, Zhao G, Su WK, Shuai Q. Enzyme-Responsive Nanoparticles for Anti-tumor Drug Delivery. Front Chem. 2020;8:1–20. doi:10.3389/fchem.2020.00001
13. Guo X, Shi C, Wang J, Di S, Zhou S. PH-triggered intracellular release from actively targeting polymer micelles. Biomaterials. 2013;34(18):4544–4554. doi:10.1016/j.biomaterials.2013.02.071
14. Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized Phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19(14):3312–3322. doi:10.1200/JCO.2001.19.14.3312
15. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–7803. doi:10.1200/JCO.2005.04.937
16. Wilhelm S, Tavares AJ, Dai Q, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1(5):1–12. doi:10.1038/natrevmats.2016.14
17. Christopher AMLS M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Physiol Behav. 2016;176:100–106.
18. Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–664. doi:10.1038/nrclinonc.2010.139
19. Li C, Zhang J, Zu YJ, et al. Biocompatible and biodegradable nanoparticles for enhancement of anti-cancer activities of phytochemicals. Chin J Nat Med. 2015;13(9):641–652. doi:10.1016/S1875-5364(15)30061-3
20. Zhao X, Xie N, Zhang H, Zhou W, Ding J. Bacterial Drug Delivery Systems for Cancer Therapy: “Why” and “How”. Pharmaceutics. 2023;15(9):2214. doi:10.3390/pharmaceutics15092214
21. Yue Y, Xu J, Li Y, et al. Antigen-bearing outer membrane vesicles as tumour vaccines produced in situ by ingested genetically engineered bacteria. Nat Biomed Eng. 2022;6(7):898–909. doi:10.1038/s41551-022-00886-2
22. Harimoto T, Hahn J, Chen YY, et al. A programmable encapsulation system improves delivery of therapeutic bacteria in mice. Nat Biotechnol. 2022;40(8):1259–1269. doi:10.1038/s41587-022-01244-y
23. Thomas SC, Madaan T, Kamble NS, Siddiqui NA, Pauletti GM, Kotagiri N. Engineered Bacteria Enhance Immunotherapy and Targeted Therapy through Stromal Remodeling of Tumors. Adv Healthc Mater. 2022;11(2):e2101487. doi:10.1002/adhm.202101487
24. Van Mellaert L, Barbé S, Anné J. Clostridium spores as anti-tumour agents. Trends Microbiol. 2006;14(4):190–196. doi:10.1016/j.tim.2006.02.002
25. Coley WB. Contribution to the Knowledge of Sarcoma. Ann Surg. 1891;14:199–220. doi:10.1097/00000658-189112000-00015
26. Loughlin KR, William B. Coley: his Hypothesis, His Toxin, and the Birth of Immunotherapy. Urol Clin North Am. 2020;47(4):413–417. doi:10.1016/j.ucl.2020.07.001
27. Tsung K, Norton JA. Lessons from Coley’s Toxin. Surg Oncol. 2006;15(1):25–28. doi:10.1016/j.suronc.2006.05.002
28. Pearl R. Cancer and Tuberculosis. Am J Hyg. 1929;9:97–159.
29. Lj OLD, Clarke DA, Benacerraf B. Effect of Bacillus Calmette-Guerin infection on transplanted tumours in the mouse. Nature. 1959;184(4682):291–292. doi:10.1038/184291a0
30. Zbar B, Bernstein ID, Rapp HJ, Branch B. Suppression of tumor growth at the site of infection with living Bacillus Calmette-Guérin. J Natl Cancer Inst. 1971;46(4):831–839.
31. Lamm DL, Thor DE, Harris SC, Reyna JA, Stogdill VD, Radwin HM. Bacillus Calmette-Guerin immunotherapy of superficial bladder cancer. J Urol. 1980;124(1):38–42. doi:10.1016/S0022-5347(17)55282-9
32. DeKernion JB, Huang MY, Lindner A, Smith RB, Kaufman JJ. The management of superficial bladder tumors and carcinoma in situ with intravesical bacillus Calmette-Guerin. J Urol. 1985;133(4):598–600. doi:10.1016/S0022-5347(17)49104-X
33. Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14(1):11–31. doi:10.1038/nrclinonc.2016.60
34. Sambasivarao SV. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 2013;18:1199–1216.
35. Chouaib S, Noman MZ, Kosmatopoulos K, Curran MA. Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene. 2017;36(4):439–445. doi:10.1038/onc.2016.225
36. Vaupel P, Multhoff G, Vaupel P, Multhoff G. Hypoxia-/HIF-1α-Driven Factors of the Tumor Microenvironment Impeding Antitumor Immune Responses and Promoting Malignant Progression. Oxyg Transp Tissue XL. 2018;171–175.
37. Heldin CH, Rubin K, Pietras K, Östman A. High interstitial fluid pressure - An obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806–813. doi:10.1038/nrc1456
38. Leschner S, Westphal K, Dietrich N, et al. Tumor invasion of Salmonella enterica serovar Typhimurium is accompanied by strong hemorrhage promoted by TNF-α. PLoS One. 2009;4(8):e6692. doi:10.1371/journal.pone.0006692
39. Forbes NS, Munn LL, Fukumura D, Jain RK. Sparse initial entrapment of systemically injected Salmonella typhimurium leads to heterogeneous accumulation within tumors. Cancer Res. 2003;63(17):5188–5193.
40. Jivrajani M, Nivsarkar M. Ligand-targeted bacterial minicells: futuristic nano-sized drug delivery system for the efficient and cost effective delivery of shRNA to cancer cells. Nanomedicine. 2016;12(8):2485–2498. doi:10.1016/j.nano.2016.06.004
41. Granato ET, Meiller-Legrand TA, Foster KR. The Evolution and Ecology of Bacterial Warfare. Curr Biol. 2019;29(11):R521–37. doi:10.1016/j.cub.2019.04.024
42. Baindara P, Mandal SM. Bacteria and bacterial anticancer agents as a promising alternative for cancer therapeutics. Biochimie. 2020;177:164–189. doi:10.1016/j.biochi.2020.07.020
43. Kang SR, Nguyen DH, Yoo SW, Min JJ. Bacteria and bacterial derivatives as delivery carriers for immunotherapy. Adv Drug Deliv Rev. 2022;181:114085. doi:10.1016/j.addr.2021.114085
44. Farkas-Himsley H, Hill R, Rosen B, Arab S, Lingwood CA. The bacterial colicin active against tumor cells in vitro and in vivo is verotoxin 1. Proc Natl Acad Sci U S A. 1995;92(15):6996–7000. doi:10.1073/pnas.92.15.6996
45. Lancaster LE, Wintermeyer W, Rodnina MV. Colicins and their potential in cancer treatment. Blood Cells Mol Dis. 2007;38(1):15–18. doi:10.1016/j.bcmd.2006.10.006
46. Stachowiak R, Lyzniak M, Budziszewska BK, et al. Cytotoxicity of bacterial metabolic products, including listeriolysin O, on leukocyte targets. J Biomed Biotechnol. 2012;2012:1–9. doi:10.1155/2012/954375
47. Lai XH, Arencibia I, Johansson A, et al. Cytocidal and Apoptotic Effects of the ClyA Protein from Escherichia coli on Primary and Cultured Monocytes and Macrophages. Infect Immun. 2000;68(7):4363–4367. doi:10.1128/IAI.68.7.4363-4367.2000
48. Barak Y, Schreiber F, Thorne SH, Contag CH, deBeer D, Matin A. Role of nitric oxide in Salmonella typhimurium-mediated cancer cell killing. BMC Cancer. 2010;10(1). doi:10.1186/1471-2407-10-146
49. Williams EL, Djamgoz MBA. Nitric oxide and metastatic cell behaviour. BioEssays. 2005;27(12):1228–1238. doi:10.1002/bies.20324
50. Liu X, Yin S, Chen Y, et al. LPS-induced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NF-κB, STAT3 or AP-1 activation. Mol Med Rep. 2018;17:5484–5491.
51. Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi:10.1126/science.aac4255
52. Huang X, Pan J, Xu F, et al. Bacteria-Based Cancer Immunotherapy. Adv Sci. 2021;8:1–22.
53. Bouzari S, Bakhtiari R, Ajoudanifar H, Sadat Shandiz SA. Review of the Effects of Probiotics and Their Metabolites in the Treatment of Liver Cancer: an Update on Probiotics as a New Treatment. Erciyes Med J. 2023;45:8–18.
54. Li Z, Wang Y, Liu J, et al. Chemically and Biologically Engineered Bacteria-Based Delivery Systems for Emerging Diagnosis and Advanced Therapy. Adv Mater. 2021;33(38):e2102580. doi:10.1002/adma.202102580
55. Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19(22):2645–2655. doi:10.1101/gad.1299905
56. Feng Q, Ma X, Cheng K, et al. Engineered Bacterial Outer Membrane Vesicles as Controllable Two-Way Adaptors to Activate Macrophage Phagocytosis for Improved Tumor Immunotherapy. Adv Mater. 2022;34:e2206200. doi:10.1002/adma.202206200
57. Hajam IA, Dar PA, Won G, Lee JH. Bacterial Ghosts as Adjuvants: mechanisms and Potential. Vet Res. 2017;48(1):37. doi:10.1186/s13567-017-0442-5
58. Jiménez-Jiménez C, Moreno VM, Vallet-Regí M. Bacteria-Assisted Transport of Nanomaterials to Improve Drug Delivery in Cancer Therapy. Nanomaterials. 2022;12(2):288. doi:10.3390/nano12020288
59. Yin T, Diao Z, Blum NT, Qiu L, Ma A, Huang P. Engineering Bacteria and Bionic Bacterial Derivatives with Nanoparticles for Cancer Therapy. Small. 2022;18(12):e2104643. doi:10.1002/smll.202104643
60. Germanier R, Fuerer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975;131(5):553–558. doi:10.1093/infdis/131.5.553
61. Lin IYC, Van TTH, Smooker PM. Live-attenuated bacterial vectors: tools for vaccine and therapeutic agent delivery. Vaccines. 2015;3(4):940–972. doi:10.3390/vaccines3040940
62. Low KB, Ittensohn M, Le T, et al. Lipid A mutant Salmonella with suppressed virulence and TNFa induction retain tumor-targeting in vivo. Nat Biotechnol. 1999;17(1):37–41. doi:10.1038/5205
63. Zhao M, Yang M, Ma H, et al. Targeted Therapy with a Salmonella Typhimurium Leucine-Arginine Auxotroph Cures Orthotopic Human Breast Tumors in Nude Mice. Cancer Res. 2006;66(15):7647–7652. doi:10.1158/0008-5472.CAN-06-0716
64. Fàbrega A, Vila J. Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin Microbiol Rev. 2013;26(2):308–341. doi:10.1128/CMR.00066-12
65. Li CX, Yu B, Shi L, et al. ‘obligate‘anaerobic Salmonella strain YB1 suppresses liver tumor growth and metastasis in nude mice. Oncol Lett. 2017;13(1):177–183. doi:10.3892/ol.2016.5453
66. Clairmont C, Lee KC, Pike J, et al. Biodistribution and Genetic Stability of the Novel Antitumor Agent VNP20009, a Genetically Modified Strain of Salmonella typhimurium. J Infect Dis. 2000;181(6):1996–2002. doi:10.1086/315497
67. Zhao M, Yang M, Li XM, et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci U S A. 2005;102(3):755–760. doi:10.1073/pnas.0408422102
68. Kimura H, Zhang L, Zhao M, et al. Targeted therapy of spinal cord glioma with a genetically modified Salmonella typhimurium. Cell Prolif. 2010;43(1):41–48. doi:10.1111/j.1365-2184.2009.00652.x
69. Nagakura C, Hayashi K, Zhao M, et al. Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res. 2009;29(6):1873–1878.
70. Hayashi K, Zhao M, Yamauchi K, et al. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J Cell Biochem. 2009;106(6):992–998. doi:10.1002/jcb.22078
71. Hayashi K, Zhao M, Yamauchi K, et al. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhimurium. Cell Cycle. 2009;8(6):870–875. doi:10.4161/cc.8.6.7891
72. Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci U S A. 2007;104(24):10170–10174. doi:10.1073/pnas.0703867104
73. Yu B, Yang M, Shi L, et al. Explicit hypoxia targeting with tumor suppression by creating an ‘obligate’ anaerobic Salmonella Typhimurium strain. Sci Rep. 2012;2(1):1–10. doi:10.1038/srep00436
74. Ning BT, Yu B, Chan S, Chan JL, Huang JD, Chan GCF. Treatment of Neuroblastoma with an Engineered “Obligate” Anaerobic Salmonella typhimurium Strain YB1. J Cancer. 2017;8(9):1609–1618. doi:10.7150/jca.18776
75. Toley BJ, Forbes NS. Motility is critical for effective distribution and accumulation of bacteria in tumor tissue. Integr Biol. 2012;4(2):165–176. doi:10.1039/c2ib00091a
76. Kasinskas RW, Forbes NS. Salmonella typhimurium Lacking Ribose Chemoreceptors Localize in Tumor Quiescence and Induce Apoptosis. Cancer Res. 2007;67(7):3201–3209. doi:10.1158/0008-5472.CAN-06-2618
77. Sznol M, Lin SL, Bermudes D, Zheng L, King I. Perspective. Use of preferentially replicating bacteria for the treatment of cancer. J Clin Invest. 2000;105(8):1027–1030. doi:10.1172/JCI9818
78. Uchugonova A, Zhao M, Zhang Y, Weinigel M, König K, Hoffman RM. Cancer-cell killing by engineered Salmonella imaged by multiphoton tomography in live mice. Anticancer Res. 2012;32(10):4331–4338.
79. Tu DG, Chang WW, Lin ST, Kuo CY, Tsao YT, Lee CH. Salmonella inhibits tumor angiogenesis by downregulation of vascular endothelial growth factor. Oncotarget. 2016;7(25):37513–37523. doi:10.18632/oncotarget.7038
80. Liu F, Zhang L, Hoffman RM, Zhao M. Vessel destruction by tumor-targeting Salmonella typhimurium A1-R is enhanced by high tumor vascularity. Cell Cycle. 2010;9(22):4518–4524. doi:10.4161/cc.9.22.13744
81. Kuan YD, Lee CH. Salmonella overcomes tumor immune tolerance by inhibition of tumor indoleamine 2, 3-dioxygenase 1 expression. Oncotarget. 2016;7(1):374–385. doi:10.18632/oncotarget.6258
82. Wang WK, Lu MF, Kuan YD, Lee CH. The treatment of mouse colorectal cancer by oral delivery tumor-targeting Salmonella. Am J Cancer Res. 2015;5(7):2222–2228.
83. Pizarro-Cerdá J, Cossart P. Microbe profile: listeria monocytogenes: a paradigm among intracellular bacterial pathogens. Microbiol. 2019;165(7):719–721. doi:10.1099/mic.0.000800
84. Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol. 2018;16(1):32–46. doi:10.1038/nrmicro.2017.126
85. Pizarro-Cerdá J, Kühbacher A, Cossart P. Entry of listeria monocytogenes in mammalian epithelial cells: an updated view. Cold Spring Harb Perspect Med. 2012;2(11):a010009–a010009. doi:10.1101/cshperspect.a010009
86. Lambrechts A, Gevaert K, Cossart P, Vandekerckhove J, Van Troys M. Listeria comet tails: the actin-based motility machinery at work. Trends Cell Biol. 2008;18(5):220–227. doi:10.1016/j.tcb.2008.03.001
87. Flickinger JC, Rodeck U, Snook AE. Listeria monocytogenes as a vector for cancer immunotherapy: current understanding and progress. Vaccines. 2018;6(3):48. doi:10.3390/vaccines6030048
88. Brocksted DG, Giedlin MA, Leong ML, et al. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci U S A. 2004;101(38):13832–13837. doi:10.1073/pnas.0406035101
89. Thompson RJ, Bouwer HGA, Portnoy DA, Frankel FR. Pathogenicity and Immunogenicity of a Listeria monocytogenes Strain That Requires d-Alanine for Growth. Infect Immun. 1998;66(8):3552–3561. doi:10.1128/IAI.66.8.3552-3561.1998
90. Verch T, Pan ZK, Paterson Y. Listeria monocytogenes -Based Antibiotic Resistance Gene-Free Antigen Delivery System Applicable to Other Bacterial Vectors and DNA Vaccines. Infect Immun. 2004;72(11):6418–6425. doi:10.1128/IAI.72.11.6418-6425.2004
91. Rayevskaya MV, Frankel FR. Systemic Immunity and Mucosal Immunity Are Induced against Human Immunodeficiency Virus Gag Protein in Mice by a New Hyperattenuated Strain of Listeria monocytogenes. J Virol. 2001;75(6):2786–2791. doi:10.1128/JVI.75.6.2786-2791.2001
92. Wallecha A, Maciag PC, Rivera S, Paterson Y, Shahabi V. Construction and Characterization of an Attenuated Listeria monocytogenes Strain for Clinical Use in Cancer Immunotherapy. Clin Vaccine Immunol. 2009;16(1):96–103. doi:10.1128/CVI.00274-08
93. Li Z, Zhao X, Higgins DE, Frankel FR. Conditional Lethality Yields a New Vaccine Strain of Listeria monocytogenes for the Induction of Cell-Mediated Immunity. Infect Immun. 2005;73(8):5065–5073. doi:10.1128/IAI.73.8.5065-5073.2005
94. Brockstedt DG, Bahjat KS, Giedlin MA, et al. Killed but metabolically active microbes: a new vaccine paradigm for eliciting effector T-cell responses and protective immunity. Nat Med. 2005;11(8):853–860. doi:10.1038/nm1276
95. Lauer P, Hanson B, Lemmens EE, et al. Constitutive Activation of the PrfA Regulon Enhances the Potency of Vaccines Based on Live-Attenuated and Killed but Metabolically Active Listeria monocytogenes Strains. Infect Immun. 2008;76(8):3742–3753. doi:10.1128/IAI.00390-08
96. Ikonomidis G, Paterson Y, Kos FJ, Portnoy DA. Delivery of a viral antigen to the class i processing and presentation pathway by listeria monocytogenes. J Exp Med. 1994;180(6):2209–2218. doi:10.1084/jem.180.6.2209
97. Shahabi V, Reyes-Reyes M, Wallecha A, Rivera S, Paterson Y, MacIag P. Development of a Listeria monocytogenes based vaccine against prostate cancer. Cancer Immunol Immunother. 2008;57(9):1301–1313. doi:10.1007/s00262-008-0463-z
98. Gunn GR, Zubair A, Peters C, Pan Z-K, Wu T-C, Paterson Y. Two Listeria monocytogenes Vaccine Vectors That Express Different Molecular Forms of Human Papilloma Virus-16 (HPV-16) E7 Induce Qualitatively Different T Cell Immunity That Correlates with Their Ability to Induce Regression of Established Tumors Immortalized by HPV-16. J Immunol. 2001;167(11):6471–6479. doi:10.4049/jimmunol.167.11.6471
99. Sewell DA, Shahabi V, Gunn GR, Pan ZK, Dominiecki ME, Paterson Y. Recombinant Listeria Vaccines Containing PEST Sequences Are Potent Immune Adjuvants for the Tumor-Associated Antigen Human Papillomavirus-16 E7. Cancer Res. 2004;64(24):8821–8825. doi:10.1158/0008-5472.CAN-04-1958
100. Schnupf P, Zhou J, Varshavsky A, Portnoy DA. Listeriolysin O Secreted by Listeria monocytogenes into the Host Cell Cytosol Is Degraded by the N-End Rule Pathway. Infect Immun. 2007;75(11):5135–5147. doi:10.1128/IAI.00164-07
101. Jin MP, Ng VH, Maeda S, Rest RF, Karin M. Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists. J Exp Med. 2004;200(12):1647–1655. doi:10.1084/jem.20041215
102. Wallecha A, Wood L, Pan ZK, Maciag PC, Shahabi V, Paterson Y. Listeria monocytogenes-derived listeriolysin O has pathogen-associated molecular pattern-like properties independent of its hemolytic ability. Clin Vaccine Immunol. 2013;20(1):77–84. doi:10.1128/CVI.00488-12
103. Hussain SF, Paterson Y. CD4+CD25+ regulatory T cells that secrete TGFβ and IL-10 are preferentially induced by a vaccine vector. J Immunother. 2004;27(5):339–346. doi:10.1097/00002371-200409000-00002
104. Chen Z, Ozbun L, Chong N, Wallecha A, Berzofsky JA, Khleif SN. Episomal expression of truncated listeriolysin O in LmddA-LLO- E7 vaccine enhances antitumor efficacy by preferentially inducing expansions of CD4+ FoxP3− and CD8+ T cells Zhisong. Cancer Immunol Res. 2014;2(9):911–922. doi:10.1158/2326-6066.CIR-13-0197
105. Sewell DA, Pan ZK, Paterson Y. Listeria-based HPV-16 E7 vaccines limit autochthonous tumor growth in a transgenic mouse model for HPV-16 transformed tumors. Vaccine. 2008;26(41):5315–5320. doi:10.1016/j.vaccine.2008.07.036
106. Wood LM, Pan ZK, Shahabi V, Paterson Y. Listeria-derived ActA is an effective adjuvant for primary and metastatic tumor immunotherapy. Cancer Immunol Immunother. 2010;59(7):1049–1058. doi:10.1007/s00262-010-0830-4
107. Moors MA, Auerbuch V, Portnoy DA. Stability of the Listeria monocytogenes ActA protein in mammalian cells is regulated by the N-end rule pathway. Cell Microbiol. 1999;1(3):249–257. doi:10.1046/j.1462-5822.1999.00020.x
108. Parker RC, Plummer HC, Siebenmann CO, Chapman MG. Effect of Histolyticus Infection and Toxin on Transplantable Mouse Tumors. Proc Soc Exp Biol Med. 1947;66(2):461–467. doi:10.3181/00379727-66-16124
109. Barbé S, Van Mellaert L, Theys J, et al. Secretory production of biologically active rat interleukin-2 by Clostridium acetobutylicum DSM792 as a tool for anti-tumor treatment. FEMS Microbiol Lett. 2005;246(1):67–73. doi:10.1016/j.femsle.2005.03.037
110. Liu S, Xu X, Zeng X, Li L, Chen Q, Li J. Tumor-targeting bacterial therapy: a potential treatment for oral cancer. Oncol Lett. 2014;8(6):2359–2366. doi:10.3892/ol.2014.2525
111. Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci U S A. 2001;98(26):15155–15160. doi:10.1073/pnas.251543698
112. Bettegowda C, Huang X, Lin J, et al. The genome and transcriptomes of the anti-tumor agent Clostridium novyi-NT. Nat Biotechnol. 2006;24(12):1573–1580. doi:10.1038/nbt1256
113. Agrawal N, Bettegowda C, Cheong I, et al. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc Natl Acad Sci U S A. 2004;101(42):15172–15177. doi:10.1073/pnas.0406242101
114. Zhang YL, Lü R, Chang ZS, et al. Clostridium sporogenes delivers interleukin-12 to hypoxic tumours, producing antitumour activity without significant toxicity. Lett Appl Microbiol. 2014;59(6):580–586. doi:10.1111/lam.12322
115. Kaper JB, Nataro JP, Mobley HLT. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2(2):123–140. doi:10.1038/nrmicro818
116. Acs Y, Loo JFC, Yu S, Kong SK, Chan TF. Monitoring bacterial growth using tunable resistive pulse sensing with a pore-based technique. Appl Microbiol Biotechnol. 2014;98(2):855–862. doi:10.1007/s00253-013-5377-9
117. Kubitschek HE. Cell volume increase in Escherichia coli after shifts to richer media. J Bacteriol. 1990;172(1):94–101. doi:10.1128/jb.172.1.94-101.1990
118. Liu Q, Gai Y, Chen Y, Lan X, Jiang D. Escherichia coli nissle 1917 as a novel microrobot for tumor-targeted imaging and therapy. Pharmaceutics. 2021;13(8):1226. doi:10.3390/pharmaceutics13081226
119. Henker J, Laass M, Blokhin BM, et al. The probiotic Escherichia coli strain Nissle 1917 (EcN) stops acute diarrhoea in infants and toddlers. Eur J Pediatr. 2007;166(4):311–318. doi:10.1007/s00431-007-0419-x
120. Stritzker J, Weibel S, Hill PJ, Oelschlaeger TA, Goebel W, Szalay AA. Tumor-specific colonization, tissue distribution, and gene induction by probiotic Escherichia coli Nissle 1917 in live mice. Int J Med Microbiol. 2007;297(3):151–162. doi:10.1016/j.ijmm.2007.01.008
121. He L, Yang H, Liu F, et al. Escherichia coli Nissle 1917 engineered to express Tum-5 can restrain murine melanoma growth. Oncotarget. 2017;8(49):85772–85782. doi:10.18632/oncotarget.20486
122. Zhang Y, Zhang Y, Xia L, et al. Escherichia coli Nissle 1917 targets and restrains mouse b16 melanoma and 4T1 breast tumors through expression of azurin protein. Appl Environ Microbiol. 2012;78(21):7603–7610. doi:10.1128/AEM.01390-12
123. Zschüttig A, Auerbach C, Meltke S, et al. Complete Sequence of Probiotic Symbioflor 2 Escherichia coli Strain G3/10 and Draft Sequences of Symbioflor 2 E. coli Strains G1/2, G4/9, G5, G6/7, and G8. Genome Announc. 2015;3(2):9–10. doi:10.1128/genomeA.01330-14
124. Kocijancic D, Felgner S, Frahm M, et al. Therapy of solid tumors using probiotic Symbioflor-2-restraints and potential. Oncotarget. 2016;7(16):22605–22622. doi:10.18632/oncotarget.8027
125. Zhu J, Ke Y, Liu Q, et al. Engineered Lactococcus lactis secreting Flt3L and OX40 ligand for in situ vaccination-based cancer immunotherapy. Nat Commun. 2022;13(1):7466. doi:10.1038/s41467-022-35130-7
126. Kaczmarek K, Więckiewicz J, Węglarczyk K, Siedlar M, Baran J. The Anti-Tumor Effect of Lactococcus lactis Bacteria-Secreting Human Soluble TRAIL Can Be Enhanced by Metformin Both In Vitro and In Vivo in a Mouse Model of Human Colorectal Cancer. Cancers. 2021;13(12):3004. doi:10.3390/cancers13123004
127. Heydari Z, Rahaie M, Alizadeh AM, Agah S, Khalighfard S, Bahmani S. Effects of Lactobacillus acidophilus and Bifidobacterium bifidum Probiotics on the Expression of MicroRNAs 135b, 26b, 18a and 155, and Their Involving Genes in Mice Colon Cancer. Probiotics Antimicrob Proteins. 2019;11(4):1155–1162. doi:10.1007/s12602-018-9478-8
128. Huter V, Szostak MP, Gampfer J, et al. Bacterial ghosts as drug carrier and targeting vehicles. J Control Release. 1999;61(1–2):51–63. doi:10.1016/S0168-3659(99)00099-1
129. Langemann T, Koller VJ, Muhammad A, Kudela P, Mayr UB, Lubitz W. The bacterial ghost platform system: production and applications. Bioeng Bugs. 2010;1(5):326–336. doi:10.4161/bbug.1.5.12540
130. Hajam IA, Dar PA, Appavoo E, Kishore S, Bhanuprakash V, Ganesh K. Bacterial ghosts of Escherichia coli drive efficient maturation of bovine monocyte-derived dendritic cells. PLoS One. 2015;10(12):1–15. doi:10.1371/journal.pone.0144397
131. Kraśko JA, Źilionyte K, Darinskas A, et al. Bacterial ghosts as adjuvants in syngeneic tumour cell lysate-based anticancer vaccination in a murine lung carcinoma model. Oncol Rep. 2017;37(1):171–178. doi:10.3892/or.2016.5252
132. Parti RP, Biswas D, Wang M, Liao M, Dillon JAR. A MinD mutant of enterohemorrhagic E. coli O157:H7 has reduced adherence to human epithelial cells. Microb Pathog. 2011;51(5):378–383. doi:10.1016/j.micpath.2011.07.003
133. MacDiarmid JA, Brahmbhatt H. Minicells: versatile vectors for targeted drug or si/shRNA cancer therapy. Curr Opin Biotechnol. 2011;22(6):909–916. doi:10.1016/j.copbio.2011.04.008
134. Farley MM, Hu B, Margolin W, Liu J, de Boer P. Minicells, back in fashion. J Bacteriol. 2016;198(8):1186–1195. doi:10.1128/JB.00901-15
135. MacDiarmid JA, Amaro-Mugridge NB, Madrid-Weiss J, et al. Sequential treatment of drug-resistant tumors with targeted minicells containing siRNA or a cytotoxic drug. Nat Biotechnol. 2009;27(7):643–651. doi:10.1038/nbt.1547
136. Shubhika K. Nanotechnology and medicine - The upside and the downside. Int J Drug Dev Res. 2013;5:1–10.
137. Nguyen HN, Romero Jovel S, Nguyen THK. Nanosized Minicells Generated by Lactic Acid Bacteria for Drug Delivery. J Nanomater. 2017;2017:1–10. doi:10.1155/2017/6847297
138. Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002;4(3):95–99. doi:10.1186/bcr432
139. Solomon BJ, Desai J, Rosenthal M, et al. A First-Time-In-Human Phase i Clinical Trial of Bispecific Antibody-Targeted, Paclitaxel-Packaged Bacterial Minicells. PLoS One. 2015;10(12):1–17. doi:10.1371/journal.pone.0144559
140. MacDiarmid JA, Langova V, Bailey D, et al. Targeted doxorubicin delivery to brain tumors via minicells: proof of principle using dogs with spontaneously occurring tumors as a model. PLoS One. 2016;11(4):1–23. doi:10.1371/journal.pone.0151832
141. Zhang Y, Ji W, He L, et al. E. coli Nissle 1917-Derived Minicells for Targeted Delivery of Chemotherapeutic Drug to Hypoxic Regions for Cancer Therapy. Theranostics. 2018;8(6):1690–1705. doi:10.7150/thno.21575
142. Chatterjee SN, Das J. Electron Microscopic Observations on the Excretion of Cell-wall Material. J Gen Microbiol. 1967;49(1):1–11. doi:10.1099/00221287-49-1-1
143. Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. 2019;17(1):13–24. doi:10.1038/s41579-018-0112-2
144. Gujrati V, Kim S, Kim SH, et al. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano. 2014;8(2):1525–1537. doi:10.1021/nn405724x
145. Li Y, Zhao R, Cheng K, et al. Bacterial Outer Membrane Vesicles Presenting Programmed Death 1 for Improved Cancer Immunotherapy via Immune Activation and Checkpoint Inhibition. ACS Nano. 2020;14(12):16698–16711. doi:10.1021/acsnano.0c03776
146. Santos JC, Dick MS, Lagrange B, et al. LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. EMBO J. 2018;37(6):1–19. doi:10.15252/embj.201798089
147. Dorward DW, Garon CF. DNA is packaged within membrane-derived vesicles of gram-negative but not gram-positive bacteria. Appl Environ Microbiol. 1990;56(6):1960–1962. doi:10.1128/aem.56.6.1960-1962.1990
148. Zhang Y, Fang Z, Li R, Huang X, Liu Q. Design of outer membrane vesicles as cancer vaccines: a new toolkit for cancer therapy. Cancers. 2019;11(9):1–24. doi:10.3390/cancers11091314
149. Blakemore R. Magnetotactic bacteria. Science. 1975;190(4212):377–379. doi:10.1126/science.170679
150. Dieudonné A, Pignol D, Prévéral S. Magnetosomes: biogenic iron nanoparticles produced by environmental bacteria. Appl Microbiol Biotechnol. 2019;103(9):3637–3649. doi:10.1007/s00253-019-09728-9
151. Farina M, Esquivel DMS, de Barros HGPL. Magnetic iron-sulphur crystals from a magnetotactic microorganism. Nature. 1990;343(6255):256–258. doi:10.1038/343256a0
152. Frankel RB, Blakemore RP, Wolfe RS. Magnetite in freshwater magnetotactic bacteria. Science. 1979;203(4387):1355–1356. doi:10.1126/science.203.4387.1355
153. Lisjak D, Mertelj A. Anisotropic magnetic nanoparticles: a review of their properties, syntheses and potential applications. Prog Mater Sci. 2018;95:286–328.
154. Yang W, Bai Y, Wang X, Dong X, Li Y, Fang M. Attaching biosynthesized bacterial magnetic particles to polyethylenimine enhances gene delivery into mammalian cells. J Biomed Nanotechnol. 2016;12(4):789–799. doi:10.1166/jbn.2016.2213
155. Mériaux S, Boucher M, Marty B, et al. Magnetosomes, Biogenic Magnetic Nanomaterials for Brain Molecular Imaging with 17.2 T MRI Scanner. Adv Healthc Mater. 2015;4(7):1076–1083. doi:10.1002/adhm.201400756
156. Orlando T, Mannucci S, Fantechi E, et al. Characterization of magnetic nanoparticles from Magnetospirillum Gryphiswaldense as potential theranostics tools. Contrast Media Mol Imaging. 2016;11(2):139–145. doi:10.1002/cmmi.1673
157. Erdal E, Demirbilek M, Yeh Y, et al. A Comparative Study of Receptor-Targeted Magnetosome and HSA-Coated Iron Oxide Nanoparticles as MRI Contrast-Enhancing Agent in Animal Cancer Model. Appl Biochem Biotechnol. 2018;185(1):91–113. doi:10.1007/s12010-017-2642-x
158. Alphandéry E, Idbaih A, Adam C, et al. Chains of magnetosomes with controlled endotoxin release and partial tumor occupation induce full destruction of intracranial U87-Luc glioma in mice under the application of an alternating magnetic field. J Control Release. 2017;262:259–272. doi:10.1016/j.jconrel.2017.07.020
159. Long R, Liu Y, Dai Q, Wang S, Deng Q, Zhou X. A natural bacterium-produced membrane-bound nanocarrier for drug combination therapy. Materials. 2016;9(11):1–10. doi:10.3390/ma9110889
160. Wang X, Wang J, Geng Y, et al. An enhanced anti-tumor effect of apoptin-cecropin B on human hepatoma cells by using bacterial magnetic particle gene delivery system. Biochem Biophys Res Commun. 2018;496(2):719–725. doi:10.1016/j.bbrc.2018.01.108
161. Dai Q, Long R, Wang S, et al. Bacterial magnetosomes as an efficient gene delivery platform for cancer theranostics. Microb Cell Fact. 2017;16(1):1–9. doi:10.1186/s12934-017-0830-6
162. Pum D, Toca-Herrera JL, Sleytr UB. S-Layer protein self-assembly. Int J Mol Sci. 2013;14(2):2484–2501. doi:10.3390/ijms14022484
163. Stel B, Cometto F, Rad B, De Yoreo JJ, Lingenfelder M. Dynamically resolved self-assembly of S-layer proteins on solid surfaces. Chem Commun. 2018;54(73):10264–10267. doi:10.1039/C8CC04597F
164. Ilk N, Egelseer EM, Sleytr UB. S-layer fusion proteins-construction principles and applications. Curr Opin Biotechnol. 2011;22(6):824–831. doi:10.1016/j.copbio.2011.05.510
165. Wetzer B, Pum D, Sleytr UB. S-layer stabilized solid supported lipid bilayers. J Struct Biol. 1997;119(2):123–128. doi:10.1006/jsbi.1997.3867
166. Farjadian F, Moghoofei M, Mirkiani S, et al. Bacterial components as naturally inspired nano-carriers for drug/gene delivery and immunization: set the bugs to work? Biotechnol Adv. 2018;36(4):968–985. doi:10.1016/j.biotechadv.2018.02.016
167. Shi H, Chen L, Liu Y, et al. Bacteria-Driven Tumor Microenvironment-Sensitive Nanoparticles Targeting Hypoxic Regions Enhances the Chemotherapy Outcome of Lung Cancer. International Journal of Nanomedicine. 2023;18:1299–1315. doi:10.2147/IJN.S396863
168. Li J, Dai J, Zhao L, et al. Bioactive Bacteria/MOF Hybrids Can Achieve Targeted Synergistic Chemotherapy and Chemodynamic Therapy against Breast Tumors. Adv Funct Mater. 2023;33(42):2303254. doi:10.1002/adfm.202303254
169. Xiao S, Shi H, Zhang Y, et al. Bacteria-driven hypoxia targeting delivery of chemotherapeutic drug proving outcome of breast cancer. J Nanobiotechnol. 2022;20(1):178. doi:10.1186/s12951-022-01373-1
170. Li Y, Leng Q, Zhang Y, et al. Anaerobic bacteria mediated ‘smart missile’ targeting tumor hypoxic area enhances the therapeutic outcome of lung cancer. Chem Eng J. 2022;438:135566. doi:10.1016/j.cej.2022.135566
171. Chen Q, Bai H, Wu W, et al. Bioengineering Bacterial Vesicle-Coated Polymeric Nanomedicine for Enhanced Cancer Immunotherapy and Metastasis Prevention. Nano Lett. 2020;20(1):11–21. doi:10.1021/acs.nanolett.9b02182
172. Kuerban K, Gao X, Zhang H, et al. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm Sin B. 2020;10(8):1534–1548. doi:10.1016/j.apsb.2020.02.002
173. Patyar S, Joshi R, Byrav DSP, Prakash A, Medhi B, Das BK. Bacteria in cancer therapy: a novel experimental strategy. J Biomed Sci. 2010;17(1):1–9. doi:10.1186/1423-0127-17-21
174. Xie S, Zhao L, Song X, Tang M, Mo C, Li X. Doxorubicin-conjugated Escherichia coli Nissle 1917 swimmers to achieve tumor targeting and responsive drug release. J Control Release. 2017;268:390–399. doi:10.1016/j.jconrel.2017.10.041
175. Quispe-Tintaya W, Chandra D, Jahangir A, et al. Nontoxic radioactive Listeria at is a highly effective therapy against metastatic pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110(21):8668–8673. doi:10.1073/pnas.1211287110
176. Chandra D, Selvanesan BC, Yuan Z, et al. 32-Phosphorus selectively delivered by listeria to pancreatic cancer demonstrates a strong therapeutic effect. Oncotarget. 2017;8(13):20729–20740. doi:10.18632/oncotarget.15117
177. Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17(11):657–674. doi:10.1038/s41571-020-0410-2
178. Qing S, Lyu C, Zhu L, et al. Biomineralized Bacterial Outer Membrane Vesicles Potentiate Safe and Efficient Tumor Microenvironment Reprogramming for Anticancer Therapy. Adv Mater. 2020;32:1–14.
179. Chen W, Wang Y, Qin M, et al. Bacteria-Driven Hypoxia Targeting for Combined Biotherapy and Photothermal Therapy. ACS Nano. 2018;12(6):5995–6005. doi:10.1021/acsnano.8b02235
180. Gujrati V, Prakash J, Malekzadeh-Najafabadi J, et al. Bioengineered bacterial vesicles as biological nano-heaters for optoacoustic imaging. Nat Commun. 2019;10(1):1–10. doi:10.1038/s41467-019-09034-y
181. Chen QW, Liu XH, Fan JX, et al. Self-Mineralized Photothermal Bacteria Hybridizing with Mitochondria-Targeted Metal–Organic Frameworks for Augmenting Photothermal Tumor Therapy. Adv Funct Mater. 2020;30. doi:10.1002/adfm.201909806
182. Zhuang Q, Xu J, Deng D, et al. Bacteria-derived membrane vesicles to advance targeted photothermal tumor ablation. Biomaterials. 2021;268:120550. doi:10.1016/j.biomaterials.2020.120550
183. Zheng DW, Chen Y, Li ZH, et al. Optically-controlled bacterial metabolite for cancer therapy. Nat Commun. 2018;9(1):1–12. doi:10.1038/s41467-018-03233-9
184. Luo Y, Xu D, Gao X, et al. Nanoparticles conjugated with bacteria targeting tumors for precision imaging and therapy. Biochem Biophys Res Commun. 2019;514(4):1147–1153. doi:10.1016/j.bbrc.2019.05.074
185. Wang H, Chen T, Wan L, et al. Attenuated Salmonella engineered with an apoptosis-inducing factor (AIF) eukaryotic expressing system enhances its anti-tumor effect in melanoma in vitro and in vivo. Appl Microbiol Biotechnol. 2020;104(8):3517–3528. doi:10.1007/s00253-020-10485-3
186. Mateos-Chávez AA, Muñoz-López P, Becerra-Báez EI, et al. Live Attenuated Salmonella enterica Expressing and Releasing Cell-Permeable Bax BH3 Peptide Through the MisL Autotransporter System Elicits Antitumor Activity in a Murine Xenograft Model of Human B Non-hodgkin’s Lymphoma. Front Immunol. 2019;10:1–22. doi:10.3389/fimmu.2019.02562
187. Samadi M, Majidzadeh-a K, Salehi M, et al. Engineered hypoxia-responding Escherichia coli carrying cardiac peptide genes, suppresses tumor growth, angiogenesis and metastasis in vivo. J Biol Eng. 2021;2:1–15.
188. Huang W, Shu C, Hua L, et al. Modified bacterial outer membrane vesicles induce autoantibodies for tumor therapy. Acta Biomater. 2020;108:300–312. doi:10.1016/j.actbio.2020.03.030
189. Swofford CA, Van Dessel N, Forbes NS. Quorum-sensing Salmonella selectively trigger protein expression within tumors. Proc Natl Acad Sci USA. 2015;112(11):3457–3462. doi:10.1073/pnas.1414558112
190. Chowdhury S, Castro S, Coker C, Hinchliffe TE, Arpaia N, Danino T. Programmable Bacteria Induce Durable Tumor Regression and Systemic Antitumor Immunity. Nat Med. 2019;25(7):1057–1063. doi:10.1038/s41591-019-0498-z
191. Yaghoubi A, Khazaei M, Jalili S, et al. Bacteria as a Double-Action Sword in Cancer. Biochim Biophys Acta Rev Cancer. 2020;1874:188388.
192. Li J, Zakariah M, Malik A, et al. Analysis of Salmonella Typhimurium Protein-Targeting in the Nucleus of Host Cells and the Implications in Colon Cancer: an in-Silico Approach. Infect Drug Resist. 2020;13:2433–2442.
193. Shi Y, Zheng W, Yang K. Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. J Exp Med. 2020;217(5):e20192282. doi:10.1084/jem.20192282
194. Lee SH, Cho SY, Yoon Y, et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat Microbiol. 2021;6(3):277–288. doi:10.1038/s41564-020-00831-6
195. Xie S, Zhang P, Zhang Z, et al. Bacterial navigation for tumor targeting and photothermally-triggered bacterial ghost transformation for spatiotemporal drug release. Acta Biomater. 2021;131:172–184. doi:10.1016/j.actbio.2021.06.030
196. Toso JF, Gill VJ, Hwu P, et al. Phase I Study of the Intravenous Administration of Attenuated Salmonella typhimurium to Patients With Metastatic Melanoma. J Clin Oncol. 2002;20(1):142–152. doi:10.1200/JCO.2002.20.1.142
197. Gniadek TJ, Augustin L, Schottel J, et al. A Phase I, dose escalation, single dose trial of oral attenuated salmonella typhimurium containing human IL-2 in patients with metastatic gastrointestinal cancers. J Immunother. 2020;43(7):217–221. doi:10.1097/CJI.0000000000000325
198. Le DT, Brockstedt DG, Nir-Paz R, et al. A Live-attenuated Listeria Vaccine (ANZ-100) and a Live- attenuated Listeria Vaccine Expressing Mesothelin (CRS-207) for Advanced Cancers: Phase 1 Studies of Safety and Immune Induction. Clin Cancer Res. 2012;18:858–868.
199. Le DT, Wang-Gillam A, Picozzi V, et al. Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes –Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. J Clin Oncol. 2015;33(12):1325–1333. doi:10.1200/JCO.2014.57.4244
200. Le DT, Picozzi VJ, Ko AH, et al. Results from a Phase IIb, Randomized, Multicenter Study of GVAX Pancreas and CRS-207 Compared with Chemotherapy in Adults with Previously Treated Metastatic Pancreatic Adenocarcinoma (ECLIPSE Study). Clin Cancer Res. 2019;25(18):5493–5502. doi:10.1158/1078-0432.CCR-18-2992
201. Hassan R, Alley E, Kindler H, et al. Clinical Response of Live-Attenuated, Listeria monocytogenes Expressing Mesothelin (CRS-207) with Chemotherapy in Patients with Malignant Pleural Mesothelioma. Clin Cancer Res. 2019;25(19):5787–5798. doi:10.1158/1078-0432.CCR-19-0070
202. Karbach J, Neumann A, Brand K, et al. Phase I Clinical Trial of Mixed Bacterial Vaccine (Coley’s Toxins) in Patients with NY-ESO-1 Expressing Cancers: immunological Effects and Clinical ActivityEffects of Mixed Bacterial Vaccine. Clin Cancer Res. 2012;18(19):5449–5459. doi:10.1158/1078-0432.CCR-12-1116
203. Steinberg GD, Shore ND, Karsh LI. Immune response results from vesigenurtacel-l (HS-410) in combination with BCG from a randomized phase 2 trial in patients with non-muscle invasive bladder cancer (NMIBC). J Clin Oncol. 2017;35(15_suppl):4531. doi:10.1200/JCO.2017.35.15_suppl.4531
204. Drake CG, Pachynski RK, Subudhi SK, et al. Safety and preliminary immunogenicity of JNJ-64041809, a live-attenuated, double-deleted Listeria monocytogenes-based immunotherapy, in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2022;25(2):219–228. doi:10.1038/s41391-021-00402-8
205. Janku F, Zhang HH, Pezeshki A, et al. Intratumoral Injection of Clostridium novyi-NT Spores in Patients with Treatment-refractory Advanced Solid Tumors. Clin Cancer Res. 2021;27(1):96–106. doi:10.1158/1078-0432.CCR-20-2065
206. Lucca I, Derré L, Cesson V, et al. Intravesical Ty21a Treatment of Non-muscle-invasive Bladder Cancer Shows a Good Safety Profile. Eur Urol Open Sci. 2022;45:55–58. doi:10.1016/j.euros.2022.09.004
207. Zhao AZ, Li F, Mai J, et al. Effects of oncolytic bacteria, SGN1, against a broad spectrum of malignancies in pre-clinical and clinical studies. J Clin Oncol. 2023;41(16_suppl):e15184. doi:10.1200/JCO.2023.41.16_suppl.e15184
208. Liu C, Han Y, Kankala R, Wang S, Chen A. Subcellular Performance of Nanoparticles in Cancer Therapy. Int J Nanomed. 2020;15:675–704. doi:10.2147/IJN.S226186
209. Forbes NS, Coffin RS, Deng L, et al. White paper on microbial anti-cancer therapy and prevention. J Immunother Cancer. 2018;6(1):1–24. doi:10.1186/s40425-018-0381-3
210. Gurbatri CR, Lia I, Vincent R, et al. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci Transl Med. 2020;12(530). doi:10.1126/scitranslmed.aax0876
211. Kim OY, Dinh NTH, Park HT, Choi SJ, Hong K, Gho YS. Bacterial protoplast-derived nanovesicles for tumor targeted delivery of chemotherapeutics. Biomaterials. 2017;113:68–79. doi:10.1016/j.biomaterials.2016.10.037
212. Aly RG, El-Enbaawy MI, Abd El-Rahman SS, Ata NS. Antineoplastic activity of Salmonella Typhimurium outer membrane nanovesicles. Exp Cell Res. 2021;399(1):112423. doi:10.1016/j.yexcr.2020.112423
213. Gao J, Wang S, Dong X, Wang Z. RGD-expressed bacterial membrane-derived nanovesicles enhance cancer therapy via multiple tumorous targeting. Theranostics. 2021;11(7):3301–3316. doi:10.7150/thno.51988
214. Kaczmarek K, Więckiewicz J, Węglarczyk K, Siedlar M, Baran J. The anti-tumor effect of Lactococcus lactis bacteria-secreting human soluble trail can be enhanced by metformin both in vitro and in vivo in a mouse model of human colorectal cancer. Cancers. 2021;13(12):1–25.
215. Boucher M, Geffroy F, Prévéral S, et al. Genetically tailored magnetosomes used as MRI probe for molecular imaging of brain tumor. Biomaterials. 2017;121:167–178. doi:10.1016/j.biomaterials.2016.12.013
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
