Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Awareness, Perception, and Utilization of Skin Lightening Agents Among Females of Asmara, Eritrea: A Cross-Sectional Study
Authors Tesfamariam S , Bahta M , Weldemariam DG , Tesfamariam EH , Yemane H , Bahta I, Russom M
Received 27 December 2022
Accepted for publication 21 April 2023
Published 8 May 2023 Volume 2023:16 Pages 1191—1202
DOI https://doi.org/10.2147/CCID.S396686
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Sirak Tesfamariam,1 Merhawi Bahta,1 Dawit G Weldemariam,2 Eyasu H Tesfamariam,3 Hermella Yemane,1 Iyassu Bahta,1 Mulugeta Russom1,4,5
1National Medicines and Food Administration, Ministry of Health, Asmara, Eritrea; 2Pharmacy, Hazhaz Zonal Referral Hospital, Asmara, Eritrea; 3Department of Statistics, Eritrean Institute of Technology, Mai-nefhi, Eritrea; 4Department of Medical Informatics, Erasmus Medical Center, Rotterdam, Netherlands; 5European Programme for Pharmacovigilance and Pharmacoepidemiology, University of Bordeaux, Bordeaux, France
Correspondence: Merhawi Bahta, Tel +2917294279, Email [email protected]
Background: The use of skin lightening agents (SLAs) is common among African females with black skin color. Although they usually contain harmful ingredients and can cause complications, their use remains to be a common practice. This study was conducted to assess the awareness, perception, and utilization of SLAs among females residing in Asmara, Eritrea.
Methods: A cross-sectional analytical study using a quantitative approach was conducted in representative samples of all beauty salons available in Asmara from May to July, 2021. The study participants were selected using two-stage stratified cluster sampling and data were collected through a face-to-face interview using a structured questionnaire. Descriptive analysis and logistic regression, at bivariate and multivariate level, were performed.
Results: The study enrolled 721 females and 684 completed the study. The majority of the respondents had the perception that SLAs can make someone light colored (84.4%), look beautiful (67.8%), trendy and fashionable (55.0%), and white skin is more attractive than dark skin (58.8%). About two-thirds (64.2%) reported they had previously used SLAs, mainly influenced by friends (60.5%). Approximately 46% were current users, while 53.6% stopped it mainly due to adverse effects, fear of adverse effects and ineffectiveness. A total of 150 products including natural ingredients were mentioned being used to lighten the skin, and Aneeza, Natural face, and Betamethasone containing brands were among the top used products. The occurrence of at least one adverse effect due to the use of SLAs was 43.7%, while 66.5% were satisfied with the use of SLAs. Additionally, employment status and perception of SLAs were found to be determinants of being a current user.
Conclusion: Utilization of SLAs, including products containing harmful or medicinal ingredients, was prevalent among the females of Asmara city. Thus, coordinated regulatory interventions are recommended to tackle unsafe practices and raise public awareness to promote the safe use of cosmetics.
Keywords: skin whitening, cosmetics, beauty salon, face-to-face interview
Introduction
Skin lightening agents (SLAs) are used to depigment the skin get a lighter skin color, and their use is common among females of African communities with black skin color.1–5 Although some skin lightening products are medically indicated for the treatment of hyperpigmentation,3 they are mostly used for cosmetic reasons and practiced without the guidance or consultation of health-care professionals.6 One of the main possible reasons for the use of SLAs is the cultural/social perception that equates beauty with fairer skin.7–9 Other social or cultural beliefs including the views that lighter skin complexion is attractive,10,11 more fashionable,10,11 reflective of high social status,1,10 and important for finding a marriage partner or job7,10,12–14 also motivate females to use these products. Besides, the consumers’ buying behavior is also affected by advertisements that grab attention and persuade consumers by presenting the products as visually capturing, unique, and colorful.15–18
Skin lightening products usually contain toxic chemicals/ingredients,1,7,13 and long-term exposure to these agents can be harmful.7,19 The most common harmful active ingredients are hydroquinone, mercurials, and potent broad-spectrum corticosteroid preparations.9,20,21 Even though these agents are present in other cosmetic products, they are found in relatively high concentrations in skin lightening products.13,22–26 Moreover, several products that do not indicate the presence of mercury, hydroquinone, and steroids on their labels can also have undeclared high concentrations of these ingredients,26,27 even in countries with strict control.7,26,28,29 Many complications are associated with these agents such as hyperpigmentation, exogenous ochronosis, impaired wound healing and wound dehiscence, nephropathy, steroid addiction syndrome, predisposition to infections, and endocrinologic complications.1,2,7,10,13,17,30,31 Despite all the complications and harm, the use of SLAs remains to be a common practice mainly due to inadequate knowledge of their harmful effects.10,32
Taking into account the seriousness of complications that could be caused by the use of the above-mentioned ingredients, their presence in the market without strict regulations is a public health concern. Medical concerns about the safety of SLAs have resulted in more stringent regulations and prohibitions on the production and trade of cosmetic products containing harmful ingredients.7,26 In Eritrea, although the regulation of cosmetics is stipulated in “Proclamation No. 36/1993: a proclamation to control drugs, medical supplies, cosmetics and sanitary items”, the marketing and use of cosmetic products remains uncontrolled owing to lax implementation. Anecdotal reports also indicate that the use of SLAs is becoming common among females of Asmara. This study was, therefore, conducted to assess the awareness, perception and utilization of SLAs among females of the capital city of Eritrea, Asmara.
Methods
Study Design and Setting
A cross-sectional analytical study, using a quantitative approach, was conducted from May to July of 2021 in representative samples of all beauty salons available in Asmara.
Asmara, the capital city of Eritrea, is divided into five sub-zones and 13 administrative areas. During the study period, as per the Ministry of Trade and Industry, there were 603 beauty salons, of which, the study was conducted in 36 randomly selected beauty salons.
Target and Study Population
All females above 18 years old and residing in Asmara were the target population of the study. The study population were those females who attended the selected beauty salons during the data collection period; were willing to participate in the study; and were mentally capable to communicate either in Tigrigna (a local language) or English.
Beauty salons that were closed during the study period and visitors of beauty salons who were not residents of Asmara were excluded from the study.
Sampling Technique
The study participants were selected using a two-stage stratified cluster sampling to minimize the introduction of sampling bias. The strata in this study were the five sub-zones of Asmara, namely, North East, North West, City center, South East, and South West. The first stage of the sampling was selection of beauty salons from each sub-zone. The second stage was the random selection of female visitors from the selected beauty salons.
Sample Size
The necessary sample size (n) was computed using sample size for one proportion formula (n = Z2*P(1-P)/d2), taking the proportion (P) with utilization as 0.5 (for no other similar studies were conducted previously), Z statistic for 95% level of confidence as 1.96, and degree of precision of 0.05 (in proportion of one).33 The initial sample size became 385. After introducing design effect of 1.7 and non-response rate of 10%, the final sample size was 721.
Sample Allocation
To optimize the heterogeneity of the participants from the beauty salons, an average of 20 females were selected from each beauty salon. Hence, the total number of beauty salons needed for the study was 36. The number of beauty salons selected from each sub-zone was kept to be proportional to the size of the sub-zone (Table 1).
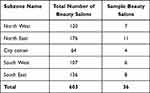 |
Table 1 Number of Beauty Salons in Each Subzone and Their Respective Samples |
Data Collection Tool and Approach
A self-developed, structured questionnaire (Supplementary File 1), designed to assess participants’ awareness, perception, and utilization of SLAs was used to collect data. The data collection tool comprised questions that explored participants’ socio-demographic information; awareness of SLAs and their potential adverse effects; perception on use of these agents in 5-point Likert scale; and practice patterns while using the products, triggering factors for use, and sources of the cosmetic products.
The data was collected through face-to-face structured interviews. Eight experienced pharmacists conducted the interviews and two supervisors were enrolled to coordinate the data collection process, and ensure data quality.
The data collectors were adequately trained on how to conduct face-to-face interviews, principles of data collection, and the nature of the study and the questionnaire. The two full-day training session also comprised practice interviews among the data collectors. To further ensure the comprehensiveness, feasibility and robustness of the data collection tool and capability of the data collectors, a three-day pre-test was conducted among 32 individuals in 16 beauty salons in Asmara, which were not included in the real study.
The study interviews were conducted after explaining the aim of the study to the selected participants and obtaining informed consent. Data collectors conducted the interview inside the selected beauty salons at a convenient place while maintaining the privacy of the participants. All ethical and professional considerations were followed throughout the study to keep the data strictly confidential.
After each day of data collection, the data collectors submitted the filled questionnaires to be checked for its completeness by the supervisors. All mistakes were corrected immediately to ensure the collected data were of good quality.
Controlling Bias and Confounders
Sampling Bias
The awareness, perception, and utilization of SLAs could be affected by the residential areas of the participants. In response to this influence, a two-stage stratified cluster sampling was used, and weights were computed to ensure the correct representativeness of the beauty salon in each sub-zone.
Selection Bias
To avoid selection of the most accessible beauty salon which in turn could affect the generalizability of the study findings, all beauty salons were given a chance to participate regardless of their location or accessibility. Similarly, some of the selected participants might be hesitant to participate in the study; while others might be eager to participate in the study. In such situations, the data collectors might tend to leave the selected but hesitant participants, and enroll those who are more willing to participate which could introduce selection bias. Additionally, some participants might look like chronic users of SLAs and be assumed to provide detailed information. To avoid such bias, data collectors were strictly instructed and supervised to enroll the randomly selected participants only.
Recall Bias
Some participants might face difficulties to recall past histories in regard to the use of SLAs. Thus, enough time was given to the participants to remember past details; in addition, the choices provided in the utilization section of the questionnaire were read to respondents to minimize recall bias.
Statistical Analysis
The filled questionnaires were manually cleaned and then entered into Census and Survey processing system (CSPro, Version 7.3) software package. After giving appropriate training on the data entry process, the data was entered by four professionals. The data was double entered to minimize keying errors and it was completed after ensuring 100% verification.
The data analysis was carried out using SPSS ver. 26. Descriptive analysis was performed using mean (SD) and median (IQR) as appropriate for continuous variables and frequency (percentage) for categorical variables. Due to the two-stage sampling process employed, weights were assigned for each beauty salon and incorporated throughout the analysis. The factors affecting the usage of SLAs were initially identified using bivariate logistic regression. Subsequently, the significant variables were retained at multiple logistic regression and adjusted odds ratios (95% CI) were computed. Results were deemed to be statistically significant when p-value is <0.05.
Ethical Considerations
Ethical clearance was obtained from the Research Proposal Review and Ethical Clearance Committee of the Ministry of Health, Eritrea [Reference: 07/08/20]. At the initial step, after thoroughly explaining the objectives of the study, permission to conduct the study was taken from the managers of the beauty salons. Prior to the data collection, the eligible study participants were briefed on the objectives of the study and written informed consent was taken. The names and other personal identifiers of the study participants were anonymized and kept strictly confidential. Besides, this study was conducted in accordance with the Declaration of Helsinki.
Operational Definition
Skin lightening agents (SLAs): Any product, including natural products, used with the intention to lighten the skin or packaged product with label claiming to lighten the skin.
Results
A total of 721 females were selected for the study. Among the selected individuals, 21 refused to participate and 16 were excluded due to missing information related to the products and/or active ingredients, resulting in a response rate of 94.9%. The majority of the respondents were of Tigrigna ethnic group (95%), Christians (87.1%), married or previously been married (58.6%), employed (53.2%), and reached secondary school (57.4%). About one-third of the respondents were residents of the Northeast of Asmara (35.4%). Most of the study population claimed to have no chronic illness (92.2%). The common chronic illnesses recorded, however, were asthma, hypertension, and diabetes. The details of demographic characteristics of the study population are summarized in Table 2.
 |
Table 2 Demographic Characteristics of the Study Participants |
General Awareness About SLAs and Their Adverse Effects
Most of the respondents (94.9%) had heard about skin lightening products. The respondents came to know about these products from a number of sources, mainly their friends (60.6%) (Figure 1). Most of the respondents (95.6%) claimed that SLAs can cause adverse effects and more than 12 conditions were reported as possible adverse effects. Acne or dark spots (61.6%), skin thinning (47.9%), hyperpigmentation (37.3%), burning sensation or skin sensitivity (26.7%), rashes (19.4%), skin injury (15.1%), depigmentation (12.7%), fragile skin (12.6%), skin cancer (7.5%), inflammation (7.3%), withdrawal syndrome (4.6%), and hirsutism (3.0%) were the commonly recognized untoward effects.
 |
Figure 1 Source of information for the awareness about skin lightening products. *Mobile applications and books. |
Perception of SLAs and Their Effects
Out of the possible 55 points, the median perception score of the respondents was 34 (IQR = 8, range = 15 to 54). More than half of the respondents agreed SLAs can make someone light skinned, beautiful, trendy and fashionable, and that white skin is more attractive than dark skin. Besides, majority of the respondents believed that using SLAs cannot help someone acquire a marriage partner, achieve and succeed in their career, and attain high social class. The percentage distribution of the perception items is detailed in Table 3.
 |
Table 3 Perception Responses of the Study Population Towards SLAs and Their Effects |
Utilization of SLAs
About two-thirds (64.2%) of the study population reported that they had used skin lightening products at least once in their lifetime. Of those who had history of use, 46.4% were current users while 53.6% informed they had stopped using skin lightening products due to several reasons such as exposure to harmful effects, experiencing no change on the skin, fear of side-effects, and loss of interest on the use. Friends (60.5%), personal choice (47.7%), relatives (10.1%), mass media (4.8%), dermatologists (2.7%), and other health-care professionals (0.5%) were found to be the major influencers for the use of skin lightening products. The most common reasons reported for use of such products were to change the skin color (64.4%) and look more beautiful (45.2%). Other reasons reported include to harmonize the skin tone (23.2%), treat acne (18.9%), have softer skin (16.0%), treat melasma (10.7%), remove skin marks (2.5%), and when prescribed by health-care professionals (1.3%).
On average, the respondents who had used skin lightening products started the practice at the age of 22.53 years (SD = 4.7, range = 13 to 45) and most of them (84.6%) started to use these products between 18 and 29 years of age. One-tenth of the respondents also claimed that they started the use of the products before the age of 18 years (8.4%). The mean duration of SLAs use was 4-years (SD = 5.6; range = 0 to 38) with the majority of respondents (67.2%) used SLAs for longer than 1-year. Besides, about half (51.7%) of the respondents used these products on a daily basis while 30.8% used them every other day, 25.5% rarely, 14.1% weekly, 3.4% once and 0.9% more than once per day.
A total of 150 products including natural ingredients were mentioned as being used for the purpose of skin lightening. More than half of the respondents (57.6%) used one product and the remaining 42.4% claimed to use two products or more. The most commonly used products as reported by the study population were Aneeza (12.1%), Natural face (10.9%) and Betamethasone containing various brands (10.7%). The top 10 commonly used products are included in Figure 2 and the list of all packaged products and natural ingredients along with their lightening effects are encompassed in the Supplementary File 2.
The main source of the products was cosmetic shops (84.8%). Other sources reported include supermarkets (9.2%), homemade (4.4%), abroad (4.4%), pharmacy outlets (4.3%), and friends (3.2%). The types of skin lightening products used were dominated by creams (87.9%) and followed by soap (12.9%), locally concocted (8.5%), powder (6.2%), home-made remedies (7.6%) and scrub (2.7%). The criteria for preference of products were mainly based on friend’s recommendation (64.4%); prior testing of the product (24.3%); cosmetic shop attendant’s recommendation (22.8%); checking ingredient (12.3%) and brand name (7.6%); previous knowledge and experience on the product (4.9%); advertisements (3.9%), and health-care professionals’ recommendation (2.4%).
Most of those who had used SLAs (96.4%) recognized the name of the product(s) used, but only 19.9% of those who had used were able to identify the ingredients contained in the products. Of those who had used SLAs, 45.9% stated that they read the product information before using the product. Direction of use (63.4%) and expiry date (58.5%) were the types of information commonly read by the respondents. Other types of information read by the respondents include use/indication (35.2%), ingredients (34.3%), name of the product (29.7%) and warnings (8.2%). When the products’ ingredients were assessed against the label of the products, of those who had practiced skin lightening products, 26.6% were found to use at least one harmful ingredient containing product. Bethamethasone (61.2%), hydroquinone (25.1%), clobetasole propionate (6.2%), and a combination of these products (7.5%) were the identified harmful ingredients.
The occurrence of at least one adverse effect due to the use of SLAs was 43.7%. Acne or dark spot (52.7%) and burning sensation or sensitivity (30.1%) were the most frequently experienced adverse effects (Table 4). After encountering adverse effects, three-fourth (74.7%) discontinued the use of the skin lightening products, 23.5% altered to other skin lightening products, 12.4% continued the use of similar products despite the adverse effect, 12.3% went to hospital, 7.7% used cosmetic products other than skin lightening, and 6.0% used medications to treat the adverse effects.
 |
Table 4 Reported Adverse Effects Due to Use of SLAs |
From those who had used SLAs, two-thirds were either fully satisfied (45.7%) or partially satisfied (20.8%) with the use of these products. However, about 70% of the participants stated that they would not recommend others to use SLAs. The reasons stated for not recommending the products to others were “the negative effects outweigh benefits (38.4%)”, “previous bad experience (32.1%)”, “not meddling on others” choices (14.1%), “using skin lightening products has no benefit (13.4%)”, “no specific reason (6.7%)”, “preference to natural beauty or products (5.1%)”, and “dissatisfaction with the results (1.2%)”. Moreover, out of the study population, one-fourth (25.4%) reported that they have future plans to use skin lightening products. The remaining 60.9% stated they had decided to no longer use such products, while 13.8% were unsure at the moment.
Factors Affecting the Current Utilization of SLAs
To find the factors that affect the current use of SLAs among the study population, all demographic characteristics and perception score were tested using logistic regression. From the tested independent variables, age, ethnicity, educational level, marital status, religion, and residence of the respondents were found to have no influence on the use of SLAs. On the other side, employment, perception score, pregnancy, and chronic illness were at least marginally significant at univariate level. To rule out the confounding factors and find the true influence(s), these variables were tested using multivariate logistic regression (Table 5).
 |
Table 5 Multivariable Association of Current Use of SLAs with Demographic Characteristics and Perception Score |
Employed females were almost twice (AOR: 1.678; CI: 1.158–2.427; p-value = 0.006) more likely to be current users of SLAs than unemployed respondents. To establish association between perception and utilization of SLAs, a perception that SLAs cannot make someone beautiful, trendy, fashionable, look more like the whites and cannot help someone to acquire popularity, marriage partner, and succeed in career was considered as a positive perception. Consequently, positive perception towards SLAs was found to have a protective effect from being a current user. Accordingly, with the increment of positive perception by one score, the likelihood of being a current user of SLAs decreases by 4.5% (AOR: 0.96, CI: 0.93–0.98; p = 0.001).
Discussion
The study showed widespread use of SLAs among females attending beauty salons in Asmara. Similar studies done in Asian and African countries also suggest that the use of skin lightening cosmetic products is a common practice.4,18,34–37 In this study, almost half of the respondents who had previously used SLAs were current users, whereas two-thirds of the current users started less than 5-years before the data was gathered. Even though baseline data on the use of skin lightening products are not available, the use of these products among females in Asmara seems to be a current trend. More than half of the respondents also claimed to have started using such products before the age of 22 and mostly under the influence of friends similar to studies conducted in South Africa, Nigeria and Somaliland.3,17,38 Since they were mostly influenced by their peers and probably not properly informed on the use, it is undoubtedly not a safe practice.
The variety of products used with the intention to lighten the skin was extensive. Despite the fact that most of the respondents knew the name of the product(s) they were using, only a few of them could exactly identify the active ingredients despite the harmful nature of some ingredients. The ingredients commonly used to enhance the skin lightening effect are known to be associated with several skin diseases,39 thus using such products without knowing the safety of ingredients can be considered a risky practice. In addition, the habit of reading product information before use was not satisfactory. This suggests the target population’s eagerness to use any product with skin lightening effect, and lack of deep understanding of the products and their possible negative health outcomes. The practice was mainly not information-based, but peer-pressure driven which necessitates planned community-based awareness raising interventions mainly targeting young females.
The present study showed that the participants have a perception that the use of SLAs is associated with being trendy, popular, and beautiful. Since consumers’ buying behavior is influenced by their perception on purchasing the product,8 the findings of this study intimate that the use of SLAs among the respondents will continue without seeking guidance from health-care professionals. The perception of the respondents on SLAs was also found to statistically influence their utilization. For this reason, tailored educational interventions that can shape the community’s perception on beauty in general and fair skin color in particular could be important. Besides, employment status was found to influence the utilization of SLAs. Given the fact that unemployed females were less likely to be current users, being unable to afford the products could be one reason that hinders the utilization. In this study, the main sources of the products were cosmetic shops followed by supermarkets and pharmacies, similar to the findings of several related studies.4,9,36,40 This indicates that despite having negative health impacts, cosmetics containing pharmaceutical or harmful ingredients are easily accessible. Although the motivation for selling such products can be attained through qualitative study, it is most probably due to weak regulation by concerned authorities, monetary gains, and lack of awareness.
The fact that skin lightening cosmetic products lack direct regulations, contain harmful and/or medicinal substances such as corticosteroids and hydroquinone, and coupled with the existing perception that equates beauty with fair skin, the utilization is predicted to continue and harm the society. As such, this study has outlined several programmatic and policy implications. A deeper understanding of individual and social drivers for the wider use of SLAs is important to develop tailored interventions. The Division of Health Promotion of the Ministry of Health in collaboration with the Ministry of Information, regulatory authorities and other stakeholders are recommended to prepare promotional materials, and conduct continuous sensitization and awareness raising programs to curb the problem. Uncontrolled availability of cosmetic products, especially those containing of-concern harmful and medicinal ingredients, was observed in the study. This poses a health risk to the public and it is recommended that the National Medicines and Food Administration – The national regulatory authority of Eritrea mandated to regulate cosmetic products among others – should work in harmony with other stakeholders to tackle the problems associated with the use of such products. Marketing of products with hidden or undeclared harmful and/or prohibited ingredients is also a common observation.21 Hence, the authors recommend further quality control testing to discover undeclared ingredients and their amount in the commonly used products. This is expected to unravel the masked burden of the problem and impose necessary regulations and cosmetic product safety guidelines.
Limitations of the Study
Since the study was conducted in a single city of the country, the findings may not reflect the situation of the whole country. Even though several steps were taken to minimize information bias (especially recall bias), bias associated with under-reporting of adverse effects, and difficulty in remembering products used long ago cannot be eliminated. The adverse effects were self-reported by the respondents and thus, could not be validated. In addition, the extent of harmful ingredients contained in the products was only discovered by mere review of the label claim. As the products were not laboratory tested, the result presented in the study may not be entirely reflective of the actual presence of harmful ingredients across the products.
Conclusion
The use of SLAs was found to be prevalent among females residing in Asmara and an extensive number of products with skin lightening effect including products containing harmful or medicinal ingredients were disclosed. Therefore, the regulatory authority in collaboration with relevant stakeholders should pay attention to this significant public health concern. It is recommended that the interventions institute a regulatory mechanism to tackle the unsafe aspects and raise public awareness of the safe use of cosmetics.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on request.
Acknowledgments
The authors sincerely thank the data collectors and ERIPA’s support in conducting the study. The authors’ sincere gratitude also goes to the Ministry of Trade and Industry for providing data of all the beauty salons in Asmara and to the owners of the beauty salons for providing spaces for data collection (interviewing the respondents). Finally, the authors thank the respondents for their invaluable time and information.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The data collection was supported by funding from Eritrean Pharmaceutical Association (ERIPA).
Disclosure
The authors declare that they have no competing interests.
References
1. Ahmed AE, Hamid ME. Use of skin-whitening products by Sudanese undergraduate females: a survey. J Racial Ethnic Health Disparities. 2017;4(2):149–155. doi:10.1007/s40615-016-0212-5
2. Ladizinski B, Mistry N, Kundu RV. Widespread use of toxic skin lightening compounds: medical and psychosocial aspects. Dermatol Clin. 2011;29(1):111–123. doi:10.1016/j.det.2010.08.010
3. Dlova N, Hamed S, Tsoka‐Gwegweni J, Grobler A. Skin lightening practices: an epidemiological study of South African women of African and Indian ancestries. Br J Dermatol. 2015;173:2–9. doi:10.1111/bjd.13556
4. Lartey M, Krampa FD, Abdul‐Rahman M, et al. Use of skin‐lightening products among selected urban communities in Accra, Ghana. Int J Dermatol. 2017;56(1):32–39. doi:10.1111/ijd.13449
5. Peltzer K, Pengpid S, James C. The globalization of whitening: prevalence of skin lighteners (or bleachers) use and its social correlates among university students in 26 countries. Int J Dermatol. 2016;55(2):165–172. doi:10.1111/ijd.12860
6. Sagoe D, Pallesen S, Dlova NC, Lartey M, Ezzedine K, Dadzie O. The global prevalence and correlates of skin bleaching: a meta‐analysis and meta‐regression analysis. Int J Dermatol. 2019;58(1):24–44. doi:10.1111/ijd.14052
7. Darj E, Infanti JJ, Ahlberg BM, Okumu J. “The fairer the better?” Use of potentially toxic skin bleaching products. Afr Health Sci. 2015;15(4):1074–1080. doi:10.4314/ahs.v15i4.4
8. Islam M, Hani FF. Hopes and reality: consumers’ purchase intention towards whitening cream. Future Bus J. 2021;7(1):1–12. doi:10.1186/s43093-021-00098-1
9. Pollock S, Taylor S, Oyerinde O, et al. The dark side of skin lightening: an international collaboration and review of a public health issue affecting dermatology. Int J Womens Dermatol. 2021;7(2):158–164. doi:10.1016/j.ijwd.2020.09.006
10. Dlova N, Hamed SH, Tsoka‐Gwegweni J, Grobler A, Hift R. Women’s perceptions of the benefits and risks of skin‐lightening creams in two South African communities. J Cosmet Dermatol. 2014;13(3):236–241. doi:10.1111/jocd.12104
11. Kpanake L, Muñoz Sastre MT, Mullet E. Skin bleaching among Togolese: a preliminary inventory of motives. J Black Psychol. 2010;36(3):350–368. doi:10.1177/0095798409353759
12. Hossain M. Attitudes of the female university students towards skin whitening in Bangladesh. Epidemiol Int J. 2020;4. doi:10.23880/EIJ-16000134
13. Majeed T, Shah SH, Anjum I. Estimation of mercury and hydroquinone content in skin whitening creams and the potential risks to the health of women in Lahore. Pakistan. 2021;31(1):33–41.
14. Nyoni-Kachambwa P, Naravage W, James NF, Van der Putten M. A preliminary study of skin bleaching and factors associated with skin bleaching among women living in Zimbabwe. African Health Sci. 2021;21(1):132–139. doi:10.4314/ahs.v21i1.18
15. Bt Mohammad HM. Factors that influence and stimulate the consumer buying behavior towards skin whitening product on social media advertisement. 2018.
16. Malik SS, Mashhood AA, Tahir M, Minhas IJ, Sadiq S. Motivational factors and user awareness in patients using skin lightening creams reporting to dermatology out patient department in Combined Military Hospital Rawalpindi. PAFMJ. 2021;71(6):1925–1929. doi:10.51253/pafmj.v6i6.5680
17. Yusuf MA, Mahmoud ND, Rirash FR, Stoff BK, Liu Y, McMichael D. Skin lightening practices, beliefs, and self-reported adverse effects among female health science students in Borama, Somaliland: a cross-sectional survey. International Journal of Women’s Dermatology. 2019;5(5):349–355. doi:10.1016/j.ijwd.2019.08.006
18. Alrayyes SF, Alrayyes SF, Farooq Dar U. Skin‐lightening practices behind the veil: an epidemiological study among Saudi women. J Cosmet Dermatol. 2020;19(1):147–153. doi:10.1111/jocd.12972
19. Rusmadi SZ, Ismail S, Norkhadijah S, Praveena SM. Preliminary study on the skin lightening practice and health symptoms among female students in Malaysia. J Environ Public Health. 2015;2015:1–6. doi:10.1155/2015/591790
20. Alam M. Fitzpatrick’s dermatology in general medicine. Arch Dermatol. 2004;140(3):372. doi:10.1001/archderm.140.3.372-A
21. Arshad M, Sadef Y, Shakoor MB, et al. Quantitative estimation of the hydroquinone, mercury and total plate count in skin-lightening creams. Sustainability. 2021;13(16):8786.
22. Del Giudice P, Yves P. The widespread use of skin lightening creams in Senegal: a persistent public health problem in West Africa. Int J Dermatol. 2002;41(2):69–72. doi:10.1046/j.1365-4362.2002.01335.x
23. Iwegbue CM, Bassey FI, Tesi GO, Onyeloni SO, Obi G, Martincigh BS. Safety evaluation of metal exposure from commonly used moisturizing and skin-lightening creams in Nigeria. Regulat Toxicol Pharmacol. 2015;71(3):484–490. doi:10.1016/j.yrtph.2015.01.015
24. Ly F, Soko AS, Dione DA, et al. Aesthetic problems associated with the cosmetic use of bleaching products. Int J Dermatol. 2007;46:15–17. doi:10.1111/j.1365-4632.2007.03456.x
25. Mahe A, Ly F, Aymard G, Dangou JM. Skin diseases associated with the cosmetic use of bleaching products in women from Dakar, Senegal. Br J Dermatol. 2003;148(3):493–500. doi:10.1046/j.1365-2133.2003.05161.x
26. Maneli M, Wiesner L, Tinguely C, et al. Combinations of potent topical steroids, mercury and hydroquinone are common in internationally manufactured skin‐lightening products: a spectroscopic study. Clin Exp Dermatol. 2016;41(2):196–201. doi:10.1111/ced.12720
27. Gbetoh MH, Amyot M. Mercury, hydroquinone and clobetasol propionate in skin lightening products in West Africa and Canada. Environ Res. 2016;150:403–410. doi:10.1016/j.envres.2016.06.030
28. Liu Y, Krueger LD, Nguyen HP. Regulation of skin lightening agents in the United States and implications for public health. J Am Acad Dermatol. 2021;85(6):1608–1610. doi:10.1016/j.jaad.2020.12.007
29. Juliano CC. Spreading of dangerous skin-lightening products as a result of colourism: a review. Appl Sci. 2022;12(6):3177.
30. Ly F, Diousse P, Ndiaye C, et al. Cutaneous squamous cell carcinomas (SCC) associated with cosmetic skin whitening: 8 cases reported in Senegal.
31. Olumide YM, Akinkugbe AO, Altraide D, et al. Complications of chronic use of skin lightening cosmetics. Int J Dermatol. 2008;47(4):344–353. doi:10.1111/j.1365-4632.2008.02719.x
32. Alghamdi WA, Khalifah NA, Alshammari HS, Althamin FA, Althamin BA, Alsultan HH. The misuse of topical creams among females in Saudi Arabia 2020. Med Sci. 2021;25(115):2203–2212.
33. Naing L, Winn T, Rusli B. Practical issues in calculating the sample size for prevalence studies. Archiv Orofacial Sci. 2006;1:9–14.
34. Alrayyes SF, Alrayyes SF, Farooq UD. Skin-lightening patterns among female students: a cross-sectional study in Saudi Arabia. Int J Womens Dermatol. 2019;5(4):246–250. doi:10.1016/j.ijwd.2019.04.026
35. Blay YA. Skin bleaching and global white supremacy: by way of introduction. J Pan African Stud. 2011;4(4):4–46.
36. Kouotou EA, Nansseu JRN, Adegbidi H, Zoa Mebara TCJ, Ndjitoyap Ndam EC. Skin whitening among Cameroonian female university students: knowledge, attitudes, practices and motivations. BMC Womens Health. 2017;17(1):1–5. doi:10.1186/s12905-017-0385-z
37. Rahiman F, Davids LM, Thomas A. A survey evaluating knowledge, perception, and use of skin lightening products among South African students. Int J Womens Dermatol. 2021;7(5):766–768. doi:10.1016/j.ijwd.2021.07.006
38. Amodu M. Knowledge, attitude and practice of skin whitening among female university students in Northeastern Nigeria. Open Access Library J. 2018;5(04):1.
39. Masub N, Khachemoune A. Cosmetic skin lightening use and side effects. J Dermatol Treat. 2022;33(3):1287–1292. doi:10.1080/09546634.2020.1845597
40. Sendrasoa F, Ranaivo I, Andrianarison M, et al. Misuse of topical corticosteroids for cosmetic purpose in Antananarivo, Madagascar. Biomed Res Int. 2017;2017:1–4. doi:10.1155/2017/9637083
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

