Back to Journals » Patient Preference and Adherence » Volume 17
Awareness of HBV Functional Cure and Attitude Toward Related Clinical Trials Among Patients with Chronic Hepatitis B in China
Authors Duan Z, Dong J, Liu Y, Zhou H, Duan S, Liu W, Liang R , Ding Y
Received 25 May 2023
Accepted for publication 11 August 2023
Published 22 August 2023 Volume 2023:17 Pages 2063—2072
DOI https://doi.org/10.2147/PPA.S422916
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Qizhi (Cathy) Yao
Zhongping Duan,1 Jinling Dong,1 Ying Liu,2 Haiyang Zhou,2 Shuli Duan,2 Weihong Liu,3 Rico Liang,3 Yue Ding3
1Fourth Department of Hepatology Center, Beijing You’an Hospital, Capital Medical University, Beijing, People’s Republic of China; 2iGandan, the Portal for All Liver Disease Caring, Beijing, People’s Republic of China; 3Brii Biosciences Inc., Beijing, People’s Republic of China
Correspondence: Rico Liang, Brii Biosciences Inc, 3F, Building 7, Zhongguancun Dongsheng International Science Park, No. 1 North. Yongtaizhuang Road, Haidian District, Beijing, 100192, People’s Republic of China, Tel/Fax +86 10 6587 6121, Email [email protected]
Purpose: HBV functional cure is an optimal treatment goal for chronic hepatitis B (CHB) at present and numerous new drugs aiming for HBV functional cure are in development. We carried out an internet-based survey to understand the treatment status, unmet needs, awareness of HBV functional cure and attitude toward related clinical trials among CHB patients in China.
Patients and Methods: An internet-based anonymous survey was conducted on CHB patients who reside in mainland China. Determinants of awareness and attitude were identified by logistic regression models.
Results: Of the 1220 CHB patients who completed the survey questionnaire, 11.1% (135/1220) were aware of HBV functional cure and 50.2% (612/1220) answered “definitely will” to participate in related clinical trials. Participants who knew their HBsAg level (HBsAg< 1500 IU/mL: OR=3.03, 95% CI: 1.87– 4.92; HBsAg≥ 1500 IU/mL: OR=2.57, 95% CI: 1.35– 4.88), who expected to achieve HBsAg loss with treatment (OR=1.63, 95% CI: 1.07– 2.50) and who were dissatisfied with current treatment due to the failure of achieving HBsAg loss (OR=1.67, 95% CI: 1.10– 2.53) had better awareness of HBV functional cure. Participants who had HBsAg level less than 1500 IU/mL (OR=1.45, 95% CI: 1.05– 1.99), treatment with pegylated interferon alpha with or without nucleos(t)ide (OR=1.68, 95% CI: 1.11– 2.53) and better awareness of HBV functional cure (OR=1.62, 95% CI: 1.01– 2.61) were more likely to say “definitely will” to participate in related clinical trials.
Conclusion: Chinese CHB patients reported a low awareness of HBV functional cure. Although CHB patients in China reported a low rate of HBV functional cure awareness, they had a high acceptance of related clinical trials.
Keywords: CHB, HBV functional cure, awareness, related clinical trials
Introduction
Hepatitis B virus (HBV) infection is a global public health concern, and patients with HBV infection are at high risk of progressive liver diseases and hepatocellular carcinoma (HCC). China has the largest chronic hepatitis B (CHB) burden with an estimated 7.8% hepatitis B surface antigen (HBsAg) seroprevalence rate and 11.4 per 100,000 all-age death rate for HBV-related diseases in 2019.1 Increasing levels of HBV DNA, HBsAg, and ALT were associated with an increasing risk of HCC.2 Studies have reported that HBsAg seroclearance can further reduce HCC risk after complete viral suppression and was associated with improved patient outcomes.3,4 However, current CHB standard of care (SoC) treatment can only achieve a low rate of HBsAg seroclearance.5–7
HBV functional cure, defined as sustained, undetectable HBsAg and HBV DNA in serum with or without seroconversion to hepatitis B surface antibody (anti-HBs) after completion of a finite course of treatment, was first officially proposed as the goal of new HBV therapies in 2016 AASLD-EASL endpoint workshop and further discussed in 2019 AASLD-EASL endpoint conference.8,9 Many new drugs aiming for HBV functional cure such as direct-acting antivirals, viral antigen production/release inhibitors, and immunomodulators are in development, many of which are being or have been tested in Phase II clinical trials with some promising readouts.10
Although the benefits of HBV functional cure have been widely accepted by professionals in hepatology and clinical development of cure regimens are prosperous, patients’ awareness and acceptance were barely reported. To better understand CHB patients’ medical needs and to support a patient-centric strategy in HBV functional cure drug development, we conducted an internet-based anonymous survey to find out current treatment status, awareness of HBV functional cure and willingness to participate in related clinical trials among CHB patients in China.
Materials and Methods
Recruitment and Data Collection
Patient recruitment, survey questionnaire distribution and data collection were conducted by iGandan, an online platform for liver disease care in China. From October 2022 to February 2023, 80 iGandan-registered doctors from 75 hospitals across China distributed the questionnaire to their patients. The questionnaire featured 29 questions and took about 10 min to complete, aiming to assess the patients’ current CHB treatment status, CHB impact on life, awareness of HBV functional cure and their attitude toward related clinical trials.
Consent Formation and Ethical Procedures
For eligibility, participants had to be at least 18 years of age, living in mainland China, able to complete the survey questionnaire independently and chronically HBV infected. All participants were informed of the purpose of the anonymous survey by reading the online consent before starting answering the questions. This study was approved by the institutional ethics committee of Beijing You’an hospital, Capital Medical University, Beijing, China (no. [2023]002) and conducted by the Declaration of Helsinki.
Participants Characteristics Measurement
Participants were asked about demographics, disease characteristics, CHB impact on life, current treatment status, definition of HBV functional cure and willingness to participate in functional cure related clinical trials. To evaluate the willingness, participants were asked to give a point ranging from 1 to 5 representing from the lowest willingness to the highest willingness. In the analysis, we grouped the answers into three categories as “definitely will” (5 points), “probably will” (4 points) and “will not” (1–3 points).
Statistical Analysis
Descriptive statistics were used to analyze participants’ demographics, baseline characteristics, CHB impact on life, current treatment status and functional cure awareness. Possible factors associated with better functional cure awareness and higher willingness to participate in related clinical trials were also analyzed.
Binary logistic regression was performed with function cure awareness as dependent variable, and age, location, education, family income, CHB duration, HBsAg level, CHB treatment, reason of satisfaction on current therapies and reason of dissatisfaction on current therapies as independent variables. Multinomial logistic regression was performed with willingness to participate in functional cure related clinical trials as dependent variable, and age, location, education, family income, CHB duration, HBsAg level, CHB treatment, reason of satisfaction on current therapies, reason of dissatisfaction on current therapies and function cure awareness as independent variables. All statistical analyses were conducted using SAS version 9.4.
Results
Demographics and CHB Impact on Life
In total, 1220 out of 1401 participants completed the questionnaire (87.1% response-rate). Table 1 shows the demographics of the participants. They came from 31 geographic regions across mainland China (22 provinces, 4 municipalities and 5 autonomous regions). The most represented regions were Henan province (n=118), Hebei province (n=82), Fujian province (n=79), Sichuan province (n=68), Xinjiang Uygur autonomous region (n=64) and Shanxi province (n=64). A full list of region distribution can be found in Table S1. 61.7% of the participants were 30–50 years old, 49.1% were less well educated with a high school or below diploma and 48.4% had a relatively low family income of less than 5000 CNY (~720 USD) per month. Regarding health insurance and expense on CHB treatment, most of the participants had national insurance (85.3%) and 81.4% of them spent less than 1000 CNY (~150 USD) per month. For CHB's impact on life, as shown in Figure 1, the three mostly reported were long-term health concern (73.9%), emotional impact (58.4%) and financial burden (39.8%).
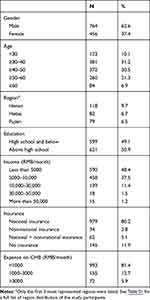 |
Table 1 Demographics of the Study Participants |
 |
Figure 1 The impact of CHB on life. A larger percentage represents more participants felt affected. |
Disease Characteristics and Status of Treatment
The average CHB duration of the participants was 13.2 years. Among the participants who knew their disease baselines, 55.6% were HBeAg positive, 75.8% were HBV DNA undetectable, and 57.4% had HBsAg level less than 1500 IU/mL.
At the time of the survey, 85.5% of the participants were on SoC treatment, in which 82.6% were treated with nucleos(t)ide analogs (NUCs) alone, 5.2% with pegylated interferon alpha (peg-IFN-α) alone and 12.2% with peg-IFN-α plus NUCs. The participants were asked to give a score ranged 1–5 representing from very dissatisfied to very satisfied with their current treatment. The average score was 4.2 points, suggesting most of the participants felt satisfied with their current treatment. Figure 2a and b list the reasons for satisfaction and dissatisfaction of current treatment, respectively. Convenience (61.5%), safety (46.9%) and liver function normalization (40.5%) were the three most mentioned reasons for satisfaction, while long-term treatment (71.2%) was the main complaint. Reasons for dissatisfaction are shown in Figure 2b. Furthermore, we asked the participants about their ideal HBV functional cure treatment. 68.6% reported they expected a short-term treatment of less than 2 years.
HBV Functional Cure Awareness and Related Clinical Trials Participation Willingness
Participants who knew the two key aspects of HBV functional cure, sustainable HBsAg seroclearance and undetectable HBV DNA for at least 6 months, were classified as aware of HBV functional cure. Only 11.1% (135/1220) of the participants and 11.4% (119/1043) of the on-treatment participants were aware of HBV functional cure. Comparing to their peers, the participants who knew their HBsAg level (HBsAg<1500 IU/mL: OR=3.03, 95% CI: 1.87–4.92; HBsAg≥1500 IU/mL: OR=2.57, 95% CI: 1.35–4.88), who expected to achieve HBsAg loss by treatment (OR=1.63, 95% CI: 1.07–2.50) and who were dissatisfied with current therapies due to failure of achieving HBsAg loss (OR=1.67, 95% CI: 1.10–2.53) were more likely to be aware of HBV functional cure, as shown in Table 2.
 |
Table 2 Association Between Functional Cure Awareness and Patient Characteristics |
Of the 1220 participants included in the analysis, 50.2% answered “definitely will” to participate in functional cure related clinical trials and 18.0% answered “probably will.” The considerations were mainly based on safety and efficacy of the study drug. Table 3 shows the association between willingness to participate in HBV functional cure related clinical trials and patient characteristics. High willingness was associated with HBsAg level less than 1500 IU/mL (OR=1.45, 95% CI: 1.05–1.99), peg-IFN-α treatment with or without NUCs (OR=1.68, 95% CI: 1.11–2.53), age 40–50 (OR=1.87, 95% CI: 1.06–3.30) and HBV functional cure awareness (OR=1.62, 95% CI: 1.01–2.61).
 |
Table 3 Association Between Willingness to Participate in HBV Functional Cure Related Clinical Trials and Patient Characteristics |
Discussion
To our knowledge, this is the first study to evaluate CHB patients’ awareness of HBV functional cure and their acceptance of related clinical trials with a national online survey. The 1220 CHB participants represented all of the geographic regions in mainland China.
HBV functional cure is a relatively new concept, which was first officially brought up in 2016.8 Although there is some difference in the definition of HBV functional cure from different literature, two key points were sustained HBsAg loss and undetectable HBV DNA for at least 6 months after a finite course of treatment.8,9,11 Many studies demonstrated that HBV functional cure was associated a decreased HCC risk especially in non-cirrhotic CHB patients.3,4,12 HBV functional cure has become an optimal treatment goal in clinics and many research resources have been invested for the development of novel therapies aiming for HBV functional cure. However, our study indicated that only a small percent of CHB patients were aware of HBV functional cure: 11.1% of all patients and 11.4% of on-SoC treatment patients, which contrasted with the perception of hepatitis professionals. And according to the survey, most of the on-SoC treatment participants reported being satisfied with their current treatment. The average satisfaction score was 4.2 points. Convenience, safety, and liver function normalization are the three most mentioned advantages of current treatment. On the other hand, this study showed that the concept of a finite course of treatment matched 68.6% of participants’ ideal HBV functional cure treatment, indicating a clear unmet need.
Our study has revealed three factors associated with HBV functional cure awareness: knowing of HBsAg level, expectation of achieving HBsAg loss with treatment and dissatisfaction of current therapies due to the failure of achieving HBsAg loss. This association is not surprising since HBsAg loss is a hallmark of HBV functional cure. Although HBV DNA undetectable is also an important indicator of HBV functional cure, a patient who has achieved HBsAg loss would have undetectable HBV DNA at large except those with occult HBV infection (OBI). However, the prevalence of OBI in the general population is very low.13
Our study also analyzed patients’ willingness to participate in HBV functional cure related clinical trials. We found that participants with HBsAg less than 1500 IU/mL, peg-IFN-α treatment with or without NUC, awareness of HBV functional cure would say “definitely will” to clinical trials. Chinese physicians in HBV area have done many studies to explore the use of peg-IFN-α to achieve HBV functional cure for many years, and CHB patients with HBsAg less than 1500 IU/mL are the target population of these studies. OSST14 and NEW SWITCH15 demonstrated that a HBsAg loss rate of 22.2–26.5% at week 48 can be achieved in HBeAg-negative patients with HBsAg less than 1500 IU/mL by switching to peg-IFN-α from NUC. The 2019 Chinese expert consensus on roadmap to functional cure highly recommends that patients with HBeAg seroclearance and HBsAg less than 1500 IU/mL employ NUC and peg-IFN-α sequential combination therapy to achieve functional cure.16 The Everest project, a very large real-world cohort study in China in exploring 48-week peg-IFN-α with or without NUC for HBV functional cure, is designed to include ~30,000 HBeAg-negative CHB patients with HBsAg less than 1500 IU/mL in more than 400 hospitals across mainland China and 25,285 cases have been enrolled as of September 2022.17 Therefore, it is possible that the participants with HBsAg less than 1500 IU/mL might have heard of HBV functional cure studies, have better understanding of these studies, and even have joined in these studies before. Similarly, participants who were on peg-IFN-α treatment at the time of the survey may have been in an attempt to achieve HBV functional cure. Thus, it is reasonable for them to have a high acceptance of functional cure related clinical trials. Interestingly, we also found that participants of 40–50 years old would say “definitely will” to clinical trials compared to those less than 30. Moreover, participants of other age groups (30–40 and >50) in our analysis also had a trend of increased willingness. This might be due to the fact that most CHB patients of an age less than 30 are in a phase of immune tolerance that treatment was not recommended by international guidelines.18–20
There are some limitations in our study. This survey is a cross-sectional study bearing inherent recall and social desirability biases. We had mitigated these biases by adopting a self-administering approach instead of responding to interviewer’s questions. Moreover, although the surveyed population was not a random sampling population, this study, as an exploratory study, has generated useful results that could be of reference for future planning of HBV functional cure drug development.
Conclusion
HBV functional cure is currently the optimal goal of CHB treatment to reduce HCC risk to the lowest extent with a finite course of medications. According to our survey, although Chinese CHB patients appear to have a low awareness of HBV functional cure, they have a high willingness to participate in functional cure related clinical trials and an unmet need for a finite, short-term treatment. Education will be valuable in the future to help them better understand surrogate biomarkers of CHB treatment and benefits of HBV functional cure. Subsequently, improvements in functional cure awareness might help accelerate clinical trial enrollment as well as functional cure regimen acceptance in the market.
Acknowledgments
We thank all the survey participants for their contributions to this study and Yao Zhang for manuscript reviewing and proofreading. Funding for the study and editorial support were provided by Brii Biosciences Inc. Beijing, China.
Disclosure
The authors report no conflicts of interest in this work.
References
1. GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Gastroenterol Hepatol. 2022;7(9):796–829.
2. Lee MH, Yang HI, Liu J, et al. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles. Hepatology. 2013;58(2):546–554. doi:10.1002/hep.26385
3. Yip TC, Wong GL, Chan HL, et al. HBsAg seroclearance further reduces hepatocellular carcinoma risk after complete viral suppression with nucleos(t)ide analogues. J Hepatol. 2019;70(3):361–370. doi:10.1016/j.jhep.2018.10.014
4. Anderson RT, Choi HSJ, Lenz O, et al. Association between seroclearance of hepatitis B surface antigen and long-term clinical outcomes of patients with chronic hepatitis B virus infection: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19(3):463–472. doi:10.1016/j.cgh.2020.05.041
5. Marcellin P, Wong DK, Sievert W, et al. Ten-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B virus infection. Liver Int. 2019;39(10):1868–1875. doi:10.1111/liv.14155
6. Hou J, Ning Q, Duan Z, et al. 3-year Treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for chronic HBV infection in China. J Clin Transl Hepatol. 2021;9(3):324–334. doi:10.14218/JCTH.2020.00145
7. Hou J, Wang G, Wang F, et al. Guideline of prevention and treatment for chronic hepatitis B (2015 update). J Clin Transl Hepatol. 2017;5(4):297–318. doi:10.14218/JCTH.2016.00019
8. Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: from discovery to regulatory approval. Hepatology. 2017;66(4):1296–1313. doi:10.1002/hep.29323
9. Cornberg M, Lok AS, Terrault NA, Zoulim F; The 2019 EASL-AASLD HBV Treatment Endpoints Conference Faculty. Guidance for design and endpoints of clinical trials in chronic hepatitis B - report from the 2019 EASL-AASLD HBV treatment endpoints conference. J Hepatol. 2020;72(3):539–557. doi:10.1016/j.jhep.2019.11.003
10. Jeng WJ, Lok ASF. What will it take to cure hepatitis B? Hepatol Commun. 2023;7(4):e0084. doi:10.1097/HC9.0000000000000084
11. Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2022). Chin J Hepatol. 2022;30(12):1309–1331.
12. Yip TC, Lai MS, Hui VM, et al. Similar risk of hepatocellular carcinoma in non-cirrhotic patients who archived HBsAg seroclearance before the age of 50 and the general population.
13. Im YR, Jagdish R, Leith D, et al. Prevalence of occult hepatitis B virus infection in adults: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(10):932–942. doi:10.1016/S2468-1253(22)00201-1
14. Ning Q, Han M, Sun Y, et al. Switching from entecavir to pegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial). J Hepatol. 2014;61(4):777–784. doi:10.1016/j.jhep.2014.05.044
15. Hu P, Shang J, Zhang W, et al. HBsAg loss with peg-interferon alfa-2a in hepatitis B patients with partial response to nucleos(t)ide analog: new switch study. J Clin Transl Hepatol. 2018;6(1):25–34. doi:10.14218/JCTH.2017.00072
16. Ning Q, Wu D, Wang GQ, et al. Roadmap to functional cure of chronic hepatitis B: an expert consensus. J Viral Hepat. 2019;26(10):1146–1155. doi:10.1111/jvh.13126
17. Gan W. Functional cure based on pegylated interferon α-2b therapy in nucleoside analog-suppressed HBeAg negative chronic hepatitis B: a multicenter real-world study (Everest Project in China) – 4 year data update.
18. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi:10.1002/hep.29800
19. Lampertico P, Agarwal K, Berg T; European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi:10.1016/j.jhep.2017.03.021
20. Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi:10.1007/s12072-015-9675-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

