Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 13
Automated oxygen control with O2matic® during admission with exacerbation of COPD
Authors Hansen EF , Hove JD , Sandau Bech C , Stæhr Jensen JU , Kallemose T , Vestbo J
Received 15 August 2018
Accepted for publication 7 November 2018
Published 14 December 2018 Volume 2018:13 Pages 3997—4003
DOI https://doi.org/10.2147/COPD.S183762
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Video abstract presented by Charlotte Sandau Bech.
Views: 1869
Ejvind Frausing Hansen,1 Jens Dahlgaard Hove,1 Charlotte Sandau Bech,1 Jens-Ulrik Stæhr Jensen,2 Thomas Kallemose,3 Jørgen Vestbo4
1Medical Unit, Amager and Hvidovre Hospital, Copenhagen, Denmark; 2Medical Department, Herlev and Gentofte Hospital, Copenhagen, Denmark; 3Clinical Research Center, Amager and Hvidovre Hospital, Copenhagen, Denmark; 4School of Biological Sciences, University of Manchester and Manchester University NHS Foundation Trust, Manchester, UK
Purpose: It is a challenge to control oxygen saturation (SpO2) in patients with exacerbations of COPD during admission. We tested a newly developed closed-loop system, O2matic®, and its ability to keep SpO2 within a specified interval compared with manual control by nursing staff.
Patients and methods: We conducted a crossover trial with patients admitted with an exacerbation of COPD and hypoxemia (SpO2 ≤88% on room air). Patients were monitored with continuous measurement of SpO2. In random order, they had 4 hours with manually controlled oxygen and 4 hours with oxygen delivery controlled by O2matic. Primary outcome was time within a prespecified SpO2 target interval. Secondary outcomes were time with SpO2 <85%, time with SpO2 below target but not <85%, and time with SpO2 above target.
Results: Twenty patients were randomized and 19 completed the study. Mean age was 72.4 years and mean FEV1 was 0.72 L (33% of predicted). Patients with O2matic-controlled treatment were within the SpO2 target interval in 85.1% of the time vs 46.6% with manually controlled treatment (P<0.001). Time with SpO2 <85% was 1.3% with O2matic and 17.9% with manual control (P=0.01). Time with SpO2 below target but not <85% was 9.0% with O2matic and 25.0% with manual control (P=0.002). Time with SpO2 above target was not significantly different between treatments (4.6% vs 10.5%, P=0.2). Patients expressed high confidence and a sense of safety with automatic oxygen delivery.
Conclusion: O2matic was able to effectively control SpO2 for patients admitted with an exacerbation of COPD. O2matic was significantly better than manual control to maintain SpO2 within target interval and to reduce time with unintended hypoxemia.
Keywords: oxygen therapy, oxygen saturation, hypoxia, hyperoxia, closed-loop
Introduction
Treatment with oxygen supplementation is a central part of the treatment of exacerbations of COPD, as many patients during a hospital admission will have acute hypoxemic respiratory failure or worsening of chronic respiratory failure. The reason for hypoxemia is mainly deterioration in the ventilation–perfusion mismatch, whereas hypercapnia does not occur as long as alveolar ventilation is maintained. There is general consensus that oxygen supplementation should be controlled to ensure an oxygen saturation (SpO2) of 88%–92% for most patients. This is recommended by the Global Initiative for Chronic Obstructive Lung Disease and in guidelines for treatment of acute hypoxemic respiratory failure.1,2 The evidence, however, for this recommendation is sparse due to lack of controlled studies of different levels of oxygenation in patients with COPD. One study in a prehospital setting found that controlled oxygen, aiming at an SpO2 of 88%–92%, compared with liberal oxygen dosing of 8–10 L/minute reduced mortality in the COPD population by 78%.3 Furthermore, patients who received titrated oxygen were significantly less likely to develop respiratory acidosis due to acute hypercapnia. In a retrospective study of 680 patients presenting with an exacerbation of COPD, the risk of serious adverse outcome was increased for both patients admitted with an SpO2 <88% and for patients with an SpO2 >96%.4 Control of SpO2 during admission is a time-consuming task for the nursing staff, and it has been suggested that closed-loop control of oxygenation could reduce the burden of the nursing staff and increase patient safety by better control of SpO2. In a controlled study with a closed-loop system, FreeO2® (OxyNov Inc., Québec, Canada), time within SpO2 target interval was increased from 51% to 81% by closed-loop control compared with manual control.5 The results for FreeO2 were confirmed in a shorter study of 3 hours with 187 patients with acute hypoxemic respiratory failure due to different conditions in the emergency ward.6 In this study, the fraction of time within target SpO2 interval was 81% for closed-loop control and 52% for manual control.
Since 2011, we have worked on a closed-loop system, O2matic® (O2matic Ltd., Herlev, Denmark), and in this study we present the first data from clinical testing in a population admitted to hospital with an exacerbation of COPD. The aim of our study was to examine the ability of O2matic to keep SpO2 within a target interval compared to manual control by nursing staff and to examine if O2matic could reduce time with hypoxemia and time with hyperoxia compared to manual control. Furthermore, we wanted to evaluate the perception of the patients and their sense of safety in regard to automated oxygen control.
Methods
Study design
From May to August 2018, we recruited patients at two pulmonary centers in Copenhagen. Twenty patients were recruited and entered the study in a crossover design with 4 hours of oxygen delivery with O2matic vs 4 hours of manually controlled oxygen delivery by nursing staff. The study was conducted in accordance with the Declaration of Helsinki and was approved by The Danish Medicines Agency, The regional Ethics Committee (H-17040114), and the regional data safety board (VD-2018–44, 6248). The study was registered at ClinicalTrials.gov (NCT03464695).
Equipment
The O2matic oxygen controller is a closed-loop system that, based on continuous monitoring of pulse and SpO2 by a standard wired pulse oximeter, adjusts oxygen flow to the patient (Figure 1). The algorithm in O2matic samples the last 15 seconds of input from the pulse oximeter and calculates increments or decrements in oxygen flow every second based on the last 15 seconds’ average. Increments and decrements are proportionally increased relative to the difference between actual SpO2 and target SpO2. Maximal oxygen flow can be specified to fit the actual condition and the device used for delivering oxygen to the patient (mask or nasal cannula with or without humidifier). O2matic allows for flow up to 15 L/minute in automatic mode, but in the actual study most patients had a setting with acceptable flow range from 0 to 6 L/minute and oxygen delivered with a standard bore nasal cannula. If minimal SpO2 cannot be maintained with the maximal oxygen flow allowed, an alarm will sound, which will intensify if SpO2 drops >3% below target interval or below 85%. Alarms will also be visible and audible if pulse rate drops <45 or exceeds a user-defined maximum. Alarm delay for loss of signal from the oximetry sensor can be individualized from 0 to 5 minutes, to avoid repeated alarms due to signal loss of shorter duration.
  | Figure 1 The O2matic® device. |
Patients
Patients were eligible for the study if they had a confirmed diagnosis of COPD and were admitted with an exacerbation of COPD with an estimated length of stay >48 hours. Inclusion required an SpO2 ≤88% on room air. Patients were excluded if they had any other significant respiratory or cardiac condition causing hypoxemia or if they had severe ongoing malignancy. Patients deemed at high risk for need of mechanical ventilation were not included in the study. All patients provided written informed consent.
Study intervention
Automated oxygen delivery by O2matic and manually controlled oxygen were delivered in random order, each for 4 hours with at least 16 hours between interventions (Figure 2). Allocation to the sequence of the two interventions was done from sealed envelopes with randomized sequences. In automatic mode, standard SpO2 interval was set to 88%–92% and oxygen flow to 0–6 L/minute, which is the flow that normally can be provided with a standard bore nasal cannula. Both SpO2 interval and oxygen interval could be customized to individual needs. In the manually controlled mode, patients received oxygen from O2matic operating in a manual mode, so the nursing staff used the device as a manual-operated flowmeter. In this mode, the SpO2 and pulse rate were not visible on the screen, so they had to apply another pulse oximeter to read SpO2 and afterward adjust oxygen delivery. In both groups, patients were monitored manually at intervals defined by their Early Warning Score, which is a composite measure of pulse rate, blood pressure, SpO2, oxygen flow, respiratory rate, temperature, and level of consciousness. All events during the two 4 hour periods were handled by the nursing staff with no supervision or interventions from study investigators. Patients were advised to be either in bed or seated in a chair during the study, but they were free to remove the oximetry sensor during meals and visits to bathroom. Neither patients nor clinicians or nursing staff were blinded to the intervention. Oxygen flow was delivered without humidification by standard nasal cannula.
  | Figure 2 Study design. |
Study outcome
The O2matic device logs SpO2, pulse rate, and oxygen flow averaged for each 15 seconds. The primary outcome was the fraction of time within the target SpO2 interval determined from the log-file. Secondary outcomes were as follows:
- Fraction of time with severe hypoxia, defined as SpO2 <85%
- Fraction of time with hypoxia, but not severe hypoxia, which was defined as SpO2 below interval, but not <85%
- Fraction of time with hyperoxia, defined as SpO2 above interval.
Time without signal from the oximetry sensor was deducted before calculation of fraction of time within target or outside target. In case of hyperoxia without oxygen supply, this was counted as time with hyperoxia even if the device had no means to adjust oxygen flow to reduce hyperoxia.
Patients’ sense of comfort and security was measured with a not validated questionnaire with four questions:
- To which degree did you trust that the oximetry sensor stayed in place on your finger?
- To which degree did you trust that the device provided you with the right amount of oxygen?
- To which degree did the equipment limit your movements?
- To which degree did you feel safe regarding the oxygen treatment in the 4 hours where O2matic controlled the oxygen supply?
All questions could be answered with “Not at all”, “A little”, “Quite a bit”, or “Very much”.
Statistical analysis
Based on an anticipated difference in fraction of time within target SpO2 interval of at least 20 percentage points and a standard deviation of 28 percentage points, we needed 15 patients to obtain a power of 80% and α of 0.05. To allow for dropout, we intended to include 20 patients. Data were tested for normality with Q–Q plots. Data that passed the normality test were compared with a paired t-test, whereas Wilcoxon signed rank test for related samples was used as a nonparametric test. All results were considered significant with P-values <0.05. Data were analyzed with IBM SPSS statistical package version 22.
Results
Admitted patients at the two centers were screened for inclusion and exclusion criteria upon arrival at the respiratory ward. Twenty-one patients fulfilled all inclusion criteria and none of the exclusion criteria. Only one patient did not want to participate; thus, 20 patients were randomized from May to August 2018. One patient was excluded before data were obtained due to wrongful inclusion. The remaining 19 patients completed both interventions in the study. The baseline characteristics of the patients are shown in Table 1. All patients were in GOLD group D. The average number of serious comorbidities was 0.7. Four patients (20%) were at long-term oxygen treatment before the admission. Six patients (30%) had pneumonia at admission. At inclusion, the average SpO2 was 90% with average supplemental oxygen dosing of 1.7 L/minute. One patient had at inclusion a need for 5 L/minute and acceptable flow range was set to 0–15 L/minute to accommodate for this. All other patients had lower oxygen need, and flow range was set to 0–6 L/minute. Outcomes with deviation from the normal distribution were analyzed by both parametric and nonparametric tests and, in all cases, let to the same interpretation of the results.
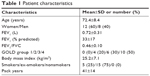  | Table 1 Patient characteristics |
On average, patients received oxygen with O2matic in automatic mode for 267 minutes compared to 250 minutes in manual mode (P=0.6). Average oxygen flow rate was 2.3 L/minute in automatic mode and 1.8 L/minute in manual mode (P=0.1). Average pulse rate was similar in the two periods (90/minute vs 88/minute, P=0.2). There was a loss of signal from the pulse oximeter in 2.9% of the time in automated mode and 4.5% of the time in manual mode (P=0.6). Loss of signal was caused either by patients not wearing the pulse oximeter probe during visits to bathroom, meals etc., or loss of signal even when the probe was in place. During periods with loss of signal, oxygen flow continued at the level used immediately before loss of signal.
Primary outcome
The O2matic device kept SpO2 within prespecified interval for 85.1% of the time vs 46.6% in manual mode, with a mean difference of 38.5% (CI: 27.8%–49.3%, P<0.001). The target SpO2 interval could be set individually at physician’s discretion. Fourteen patients had a target SpO2 interval of 88%–92% whereas six patients had a specified target interval of 90%–94%. The fraction of time within SpO2 interval is shown in Figure 3.
  | Figure 3 Fraction of time with different levels of SpO2 for O2matic® (blue bars) and manual control (red bars). |
Secondary outcomes
Fraction of time with very low SpO2 (<85%) was 1.3% in automatic mode and 17.9% in manual mode, with a mean difference of 16.6% (CI: 4.0%–29.3%, P=0.01). Fraction of time with SpO2 below target but not <85% was 9.0% in automatic mode and 25.0% in manual mode, with a mean difference of 16.0% (CI: 6.9%–25.1%, P=0.002). Fraction of time with high SpO2 above target interval was 4.6% in automatic mode and 10.5% in manual mode, with a mean difference of 5.9% (CI: −3.5% to 15.3%, P=0.2). All results for fraction of time within different intervals are shown in Figure 3.
Safety
In one instance audible and visible alarms for no power supply and low battery were ignored resulting in shutdown of oxygen supply after 2 hours of battery mode, which is in accordance with specifications for battery durability in O2matic. The patient was on low-dose oxygen and no harm was reported, but equipment alarms were afterward adjusted to flash on the screen if there is a lack of power supply and warn that there could be imminent shutdown. No other safety issues were observed.
Other outcomes
Out of the 19 patients completing the study, 13 completed an interviewer-administered questionnaire about how they felt regarding the automated oxygen treatment with O2matic. Missing data on six subjects was due to lack of follow-up when study was completed as there was no investigator available to administer the questionnaire. Eight (62%) expressed very high confidence in getting the right amount of oxygen, four (31%) expressed quite a bit confidence, and one (8%) did not know. None expressed little or no confidence. Limitation of movements due to the wired pulse oximeter was an issue. Three (23%) felt very much limited, six (46%) felt a little limited, three (23%) felt no limitation, and one did not answer. Eleven (85%) felt very safe regarding the risk of misplacing the pulse oximeter probe during rest and two (15%) felt quite a bit safe. None felt only a little or not safe. Overall, sense of safety with the concept of automated oxygen delivery was very high. Twelve (92%) expressed that they felt very safe, and one (8%) felt quite a bit safe. None felt only a little or not safe.
Discussion
This study reports the first clinical data on closed-loop control of oxygen supply with O2matic to patients admitted with an exacerbation of COPD. The data show that automated oxygen supply is feasible and superior to manual control by nursing staff in maintaining SpO2 within the prescribed interval. Especially, episodes with very low SpO2 (<85%) and low SpO2 (below target but not <85%) were significantly reduced with O2matic compared with manual control, whereas episodes with high SpO2 (above target interval) showed a trend to reduction from 10.5% to 4.6%. These results are well in line with previous studies on closed-loop oxygen control. The FreeO2 device from Canadian company OxyNov has shown similar figures for time within prescribed interval for patients with COPD and for patients with acute hypoxemia in the emergency ward.5,6 However, the figures are not necessarily comparable, as our study was a daytime crossover study in a pulmonary ward, whereas the studies with FreeO2 were with a parallel design and either of longer duration including nighttime or in an emergency room setting. Closed-loop control of oxygenation has also been shown to be feasible in other settings such as neonates, during exercise, and in a home setting.7–13
We were not able to demonstrate a reduction in oxygen consumption with O2matic compared to manual control. Average flow rate was 2.3 L/minute with O2matic and 1.8 L/minute with manual control, which was not significantly different. However, out study was not powered to study this outcome. In the two studies of FreeO2, there was a tendency toward a reduction in oxygen consumption in one study from 1.2 L/minute to 0.7 L/minute (P=0.06), and no overall difference in another study where average flow rate was 4.6 L/minute with FreeO2 and 4.2 L/minute with manual control.5,6 The tendency in our study toward lower flow rate with manual control than with O2matic is consistent with the patients being hypoxemic in a large proportion of the time with manual control. Thus, actual oxygen flow rates with manual control were often inadequate to maintain the prescribed SpO2.
It is well documented that prescription practices for oxygen supplementation and adherence to prescriptions are very poor, probably reflecting unawareness about the necessity of accurate oxygen prescription and therapy.14 An audit in 2013 by the British Thoracic Society found that only 55% of patients who were administered oxygen during an admission had a written prescription.15 However, this was an improvement from 2008, where only 32% of patients supplied with oxygen had a written prescription. In an Australian audit, only 3% of in-patients with an exacerbation of COPD had a written oxygen prescription despite 79% of the patients receiving oxygen supplementation.16 In a large European audit in 2010–2011 of 16,018 patients with an exacerbation in COPD, it was found that 10.1% received inappropriate oxygen therapy, either with high flow oxygen or no oxygen despite being hypoxic.17
Patients’ acceptance of automated oxygen delivery in our study was very high, and in general, the patients expressed very high confidence in the concept and felt secure that they received the right amount of oxygen. However, limitation of movements due to the wired pulse oximeter was an issue for some patients. As our study was limited to two times 4 hours of continuous SpO2 monitoring during daytime, longer studies including nighttime are needed to properly evaluate patient experience with continuous monitoring and oxygen control.
Our study was a crossover study and thus did not allow for examining outcomes such as time for weaning from oxygen and duration of admission. Other studies have shown that closed-loop control of oxygen probably allows for faster weaning from oxygen and shorter admission time and thus could be very cost-effective compared with manual control by nursing staff.5,6 We found that the crossover design was ideal as no carryover effect was to be expected in relation to the treatment outcome which was SpO2 in response to oxygen treatment. Still we used a washout period of 16 hours, primarily to secure that O2 titration in the active arm did not influence the dosing of O2 in the manual arm. Furthermore, to evaluate if there was a systematic change in the condition from day 1 to day 2 of the intervention, we examined for a period effect with oxygen flow and SpO2 within target interval on day 1 and day 2 and found no significant differences in those two parameters.
It was not possible to blind our study, as the nursing staff had to manually adjust oxygen in the control arm. This difference was also visible to the patients due to the manual adjustments in one arm, and due to difference in O2matic user screen setup, where pulse rate and SpO2 were not visible in the control arm and the O2matic was used as a manual flowmeter. However, as our main outcome was a physiological parameter (SpO2), the lack of blinding is not considered to have influenced the results regarding SpO2 data, but it could possibly confound the questionnaire data. Time outside target saturation interval for manual control was comparable with results from other studies.5,6
A relevant issue is the clinical importance of maintaining SpO2 within a rather narrow interval. There is a lack of controlled studies of the outcome in clinical terms of prescribing and adhering to different oxygen dosing regimes for patients with an exacerbation of COPD, but a low as well as a high SpO2 on admission has been associated with worse outcome in terms of mortality or other serious adverse outcome.3,4 It seems reasonable to assume that the findings from studies at prehospital level and on admission to some extent can be extrapolated to similar conditions during an admission, but further studies are needed to evaluate clinical outcomes related to episodes with prolonged hypoxemia and hyperoxia. We did not evaluate the effect of closed-loop control of oxygen on arterial CO2 tension (PaCO2) in our study. An increase in arterial oxygen tension is known to increase PaCO2 due to the Haldane effect and due to increased dead-space ventilation, caused by reversal of hypoxemic pulmonary vasoconstriction and worsening in ventilation-perfusion mismatch.18 However, the recommended strategy for avoiding CO2 retention is to eliminate hyperoxia and control SpO2 in the interval 88%–92%.2 This makes CO2 retention more unlikely when SpO2 is better controlled, as is the case in our study with O2matic.
Conclusion
We found that O2matic was able to effectively control SpO2 for patients admitted with an exacerbation of COPD. O2matic was significantly better than manual control by nursing staff to maintain SpO2 within the specified interval and to reduce time with unintended hypoxemia. The patients accepted automated oxygen control without reservations and felt safe about getting the right amount of oxygen. Whether such a strategy for oxygen therapy during admission can reduce admission length, and possibly improve survival among COPD patients, remains to be determined.
Data sharing statement
Individual data from this study will not be shared due to pending patent application.
Acknowledgments
The development of the first prototype of O2matic and the preclinical testing were partially funded by The Capital Region of Denmark. The development of the final version of O2matic and the clinical testing presented in this study were funded by Innovation Fund Denmark. The grant was used in a partnership between The Capital Region of Denmark, Pactor Ltd. (IT-company) and GreenDale Medical Engineering AG (Medical Documentation). Technical University Denmark, Department of Electrical Engineering (Automation and Control) has participated throughout the development of O2matic with technical support and in the design of the algorithm for oxygen control. The Danish Lung Association has provided invaluable support during all phases of the development and trial.
Author contributions
All authors participated in the critical appraisal of the data and in writing and revision of the paper. EFH was the principal investigator on the study and JDH, J-USJ, and CSB were coinvestigators. TK was mainly responsible for the statistical analyses. JV was the independent supervisor for the study. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
EFH and JDH are co-inventors of O2matic® and both have participated in the development and testing of the device since 2011. The partnership, which was built during the development funded by Innovation Fund Denmark, formed a new company (O2matic Ltd., Herlev, Denmark) and both EFH and JDH participate as shareholders in O2matic Ltd. Jørgen Vestbo is supported by the NIHR Manchester Biomedical Research Center. The authors report no other conflicts of interest in this work.
References
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of COPD; 2018. Available from: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf. Accessed December 02, 2018. | ||
British Thoracic Society Emergency Oxygen Guideline Development Group. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72:i1–i90. | ||
Austin MA, Wills KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:c5462. | ||
Cameron L, Pilcher J, Weatherall M, Beasley R, Perrin K. The risk of serious adverse outcomes associated with hypoxaemia and hyperoxaemia in acute exacerbations of COPD. Postgrad Med J. 2012;88(1046):684–689. | ||
Lellouche F, Bouchard PA, Roberge M, et al. Automated oxygen titration and weaning with FreeO2 in patients with acute exacerbation of COPD: a pilot randomized trial. Int J Chron Obstruct Pulmon Dis. 2016;11:1983–1990. | ||
L’Her E, Dias P, Gouillou M, et al. Automatic versus manual oxygen administration in the emergency department. Eur Respir J. 2017;50(1):1602552. | ||
Claure N, Gerhardt T, Everett R, Musante G, Herrera C, Bancalari E. Closed-loop controlled inspired oxygen concentration for mechanically ventilated very low birth weight infants with frequent episodes of hypoxemia. Pediatrics. 2001;107(5):1120–1124. | ||
Claure N, D’Ugard C, Bancalari E. Automated adjustment of inspired oxygen in preterm infants with frequent fluctuations in oxygenation: a pilot clinical trial. J Pediatr. 2009;155(5):640–645. | ||
Hallenberger A, Poets CF, Horn W, et al. Closed-loop automatic oxygen control (CLAC) in preterm infants: a randomized controlled trial. Pediatrics. 2014;133(2):e379–e385. | ||
Lal M, Tin W, Sinha S. Automated control of inspired oxygen in ventilated preterm infants: crossover physiological study. Acta Paediatr. 2015;104(11):1084–1089. | ||
Cirio S, Nava S. Pilot study of a new device to titrate oxygen flow in hypoxic patients on long-term oxygen therapy. Respir Care. 2011;56(4):429–434. | ||
Lellouche F, L’Her E, Bouchard PA, Brouillard C, Maltais F. Automatic oxygen titration during walking in subjects with COPD: a randomized crossover controlled study. Respir Care. 2016;61(11):1456–1464. | ||
Rice KL, Schmidt MF, Buan JS, Lebahn F, Schwarzock TK. AccuO2 oximetry-driven oxygen-conserving device versus fixed-dose oxygen devices in stable COPD patients. Respir Care. 2011;56(12):1901–1905. | ||
Cousins JL, Wark PA, McDonald VM. Acute oxygen therapy: a review of prescribing and delivery practices. Int J Chron Obstruct Pulmon Dis. 2016;11:1067–1075. | ||
O’Driscoll BR. British Thoracic Society. Emergency oxygen audit 2013. Available from: https://www.brit-thoracic.org.uk/document-library/audit-and-quality-improvement/audit-reports/bts-emergency-oxygen-audit-report-2013/. Accessed August 15, 2018. | ||
Pretto JJ, McDonald VM, Wark PA, Hensley MJ. Multicentre audit of inpatient management of acute exacerbations of chronic obstructive pulmonary disease: comparison with clinical guidelines. Intern Med J. 2012;42(4):380–387. | ||
Roberts CM, Lopez-Campos JL, Pozo-Rodriguez F, Hartl S; European COPD Audit team. European hospital adherence to GOLD recommendations for chronic obstructive pulmonary disease (COPD) exacerbation admissions. Thorax. 2013;68(12):1169–1171. | ||
Abdo WF, Heunks LM. Oxygen-induced hypercapnia in COPD: myths and facts. Crit Care. 2012;16(5):323. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
