Back to Journals » Infection and Drug Resistance » Volume 12
Augmented renal clearance is associated with inadequate antibiotic pharmacokinetic/pharmacodynamic target in Asian ICU population: a prospective observational study
Authors Wu CC , Tai CH, Liao WY , Wang CC , Kuo CH , Lin SW , Ku SC
Received 23 April 2019
Accepted for publication 16 July 2019
Published 16 August 2019 Volume 2019:12 Pages 2531—2541
DOI https://doi.org/10.2147/IDR.S213183
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eric Nulens
Chien-Chih Wu,1,2 Chih-Hsun Tai,1 Wen-You Liao,2 Chi-Chuan Wang,2 Ching-Hua Kuo,2 Shu-Wen Lin,1,3 Shih-Chi Ku4
1Department of Pharmacy, National Taiwan University Hospital, College of Medicine, National Taiwan University, Taipei, Taiwan; 2School of Pharmacy, College of Medicine, National Taiwan University, Taipei, Taiwan; 3Graduate Institute of Clinical Pharmacy, College of Medicine, National Taiwan University, Taipei, Taiwan; 4Division of Chest Medicine, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
Correspondence: Shih-Chi Ku
Division of Chest Medicine, Department of Internal Medicine, National Taiwan University Hospital, 7 Chung Shan S. Road, Taipei, Taiwan
Tel +886 22 356 2905
Fax +886 22 358 2867
Email [email protected]
Background: Augmented renal clearance (ARC) is common in critically ill patients and could result in subtherapeutic antibiotic concentration. However, data in the Asian population are still lacking. The aim of this study was to explore the incidence and risk factors of ARC and its effect on β-lactam pharmacokinetics/pharmacodynamics (PK/PD) in Asian populations admitted to a medical ICU. In addition, we evaluated the appropriateness of using three estimated glomerular filtration (eGFR) formulas [Cockcroft–Gault (CG), Modification of Diet in Renal Disease (MDRD), and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)] as screening tools.
Methods: We measured 2-, 8-, and 24-hr creatinine clearance (CLCr) and calculated eGFR by using three formulas for each. ARC was defined as CLCr24hr >130 mL/min/1.73 m2. Concentrations at the mid-dosing interval and prior to the next dose were collected if patients received the β-lactam antibiotic of piperacillin/tazobactam, cefepime, and meropenem, to determine the PK/PD index of fT > MIC. Multiple logistic regression analysis was conducted to identify the risk factors for ARC. Pearson correlation coefficient and the Bland and Altman method were applied to assess the accuracy of CLCr2hr, CLCr8hr, and eGFR for predicting ARC.
Results: Of 100 patients, 46 (46%) manifested ARC. Younger age (<50 years) and lower Sequential Organ Failure Assessment score increased the likelihood of ARC. ARC resulted in a low chance of achieving 50% fT >4MIC (33% vs 75%, p<0.01), 100% fT > MIC (23% vs 69%, p<0.01), and 100% fT >4MIC (3% vs 25%, p<0.02). CLCr8hr wielded the best correlation and agreement with CLCr24hr. eGFRCG was the most appropriate screening tool, and the optimal cutoff value for detecting ARC was 130.5 mL/min/1.73 m2.
Conclusion: ARC is associated with inadequate β-lactam PK/PD target in Asian ICU.
Keywords: augmented renal clearance (ARC), critical care, glomerular filtration rate, pharmacokinetic/pharmacokinetics, β-lactam antibiotic
Introduction
Pharmacokinetic (PK)/pharmacodynamic (PD) profiles of drugs differ considerably between critically ill patients and healthy subjects. In patients with profound shock, tissue hypoperfusion causes organ damage, which could reduce drug elimination, and thus reducing the drug dosage is necessary.1 By contrast, systemic inflammatory response syndrome, resuscitation, and vasopressor use increase cardiac output, which may increase organ perfusion and lead to excessive drug elimination.2 Augmented renal clearance (ARC) is a state of increased renal drug excretion that has been described and is defined by a creatinine clearance >130 mL/min/1.73 m2.3 ARC leads to a low concentration of antibiotics such as β-lactam, which may be associated with poor clinical outcomes.4 The incidence of ARC in the intensive care units (ICUs) is approximately 30–65% in previous studies.3,5,6 Until now, only one ARC study was conducted in the Japanese population; however, it only demonstrates the incidence of ARC without exploring its effect on antibiotics.7 Studies on the occurrence of ARC and its influence on the PK/PD of β-lactam antibiotics in Asian populations admitted to medical ICU are lacking.
The gold standard measurement for the assessment of renal function is glomerular filtration rate (GFR) with exogenous markers such as inulin or radiocontrast agents like iohexol or iothalamate.8 The advantage of radiocontrastis that it only needs to collect blood sample, which is more convenient than inulin use which needs both plasma and urine sample.8 However, exogenous markers are still not routinely used in the ICU because it is more complex and expensive.9 Measurement of 24-hr creatinine clearance (CLCr24hr) is a surrogate for exogenous markers, but it is not useful for meeting the need for timely detection of ARC in clinical practice. Recent studies have shown that 2-hr creatinine clearance (CLCr2hr) and 8-hr creatinine clearance (CLCr8hr) measurements had a strong correlation with CLCr24hr measurement in patients with trauma and undergoing surgery and could be optimal surrogates for detecting ARC.10–12 Clinically, estimated GFR (eGFR) is most commonly calculated using serum creatinine (SCr) and different formulas such as Cockcroft–Gault (CG), Modification of Diet in Renal Disease (MDRD), and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI).13–15 Although studies have shown that these formulas could be effective screening tools for identifying ARC, they are still not validated in medical ICU patients.5,7
Therefore, the primary aims of this study were to explore the incidence and risk factors for ARC and its effect on the PK/PD of β-lactam antibiotics. In addition, we evaluated the accuracy of CLCr2hr, CLCr8hr, and GFR estimated using three commonly used formulas for medical ICU patients. Finally, we clarified the association between ARC and eGFR by using the CG, MDRD, and CKD-EPI methods, separately, to elucidate whether they could be suitable screening tools for patients with ARC admitted to the medical ICU.
Methods
Study population
This prospective observational study was conducted from August 2017 to May 2018 at National Taiwan University Hospital (NTUH), which is a tertiary referral center with a 39-bed medical ICU in northern Taiwan. This study was approved by the Institutional Review Board (201605033RIND) of NTUH, and written informed consent was acquired from all participants or a legal representative. This study was conducted in accordance with the Declaration of Helsinki. We enrolled adult patients (≥20 years) without chronic kidney disease who had a Foley catheter and stayed in the medical ICU for more than 24 hrs. Patients were excluded if they suffered from acute kidney injury, which was defined by SCr increase at least 0.3 mg/dL within 48 hrs, or received renal replacement therapy for acute illness.16
A standardized case report form was used to collect data concerning demographic characteristics (sex, age, height, and weight), SCr on admission, enrollment day and 1 day after the enrollment day, Charlson comorbidity index (CCI) scores and underlying diseases, Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II scores (24 hrs within ICU admission), Sequential Organ Failure Assessment (SOFA) scores on the date of enrollment, ICU admission indication, and ICU mortality. If patients were prescribed a β-lactam antibiotic, namely piperacillin (PIP)/tazobactam (TZP), cefepime (FEP), or meropenem (MEM), the dose, frequency, infusion time, duration, and microbiologic data were also recorded.
ARC evaluation
Urine samples were collected at 0–2, 0–8, and 0–24 hrs after enrollment. Urine creatinine (UCr) at 2, 8, and 24 hrs and SCr on enrollment day and the next day were measured using a colorimetric method. Serum creatinine on enrollment date was used to calculate CLCr2hr and CLCr8hr. Average of SCr on enrollment date and the next day was used for CLCr24hr calculation.
The CLCr2hr, CLCr8hr, and CLCr24hr were calculated as follows:
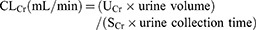
Creatinine clearance was corrected to a standard value of 1.73 m2 body surface area (BSA) and calculated as follows:
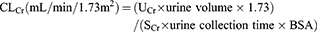
BSA was calculated using the Dubois method:17 BSA =0.20247⨰height (m)°.725⨰weight (kg)°.425. ARC was defined as CLCr24hr >130 mL/min/1.73 m2.
The GFR was estimated using the CG, MDRD, and CKD-EPI formulas.13–15 These formulas are as follows:
CG method (mL/min/1.73 m2):
GFRCG (mL/min/1.73 m2) = (140−age)⨰weight (kg)⨰1.73/(72⨰SCr⨰BSA); a correcting factor of 0.85 was used for female sex.
MDRD method:
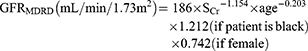
CKD-EPI method:
GFRCKD-EPI (mL/min/1.73 m2) = A × (SCr/B)C ×0.993age × (1.159 if black), where A, B, and C are the following:
Female: A =144, B =0.7, C = −0.329 if SCr ≤0.7 or C = −1.209 if SCr >0.7
Male: A =141, B =0.9, C = −0.411 if SCr ≤0.9 or C = −1.209 if SCr >0.9
β-lactam antibiotic sampling
Blood samples were collected if patients received TZP, FEP, or MEM. Empirical dose selection was performed at the discretion of the treating physician or as suggested by a clinical pharmacist and depended on the severity of illness and infection site. The usual dose of TZP, FEP, or MEM is 4.5 g q6h, 2 g q8h, and 1 g q8h for CLCr >40, 60, or 50 mL/min, respectively, in our institution. Two blood samples were drawn at the mid-dosing interval and immediately prior to the next dose after at least four prior doses had been administered and within the 24-hr urine collection period, a sequence which was used to ensure that all samples were collected during the steady state. Total plasma concentrations of each antibiotic were determined, and free plasma concentrations of each antibiotic were calculated by multiplying the unbound fraction of each antibiotic (PIP: 70%, FEP: 80%, MEM: 98%).
β-lactam antibiotic assay
PIP, FEP, and MEM and their internal standard, piperacillin-d5 (PIP-d5), cefepime-d3 (FEP-d3), and meropenem-d6 (MEM-d6) were prepared separately in methanol at concentrations of 1000 μg mL−1 as stock solutions. Working solutions were prepared by diluting the stock solutions in 50% MeOH. Aliquots of the working solutions containing PIP, FEP, and MEM were added in plasma containing internal standard to obtain solutions at concentration of 300, 500, 1000, 5000, 25,000, 75,000, 150,000, and 250,000 ng mL−1 to construct the calibration curves. To prepare the sample solution, a 10-μL aliquot of plasma was diluted with 90 μL of methanol-containing internal standard, and the final concentration of the internal standard was 100 ng mL−1. The deproteinized sample was centrifuged at 15,000 rpm for 5 mins. Two hundred microliters of deionized water was added to 50 μL of supernatant, which was then filtered through a 0.22-μm syringe filter (RC-4, Sartorius, Göttingen, Germany) prior to the LC-ESI-MS analysis.
The instrument used for PIP, FEP, and MEM quantification was an Agilent 1290 U-HPLC system coupled with an Agilent 6460 triple quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). The injection volume was 5 μL. Separation was performed using a Kinetex™ column (2.1×50 mm, 2.6 μm; Phenomenex, Torrance, CA, USA). The analytical column was maintained at 40°C. The mobile phase consisted of 0.1% aqueous formic acid (solvent A) and 0.1% formic acid in ACN (solvent B). The flow rate was 0.3 mL min−1. The gradient profile was as follows: 0–1 min, 2% B; 1–2 mins, 2–25% B; 3–4 mins, 50–70% B; 4–5 min, 70–100% B; 5–7 mins, 100% B. The sample reservoir was maintained at 4°C. The JetStream electrospray ionizer (Agilent Technologies) was employed as the ion source. The positive electrospray ionization mode was used with the following parameters: 350°C for the drying gas temperature, 7 L min−1 for the drying gas flow, 35 psi for the nebulizer flow, 350°C for the sheath gas temperature, 11 L min−1 for the sheath gas flow, and 4000 V for the capillary voltage. The mass spectrometer was configured in the multiple reaction monitoring mode and monitored the transitions of m/z 518.2 → 143.1 for PIP and 523.2 → 148.1 for PIP-d5, 481.1 → 86.0 for FEP, 484.2 → 89.1 for FEP-d3, 384.2 → 68.0 for MEM, and 390.2→114.0 for MEM-d6.
Quality control samples were arranged within every 10 sample analysis. The precision and accuracy of the quantification method were 2.6–7.6% and 91.9–102.2%, respectively. The regression coefficients of the calibration curves within the range of 500–150,000 ng mL−1 were all higher than 0.995. The limits of detection of PIP, FEP, and MEM were 100, 150, and 100 ng mL−1, respectively. The lower limit of quantitation of each drug was 500 ng mL−1.
PK and PD analysis
For β-lactam antibiotics, 50% of the time above the minimum inhibitory concentration (fT > MIC) is the minimum requirement to ensure clinical efficacy.18 According to a recent report, a more stringent target of 50% fT >4MIC, 100% fT > MIC, or 100% fT >4MIC was associated with a more favorable clinical outcome.18,19 Therefore, we analyzed the results either in terms of a conservative 50% fT > MIC or a more aggressive target of 50% fT >4MIC, 100% fT > MIC, and 100% fT >4MIC. Because Pseudomonas aeruginosa is the most virulent pathogen in the medical ICU and causes high rates of ICU mortality, we adopted the clinical breakpoint of this pathogen as the target MIC.20 The MIC thresholds for this pathogen are as follows: ≤16 mg/L for TZP, ≤8 mg/L for FEP, and ≤2 mg/L for MEM. We classified each patient as having an adequate or inadequate PK/PD target (attainment: 50% fT > MIC, 50% fT >4MIC, 100% fT >MIC, and 100% fT >4MIC) according to the percentage of time during which serum drug concentrations remained above the clinical breakpoint for P. aeruginosa.
Statistical analysis
Continuous and categorical data were described as median with interquartile and number with percentage, respectively. The Mann–Whitney U test was used to assess continuous data, and either a Chi-square or Fisher’s exact test was used for assessing categorical data. A multivariate logistic regression model (single step, forced entry) was constructed to identify the predictors of ARC by using variables for which the p-value was <0.1 in the univariate analysis. Based on a previous study, age was a significant predictor for ARC, especially when age was <50 years.21 Therefore, we set age as a dichotomous variable (<50 or ≥50) when constructing the regression model. Goodness of fit was assessed using the Hosmer–Lemeshow statistic.22
The correlations between CLCr2hr, CLCr8hr, GFRCG, GFRMDRD, GFRCKD-EPI, and CLCr24hr were assessed using the Pearson correlation coefficient (r). Bias was defined as the mean difference between CLCr2hr, CLCr8hr, GFRCG, GFRMDRD, GFRCKD-EPI, and CLCr24hr. The Bland and Altman method was used to assess bias and 95% limits of agreement (bias ±1.96 SD) among CLCr2hr, CLCr8hr, GFRCG, GFRMDRD, GFRCKD-EPI, and CLCr24hr.23 A receiver operating curve (ROC) analysis was performed to examine the accuracy of GFRCG, GFRMDRD, and GFRCKD-EPI for predicting ARC occurrence.
A p-value of ≤0.05 was considered statistically significant. The statistical analysis was performed using SPSS 18 (SPSS Inc, Chicago, IL, USA).
Results
We enrolled 100 patients who were consecutively admitted to the medical ICU, of which 46 patients (46%) had ARC (Table 1). No significant differences in demographics, except age and SCr, were observed between these two groups. The ARC group was significantly younger than the non-ARC group (52 vs 64 years, p=0.02) and had a lower value of SCr (0.4 vs 0.6 mg/dL, p<0.001). The use of a loop diuretic was lower in the ARC group than that in the non-ARC group (15% vs 33%, p=0.04). The ICU mortality was not significantly different in ARC and non-ARC group (26% vs 32%) for patients who had cultures yielding β-lactam-susceptible pathogens.
 |
Table 1 Demographic and clinical characteristics of enrolled patients |
A multivariate analysis was performed to examine the determinants associated with ARC. Younger patients (<50 years) with a lower SOFA score exhibited a significantly increased risk of ARC. By contrast, loop diuretic use reduced its occurrence (Table 2).
 |
Table 2 Multivariate logistic regression analysis with ARC as the dependent variable |
Sixty-two patients received β-lactam antibiotics and were included in the PK/PD analysis. No significant difference was observed between the ARC and non-ARC groups with respect to achieving a conservative target of 50% fT > MIC (90% vs 100%). However, if the target was more stringent, patients with ARC were less likely to achieve this target compared with those without ARC (33% vs 75% for 50% fT >4MIC, p<0.01; 23% vs 69% for 100% fT > MIC, p<0.01; 3% vs 25% for 100% fT >4MIC, p<0.02; Figure 1). More patients achieved the PK/PD target of 100% fT > MIC in patients with ICU survival than those with ICU mortality (64% vs 23%, p<0.01).
 |
Figure 1 Targets of pharmacokinetic and pharmacodynamic attainment. *p<0.05. (A) piperacillin/tazobactam, (B) cefepime, (C) meropenem, (D) all β-lactam. |
Compared with CLCr24hr, the r values of CLCr2hr, CLCr8hr, GFRCG, GFRMDRD, and GFRCKD-EPI were 0.80, 0.88, 0.72, 0.58, and 0.58, respectively (Figure 2). In addition, the bias and 95% limits of agreement were 9 (−107 to 125), 9 (−58 to 76), 3 (−103 to 109), 47 (−139 to 233), and −25 (−133 to 83) mL/min/1.73 m2 for CLCr2hr, CLCr8hr, GFRCG, GFRMDRD, and GFRCKD-EPI, respectively (Figure 3).
The ROC analysis was conducted to evaluate the performance of ARC by using GFRCG, GFRMDRD, and GFRCKD-EPI (Table 3). The area under the curve (AUC) with 95% CI and the cutoff values of GFRCG, GFRMDRD, and GFRCKD-EPI were 0.87 (0.80–0.94), 0.84 (0.76–0.91), and 0.84 (0.77–0.92) and 130.5, 180.5, and 118.5 mL/min/1.73 m2, respectively. The sensitivity and specificity of GFRCG, GFRMDRD, and GFRCKD-EPI were 78.3% and 85.2%, 67.4% and 85.2%, and 73.9% and 83.3%, respectively.
 |
Table 3 Performance of various formulas of eGFR estimation to predict ARC using the receiver operating curve |
Discussion
To the best of our knowledge, this is the first study of ARC that focused on Asian ICU population to demonstrate its effect on the β-lactam concentration. Previous studies have shown that the incidence of ARC in the ICU was approximately 30–65%. The incidence of ARC in our study was 46%, which is comparable to that in previous studies. Young age and low disease severity were identified as independent factors for ARC in our study, which was compatible with previous studies that determined that ARC was more common in relatively young patients and patients with a relatively low disease severity because of higher physiological reserves.3,5,24 When patients are older or have a severe illness, physiological reserves decrease, which may lead to renal hypoperfusion, reducing the risk of ARC. By contrast, patients receiving diuretics are less likely to manifest ARC. Studies that used mannitol have found that postsurgery patients and patients with severe injuries demonstrate an increase in GFR, but this result was not found for furosemide use.25,26 In addition, furosemide did not increase the renal blood flow or exhibit the potential to reduce intravascular volume, both of which may reduce the risk of ARC.27 Besides, diuretic use may be an early sign of ongoing kidney dysfunction, which implies the risk of acute kidney injury instead of ARC.
ARC can be the consequence of the inflammatory state and therapeutic intervention provided, which is commonly seen in critically ill patients.2 Sepsis is the leading cause of mortality in critically ill patients; as a result, optimal antibiotic PK/PD is paramount for achieving favorable outcomes.28 For the class of β-lactam antibiotics, attaining a higher percentage of fT > MIC during treatment was associated with a more favorable clinical outcome; therefore, 100% fT > MIC was recommended as the target for treating severe infections in patients with severe illnesses.18 The optimal goals of antibiotic PK/PD in ICU patient groups are markedly different from those in the general population and may vary in different clinical situations.1 In patients with acute kidney injury, the kidney reduces antibiotic excretion, and thus a reduced antibiotic dosage is required. Yet, ARC reflects supranormal renal excretion of substances, which could lead to subtherapeutic drug exposure if the drug is primarily eliminated through the kidney, thereby reducing the chance to achieve higher PK/PD targets such as 100% fT > MIC of β-lactam antibiotics.4,29 Previous studies have demonstrated that ARC is associated with therapeutic failure and recurrent infection by subtherapeutic antibiotic exposure in surgical and trauma ICU.4,30 Therefore, patients with ARC require higher antibiotic doses and strategies of administration such as extended or continuous infusion to achieve adequate PK/PD targets.31 Our results demonstrated that patients with ARC had a lower chance of achieving 100% fT > MIC than those without ARC.29 However, it did not lead to worse clinical outcome in patients with ARC. The lower mortality in the ARC group may be attributable majorly to their young age and lower disease severity instead of the influence of antibiotic PK/PD. Further prospective study to explore the relationship of ARC on clinical outcome in medical ICU population is still warranted.
Timely assessment of renal function to detect ARC is pivotal for dose adjustment of antibiotics and improving clinical outcomes. The CLCr24hr is the most commonly used method to evaluate renal function; however, it has a long calculation time. Studies have shown that CLCr2hr and CLCr8hr have a strong correlation with CLCr24hr in critically ill patients and patients with trauma.11,12 Our findings demonstrate that CLCr8hr has the best correlation and agreement with CLCr24hr in medical ICU patients.
Compared with CLCr8hr, estimated GFR by formula had a weaker correlation and agreement with CLCr24hr. This conclusion is consistent with those of previous studies that indicated that the renal function estimated using formulas was not reliable for accurately evaluating renal function in ICU patients.6,32,33 Our data show that GFRCG and GFRMDRD would overestimate renal function, and the finding is consistent with that of Grootaert et al but not with that of Baptista et al.6,33 This difference could be explained by the heterogeneity of the patient population. The age, body weight, disease severity, and diagnosis were different in these studies. The cautious use of GFRCG and GFRMDRD formulas for estimating eGFR in critically ill patients is highly recommended.
Despite the lack of accuracy of assessing renal function by using eGFR, it could be used as a screening tool for ARC. Considering the AUCs as well as the sensitivity and the specificity of these formulas, GFRCG, with a cutoff value of 130.5 mL/min/1.73 m2, was found to be slightly more efficient than GFRMDRD and GFRCKD-EPI. Because of the poor correlation of eGFR and measured creatinine clearance, measured creatinine clearance should be used to adjust the drug dosage after identifying patients with ARC. We determined that CLCr8hr could be applied in medical ICU patients because of its good correlation, low bias, and appropriate precision compared with CLCr24hr. The short duration needed for urine collection means it could provide a timely assessment of renal function and enable the prompt adjustment of drug dose to achieve higher antibiotic PK/PD.
This study had several limitations. First, the measurement of GFR with exogenous substances such as inulin or radiocontrast agents is the gold standard for the assessment of renal function, but it is not routinely performed due to practical limitations.9 Carlier et al compared different equations to assess GFR and reported that CLCr24hr had the strongest correlation to inulin clearance in ICU patients.10 Therefore, using CLCr24hr as the surrogate might be reasonable. Second, this was a single-center observational study that included a limited number of study participants. Further, large prospective studies are warranted to confirm the results demonstrated by this study. Third, we only measured the total concentration of β-lactam instead of the free-form concentration, which is the active moiety because of technical limitation. Therefore, we used the unbound fraction of each antibiotic to calculate the free-form concentration, which is more accurate to evaluate PK/PD target than that by total concentration. Last, due to the small sample size, we cannot demonstrate the causal relationship between PK/PD of antibiotics and the clinical outcomes.
Conclusion
ARC was common in critically ill Asian patients admitted to the medical ICU, especially in patients aged <50 years and with a relatively low disease severity. Patients with ARC have a low chance of achieving adequate PK/PD targets. GFRCG could be a more efficient screening tool for ARC. If medical ICU patients are suspected to have ARC, CLCr8hr could further be applied to assess renal function adequately and in a timely manner, and the antibiotic dose could be accordingly adjusted.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board (201605033RIND) of NTUH, and written informed consent was acquired from all participants or a legal representative before enrollment. This study was conducted in accordance with the Declaration of Helsinki.
Data sharing statement
The datasets used and/or analyzed during the current study can be obtained from the corresponding author on reasonable request.
Acknowledgments
The authors acknowledge the staff of the medical ICU at NTUH for urine sample collection. This study was supported by research grants from National Taiwan University Hospital (NTUH 106-S3390). The funders played no role in the study design, data collection and analysis, preparation of the manuscript or decision to publish.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Roberts JA, Abdul-Aziz MH, Lipman J, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14(6):498–509. doi:10.1016/S1473-3099(14)70036-2
2. Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet. 2010;49(1):1–16. doi:10.2165/11318140-000000000-00000
3. Udy AA, Baptista JP, Lim NL, et al. Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations. Crit Care Med. 2014;42(3):520–527. doi:10.1097/CCM.0000000000000029
4. Carrie C, Petit L, d’Houdain N, et al. Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of beta-lactams administered by continuous infusion: a prospective observational study. Int J Antimicrob Agents. 2018;51(3):443–449. doi:10.1016/j.ijantimicag.2017.11.013
5. Ruiz S, Minville V, Asehnoune K, et al. Screening of patients with augmented renal clearance in ICU: taking into account the CKD-EPI equation, the age, and the cause of admission. Ann Intensive Care. 2015;5(1):49. doi:10.1186/s13613-015-0090-8
6. Grootaert V, Willems L, Debaveye Y, Meyfroidt G, Spriet I. Augmented renal clearance in the critically ill: how to assess kidney function. Ann Pharmacother. 2012;46(7–8):952–959. doi:10.1345/aph.1Q708
7. Kawano Y, Morimoto S, Izutani Y, et al. Augmented renal clearance in Japanese intensive care unit patients: a prospective study. J Intensive Care. 2016;4:62. doi:10.1186/s40560-016-0187-7
8. Traynor J, Mactier R, Geddes CC, Fox JG. How to measure renal function in clinical practice. BMJ. 2006;333(7571):733–737. doi:10.1136/bmj.38975.390370.7C
9. Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function–measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–2483. doi:10.1056/NEJMra054415
10. Carlier M, Dumoulin A, Janssen A, et al. Comparison of different equations to assess glomerular filtration in critically ill patients. Intensive Care Med. 2015;41(3):427–435. doi:10.1007/s00134-014-3641-9
11. Herrera-Gutierrez ME, Seller-Perez G, Banderas-Bravo E, Munoz-Bono J, Lebron-Gallardo M, Fernandez-Ortega JF. Replacement of 24-h creatinine clearance by 2-h creatinine clearance in intensive care unit patients: a single-center study. Intensive Care Med. 2007;33(11):1900–1906. doi:10.1007/s00134-007-0745-5
12. Cherry RA, Eachempati SR, Hydo L, Barie PS. Accuracy of short-duration creatinine clearance determinations in predicting 24 hr creatinine clearance in critically ill and injured patients. J Trauma. 2002;53(2):267–271. doi:10.1097/00005373-200208000-00013
13. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi:10.1159/000180580
14. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
15. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi:10.7326/0003-4819-130-6-199903160-00002
16. Kellum JA, Lameire N, Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Critical Care. 2013;17(1):204. doi:10.1186/cc12734
17. Dubois D, Dubois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. doi:10.1001/archinte.1916.00080130010002
18. Roberts JA, Paul SK, Akova M, et al. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clini Infect Dis. 2014;58(8):1072–1083. doi:10.1093/cid/ciu027
19. Carrie C, Petit L, d’Houdain N, et al. Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of beta-lactams administered by continuous infusion: a prospective observational study. Int J Antimicrob Agents. 2018;51(3):443–449. doi:10.1016/j.ijantimicag.2017.11.013
20. Shorr AF. Review of studies of the impact on Gram-negative bacterial resistance on outcomes in the intensive care unit. Crit Care Med. 2009;37(4):1463–1469. doi:10.1097/CCM.0b013e31819ced02
21. Hobbs AL, Shea KM, Roberts KM, Daley MJ. Implications of augmented renal clearance on drug dosing in critically ill patients: a focus on antibiotics. Pharmacotherapy. 2015;35(11):1063–1075. doi:10.1002/phar.1653
22. Lemeshow S, Hosmer D. Assessing the fit of the model. In: Applied Logistic Regression.
23. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310.
24. Udy AA, Morton FJ, Nguyen-Pham S, et al. A comparison of CKD-EPI estimated glomerular filtration rate and measured creatinine clearance in recently admitted critically ill patients with normal plasma creatinine concentrations. BMC Nephrol. 2013;14:250. doi:10.1186/1471-2369-14-250
25. Sward K, Valsson F, Sellgren J, Ricksten SE. Differential effects of human atrial natriuretic peptide and furosemide on glomerular filtration rate and renal oxygen consumption in humans. Intensive Care Med. 2005;31(1):79–85. doi:10.1007/s00134-004-2490-3
26. Redfors B, Sward K, Sellgren J, Ricksten SE. Effects of mannitol alone and mannitol plus furosemide on renal oxygen consumption, blood flow and glomerular filtration after cardiac surgery. Intensive Care Med. 2009;35(1):115–122. doi:10.1007/s00134-008-1206-5
27. Bradley VE, Shier MR, Lucas CE, Rosenberg IK. Renal hemodynamic response to furosemide in septic and injured patients. Surgery. 1976;79(5):549–554.
28. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi:10.1007/s00134-017-4683-6
29. Huttner A, Von Dach E, Renzoni A, et al. Augmented renal clearance, low beta-lactam concentrations and clinical outcomes in the critically ill: an observational prospective cohort study. Int J Antimicrob Agents. 2015;45(4):385–392. doi:10.1016/j.ijantimicag.2014.12.017
30. Carrie C, Bentejac M, Cottenceau V, et al. Association between augmented renal clearance and clinical failure of antibiotic treatment in brain-injured patients with ventilator-acquired pneumonia: a preliminary study. Anaesth Crit Care Pain Med. 2018;37(1):35–41. doi:10.1016/j.accpm.2017.06.006
31. Sjovall F, Alobaid AS, Wallis SC, Perner A, Lipman J, Roberts JA. Maximally effective dosing regimens of meropenem in patients with septic shock. J Antimicrob Chemother. 2018;73(1):191–198. doi:10.1093/jac/dkx330
32. Baptista JP, Udy AA, Sousa E, et al. A comparison of estimates of glomerular filtration in critically ill patients with augmented renal clearance. Critical Care. 2011;15(3):R139. doi:10.1186/cc10324
33. Baptista JP, Neves M, Rodrigues L, Teixeira L, Pinho J, Pimentel J. Accuracy of the estimation of glomerular filtration rate within a population of critically ill patients. J Nephrol. 2014;27(4):403–410. doi:10.1007/s40620-013-0036-x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


