Back to Journals » Journal of Inflammation Research » Volume 16
Aucubin Alleviates Intervertebral Disc Degeneration by Repressing NF-κB-NLRP3 Inflammasome Activation in Endplate Chondrocytes
Authors Zou K , Ying J, Xu H , Zeng Q, Huang H, Chen W, Li X, Wang P, Jin H, Li J, Wu Y
Received 13 September 2023
Accepted for publication 30 November 2023
Published 6 December 2023 Volume 2023:16 Pages 5899—5913
DOI https://doi.org/10.2147/JIR.S439981
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Kaiao Zou,1,2,* Jun Ying,3,* Huihui Xu,1,2 Qinghe Zeng,1,2 Haipeng Huang,1,2 Wenzhe Chen,1,2 Xuefeng Li,1,2 Pinger Wang,1 Hongting Jin,1 Ju Li,3 Yungang Wu4
1Institute of Orthopaedics and Traumatology of Zhejiang Province, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310006, People’s Republic of China; 2The First College of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 3Department of Orthopaedics, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, People’s Republic of China; 4Department of the Orthopedics of TCM, First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang Province, 325000, People’s Republic of China
*These authors have contributed equally to this work
Correspondence: Yungang Wu; Ju Li, Email [email protected]; [email protected]
Background: Intervertebral disc degeneration (IDD) is a prevalent degenerative disease and often recognized as the primary cause of lower back pain (LBP). Aucubin (Au) is a natural compound with anti-inflammatory properties in various diseases. The present study aimed to confirm the therapeutic effect of Au on IDD and explore its potential mechanism in vivo and in vitro.
Methods: The process of IDD was simulated using the lumbar spine instability (LSI) model. In vivo, the therapeutic effect of Au on LSI-induced mice was evaluated by micro-CT and histomorphometry. Additionally, immunohistochemistry was applied to detect the cartilage metabolism and inflammasome activation in endplate. In vitro, the cytotoxicity of Au on ATDC5 cells was detected by Cell Counting Kit-8 (CCK-8), and the biological effects of Au were evaluated by Quantitative Real-time PCR (qRT-PCR) and Western blotting.
Results: Micro-CT analysis showed that Au administration significantly alleviated LSI-induced disc volume narrowing and endplate cartilage degeneration, which was further supported by Alcian Blue Hematoxylin/Orange G (ABH/OG) staining. Immunohistochemistry results verified that Au could increase the expression of Col2α 1 and Aggrecan, reduce the expression of Mmp-13, and attenuate the degradation of the endplate extracellular matrix (ECM). Mechanistically, we found that Au treatment, both in vivo and in vitro, significantly inhibited NF-κB-NLRP3 inflammasome activation in chondrocytes as determined by the decreased expression of p-P65, NLRP3, and Caspase-1.
Discussion: Taken together, our findings have demonstrated for the first time that Au treatment ameliorated the degeneration of cartilage endplates in IDD may by inhibiting NF-κB-NLRP3 inflammasome activation in chondrocytes and provided a potential candidate for the treatment of IDD.
Keywords: Aucubin, cartilage endplate, intervertebral disc degeneration, NF-κB-NLRP3, inflammasome
Introduction
Low back pain (LBP) is a frequent disease that suffered people of all ages, and its prevalence increases with age.1,2 Although the etiology of LBP remains largely unknown, intervertebral disc degeneration (IDD) is recognized as its major cause and target for treatment.3 IDD is a very complex pathological process that involves multiple stress-induced tissue injuries, and is also strongly associated with age.4 The prevalence of IDD is increasing due to the aging of the population.5 The intervertebral disc (IVD) is one of the elements comprising the lumbar spine. It is located between two adjacent vertebrae and consists of a central nucleus pulposus (NP) surrounded by a fibrous ring and cartilaginous endplate (CEP) envelope.6 Besides, it is capable of cushioning pressure from different directions and is prone to degradation accordingly.7 The process of IDD consists of gradual structural changes accompanied by an anabolic-catabolic imbalance.8 Cartilage extracellular matrix (ECM) degradation, immune response, apoptosis and pyroptosis of IVD cells are major molecular changes during IDD, ultimately causing alterations in the level of tissue.9–11 There are multiple causative factors for IDD pathology, and the search for more effective interventions is particularly urgent.
The CEP is a layer of hydrated cartilage tissue dominated by chondrocytes.12 Intervertebral cartilage cushions mechanical stress and has an important protective role for the spine. Degradation of ECM and calcification of intervertebral cartilage are the main features of IDD. However, Inflammatory factors including tumor necrosis factor alpha (TNF-α), and matrix metalloproteinases (Mmps) released by chondrocytes during IDD accelerate ECM degradation.13 Pyroptosis is a type of programmed cell death, but excessive pyroptosis tends to cause cellular as well as ECM abnormalities. Highly active NF-κB signaling contributes to programmed chondrocyte death such as apoptosis and pyroptosis, which exacerbates CEP calcification.14,15 The NLR pyrin domain containing 3 (NLRP3) inflammasome is a multiprotein complex composed of NLRP3 and the inflammatory protease Caspase-1, in addition, hyperactivation of NLRP3 inflammasome implicated in a wide of inflammatory disorders.16,17 Besides, the NLRP3 is downstream of the NF-κB signaling pathway, and activation of the NLRP3 inflammasome leads to IDD by promoting pyroptosis in NP cell, and ECM degradation in IVD.18,19 Therefore, endplate chondrocyte pyroptosis due to upregulation of NLRP3 by NF-κB activation may be an important causative factor for ECM degradation and cartilage calcification, and effective intervention in chondrocyte pyroptosis may be critical for treating IDD.
Aucubin (Au) is a natural compound that is widely found in Chinese herbs such as Cortex Eucommiae, Radix et Rhizoma Dioscoreae, Plantago Ovata, and many others. In past research has revealed that Au has potent anti-inflammatory effects.20–22 In particular, Au inhibits NF-κB activity in chondrocytes and alleviates inflammatory matrix degradation in articular cartilage.23,24 Furthermore, some previous researches have demonstrated Au could inhibit TNF-α and IL-1β induced nucleus pulposus cell ECM degradation in vitro.25 These findings indicate that Au may have a protective role against IVD in pathological conditions.
In summary, we presumed that Au could modify inflammation-mediated chondrocyte pyroptosis and be a potential therapeutic approach for IDD. In the present study, we emphasized the therapeutic effects of Au on IDD and investigated the potential mechanisms in vivo and in vitro.
Materials and Methods
Mice
Ten-weeks-old male C57BL/6J mice were purchased from the Laboratory Animal Research Center of Zhejiang Chinese Medical University (Hangzhou, China). All experimental protocols for mice were approved by the Animal Experimentation Ethics Committee of Zhejiang Chinese Medical University and complied with the guidelines of the Care and Use of Laboratory Animals (LY23H270005).
In vivo Studies
Aucubin (Aucubin, MUST BIO-TECHNOLOGY CAS: 479-98-1, purity ≥ 98.0%, ChengDu, China), an iridoid glycoside from natural plants, has antioxidative and anti-inflammatory bioactivities (Figure 1A). We employed lumbar spine instability (LSI) surgery to establish a mouse model of IDD.26–28 As reported in previous studies,29 mice were anesthetized with sodium pentobarbital, and the supraspinous ligament, the interspinous ligament, and the spinous processes between the lumbar 3 (L3) to lumbar 5 (L5) were excised with a machete. Meanwhile, mice subjected to sham surgery group were only resected for paraspinal muscle tissue. Finally, antibiotics were used to suture the incision to prevent infection. Mice in the Au group were injected intraperitoneally with Au (10 mg/kg/day) for 8 weeks. Equal amounts of normal saline as Vehicle were administered to mice in the sham-operated and model groups. Groups of mice were euthanized at 4 and 8 weeks after surgery. (Figure 1B). Six mice were used in each group (Sham, LSI+Vehicle, LSI+Au).
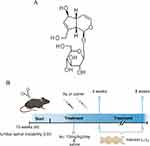 |
Figure 1 Experimental model diagram. (A) Au chemical structure formula. (B) LSI Surgery and Au Treatment Timeline. |
Micro-CT Analyses
All isolated lumbar spine samples were scanned by a high-resolution MIcro-CT scanner (SkyScan, 1176) with scanning parameters set at 45 kV, 500 μA, and 780 ms exposure time. Successive vertebrae from L3-L5 were scanned and three-dimensional images were generated by visualization software (CTVolx, v3.0, SkyScan). Five contiguous coronary images of the regions of interest were used for three-dimensional reconstruction. The invisible space between L4 and L5 was defined as the ROI for the analysis of the IVD.
Histological Analyses
About histological staining, vertebral samples were fixed in 4% paraformaldehyde (PFA) for 3 days. After fixation, samples were decalcified in 14% Ethylene Diamine Tetra acetic Acid (EDTA) solution (pH = 7.2) for 14 days. Subsequently, samples were embedded in paraffin and sectioned at 3 μm in thickness. The slices after dewaxing and rehydrating were stained with Alcian Blue Hematoxylin/ Orange G (ABH/OG) to assess histopathology and endplate scoring.
Immunohistochemical Staining
For immunohistochemistry, the slices after dewaxing and rehydrating were antigen restoration in 60 °C with 0.01 M sodium citrate for 4 h. After that, the slices were incubated with 0.3% Triton X-100 for 10 min at room temperature and then blocked with an endogenous peroxidase blocker (ZSGB-BIO, PV-6001, Beijing, China) for 15 min. The slices were then treated with primary antibody overnight at 4 °C, treated for reaction enhancer 15 min and then with a homologous secondary antibody for 20 min the next day. Positive expression highlighted by DAB (ZSGB-BIO, ZLI-9018, Beijing) staining. These are the primary antibodies used in this study: Collagen II (Abcam, ab34712), MMP-13 (Abcam, ab39012), Aggrecan (Abcam, ab216965), p-P65 (Arigo, ARG51518), TNF-α (Arigo, ARG56080), NLRP3 (HuaBio, ET1610-93), Casapase-1 (HuaBio, ET1608-69).
Cell Culture and Viability Assay
In vitro, the ATDC5 chondrocyte cell line (RIKEN Cell Bank, Tsukuba, Japan) was used and cultured in Dulbecco’s Modified Eagle Medium/F12 (DMEM/F12) (Gibco, USA) medium supplemented with 10% fetal bovine serum (FBS), 100 μg/mL streptomycin, and 100 U/mL penicillin (Gibco, USA). Cells were cultured at 37 °C with 5% CO2. The cytotoxic effect of Aucubin on chondrocytes was evaluated using Cell Counting Kit-8 (CCK-8, Bioss, Beijing, China). ATDC5 cells were seeded in 96-well plates at a density of 8000 cells per well, and the cells were treated with different doses (0, 1, 5, 10, 20, 50, 100, 200 μM) of Au for 12 and 24 h after adhesion. Finally, the absorption of each well was analyzed at 450 nm using a microwell plate reader.
Quantitative RT-PCR
The total RNA of ATDC5 cells was extracted with TRIzol reagent after the cells were treated with 10 ng/mL TNF-α and different doses of Au. The cells were then subjected to Real-time polymerase chain reaction (qRT-PCR) using Taq Pro Universal SYBR qPCR Master Mix (Vazyme, Q712-02). Quantitative analysis was performed using QuantStudio™ Real-Time PCR software. The β-actin was used as a quantitative analysis reference gene. Table 1 lists the primer sequences for the target genes used in this study.
 |
Table 1 The primer sequences for the target genes. |
Western Blot Analysis
Cell lysates were prepared by adding phosphatase and protease inhibitors (Roche Diagnostics, Basel, Switzerland) to the RIPA lysis buffer. All Cells added to the lysate were lysed on ice for more than 30 minutes. Total proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto 0.22 μm NC membranes (MilliporeSigma, Burlington, MA, USA). The target proteins were quantified with β-actin (Proteintech, HRP-60008) before detection. Membranes with 5% skimmed milk (Beyotime, P0216) were blocked for 1 h at room temperature (RT = 25 °C) and then incubated with primary antibodies. Next day, the membranes were washed with Tris-buffered saline (TBS)-0.1% Tween 20 (TBST) and subsequently incubated with a horseradish peroxidase-conjugated secondary goat anti-rabbit IgG antibody for 1 hour at room temperature. After washing in TBST, protein immunoreactivity was detected using an ECL Reagent (4A Biotech, Beijing, China).
Statistical Analysis
In the present study, GraphPad Prism 8.2 software (San Diego, CA) was used for all statistical analyses. Data were presented as mean ± standard deviation (S.D.). Comparisons between the two groups above were statistically analyzed by one-way analysis of variance (ANOVA), and a p value of less than 0.05 was considered a statistically significant difference.
Results
Au Attenuated of LSI-Induced Disc Degeneration
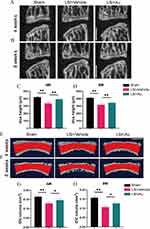
The intervertebral disc serves as an essential tissue for cushioning stress and is extremely resilient. However, degenerative discs undergo hardening that makes it difficult to return to the original level after prolonged exposure to mechanical forces transmitted from the vertebral. Therefore, we induced IDD using LSI and investigated the disc height and disc volume using Micro-CT assays. Sagittal plane images showed that the LSI procedure decreased the disc height between L4 and L5, in particular the posterior region. However, the loss of height can be partially mitigated by the treatment of Au (Figure 2A–D). Moreover, in an attempt to further observe the volume changes of the intervertebral disc, we performed a 3-dimensional reconstruction of the L4-L5 intervertebral disc and endplates, the red area represents IVD (Figure 2E and F). Similarly, Au ameliorated the LSI-induced disc volume narrowing (Figure 2G and H). This evidence indicated that Au administration was effective in alleviating LSI-induced IDD.
Au Suppressed Calcification and Degeneration of Cartilage Endplates in LSI-Induced Mice
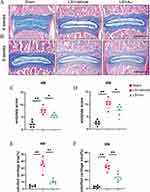
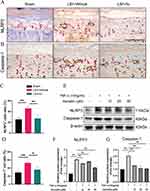
Based on the Micro-CT results, Au improved disc degeneration and effectively slowed the progression of IDD. The cartilaginous endplate is an important source of nutrient supply to IVD. Aiming to further explore the histologic effects of Au on IDD, we explored the effects of Au on CEP using ABH/OG staining, which was able to distinguish cartilaginous and calcified tissues clearly. In tissue sections, samples from the Au-treated group showed reduced cartilage matrix loss and suppressed endplate calcification at 4 weeks (Figure 3A). At 8 weeks, samples from the mice received Au treatment had significantly lower areas of calcification compared to vehicle-treated mice (Figure 3B). Additionally, the cartilage endplate score was much lower in the Au treatment group than that in the LSI surgical induction group after both 4 and 8 weeks (Figure 3C and D). Consistently, and it was clear that the calcified endplate areas at both 4 and 8 weeks were significantly decreased following Au treatment (Figure 3E and F). From the analysis of the histological examination, it was evident that Au treatment could alleviate endplate calcification and degeneration.
Au Alleviated LSI-Induced Metabolic Disturbances in Endplate Cartilage Matrix
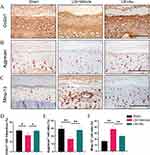
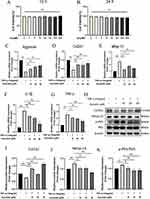
Since the homeostasis of cartilage is determined by the balance of anabolism as well as catabolism, we then examined the expression of Col2α1 and Aggrecan as well as Mmp-13 on endplates by immunohistochemical tests (Figure 4A–C). We found that the expression of Col2α1 and Aggrecan were down-regulated in response to LSI and were rescued with Au intervention (Figure 4D and E). In contrast, the expression of Mmp-13 was increased after modeling and inhibited by Au treatment (Figure 4F). Collectively, these results provided sufficient evidence that Au treatment has maintained cartilage metabolism in LSI-induced mice.
Au Ameliorated IDD by Inhibiting NF-κB Signaling Activity
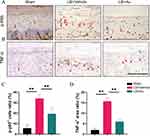
The series of immune responses induced by inflammation is an important reason for exacerbating the process of IDD. Besides, in a previous report, NF-κB signaling was activated in endplate chondrocytes during IDD pathology. We further explore whether Au ameliorated endplate degeneration by inhibiting NF-κB signaling activity in vivo. As expected, the expression of p-P65, a key activator protein for NF-κB signaling, was markedly downregulated in endplate chondrocytes in the Au-treated group (Figure 5A and C). We found that Au diminished the elevation of TNF-α expression in endplate chondrocytes induced by LSI surgery (Figure 5B and D). These data suggested Au has the potential to alleviate IDD by inhibiting NF-κB signaling activity and attenuating endplate cartilage ECM degradation.
Au Rescued TNF-α-Induced Chondrocyte Catabolism and NF-κB Signaling Activation in vitro
Given that Au attenuated endplate chondrocyte degeneration in vivo, we used ATDC5 cells in culture to investigate the protective effect of Au against TNF-α-induced chondrocyte inflammation in vitro.30 CCK-8 assay showed that treatment of Au at doses ranging from 1 to 200 μM for 12 and 24 hours had no effect on the viability of ATDC5 cells (Figure 6A and B). Therefore, we chose the centered three doses as our main intervention doses. In qRT-PCR assay, Au promoted the mRNA expression of Aggrecan and Colα1 in ATDC5 cells after TNF-α treatment, in addition to decreasing the TNF-α-induced expression of Mmp-13 (Figure 6C–E), which were highly in keeping with the immunohistochemistry results. Also, the expressions of IL-1β and TNF-α were both inhibited by Au (Figure 6F and G). Furthermore, we verified the protein expressions by Western blotting experiments (Figure 6H). Consistently, Au rescued TNF-α-induced decreased levels of Col2α1 and inhibited the Mmp-13 expression as well as P65 phosphorylation (Figure 6I and J). All of these results indicated that Au protected chondrocytes from catabolism and inflammation, at least in part, by repressing NF-κB signaling.
Au Protected Endplate Chondrocyte Against NLRP3 Inflammasome Activation
Active NF-κB signaling tends to activate a number of downstream inflammatory factors thereby exacerbating the inflammatory response. NLRP3 as an inflammasomes downstream of NF-κB signaling has been shown to cite as a key protein in the initiation of cellular pyroptosis. Meanwhile, from recent studies, we focused on the effects of endplate chondrocyte pyroptosis on IDD.31 Moreover, the accumulated NLRP3 inflammasome activates the protease Caspase-1 to induce pyroptosis, which is a key component of cellular pyroptosis.32 Therefore, we evaluated the expression of NLRP3 and Caspase-1 on the endplates with immunohistochemical staining (Figure 7A and B). The results showed that NLRP3 and Caspase-1 expression in the Au treatment group was lower than that in the LSI surgery group (Figure 7C and D). To verify the reliability of this mechanism in vitro, we intervened ATDC5 cells with the same dose as in previous data and confirmed the protein expressions by Western blotting (Figure 7E). These data showed that Au treatment substantially attenuated the TNF-α-induced elevation of NLRP3 and Capase1 expression (Figure 7F and G). Taken together, we have demonstrated that Au alleviated IDD by suppression the NF-κB-NLRP3 inflammasome activation in endplate chondrocyte.
Discussion
The prevalence of LBP is at the forefront of the morbidity of chronic diseases.33 LBP can lead to severe, lifelong disability, with the attendant high cost of treatment.34,35 Moreover, the etiology of LBP is multifactorial, and disc degeneration is undoubtedly a crucial factor. Although many treatments have been applied to IDD, treatments that are more cost-effective and specific to the source of the pain need to be investigated.36 Here, our data demonstrated, for the first time, Au suppresses ECM degradation and retards endplate calcification of by suppressing the NF-κB-NLRP3 inflammasome activation. The data from this study suggest that Au is a potential drug for the therapy of IDD.
In the context of this study, we used a mouse model of LSI that is similar in imaging and histopathologic examination to the human manifestations of IDD.26,27 We successfully reproduced the phenotype of IDD in mice using LSI surgery. For example, compared with the Sham group, lumbar spine Micro-CT at 4 and 8 weeks postoperatively showed the IVD height narrowing, and 3D reconstruction demonstrated a reduction in the IVD volume. In parallel, ABH/OG staining also revealed a distinct endplate cartilage calcification layer, these phenotypes are in accordance with prior findings.37,38 Of note, all of these manifestations of IDD partially reversed with Au treatment. Previous studies have found that endplate cartilage is the sole source of nutrients for IVD and its inflammation exacerbates IVD degeneration.39–41 This seems to be further evidence that Au mitigated IDD by relieving inflammation and degradation of CEP.
Recently, increasing research focuses on endplate cartilage degeneration.42 The IVD is avascular tissue, and the CEP adjacent to it is its only source of nutrients.43 In addition to the destruction of the nucleus pulposus, which is the main structure of the IVD, recent, studies have shown that degeneration of the fibrous ring and cartilage endplates is also a major factor in the development of IDD.44 Degeneration of the vertebral endplates is manifested early by calcification of the cartilage, a change that is also considered to be the initial pathologic manifestation.45 However, previous studies have shown that TNF-α can induce the expression of transcription factors associated with osteogenesis in CEPs, which is similar to our findings.12 TNF-α may be a main cause of endplate cartilage calcification. In the present study, ABH/OG staining revealed significant areas of calcification in the L4-L5 inferior endplate at 4 and 8 weeks after surgery. Immunohistochemical results suggest decreased matrix synthesis and increased matrix degradation. In addition, since matrix metabolism is closely related to cartilage calcification.46 Therefore, we supposed that Au could slow the progression of IDD by restoring the metabolic homeostasis of endplate cartilage.
NF-κB signaling is involved in IDD pathophysiology through a variety of roles, including the promotion of gene transcription and protein translation involved in inflammation and catabolism.47–49 It has been widely reported that Au could suppress NF-κB signaling to modulate the inflammatory response.50,51 From our findings on IDD, we suggest that disruption of chondrocyte pyroptosis and matrix metabolic homeostasis is caused by aberrant activation of the NF-κB signaling. NLRP3/Caspase-1 axis activation plays an important role in neuroinflammation and neuronal injury in various neurological diseases.52,53 Previous studies have identified the effect of pyroptosis in nucleus pulposus cells during IDD,14,54,55 and recently, Caspase-1 and NLRP3 expression has been reported to be upregulated in CEP of patients with degenerative disease.56 Consistently, as both in vivo and in vitro studies presented in the current study, Au inhibited the phosphorylation of P65 and reduced the activity of NF-κB signaling, thereby decreased the expression of NLRP3 and Caspase-1, suggested that Au reduced chondrocyte pyroptosis by decreasing the activation of NF-κB signaling. Besides, the expressions of Col2α1 and Aggrecan were restored in the Au-treated group, while the expression of Mmp-13 and TNF-α and IL-1β was decreased. Thus, Au may protect CEP degeneration by reducing pyroptosis and alleviating severe inflammation. Although the anti-inflammatory effects of Au have been extensively studied in many other diseases, including neuronal injury,57 myocardial damage,58 brain injury59 and osteoarthritis,24 our study demonstrates, for the first time, that Au attenuates the inflammatory response and pyroptosis of endplate chondrocytes in IDD.
Inevitably, this study still has some limitations. Due to the limitations of the experimental facilities and other objective conditions, we were unable to determine the effect of Au on behavioral assessments such as gait analysis and spontaneous activity in this study. In addition, our findings do not completely exclude the possibility that Au may mitigate IDD by attenuating chondrocyte pyroptosis through pathways other than NF-κB signaling.
Conclusion
In summary, our study shows for the first time that Au could alleviate LSI-induced cartilage degeneration as well as IDD by inhibiting NF-κB signaling activity and attenuating endplate chondrocyte pyroptosis and suggested its potential pharmacological effects on IDD.
Data Sharing Statement
The data used to provide support for the results of this study can be obtained from the corresponding authors.
Acknowledgments
We appreciate the great help from the Public Platform of Medical Research Center, Academy of Chinese Medical Science, Zhejiang Chinese Medical University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Scientific research project of Wenzhou Science and Technology Bureau (Y20210243).
Disclosure
All authors declare that they have no conflict of interest.
References
1. Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356–2367. doi:10.1016/S0140-6736(18)30480-X
2. Chou R. Low Back Pain. Ann Intern Med. 2021;174(8):ITC113–ITC128. doi:10.7326/AITC202108170
3. Vergroesen PP, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23(7):1057–1070. doi:10.1016/j.joca.2015.03.028
4. Wang F, Cai F, Shi R, Wang XH, Wu XT. Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthritis Cartilage. 2016;24(3):398–408. doi:10.1016/j.joca.2015.09.019
5. Qian J, Ge J, Yan Q, Wu C, Yang H, Zou J. Selection of the optimal puncture needle for induction of a rat intervertebral disc degeneration model. Pain Physician. 2019;22(4):353–360.
6. Li G, Zhang W, Liang H, Yang C. Epigenetic regulation in intervertebral disc degeneration. Trends Mol Med. 2022;28(10):803–805. doi:10.1016/j.molmed.2022.07.007
7. Vlaeyen JWS, Maher CG, Wiech K, et al. Low back pain. Nat Rev Dis Primers. 2018;4(1):52. doi:10.1038/s41572-018-0052-1
8. Francisco V, Pino J, Gonzalez-Gay MA, et al. A new immunometabolic perspective of intervertebral disc degeneration. Nat Rev Rheumatol. 2022;18(1):47–60. doi:10.1038/s41584-021-00713-z
9. Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10–14. doi:10.2106/JBJS.F.00019
10. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. doi:10.1038/nrrheum.2013.160
11. Sun K, Jiang J, Wang Y, et al. The role of nerve fibers and their neurotransmitters in regulating intervertebral disc degeneration. Ageing Res Rev. 2022;81:101733. doi:10.1016/j.arr.2022.101733
12. Shao Y, Sun L, Yang G, et al. Icariin protects vertebral endplate chondrocytes against apoptosis and degeneration via activating Nrf-2/HO-1 pathway. Front Pharmacol. 2022;13:937502. doi:10.3389/fphar.2022.937502
13. Fontana G, See E, Pandit A. Current trends in biologics delivery to restore intervertebral disc anabolism. Adv Drug Deliv Rev. 2015;84:146–158. doi:10.1016/j.addr.2014.08.008
14. Gong Y, Qiu J, Jiang T, et al. Maltol ameliorates intervertebral disc degeneration through inhibiting PI3K/AKT/NF-kappaB pathway and regulating NLRP3 inflammasome-mediated pyroptosis. Inflammopharmacology. 2023;31(1):369–384. doi:10.1007/s10787-022-01098-5
15. Zehra U, Tryfonidou M, Iatridis JC, Illien-Junger S, Mwale F, Samartzis D. Mechanisms and clinical implications of intervertebral disc calcification. Nat Rev Rheumatol. 2022;18(6):352–362. doi:10.1038/s41584-022-00783-7
16. Lu A, Magupalli VG, Ruan J, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156(6):1193–1206. doi:10.1016/j.cell.2014.02.008
17. Wang L, Hauenstein AV. The NLRP3 inflammasome: mechanism of action, role in disease and therapies. Mol Aspects Med. 2020;76:100889. doi:10.1016/j.mam.2020.100889
18. Chen F, Jiang G, Liu H, et al. Melatonin alleviates intervertebral disc degeneration by disrupting the IL-1beta/NF-kappaB-NLRP3 inflammasome positive feedback loop. Bone Res. 2020;8(1):10. doi:10.1038/s41413-020-0087-2
19. Chao-Yang G, Peng C, Hai-Hong Z. Roles of NLRP3 inflammasome in intervertebral disc degeneration. Osteoarthritis Cartilage. 2021;29(6):793–801. doi:10.1016/j.joca.2021.02.204
20. Gao F, Li H, Feng Y, Tian W, Cao R, Fu K. Aucubin ameliorates the LPS-induced inflammatory response in bovine endometrial epithelial cells by inhibiting NF-kappaB and activating the Keap1/Nrf2 signalling pathway. Reprod Domest Anim. 2021;56(7):972–982. doi:10.1111/rda.13939
21. Park KS, Chang IM. Anti-inflammatory activity of aucubin by inhibition of tumor necrosis factor-alpha production in RAW 264.7 cells. Planta Med. 2004;70(8):778–779. doi:10.1055/s-2004-827211
22. Park KS. Aucubin, a naturally occurring iridoid glycoside inhibits TNF-alpha-induced inflammatory responses through suppression of NF-kappaB activation in 3T3-L1 adipocytes. Cytokine. 2013;62(3):407–412. doi:10.1016/j.cyto.2013.04.005
23. Zhang Y, Tang LD, Wang JY, et al. Anti-inflammatory effects of aucubin in cellular and animal models of rheumatoid arthritis. Chin J Nat Med. 2022;20(6):458–472. doi:10.1016/S1875-5364(22)60182-1
24. Wang SN, Xie GP, Qin CH, et al. Aucubin prevents interleukin-1 beta induced inflammation and cartilage matrix degradation via inhibition of NF-kappaB signaling pathway in rat articular chondrocytes. Int Immunopharmacol. 2015;24(2):408–415. doi:10.1016/j.intimp.2014.12.029
25. Yang S, Li L, Zhu L, et al. Aucubin inhibits IL-1beta- or TNF-alpha-induced extracellular matrix degradation in nucleus pulposus cell through blocking the miR-140-5p/CREB1 axis. J Cell Physiol. 2019;234(8):13639–13648. doi:10.1002/jcp.28044
26. Oichi T, Taniguchi Y, Soma K, et al. A mouse intervertebral disc degeneration model by surgically induced instability. Spine. 2018;43(10):E557–E564. doi:10.1097/BRS.0000000000002427
27. Mao J, Wang D, Wang D, et al. SIRT5-related desuccinylation modification of AIFM1 protects against compression-induced intervertebral disc degeneration by regulating mitochondrial homeostasis. Exp Mol Med. 2023;55(1):253–268. doi:10.1038/s12276-023-00928-y
28. Fu F, Bao R, Yao S, et al. Aberrant spinal mechanical loading stress triggers intervertebral disc degeneration by inducing pyroptosis and nerve ingrowth. Sci Rep. 2021;11(1):772. doi:10.1038/s41598-020-80756-6
29. Bian Q, Jain A, Xu X, et al. Excessive activation of TGFbeta by spinal instability causes vertebral endplate sclerosis. Sci Rep. 2016;6(1):27093. doi:10.1038/srep27093
30. Xue J, Hu B, Xing W, et al. Low expression of miR-142-3p promotes intervertebral disk degeneration. J Orthop Surg Res. 2021;16(1):55. doi:10.1186/s13018-020-02194-4
31. Zhao K, An R, Xiang Q, et al. Acid-sensing ion channels regulate nucleus pulposus cell inflammation and pyroptosis via the NLRP3 inflammasome in intervertebral disc degeneration. Cell Prolif. 2021;54(1):e12941. doi:10.1111/cpr.12941
32. Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18(9):2114–2127. doi:10.1038/s41423-021-00740-6
33. Raftery MN, Sarma K, Murphy AW, De la Harpe D, Normand C, McGuire BE. Chronic pain in the Republic of Ireland--community prevalence, psychosocial profile and predictors of pain-related disability: results from the Prevalence, Impact and Cost of Chronic Pain (PRIME) study, part 1. Pain. 2011;152(5):1096–1103. doi:10.1016/j.pain.2011.01.019
34. Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222.
35. Belitskaya-Levy I, Clark JD, Shih MC, Bair MJ. Treatment preferences for chronic low back pain: views of veterans and their providers. J Pain Res. 2021;14:161–171. doi:10.2147/JPR.S290400
36. Mohd Isa IL, Mokhtar SA, Abbah SA, Fauzi MB, Devitt A, Pandit A. Intervertebral disc degeneration: biomaterials and tissue engineering strategies toward precision medicine. Adv Healthc Mater. 2022;11(13):e2102530. doi:10.1002/adhm.202102530
37. Guo Q, Zhu D, Wang Y, et al. Targeting STING attenuates ROS induced intervertebral disc degeneration. Osteoarthritis Cartilage. 2021;29(8):1213–1224. doi:10.1016/j.joca.2021.04.017
38. Bian Q, Ma L, Jain A, et al. Mechanosignaling activation of TGFbeta maintains intervertebral disc homeostasis. Bone Res. 2017;5(1):17008. doi:10.1038/boneres.2017.8
39. Luo L, Jian X, Sun H, et al. Cartilage endplate stem cells inhibit intervertebral disc degeneration by releasing exosomes to nucleus pulposus cells to activate Akt/autophagy. Stem Cells. 2021;39(4):467–481. doi:10.1002/stem.3322
40. Zhang Y, He F, Chen Z, et al. Melatonin modulates IL-1beta-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation. Aging. 2019;11(22):10499–10512. doi:10.18632/aging.102472
41. Yang G, Liu X, Jing X, et al. Astaxanthin suppresses oxidative stress and calcification in vertebral cartilage endplate via activating Nrf-2/HO-1 signaling pathway. Int Immunopharmacol. 2023;119:110159. doi:10.1016/j.intimp.2023.110159
42. Zuo R, Wang Y, Li J, et al. Rapamycin induced autophagy inhibits inflammation-mediated endplate degeneration by enhancing Nrf2/Keap1 signaling of cartilage endplate stem cells. Stem Cells. 2019;37(6):828–840. doi:10.1002/stem.2999
43. Huang B, Liu J, Wei X, et al. Damage to the human lumbar cartilage endplate and its clinical implications. J Anat. 2021;238(2):338–348. doi:10.1111/joa.13321
44. Rade M, Maatta JH, Freidin MB, Airaksinen O, Karppinen J, Williams FMK. Vertebral endplate defect as initiating factor in intervertebral disc degeneration: strong association between endplate defect and disc degeneration in the general population. Spine. 2018;43(6):412–419. doi:10.1097/BRS.0000000000002352
45. Xiao ZF, Su GY, Hou Y, et al. Mechanics and biology interact in intervertebral disc degeneration: a novel composite mouse model. Calcif Tissue Int. 2020;106(4):401–414. doi:10.1007/s00223-019-00644-8
46. Wang X, Lu Y, Wang W, et al. Effect of different aged cartilage ECM on chondrogenesis of BMSCs in vitro and in vivo. Regen Biomater. 2020;7(6):583–595. doi:10.1093/rb/rbaa028
47. Wang L, Gu Y, Zhao H, et al. Dioscin Attenuates Interleukin 1beta (IL-1beta)-induced catabolism and apoptosis via modulating the toll-like receptor 4 (TLR4)/Nuclear Factor kappa B (NF-kappaB) signaling in human nucleus pulposus cells. Med Sci Monit. 2020;26:e923386. doi:10.12659/MSM.923386
48. Li Y, Cao L, Li J, et al. Influence of microgravity-induced intervertebral disc degeneration of rats on expression levels of p53/p16 and proinflammatory factors. Exp Ther Med. 2019;17(2):1367–1373. doi:10.3892/etm.2018.7085
49. Krupkova O, Sekiguchi M, Klasen J, et al. Epigallocatechin 3-gallate suppresses interleukin-1beta-induced inflammatory responses in intervertebral disc cells in vitro and reduces radiculopathic pain in rats. Eur Cell Mater. 2014;28:372–386. doi:10.22203/eCM.v028a26
50. Huang H, Chang YH, Xu J, et al. Aucubin as a natural potential anti-acute hepatitis candidate: inhibitory potency and hepatoprotective mechanism. Phytomedicine. 2022;102:154170. doi:10.1016/j.phymed.2022.154170
51. Jeong HJ, Koo HN, Na HJ, et al. Inhibition of TNF-alpha and IL-6 production by Aucubin through blockade of NF-kappaB activation RBL-2H3 mast cells. Cytokine. 2002;18(5):252–259. doi:10.1006/cyto.2002.0894
52. Xu XE, Liu L, Wang YC, et al. Caspase-1 inhibitor exerts brain-protective effects against sepsis-associated encephalopathy and cognitive impairments in a mouse model of sepsis. Brain Behav Immun. 2019;80:859–870. doi:10.1016/j.bbi.2019.05.038
53. Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab. 2009;29(3):534–544. doi:10.1038/jcbfm.2008.143
54. Chen D, Jiang X, Zou H. hASCs-derived exosomal miR-155-5p targeting TGFbetaR2 promotes autophagy and reduces pyroptosis to alleviate intervertebral disc degeneration. J Orthop Translat. 2023;39:163–176. doi:10.1016/j.jot.2023.02.004
55. Deng Z, Zhang Y, Zhu Y, et al. BRD9 inhibition attenuates matrix degradation and pyroptosis in nucleus pulposus by modulating the NOX1/ROS/NF-kappaB axis. Inflammation. 2023;46(3):1002–1021. doi:10.1007/s10753-023-01786-6
56. Tang P, Zhu R, Ji WP, et al. The NLRP3/Caspase-1/Interleukin-1beta axis is active in human lumbar cartilaginous endplate degeneration. Clin Orthop Relat Res. 2016;474(8):1818–1826. doi:10.1007/s11999-016-4866-4
57. Xiao S, Zhong N, Yang Q, et al. Aucubin promoted neuron functional recovery by suppressing inflammation and neuronal apoptosis in a spinal cord injury model. Int Immunopharmacol. 2022;111:109163. doi:10.1016/j.intimp.2022.109163
58. Wu QQ, Xiao Y, Duan MX, et al. Aucubin protects against pressure overload-induced cardiac remodelling via the beta(3) -adrenoceptor-neuronal NOS cascades. Br J Pharmacol. 2018;175(9):1548–1566. doi:10.1111/bph.14164
59. Wang H, Zhou XM, Wu LY, et al. Aucubin alleviates oxidative stress and inflammation via Nrf2-mediated signaling activity in experimental traumatic brain injury. J Neuroinflammation. 2020;17(1):188. doi:10.1186/s12974-020-01863-9
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.