Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Atypical Treatment Switches in Schizophrenia Patients: Drivers and Associated Outcomes
Authors Khandker R, Shepherd J , Chekani F , Qureshi Z, Bailey H , Berry M, Wright J, Massey L
Received 14 January 2022
Accepted for publication 10 May 2022
Published 18 May 2022 Volume 2022:18 Pages 1057—1067
DOI https://doi.org/10.2147/NDT.S358392
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Rezaul Khandker,1 Jason Shepherd,2 Farid Chekani,1 Zaina Qureshi,1 Hollie Bailey,2 Mia Berry,2 Jack Wright,2 Lucy Massey2
1Merck & Co., Inc., Kenilworth, NJ, USA; 2Adelphi Real World, Bollington, UK
Correspondence: Farid Chekani, Merck & Co., Inc., Kenilworth, NJ, USA, Tel +1-267-305-4070, Email [email protected]
Objective: To describe and compare demographics and outcomes among patients with schizophrenia who have switched atypical treatments versus non-switchers.
Methods: Data were extracted from the Adelphi Schizophrenia Disease Specific Programme™ conducted from January to May 2014 in the United States. Participating physicians provided information on their next 10 consulting schizophrenia patients aged ≥ 18 years; the same patients were invited to voluntarily complete a patient self-completion form (PSC). Patients were considered switchers (S) or non-switchers (NS) based on their physician-provided treatment history. S were patients who had switched, stopped or added an atypical treatment within the last 2 years. NS had no treatment changes within the last 2 years or were receiving their first-line treatment (for ≥ 3 months). Demographics, clinical characteristics and outcomes among S and NS were compared using both descriptive and multivariate statistics.
Results: One-hundred fifty physicians provided data on 1003 patients with schizophrenia (395 S, 608 NS); 500 patients completed a PSC (170 S, 330 NS). When compared with NS, S were more likely to be unemployed (p=0.0060), have a caregiver (p< 0.0001), have greater activity impairment as assessed by Work and Productivity Activity Impairment (p=0.0031), be hospitalized for schizophrenia (p< 0.0001) and have had a greater mean number of hospitalizations in the last 12 months (p=0.0012). NS vs S were more likely to have much or very much improved illness (p< 0.0001) and less severe disease (p< 0.0001) as assessed by Clinical Global Impression.
Conclusion: Despite switching drugs, some schizophrenia patients continue to have high levels of disease burden, suggesting that currently available therapies are insufficiently effective in these patients.
Keywords: schizophrenia, atypical treatment, switching, compliance, outcomes, quality of life, EQ-5D, WPAI, CGI
Introduction
Schizophrenia is a common psychotic disorder that affects over 21 million people worldwide1 and is one of the leading causes of disability globally.2 The lifetime prevalence of schizophrenia is approximately 1%.3 Despite this low prevalence, the health, social and economic burden of schizophrenia is considerable.4 The total cost of schizophrenia in the United States (US) was approximately $156 billion in 2013;5 approximately $38 billion was due to direct healthcare costs, of which $15 billion was due to costs resulting from inpatient care.5–7
Schizophrenia is a chronic disease characterized by a range of symptoms known as positive (psychotic symptoms such as delusions and hallucinations),1 negative (such as reduced emotional expression and avolition)1 and cognitive (such as disorganized speech, thought or attention).8,9 Schizophrenia symptoms generally begin in late adolescence or early adulthood and the disease is often profoundly disabling without treatment.10
Antipsychotic agents are pharmacotherapies for the treatment of schizophrenia; these consist of first-generation (typical) and second-generation (atypical) agents. Prompt initiation of pharmacotherapy after schizophrenia diagnosis is recommended1 and atypical antipsychotics are recommended as first-line therapies.11
Antipsychotic switching is a common occurrence in schizophrenia patients. Results from clinical trials indicate that approximately one third of patients switch antipsychotics within a year of treatment initiation.12,13 Analyses of pharmacy or prescription records revealed antipsychotic switches in 11% to 42% of patients.14–16 The reasons for switching are diverse and may include issues with efficacy (such as persistent symptoms, relapse or insufficient functioning achieved), tolerability (such as extrapyramidal side effects, sedation, metabolic disorders or sexual dysfunction), non-compliance, or patient or family preference.17
Although antipsychotic switching is informed by valid concerns, switching is associated with increased risk of relapse and healthcare resource utilization. A retrospective study of Medicaid claims from six US states over a period of 6 years revealed that patients with schizophrenia, bipolar disorder or major depressive disorder who switched antipsychotics had a shorter time to disease relapse, other psychiatric relapse, emergency room visit or inpatient admission when compared to patients who did not switch.18
To further explore the possible associations between antipsychotic switching and outcomes, we performed an analysis of real-world survey data to describe and compare the demographics and outcomes amongst schizophrenia patients who have switched atypical treatments versus those that did not switch. As improved efficacy and tolerability are often the goals of switching, this analysis of treatment outcomes can determine if these goals were achieved in real life.
Materials and Methods
Data for this study were drawn from the Adelphi Schizophrenia Disease Specific Programme (DSP)™ conducted from January to May 2014 in the US. DSPs are large surveys conducted in clinical practice that describe current disease management, disease-burden impact and associated treatment effects (clinical and physician-perceived). The DSP is a point-in-time survey of physicians and their patients presenting in a real-world clinical setting. The DSP methodology has been described and validated previously.19–21
Participating Physicians and Patients
Participating physicians were hospital or office-based psychiatrists who had been practicing for 2 to 40 years at time of study, saw ≥ 6 patients with schizophrenia per week and were personally responsible for treatment decisions. Participating patients were aged ≥ 18 years, had a diagnosis of schizophrenia and were not currently participating in a clinical trial.
Data Collection
Participating physicians were instructed to complete a patient record form (PRF) for the first 10 consecutive patients who they saw in their daily clinical practice who met the eligibility criteria. PRFs contained questions related to demographics, diagnosis, management, clinical status, concomitant conditions, current treatment and treatment history.
Patients were classified as switchers (S) or non-switchers (NS) on the basis of the physician-provided treatment history. S were patients who had switched, stopped or added on an atypical treatment within the last 2 years; NS patients had no treatment changes within the last 2 years or were in receipt of their first-line treatment, which they had been receiving for at least 3 months.
Disease severity and improvement were based on physician-reported Clinical Global Impression (CGI) scale on current treatment. The CGI is a brief three-item physician-rated scale that assesses illness severity, global improvement or change, and therapeutic response.22 Severity of illness was operationalized as mild illness if the patients were rated as “normal, not at all ill”, “borderline mentally ill” or “mildly ill”; moderate illness if patients were rated as “moderately ill” or “markedly ill”; and severe illness if patients were rated as “severely ill” or “among the most extremely ill patients” on the CGI. Patients rated as “very much improved” or “much improved” were considered responders; patients rated as “minimally improved” were considered partial responders; and patients rated as “no change”, “minimally worse”, “much worse” or “very much worse” were considered non-responders.
Physicians assessed patient compliance with a 5-point scale. Compliance groups were derived according to the following: “always compliant” = always compliant; “sometimes compliant” or “often compliant” = sometimes compliant; and “not at all compliant” and “rarely compliant” = non-compliant.
In the analysis of drivers of switching, high-risk drugs for weight gain (at previous regimen) were olanzapine (oral/depot), chlorpromazine, iloperidone, paliperidone (oral/depot), quetiapine and risperidone.23,24 Central nervous system (CNS) comorbidities were anxiety, depression, insomnia and stress. Cardiovascular comorbidities were hypertension, diabetes, obesity and dyslipidemia.
Each patient for whom the physician completed a PRF was then invited to complete a patient self-completion form (PSC). Upon agreement, patients provided informed consent to participate. PSCs contain questions on demographics and impact of current condition. Self-rated health was assessed by the EuroQoL 5-dimension (EQ-5D) Visual Analogue Scale (VAS) and utility score; higher scores indicate better health.25,26 Impairment was assessed by the Work and Productivity Activity Impairment (WPAI);27 higher scores indicate greater impairment. Overall life satisfaction was assessed by the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q);28 higher scores indicate better quality of life. Patients were also asked to self-rate their overall satisfaction in the past week (available responses were very poor, poor, fair, good and very good). PSCs were completed by the patient independently of the physician immediately after consultation and were returned in a sealed envelope to ensure confidentiality.
Ethics
Patients completing a PSC provided informed consent for use of their anonymized and aggregated data for research and publication in scientific journals. Physicians provided consent to participate and provide patient information during screening into the study. Data were collected such that patients and physicians could not be identified directly; all data were aggregated and de-identified before receipt. Data collection was consistent with the European Pharmaceutical Marketing Research Association guidelines29 and as such ethics committee approval was not required. The survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act 1996,30 and Health Information Technology for Economic and Clinical Health Act legislation.31
Statistical Methods
Analyses were performed using Stata 16.1 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC). Descriptive statistics used were numerical (expressed as count, mean and standard deviation) or categorical (expressed as count and percentage of patients falling into each response). Bivariate statistical tests used to compare outcomes between groups included t-tests or analysis of variance for numerical variables, Mann–Whitney U (non-parametric) tests for ordered categorical variables, and Fisher’s Exact test or chi-squared tests for non-ordered categorical variables.
Regression analyses were used to determine the effect associated with being S, after adjusting for age, gender, Body Mass Index (BMI), high risk of weight gain drugs and the presence of specified comorbid conditions. Regression type was dependent on the outcome being modeled; negative binomial being employed for count outcomes, logistic for binary outcomes and linear regression for other continuous outcomes.
Results
Physicians (n = 150) provided data on 1003 patients with schizophrenia, of which 395 were classified as S and 608 as NS. A total of 500 patients completed a PSC (170 S and 330 NS).
Patient Demographics and Reasons for Choice of Current Treatment
Mean patient age was 41.3 years and was similar between groups (S vs NS, 41.2 years vs 41.4 years; p=0.7889). Most patients were male (557/1003; 55.5%); when compared with S, a greater proportion of NS were male (56.6% vs 53.9%; p=0.4353). Mean BMI was 28.9 kg/m2 and was similar between S (28.9 kg/m2) and NS (28.8 kg/m2) (p=0.8413). S were more likely to be unemployed (62.1% vs 50.5%; p=0.0060) and have a caregiver (44.8% vs 27.8%; p<0.0001) when compared with NS (Table 1).
 |
Table 1 Patient Demographics |
Most physicians chose current treatment regimens considering effects on positive (S vs NS, 92.2% vs 92.4% of physicians; p=0.9036) or negative (72.7% vs 74.8%; p=0.4618) symptoms (Supplementary Table 1). When comparing S with NS, physicians were more likely to choose current treatment regimen based on reducing aggression (33.7% vs 27.3% of physicians; p=0.0341), reduced risk of diabetes (29.9% vs 21.7%; p=0.0045), reduced risk of elevating of plasma glucose (21.0% vs 15.1%; p=0.0173), reduced risk of weight gain (26.1% vs 19.2%; p=0.0124) and value for money (24.8% vs 18.6%; p=0.0214) (Table 2 and Supplementary Table 1).
 |
Table 2 Reasons for Choice of Current Regimen (All Drugs) |
When compared with NS, S were less frequently prescribed pre-identified high-risk drugs for weight gain at current regimen, including olanzapine (13.5% vs 16.3%; p=0.2417), quetiapine (11.4% vs 15.5%; p=0.758) and risperidone (16.0% vs 21.9%; p=0.225) (Supplementary Table 2). S were more likely to be prescribed some concomitant treatments, including benzodiazepines (23.6% vs 15.6%; p=0.0021) and mood stabilizers (18.8% vs 12.0%; p=0.0034) (Supplementary Table 3).
Outcomes
When compared with NS, a greater proportion of S were hospitalized for schizophrenia (42.9% vs 16.5%; p<0.0001) and had a greater mean number of hospitalizations in the last 12 months (1.7 vs 1.3; p=0.0012). A greater proportion of NS vs S had much or very much improved illness (68.5% vs 50.5%; p<0.0001) and were considered borderline, mildly or moderately ill (70.7% vs 53.2%; p<0.0001) as assessed by CGI. WPAI work time missed (27.5 vs 26.0; p=0.6926), impairment while working (27.1 vs 27.7; p=0.8855) and overall work impairment (42.6 vs 43.4; p=0.8645) were similar between S and NS. However, S vs NS reported greater mean WPAI activity impairment (51.0 vs 42.9; p=0.0031). EQ-5D utility score (both S and NS, 0.8; p=0.8383) and EQ-5D VAS (S vs NS, 64.8 vs 66.2; p=0.4453) were similar between groups. NS reported higher mean Q-LES-Q scores when compared with S (52.3 vs 48.7; p=0.0608). A greater proportion of S (24.0%) reported poor or very poor overall satisfaction in the past week than NS (15.6%) (p=0.1674) (Table 3).
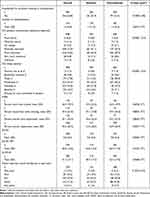 |
Table 3 Patient Clinical Characteristics and Hospitalizations |
Drivers of Switching
A logistic regression analysis was performed to identify potential drivers of treatment switching. We observed that patients were more likely to be S if they were hospitalized in the last 12 months (odds ratio [OR] 3.39, 95% confidence interval [CI] 2.29 to 5.03; p<0.001), prescribed a drug with high risk of weight gain (OR 3.09, 95% CI 2.21 to 4.34; p<0.001), had at least one other CNS comorbidity (OR 1.92, 95% CI 1.28 to 2.88; p=0.002), had gastroesophageal reflux disease (OR 2.80, 95% CI 1.53 to 5.12; p=0.001) or had severe disease (OR 3.05, 95% CI 1.56 to 5.97; p=0.001). Patients were less likely to be S with increasing number of comorbidities (OR 0.86, 95% CI 0.75 to 0.98; p=0.022) (Table 4).
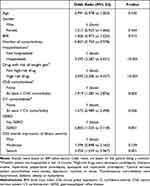 |
Table 4 Drivers of Switching |
Clinical and Humanistic Outcomes Associated with Switching
Multivariate regression analyses were performed to identify outcomes associated with S status. S were more likely to be unemployed (OR 1.47, 95% CI 1.05 to 2.07; p=0.024), have a caregiver (OR 2.03, 95% CI 1.39 to 2.97; p<0.001), have been hospitalized in the past 12 months (OR 3.77, 95% CI 2.53 to 5.60; p<0.001) and have more severe disease (coefficient 0.74, 95% CI 0.35 to 1.13; p<0.001). S were less likely to be always compliant with their current medication regimen (OR 0.53, 95% CI 0.37 to 0.75; p<0.001) or to respond to their current medication regimen (coefficient −0.57, 95% CI −0.97 to −0.17; p=0.005) (Table 5).
 |
Table 5 Multivariate Regression Analyses |
Discussion
This analysis of real-life data from schizophrenia patients and their physicians revealed that patients who switched atypical treatments in the past 2 years had significantly poorer clinical outcomes and were more likely to need caregiver support when compared with NS.
Reasons for antipsychotic switching may include poor efficacy, tolerability and compliance.17,32–34 In this study, we observed that lower proportions of S had much or very much improved illness, were considered borderline, mildly or moderately ill, and were always compliant when compared with NS. Although improved efficacy or tolerability may have been the motivation for switching, the results from this study indicate that switching was not associated with these outcomes. Additionally, we observed that S had an increased likelihood of unemployment and need for a caregiver. Schizophrenia patients experience great social difficulties, particularly with respect to employment, and this may explain why some seek to switch treatments.35 Caregiver support is also a concern for many patients, as social barriers and lack of family support may drive patients and their caregivers to try switching treatments.36 Our findings suggest that there is an absence of effective atypical antipsychotics available when switching from a previous atypical antipsychotic.
The two most common reasons for choice of current treatment regimen reported by physicians were effects on positive or negative symptoms; similar proportions of physicians based their treatment decisions on these reasons for both S and NS. In contrast, we observed that significantly greater proportions of physicians based their treatment decisions around metabolic concerns (diabetes, plasma glucose or weight gain) for S than NS. These results are consistent with the known risks of weight gain with atypical antipsychotics,23 and concerns regarding the already increased risk of metabolic syndrome and obesity in this patient population.37,38 These results also suggest that there is an unmet need for antipsychotics that can reduce the demand for switch due to these metabolic side effects.
Although we observed poorer outcomes and compliance in S vs NS, a 2005 review of atypical antipsychotic switching studies revealed that switching was generally associated with improved outcomes.39 For example, improved positive and negative symptom scale (PANSS) and CGI scores were observed after switching from either atypical or typical antipsychotics to quetiapine,40 ziprasidone41 or aripiprazole.42 A more recent observational study on 568 patients revealed that treatment switch between atypical antipsychotics was associated with significant decreases in PANSS and CGI scores, and improved compliance.34 In contrast, switching from an atypical antipsychotic or haloperidol to olanzapine was not associated with improvements in PANSS scores.43 An analysis of data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial revealed that there were no significant differences in outcomes such as symptoms, neurocognition, quality of life, neurological side effects, weight and health costs between patients who stayed on the same atypical antipsychotic after randomization and those who switched to a different atypical antipsychotic.44 Patients who switched antipsychotics (both typical and atypical) were significantly more likely to be hospitalized when compared to those who did not switch.45,46 However, it is important to note that these studies are not directly comparable to each other or to the present study due to differences in methodology and patient populations.
Logistic regression analyses revealed several drivers associated with S status. The drivers associated with the greatest risk for switching (high-risk drug of weight gain, hospitalizations or severe disease) in this study are consistent with previous observations.23 A prospective observational study in real-life clinical practice revealed that patients that switched from an atypical antipsychotic were more likely to do so because of weight gain when compared with those who switched from a typical antipsychotic.47 Patients who are hospitalized or experience persistent severe disease despite medication compliance would be candidates for antipsychotic switching, consistent with the results observed here.
It is important to note that there may be a bidirectional association between these drivers and switching. For example, a patient prescribed a drug of high weight gain risk is more likely to be a S. However, it is not clear if the patient was switched due to weight gain or other concerns surrounding a high-risk drug or if the patient was switched to a high-risk drug because they had not responded to other drugs with a lower risk of weight gain. This bidirectional relationship may be applicable to other variables examined in this study.
Although S had an increased likelihood of unemployment, hospitalization, need for caregiver, more severe disease, and poorer compliance and response, additional factors beyond treatment choice and treatment decisions may also impact these outcomes. Such factors may include demographic and clinical characteristics not captured in this survey, patient-physician relationships, support network available to the patient, and social and cultural circumstances unique to the patient. Additionally, the health insurance status of patients is variable and may affect the accessibility and quality of treatment received.48
The poorer outcomes observed in S vs NS may suggest that despite the advantages of currently available atypical antipsychotics, these therapies continue to have important shortcomings regarding efficacy and metabolic side effects. Patients treated with atypical antipsychotics may continue to experience negative and positive symptoms. Side effects associated with atypical antipsychotics may contribute significantly to cardiometabolic and endocrine side effects.49 The greater disease severity and reduced improvement observed in S vs NS, along with metabolic comorbidities acting as a driver for switching, are consistent with the shortcomings of atypical antipsychotics.
There have been reports of reduced side effects and improved function among patients switching from atypical antipsychotic polypharmacy to monopharmacy.50,51 Additionally, there is some evidence that antipsychotic dose reduction may also help to reduce side effects while maintaining efficacy.52 If considered in future, these alternatives may help to offset the potential problems associated with switching one atypical antipsychotic drug for another.
This study had some limitations. The data obtained from the DSP were not based on a true random sample of physicians or patients. Physician participation was influenced by willingness to complete the survey. Although no formal patient selection procedures were used, physicians were asked to provide information on the next 10 patients they consulted. While the point-in-time study design prevented any conclusions about causal relationships, identification of significant associations was possible. However, it was not possible to define precise temporal relationships between switching and outcomes. For example, data on response and compliance were based on physician assessment at time of survey, while switching may have occurred at any time in the previous two years. It is possible that patients who switched recently before the time of survey may not yet have achieved the full efficacy of their new regimen. While recall bias may have affected patient and physician responses to the questionnaires, this is a common limitation of surveys. However, data were collected at the time of each patient’s consultation and physicians had access to the patient’s medical history; these were both expected to have reduced the likelihood of recall bias. Although a total of 1003 PRFs were completed, only 500 patients completed PSCs. It is unclear if this introduced bias into the results.
Conclusions
Patients with schizophrenia who switched atypical antipsychotic treatments experienced worse clinical and social outcomes than those who did not. While there are a variety of factors that may drive patients or their physicians to seek a switch, we found that switching treatments does not achieve improved results for these patients. The poorer outcomes and increased need for caregiver support in S vs NS suggest that more efficacious therapies are needed to avoid switches and also to provide an effective treatment option when a switch is warranted in patients with schizophrenia.
Data Sharing Statement
All data that support the findings of this study are intellectual property of Adelphi Real World. All requests should be addressed directly to Jason Shepherd at [email protected].
Acknowledgments
The authors thank Derek Ho, PhD (ScriboMedica Ltd, Helsinki, Finland), supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, for medical writing support with the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi Schizophrenia Disease Specific Programme, sponsored by multiple pharmaceutical companies of which one was Merck Sharp & Dohme Corp. Merck Sharp & Dohme Corp. did not influence the original survey through either contribution to the design of questionnaires or data collection. The study described here using data from the Adelphi Schizophrenia Disease Specific Programme was funded by Merck Sharp & Dohme Corp.
Disclosure
RK, ZQ and FC are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA who may own stock and/or hold stock options in Merck & Co., Inc., Kenilworth, NJ, USA. The authors report no other conflicts of interest in this work.
References
1. Holder SD, Wayhs A. Schizophrenia. Am Fam Physician. 2014;90(11):775–782.
2. Vos T, Allen C, Arora M, et al.Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi:10.1016/S0140-6736(16)31678-6
3. van Os J, Kapur S. Schizophrenia. Lancet. 2009;374(9690):635–645. doi:10.1016/S0140-6736(09)60995-8
4. Chong HY, Teoh SL, Wu DB, Kotirum S, Chiou CF, Chaiyakunapruk N. Global economic burden of schizophrenia: a systematic review. Neuropsychiatr Dis Treat. 2016;12:357–373. doi:10.2147/NDT.S96649
5. Cloutier M, Aigbogun MS, Guerin A, et al. The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry. 2016;77(6):764–771. doi:10.4088/JCP.15m10278
6. dosReis S, Johnson E, Steinwachs D, et al. Antipsychotic treatment patterns and hospitalizations among adults with schizophrenia. Schizophr Res. 2008;101(1–3):304–311. doi:10.1016/j.schres.2007.12.475
7. Fitch K, Iwasaki K, Villa KF. Resource utilization and cost in a commercially insured population with schizophrenia. Am Health Drug Benefits. 2014;7(1):18–26.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.
9. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 Suppl):1–56.
10. Jones PB, Barnes TR, Davies L, et al. Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006;63(10):1079–1087. doi:10.1001/archpsyc.63.10.1079
11. Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318–378. doi:10.3109/15622975.2012.696143
12. Perez-Iglesias R, Crespo-Facorro B, Martinez-Garcia O, et al. Weight gain induced by haloperidol, risperidone and olanzapine after 1 year: findings of a randomized clinical trial in a drug-naive population. Schizophr Res. 2008;99(1–3):13–22. doi:10.1016/j.schres.2007.10.022
13. Nyhuis AW, Faries DE, Ascher-Svanum H, Stauffer VL, Kinon BJ. Predictors of switching antipsychotic medications in the treatment of schizophrenia. BMC Psychiatry. 2010;10:75. doi:10.1186/1471-244X-10-75
14. Covell NH, Jackson CT, Evans AC, Essock SM. Antipsychotic prescribing practices in Connecticut’s public mental health system: rates of changing medications and prescribing styles. Schizophr Bull. 2002;28(1):17–29. doi:10.1093/oxfordjournals.schbul.a006920
15. Menzin J, Boulanger L, Friedman M, Mackell J, Lloyd JR. Treatment adherence associated with conventional and atypical antipsychotics in a large state Medicaid program. Psychiatr Serv. 2003;54(5):719–723. doi:10.1176/appi.ps.54.5.719
16. de Smidt C, Haffmans J, Hoencamp E. Antipsychotics switching strategies in real life. A longitudinal study in clinical practice. Eur J Psychiat. 2012;26(1):41–49. doi:10.4321/S0213-61632012000100004
17. Bernardo M, Vieta E, Saiz Ruiz J, et al. Recommendations for switching antipsychotics. A position statement of the Spanish Society of Psychiatry and the Spanish Society of Biological Psychiatry. Rev Psiquiatr Salud Ment. 2011;4(3):150–168. doi:10.1016/j.rpsm.2011.07.003
18. Ayyagari R, Thomason D, Mu F, Philbin M, Carroll B. Association of antipsychotic treatment switching in patients with schizophrenia, bipolar, and major depressive disorders. J Med Econ. 2020;23(2):204–212. doi:10.1080/13696998.2019.1685818
19. Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: disease-specific programmes - a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. doi:10.1185/03007990802457040
20. Babineaux SM, Curtis B, Holbrook T, Milligan G, Piercy J. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the Disease Specific Programme. BMJ Open. 2016;6(8):e010352. doi:10.1136/bmjopen-2015-010352
21. Higgins V, Piercy J, Roughley A, et al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. 2016;9:371–380. doi:10.2147/DMSO.S120101
22. Clinical Global Impression (CGI). Available from: https://www.psywellness.com.sg/docs/CGI.pdf.
23. Dayabandara M, Hanwella R, Ratnatunga S, Seneviratne S, Suraweera C, de Silva VA. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatr Dis Treat. 2017;13:2231–2241. doi:10.2147/NDT.S113099
24. Meyer JM, Ng-Mak DS, Chuang CC, Rajagopalan K, Loebel A. Weight changes before and after lurasidone treatment: a real-world analysis using electronic health records. Ann Gen Psychiatry. 2017;16:36. doi:10.1186/s12991-017-0159-x
25. EuroQol G. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208.
26. Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. doi:10.1016/0168-8510(96)00822-6
27. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi:10.2165/00019053-199304050-00006
28. Endicott J, Nee J, Harrison W, Blumenthal R. Quality of life enjoyment and satisfaction questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321–326.
29. EphMRA code of conduct 2019; 2019. Available from: https://www.ephmra.org/media/2811/ephmra-2019-code-of-conduct-doc-f.pdf.
30. Summary of the HIPAA security rule. Available from: https://www.hhs.gov/hipaa/for-professionals/security/laws-regulations/index.html.
31. HITECH act enforcement interim final rule. Available from: https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html.
32. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi:10.1056/NEJMoa051688
33. Bobo WV. Switching antipsychotics: why, when, and how? Psychiatric times; 2013. Available from: https://www.psychiatrictimes.com/view/switching-antipsychotics-why-when-and-how.
34. Roussidis A, Kalkavoura C, Dimelis D, et al. Reasons and clinical outcomes of antipsychotic treatment switch in outpatients with schizophrenia in real-life clinical settings: the ETOS observational study. Ann Gen Psychiatry. 2013;12(1):42. doi:10.1186/1744-859X-12-42
35. Schennach R, Musil R, Möller HJ, Riedel M. Functional outcomes in schizophrenia: employment status as a metric of treatment outcome. Curr Psychiatry Rep. 2012;14(3):229–236. doi:10.1007/s11920-012-0261-3
36. Chue P, Stip E, Remington G, Kopala L. Switching atypical antipsychotics: a review. Acta Neuropsychiatr. 2004;16(6):301–313. doi:10.1111/j.0924-2708.2004.00098.x
37. Mitchell AJ, Vancampfort D, De Herdt A, Yu W, De Hert M. Is the prevalence of metabolic syndrome and metabolic abnormalities increased in early schizophrenia? A comparative meta-analysis of first episode, untreated and treated patients. Schizophr Bull. 2013;39(2):295–305. doi:10.1093/schbul/sbs082
38. Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders–a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306–318. doi:10.1093/schbul/sbr148
39. Edlinger M, Baumgartner S, Eltanaihi-Furtmuller N, Hummer M, Fleischhacker WW. Switching between second-generation antipsychotics: why and how? CNS Drugs. 2005;19(1):27–42. doi:10.2165/00023210-200519010-00003
40. De Nayer A, Windhager E, Irmansyah I, et al. Efficacy and tolerability of quetiapine in patients with schizophrenia switched from other antipsychotics. Int J Psychiatry Clin Pract. 2003;7(1):59–66. doi:10.1080/13651500310001095
41. Weiden PJ, Simpson GM, Potkin SG, O’Sullivan RL. Effectiveness of switching to ziprasidone for stable but symptomatic outpatients with schizophrenia. J Clin Psychiatry. 2003;64(5):580–588. doi:10.4088/JCP.v64n0514
42. Casey DE, Carson WH, Saha AR, et al. Switching patients to aripiprazole from other antipsychotic agents: a multicenter randomized study. Psychopharmacology. 2003;166(4):391–399. doi:10.1007/s00213-002-1344-3
43. Lindenmayer JP, Czobor P, Volavka J, et al. Olanzapine in refractory schizophrenia after failure of typical or atypical antipsychotic treatment: an open-label switch study. J Clin Psychiatry. 2002;63(10):931–935. doi:10.4088/JCP.v63n1011
44. Rosenheck RA, Davis S, Covell N, et al. Does switching to a new antipsychotic improve outcomes? Data from the CATIE trial. Schizophr Res. 2009;107(1):22–29. doi:10.1016/j.schres.2008.09.031
45. Faries DE, Ascher-Svanum H, Nyhuis AW, Kinon BJ. Clinical and economic ramifications of switching antipsychotics in the treatment of schizophrenia. BMC Psychiatry. 2009;9:54. doi:10.1186/1471-244X-9-54
46. Noordsy DL, Phillips GA, Ball DE, Linde-Zwirble WT. Antipsychotic adherence, switching, and health care service utilization among Medicaid recipients with schizophrenia. Patient Prefer Adherence. 2010;4:263–271. doi:10.2147/PPA.S6053
47. Hugenholtz GW, Heerdink ER, Meijer WE, Stolker JJ, Egberts AC, Nolen WA. Reasons for switching between antipsychotics in daily clinical practice. Pharmacopsychiatry. 2005;38(3):122–124. doi:10.1055/s-2005-864122
48. Khaykin E, Eaton WW, Ford DE, Anthony CB, Daumit GL. Health insurance coverage among persons with schizophrenia in the United States. Psychiatr Serv. 2010;61(8):830–834. doi:10.1176/ps.2010.61.8.830
49. Riordan HJ, Antonini P, Murphy MF. Atypical antipsychotics and metabolic syndrome in patients with schizophrenia: risk factors, monitoring, and healthcare implications. Am Health Drug Benefits. 2011;4(5):292–302.
50. Hori H, Yoshimura R, Katsuki A, Sugita AI, Atake K, Nakamura J. Switching to antipsychotic monotherapy can improve attention and processing speed, and social activity in chronic schizophrenia patients. J Psychiatr Res. 2013;47(12):1843–1848. doi:10.1016/j.jpsychires.2013.08.024
51. Borlido C, Remington G, Graff-Guerrero A, et al. Switching from 2 antipsychotics to 1 antipsychotic in schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(1):14–20. doi:10.4088/JCP.14m09321
52. Zhou Y, Li G, Li D, Cui H, Ning Y. Dose reduction of risperidone and olanzapine can improve cognitive function and negative symptoms in stable schizophrenic patients: a single-blinded, 52-week, randomized controlled study. J Psychopharmacol. 2018;32(5):524–532. doi:10.1177/0269881118756062
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
