Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Attrition and Its Predictors Among Adults Receiving First-Line Antiretroviral Therapy in Woldia Town Public Health Facilities, Northeast Ethiopia: A Retrospective Cohort Study
Authors Dejen D , Jara D, Yeshanew F , Fentaw Z , Mengie Feleke T, Girmaw F , Wagaye B
Received 30 January 2021
Accepted for publication 30 March 2021
Published 20 April 2021 Volume 2021:13 Pages 445—454
DOI https://doi.org/10.2147/HIV.S304657
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Demeke Dejen,1 Dube Jara,2 Fanos Yeshanew,3 Zinabu Fentaw,4 Tesfa Mengie Feleke,5 Fentaw Girmaw,6 Birhanu Wagaye3
1Care and Treatment, Amhara Regional Health Bureau CDC Project Cluster Health Facilities HIV Case Detection Linkage, Woldia, Ethiopia; 2Department of Epidemiology and Biostatics, School of Public Health, College of Medicine and Health Science, Addis Ababa University, Addis Ababa, Ethiopia; 3Department of Public Health Nutrition, School of Public Health, College of Medicine and Health Science, Wollo University, Dessie, Ethiopia; 4Department of Epidemiology and Biostatistics, School of Public Health, College of Medicine and Health Science, Wollo University, Dessie, Ethiopia; 5Amhara Regional Health Bureau CDC Project Zonal Monitoring and Evaluation, Dessie, Ethiopia; 6Department of Pharmacy, College of Health Science, Woldia University, Woldia, Ethiopia
Correspondence: Zinabu Fentaw
Department of Epidemiology and Biostatistics, School of Public Health, College of Medicine and Health Science, Wollo University, Dessie, Ethiopia
Tel +251912757286
Email [email protected]
Introduction: There is an expansion and advancement of antiretroviral therapy. However, attrition of patients from HIV care is one of the major drivers of poor performance of HIV/AIDS programs, which leads to drug resistance, morbidity and mortality. The study aimed to assess the incidence of attrition and its predictors among adults receiving first-line antiretroviral therapy.
Methods: An institution-based retrospective cohort study was conducted among 634 adults receiving antiretroviral therapy, and study participants were selected using a simple random sampling technique. Data were cleaned and entered into Epi Data version 3.1 and exported to STATA 14.1 for further analysis. The predictors of attrition were identified using bivariable and multivariable Cox Proportional hazard models; then, variables at a p-value of less than 0.25 and 0.05 were included in the multivariable analysis and statistically significant, respectively.
Results: The total time observed was found to be 1807.08 person-years of observation with a median follow-up time of 2.67 years (IQR 1.25− 4.67). The incidence rate of attrition was 8.36 (95% CI: 7.12− 9.80) per 100 person-years. Significant predictors of attrition were being young age [adjusted hazard ratio (AHR) =2.0, 95% CI, (1.11− 3.58)], nearest calendar year of ART initiation [AHR =2.32, 95% CI, (1.08– 5.01)], bedridden functional status [AHR=3.25, 95% CI, (1.33− 7.96)], WHO stage III [AHR=3.57, 95% CI, (1.58− 8.06)] and stage IV [AHR=5.46, 95% CI, (1.97− 15.13)], viral load result of ≤ 1000 [AHR=0.11, 95% CI, (0.06− 0.23)], disclosure status [AHR=2.03, 95% CI, (1.22− 3.37)] and adherence level of poor [AHR=3.19, 95 CI, (1.67− 6.09)].
Conclusion: The result of this study showed that the incidence of attrition among adults receiving antiretroviral therapy was high. However, as a standard, every client who started antiretroviral therapy should be retained. Positive predictors of attrition were young age (15– 24), recent year of ART initiation, baseline functional status, advanced WHO stage III and IV, no disclosure status, fair/poor adherence whereas, viral load result of ≤ 1000 copies/mL had a preventive effect on attrition.
Keywords: attrition, HIV/AIDS, antiretroviral therapy, retrospective cohort, Ethiopia
Introduction
Attrition is the contrary of retention on antiretroviral therapy (ART). It is the summation of the dead, lost to follow-up (LTFU), and stop from chronic ART care.1 On the contrary, retention in ART care is a continuous engagement of patients linked to HIV care, and it is one of the crucial indicators of the success of ART programs.2
Globally, over 37.9 million people were living with HIV/AIDS. Two-thirds of them were in Sub–Saharan Africa, and 24.5 million people on ART, 62% were adults. 6.2% of the world’s population lives in Eastern and Southern Africa. Like other countries, Ethiopia also adopted the Joint United Nations Program on HIV/AIDS (UNAIDS) fast track strategy of 90-90-90.3 However, of all people living with HIV, 79% knew their status, 62% were on treatment, and 53% were virally suppressed in 2018.4,5 Moreover, patients who are not retained due to loss to follow–up are likely to develop a high viral load, which increases the risk of infecting others.6,7
Countries face the dual challenge of managing and sustaining growing cohorts of patients on ART in addition to the need for increasing access to ART for the patients who still do not have access to it. The high levels of attrition undermine the proven benefits of early treatment for individuals and the prevention of onward transmission of HIV.8 Patient adherence to the treatment and attrition from care emerged as a serious public health concern to gain the desired outcome of a combined ART treatment regimen.9 Lost to follow-up or attrition from care negatively impacts the immunological benefits of ART, which leads to drug resistance, treatment failure, morbidity, hospitalization, and death.8,10–12 Despite the implementation of free highly active antiretroviral treatment, decentralization, role shifting of ART programs, appointment spacing model, fixed–dose combinations, patient monitoring, and involvement of case managers and adherence supporters significantly increased HIV/AIDS infected people’s retention, reducing morbidity and mortality through viral load suppression and improved adherence.13–18 However, almost half of all HIV patients have undetected viral loads, and as a result, the number of people who are lost to follow-up is higher than the number of people who start new care.19
The extent of non-retention of treatment and the factors that contribute to it can differ from one location to the next. It will be crucial to evaluate the magnitude and factors of attrition among people living with HIV/AIDS in order to establish successful intervention strategies and enhance their quality of life. Furthermore, the impact of new HIV/AIDS initiatives such as viral load status, ASM, and a test-and-treat approach was not considered in previous studies. As a result, further research was done to evaluate the rate of attrition and its predictors among adults on antiretroviral therapy.
Methods
Study Design and Setting
An institution-based retrospective cohort study was conducted in Woldia Town Public Health Facilities. Woldia Town is located in North Wollo Zone of Amhara regional state at 520km away from Addis Ababa. In Ethiopia, ART began as a free service in 2005. Ethiopia’s Federal Ministry of Health currently recommends ART care based on the 2018 National Guideline, which was revised from the 2014 National Guideline. A differentiated service delivery system, varying from monthly to six-month intervals, is recommended by the latest national guideline. The town report in January 2020 showed that more than 5,700 were taking ART and more than 5,570 of them were adults. The data were collected from February 1, 2020, to March 30, 2020.
Study Populations, Sample Size and Sampling Procedure
All HIV-infected patients who had ever begun antiretroviral therapy (ART) and were 15 years old or older at the time of ART initiation in Woldia Town Public Health Facilities were included in the study. The research included HIV-positive adults who had begun antiretroviral therapy (ART) in Woldia General Hospital and Woldia Health Center between February 2014 and January 2020. The sample size was estimated using Epi Info 7.2 Stat-Calc software and using some independent variables based on the following assumptions: degree of confidence=95%, power=80%, exposed: unexposed ratio=1:1.
So, a larger sample size of the minimum sample of the current study was 634. In terms of the sampling process, ART data were first exported from SMART Care to Microsoft Excel, and then study participants were chosen using a simple random sampling procedure using lottery methods based on their medical record numbers. Figure 1
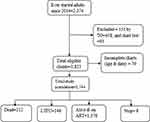 |
Figure 1 Profiles of HIV/AIDS clients in Woldia Town Public Health Facilities (February 1, 2014 to January 31, 2020) Northeast Ethiopia; 2020. |
Data Collection Tool, Methods and Measurements
The data abstraction tool was adapted from the ART intake form, register, and follow-up form. The clients’ outcome status was determined based on the ART register, SMART care, and patient follow-up card. It was recorded as dead (of any cause), lost and dropped, stopped, or retained in care. The study began when clients’ initiated ART; then, the duration of the study was the follow-up of clients to the next visit. Adult clients who were transferred out (TO) and a patient chart with missed age and date of ART initiation were excluded. The time component was taken first date of ART initiation, which was considered as a baseline date or time zero for this study. The event of interest considered as success (event = 1) if adult ART clients and (event = 0) otherwise. The outcome was labeled as lost, dead, stop, or retained. The follow-up status of clients was calculated in months and a maximum of 72 months or 6 years. If the client was active on follow-up during the data collection period, then the client was labeled as retained in care otherwise labeled as censored due to administrative censoring otherwise labeled as attrition from cares. The time to attrition or censoring was recorded in months starting from the date of ART initiation to the last date of a missed appointment, stop or death, and the last date of medication pick up in case of administrative censoring.
Data Quality Management
As a quality control measure, sampled data from the charts to be chosen was re-abstracted. The data quality was reviewed by ensuring its completeness, monitoring data collectors on a continuous basis, and keeping a close eye on data management.
Data Processing and Analysis
The primary outcome variable was attrition from care. The data were entered into Epi-Data version 3.1 and exported to STATA 14.1 for further analysis. Cox proportional hazard model assumption was checked using graphical representation (log-log plot or Kaplan–Meier and predicted survival plot), the goodness of fit like Scaled Schoenfeld residuals for each variable, Schoenfeld residuals for the global test and lastly, time vary covariates. To identify predictors of attrition Proportional Hazard Cox regression model was fitted. A significant difference in survival curves was tested by equality of survival functions using a Log rank test. The survival and hazard probability of the risk group were shown by the survival graph and the hazard failure estimated curve, respectively. All Variables were assessed with the outcome variables in the bi-variable analysis at P-value ≤0.25 were included in the multivariable Cox Proportional Hazard Model. An adjusted hazard ratio with their corresponding 95% confidence interval was presented and variables with a P-value less than 0.05 were also considered statistically significant.
Ethics Consideration
The Ethical Review Committee of the College of Medicine and Health Sciences, Wollo University had secured the ethical clearance letter. The school of public health also offered an official letter to all health facilities of the study area. The data collection was conducted following the Helsinki Declaration and confidentiality was maintained anonymously. Informed consent of the participants to review their chart was waived by the Quality Assurance Committee of the respective Health Facilities on behalf of their clients.
Result
Sociodemographic and Behavioral Characteristics
Totally, 634 patients who had a follow-up at ART clinics in public health facilities were included in the study. The mean ages of the respondents were 35.9 (SD±11.148) years. The median duration of months on ART from the start of ART till the end of follow-up period was 32 months (IQR 15−55). Of the total respondents, 345 (54.4%) of them were females, and 354 (55.8%) were urban dwellers. The majority of the respondents, 518 (81.7%), were Orthodox Christian followers, and 271 (42.7%) of them were unable to read and write. Two hundred sixty-three (41.5%) of the respondents were unemployed, and 310 (48.9%) of them were married. Three-fourth of respondents, 470 (74.1%), were disclosed to someone else, and regarding medication adherence 413 (65.1%) of them were with good adherence at the last visit of patient follow-up. Eighty-eight (13.9%) of the respondents were substance users in addition to their ART medication. Table 1
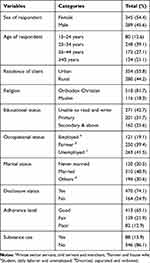 |
Table 1 Sociodemographic and Behavioral Characteristic of Adults Receiving ART Related with Attrition in Woldia Town Public Health Facilities, Northeast Ethiopia 2020, n=634 |
Baseline Clinical and Health System Factors
Out of the total respondents, 264 (41.6%) were linked to ART care from VCT, and 206 (32.5%) of them were underweight (<18.5 kg/m2) according to their BMI. Three-fourth (75.1%) of the patients started ART at working functional status and 195 (30.8%) at advanced WHO stage of either stage III or IV. Table 2
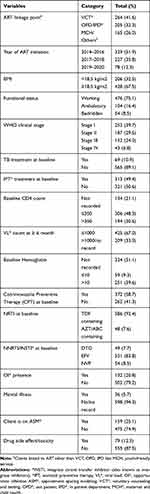 |
Table 2 Baseline Clinical and Health System Characteristics Among Adult Clients Who Were Receiving ART in Woldia Town Public Health Facilities, 2020, (n=634) |
Attrition Among Adult Patients Receiving Antiretroviral Therapy
At the end of six years, 483 (76.2%) of respondents had been retained, while 91 (14.3%) had died, 57 (9.0%) had LTFU, and 3 (0.5%) had stopped. The Kaplan-Meier estimate of attrition from care were 9.6% (7.6–12.2), 13.6% (11.2–16.6), 16.3% (13.6–19.5), 18.3% (15.4–21.6), 23.7% (20.3–27.5), 27.1% (23.4–31.3), 29.2% (25.2–33.8) and 31.8% (27.0–37.1) at 6 months, 1 year, 18 months, 2nd year, 3rd year, 4th year, 5th year and 6th year, respectively, while the retention in care was vice versa. Figure 2
 |
Figure 2 Kaplan–Meier failure estimates by failure function of attrition among adult patients on ART in Woldia Town, Northeast Ethiopia, 2020. |
Incidence of Attrition
The total time observed was found to be 1807.08 person-years of observation with a median follow-up time of 2.67 years (IQR 1.25−4.67). The overall incidence rate of attrition was estimated to be 8.36 (95% CI: 7.12−9.80) per 100 person-years (PY). Of the total incidence of attrition rate, 5.04/100 PY, 3.15/100 PY, and 0.17/100 PY attributed to death, LTFU and stop ART, respectively. The incidence of attrition had shown that differences among different levels of the WHO clinical stage. The incidence of attrition at advanced WHO clinical stage of either stage III or IV was higher than stage one (Figure 3).
 |
Figure 3 Kaplan–Meier failure estimates of attrition by baseline WHO stage among adult patients on ART in Woldia Town, Northeast Ethiopia, 2020. |
The incidence of attrition has also shown differences among different levels of adherence. The incidence of attrition among respondents with sub-optimal adherence status of fair or poor was higher than respondents with good adherence (Figure 4).
 |
Figure 4 Kaplan–Meier failure estimates of attrition by adherence level among adult patients on ART in Woldia Town Public Health Facilities, Northeast Ethiopia, 2020. |
Predictors of Attrition Among Adult ART Clients
On bivariate cox regression sex, age of respondent, year of enrollment to HIV care, BMI, functional status, baseline WHO stage, co-infection with TB at baseline, viral load count, history of CPT prophylaxis, IPT treatment, presence of at least one OI, mental illness, substance abuse, history of disclosure, adherence level and ASM were found to be associated with attrition at P value of 0.25 or lower. After conducting a bi-variable analysis, the final model was fitted using a back ward multivariable cox–regression model. Finally, seven of the predictors were found to have statistically significant with attrition during multivariable cox proportional regression analysis at 95% confidence level.
The study indicated that age of respondent was found to be significant predictor of attrition, in which the risk of attrition among young adults (15−24 years) was 2 times higher as compared to older adults (25–34 years) (AHR=2.0, 95% CI, 1.11−3.58). Respondents with recent year of enrollment had 2.3 times more likely to undergo attrition as compared to previous year of enrolment (AHR=2.32, 95% CI, 1.08–5.01). Participants who were with bedridden functional status had 3 times more risk of attrition as compared to working functional status (AHR=3.25, 95% CI, 1.33−7.96). The risk of attrition among those with advanced WHO stage was higher as compared to early stage, in which the risk of attrition among those on stage III was 3.6 (AHR=3.57, 95% CI, 1.58−8.06) and among stage IV was 5.5 (AHR=5.46, 95% CI, 1.97−15.13) times higher as compared to those on WHO stage one. The risk of attrition among those with viral load result of ≤1000 copies/mL lowers the risk of attrition by 0.11 (AHR=0.11, 95% CI, 0.06−0.23) times as compared to those with >1000 copies/mL. Disclosure status was found to have statistically significant association with attrition, in which the risk of attrition among those who had not disclosed their HIV status was 2 (AHR=2.03, 95% CI, 1.22−3.37) times higher as compared to those who had disclosed their status to anyone else. The risk of attrition among those with fair/poor adherence was higher as compared to those having good adherence, in which the risk of attrition among those who had fair adherence was 1.9 (AHR=1.86, 95% CI, 1.01−3.43) and poor adherence was 3.2 (AHR=3.19, 95CI, 1.67−6.09) times higher as compared to those having good adherence level. Table 3
 |
Table 3 Predictors of Attrition Among Patients with HIV/AIDS Receiving ART in Woldia Town Public Health Facilities, January 2020 |
Discussion
The overall incidence of attrition was 8.36/100 PY. This finding was lower as compared to studies conducted in Kenya (23.1/100 PY), Sub–Saharan Africa (14.2/100 PY), South Africa (26.4/100 PY) and India (14.1/100PY).20–23 This might be due to the practice of ASM implementation in the current study compared with the previous one, which was conducted before WHO recommendations of the ASM model24 and ongoing active tracing of clients who had missed their appointment by case managers and adherence supporters. The incidence of attrition was higher than the studies conducted in Ethiopia (6.5/100PY)25 and in Mozambique (2.2/100 PY).26 This high attrition rate might be due to the rapid scale–up of ART services without considering the package of care to the clients7 and the absence of practicing community ART services.26
Findings from this study revealed that young age (15–24) increases the hazard of attrition by 2 times as compared to 35–44 years of adults. This finding was in line with studies conducted in Kenya and Mozambique.20,27 This high hazard of attrition might be due to high loss to follow–up as a result of fear related to stigma and discrimination in addition to low psychosocial support during a health facility visit to pick up ART medication.28 Adopting innovative approaches like the provision of community ART services and creating youth-friendly services might reduce a high attrition rate, which was supported by studies conducted in Mozambique and systematic review including Sub Saharan Africa.26,27,29
In this study, initiation in recent calendar years increases the hazard of attrition by 2.32 times as compared to individuals who started ART in the previous calendar years. This finding adds consistent evidence with studies in Côte d’Ivoire, Zimbabwe, Rwanda.30–33 However, this finding was in contrast with previous studies conducted in Ethiopia, which prevents hazard of attrition by 33%,11 Haiti reduced the hazard of attrition by 63%,34 Latin America and the Caribbean reduced by 41%35 when clients were initiated ART in recent calendar years. The possible explanation behind this high attrition was high lost follow-up due to the shift of Ethiopian national ART guideline from <500 CD4 cells to test and treat and silent referral of clients towards nearby health facilities as a result of the decentralization of HIV care as well as inadequate adherence of clients at the time of ART initiation.36
Findings from this study revealed that bedridden functional status at baseline increases the hazard of attrition by 3.25 times as compared to working functional status. This finding was in line with previous studies conducted in Ethiopia,37–39 Zimbabwe, Tanzania, Uganda and Zambia.32,40,41 In this study, advanced WHO stage III and stage IV at baseline increases the hazard of attrition by 3.57 and 5.46 times, respectively, as compared to stage I. This finding was in line with studies in Eswanti, Zimbabwe, Rwanda42–44 and Myanmar.45 The possible explanation behind this would be late enrolment of individuals towards HIV care at advanced stages (stage III and IV) and referral of complicated cases to the hospital, which leads to a higher risk of mortality.33,46–48
An interesting finding from this study revealed that recorded viral load result of ≤1000 copies/mL prevented the hazard of attrition by 89% as compared to >1000 copies/mL. Viral load measurement is an indicator of successful medication adherence to clients and a suppressed viral load result is less risk of attrition.49 This finding implies the need for expansion to viral load testing sites to attain universal access to timely viral load monitoring to avoid premature switching of clients to either second- or third-line ART regimen, which is more toxic and not as effective as the first-line regimen.
The study found that individuals who had not disclosed their status to anyone were prone to the hazard of attrition by 2 times as compared to those who had disclosed their status. This result was consistent with studies conducted in Ethiopia,50 Democratic Republic of Congo51 and England.52 The possible explanation behind this result might be due to fear of using ARV medication in social places as well as forgetting to take it timely. This results from a lack of social support to collect and to take the medication regularly. This finding emphasizes applying weekly mobile phone text messages to avoid missing appointment dates, especially for clients who had not disclosed their status.53
The study showed that inadequate adherence level of fair/poor increased the hazard of attrition by 1.86 and 3.19 times, respectively. This finding was in line with studies conducted in Ethiopia,54 Nigeria and Côte d’Ivoire’.12,30 This was due to non-adherence clients have discontinued their lifelong ART treatment due to poor drug and clinical adherence, which resulted in high viral load, treatment failure, easy probability of contracting OIs, and then they would have died. Furthermore, this high hazard of attrition might be due to substance use55 and suboptimal screening of individuals with HIV for mental health as a result of the fact that mental illnesses are prevalent among clients with HIV/AIDs, which leads to forgetting to take medication timely.38 This finding implied the need for mental health integration with ART services, which leads to improving attrition for achieving the second and third 90 of UNAIDs fast–track strategy of Ethiopia. As it was secondary data analysis, the incompleteness of records was the challenge to undergo in this study.
Limitation of the Study
This study is not without limitation since it includes only patients’ medical records with complete data, so this might have made a selection bias and also shares the limitations of retrospective studies.
Conclusion
The result of this study showed that the incidence of attrition among adults receiving antiretroviral therapy was high. However, as a standard, every client who started antiretroviral therapy should be retained. The main positive predictors of attrition were Young age (15–24), recent year of ART initiation, baseline functional status, advanced WHO stage (III &IV), no disclosure status, fair/poor adherence while suppressed viral load result (≤1000 copy/mL) had a preventive effect on attrition.
Abbreviations
AHR, adjusted hazard ratio; ART, anti-retroviral therapy, ASM, appointment spacing model; HAART, highly active anti-retroviral therapy; LTFU, lost to follow-up; UNAIDS, the Joint United Nations Program on HIV/AIDS.
Data Sharing Statement
The data set for this result and conclusion of the manuscript is available and contact the corresponding author on reasonable request.
Acknowledgment
We want to acknowledge Woldia General Hospital and Woldia Health Center for providing ART data for this study.
Funding
The source of funding is self-sponsored.
Disclosure
The authors reported no conflicts of interest for this work.
References
1. Alhaj M, Amberbir A, Singogo E, et al. Retention on antiretroviral therapy during Universal test and treat implementation in Zomba district, Malawi: a retrospective cohort study. J Int AIDS Soc. 2019;22(2):1–7. doi:10.1002/jia2.25239
2. World Health Organization. Retention in HIV programmes: defining the challenges and identifying solutions; 2011.
3. Joint United Nations Programme on HIV/AIDS, UNAIDS 2017. Ending aids: progress towards the 90- 90-90Targets; 2017.
4. Joint UNAIDS. UNAIDS data 2019. Vol 268; 2019. doi:10.1126/science.7716530
5. UNAIDS. Global HIV & AIDS statistics — 2019 fact sheet; 2019.
6. Assefa Y, Kiflie A, Tesfaye D, et al. Outcomes of antiretroviral treatment program in Ethiopia: retention of patients in care is a major challenge and varies across health facilities. BMC Health Serv Res. 2011;11(1):1–7. doi:10.1186/1472-6963-11-81
7. Assefa Y, Jerene D, Lulseged S, Ooms G, Van Damme W. Rapid scale-up of antiretroviral treatment in Ethiopia: successes and system-wide effects. PLoS Med. 2009;6(4):1–4. doi:10.1371/journal.pmed.1000056
8. Gebrezgabher BB, Kebede Y, Kindie M, Tetemke D, Abay M, Gelaw YA. Determinants to antiretroviral treatment non-adherence among adult HIV/AIDS patients in northern Ethiopia. AIDS Res Ther. 2017;14(1):1–7. doi:10.1186/s12981-017-0143-1
9. Murray KR, Dulli LS, Ridgeway K, et al. Improving retention in HIV care among adolescents and adults in low- and middle-income countries: a systematic review of the literature. PLoS One. 2017;12(9):1–22. doi:10.1371/journal.pone.0184879
10. Wubshet M, Berhane Y, Worku A, Kebede Y, Diro E. High loss to followup and early mortality create substantial reduction in patient retention at antiretroviral treatment program in North-West Ethiopia. Isrn Aids. 2012;2012:1–9. doi:10.5402/2012/721720
11. Melaku Z, Lamb MR, Wang C, et al. Characteristics and outcomes of adult Ethiopian patients enrolled in HIV care and treatment: a multi-clinic observational study. BMC Public Health. 2015;15(1):1–13. doi:10.1186/s12889-015-1776-4
12. Umeokonkwo CD, Onoka CA, Agu PA, Ossai EN, Balogun MS, Ogbonnaya LU. Retention in care and adherence to HIV and AIDS treatment in Anambra State Nigeria. BMC Infect Dis. 2019;19(1):1–11. doi:10.1186/s12879-019-4293-8
13. Girum T, Wasie A, Worku A. Trend of HIV/AIDS for the last 26 years and predicting achievement of the 90- 90-90HIV prevention targets by 2020 in Ethiopia: a time series analysis. BMC Infect Dis. 2018;18(1):1–10. doi:10.1186/s12879-018-3214-6
14. Dalhatu I, Onotu D, Odafe S, et al. Outcomes of Nigeria’s HIV/AIDS treatment program for patients initiated on antiretroviral treatment between 2004–2012. PLoS One. 2016;11(11):1–25. doi:10.1371/journal.pone.0165528
15. ONUSIDA. Data 2017. Program HIV/AIDS; 2017:1–248.
16. Ministry of Health FDR of E. Country/Regional operational plan (COP/ROP); 2017:1–102.
17. Kharsay ABM, Karim QA. Differentiated care in Ethiopia the way forward; 2017:1–29.
18. FMOH Ethiopia. National consolidated guidelines for comprehensive HIV prevention, care; 2018:1–238.
19. UNAIDS. Communities at the center breaking barriers reaching people with HIV services; 2017. doi:10.2307/j.ctt1t898kc.12
20. Hassan AS, Mwaringa SM, Ndirangu KK, Sanders EJ, De Wit TFR, Berkley JA. Incidence and predictors of attrition from antiretroviral care among adults in a rural HIV clinic in Coastal Kenya: a retrospective cohort study. BMC Public Health. 2015;15(1):1–9. doi:10.1186/s12889-015-1814-2
21. Lamb MR, El-Sadr WM, Geng E, Nash D. Association of adherence support and outreach services with total attrition, loss to follow-up, and death among art patients in Sub-Saharan Africa. PLoS One. 2012;7(6):e38443. doi:10.1371/journal.pone.0038443
22. Bock P, Fatti G, Ford N, et al. Attrition when providing antiretroviral treatment at CD4 counts >500cells/μL at three government clinics included in the HPTN 071 (PopART) trial in South Africa. PLoS One. 2018;13(4):1–12. doi:10.1371/journal.pone.0195127
23. Alvarez-Uria G, Naik PK, Pakam R, Midde M. Factors associated with attrition, mortality, and loss to follow up after antiretroviral therapy initiation: data from an HIV cohort study in India. Glob Health Action. 2013;6(1):1–8. doi:10.3402/gha.v6i0.21682
24. Bekolo CE, Diallo A, Philips M, et al. Six-monthly appointment spacing for clinical visits as a model for retention in HIV care in Conakry-Guinea: a cohort study. BMC Infect Dis. 2017;17(1):1–10. doi:10.1186/s12879-017-2826-6
25. Berheto TM, Hinderaker SG, Senkoro M, et al. Body and mind: retention in antiretroviral treatment care is improved by mental health training of care providers in Ethiopia. BMC Public Health. 2018;18(1):1–8. doi:10.1186/s12889-018-5821-y
26. Decroo T, Koole O, Remartinez D, et al. Four-year retention and risk factors for attrition among members of community ART groups in Tete, Mozambique. Trop Med Int Heal. 2014;19(5):514–521. doi:10.1111/tmi.12278
27. Decroo T, Telfer B, Dores CD, et al. Effect of Community ART Groups on retention-in-care among patients on ART in Tete Province, Mozambique: a cohort study. BMJ Open. 2017;7(8):1–9. doi:10.1136/bmjopen-2017-016800
28. Evangeli M, Newell ML, Richter L, McGrath N. The association between self-reported stigma and loss-to-follow up in treatment eligible HIV positive adults in rural Kwazulu-Natal, South Africa. PLoS One. 2014;9(2):1–10. doi:10.1371/journal.pone.0088235
29. Casale M, Carlqvist A, Cluver L. Recent interventions to improve retention in HIV care and adherence to antiretroviral treatment among adolescents and youth: a systematic review. AIDS Patient Care STDS. 2019;33(6):1–17. doi:10.1089/apc.2018.0320
30. Auld AF, Ekra KA, Shiraishi RW, et al. Temporal trends in treatment outcomes for HIV-1 and HIV-2-infected adults enrolled in Côte d’Ivoire’s national antiretroviral therapy program. PLoS One. 2014;9(5):1–16. doi:10.1371/journal.pone.0098183
31. Nsanzimana S, Semakula M, Ndahindwa V, et al. Retention in care and virological failure among adult HIV+ patients on second-line ART in Rwanda: a national representative study. BMC Infect Dis. 2019;19(1):1–9. doi:10.1186/s12879-019-3934-2
32. Makurumidze R, Mutasa-Apollo T, Decroo T, et al. Retention and predictors of attrition among patients who started antiretroviral therapy in Zimbabwe’s National antiretroviral therapy programme between 2012 and 2015. PLoS One. 2020;15:e0222309. doi:10.1371/journal.pone.0222309
33. Blankley S, Gashu T, Ahmad B, et al. Lessons learned: retrospective assessment of outcomes and management of patients with advanced HIV disease in a semi-urban polyclinic in Epworth, Zimbabwe. PLoS One. 2019;14(4):1–14. doi:10.1371/journal.pone.0214739
34. Puttkammer NH, Zeliadt SB, Baseman JG, et al. Patient attrition from the HIV antiretroviral therapy program at two hospitals in Haiti. Rev Panam Salud Publica/Pan Am J Public Heal. 2014;36(4):1–19.
35. Carriquiry G, Fink V, Koethe JR, et al. Mortality and loss to follow-up among HIV-infected persons on long-term antiretroviral therapy in Latin America and the Caribbean. J Int AIDS Soc. 2015;18(1):1–10. doi:10.7448/IAS.18.1.20016
36. Kerschberger B, Schomaker M, Jobanputra K, et al. HIV programmatic outcomes following implementation of the ‘Treat-All’ policy in a public sector setting in Eswatini: a prospective cohort study. J Int AIDS Soc. 2020;23(3):1–15. doi:10.1002/jia2.25458
37. Mekuria LA, Prins JM, Yalew AW, Sprangers MAG, Nieuwkerk PT. Retention in HIV care and predictors of attrition from care among HIV-infected adults receiving combination anti-retroviral therapy in Addis Ababa. PLoS One. 2015;10(6):1–15. doi:10.1371/journal.pone.0130649
38. Gesesew HA, Ward P, Hajito KW, Feyissa T, Mohammadi L, Mwanri L. Discontinuation from antiretroviral therapy: a continuing challenge among adults in HIV care in Ethiopia: a systematic review and meta-analysis. PLoS One. 2017;12:e0169651. doi:10.1371/journal.pone.0169651
39. Tiruneh YM, Galárraga O, Genberg B, Wilson IB. Retention in care among HIV-infected adults in Ethiopia, 2005–2011: a mixed-methods study. PLoS One. 2016;11(6):2005–2011. doi:10.1371/journal.pone.0156619
40. Koole O, Tsui S, Wabwire‐Mangen F, et al. Retention and risk factors for attrition among adults in antiretroviral treatment programmes in Tanzania, Uganda and Zambia. Trop Med Int Health. 2014;19(12):1397–1410. doi:10.1016/j.physbeh.2017.03.040
41. Kwesigabo G, Mulenga M, Auld A, Agolory S. Reasons for missing antiretroviral therapy: results from a multi-country study inTanzania, Uganda, and Zambia. PLoS One. 2016;11:e0147309. doi:10.1371/journal.pone.0147309
42. Mugisha V, Teasdale CA, Wang C, et al. Determinants of mortality and loss to follow-up among adults enrolled in HIV care services in Rwanda. PLoS One. 2014;9(1):e85774. doi:10.1371/journal.pone.0085774
43. Kerschberger B, Schomaker M, Ciglenecki I, et al. Programmatic outcomes and impact of rapid public sector antiretroviral therapy expansion in adults prior to introduction of the WHO treat-all approach in rural Eswatini. Trop Med Int Heal. 2019;24(6):701–714. doi:10.1111/tmi.13234
44. Matyanga CMJ, Takarinda KC, Owiti P, et al. Outcomes of antiretroviral therapy among younger versus older adolescents and adults in an Urban clinic, Zimbabwe. Public Heal Action. 2016;6(2):1–8. doi:10.5588/pha.15.0077
45. Thida A, Tun STT, Zaw SKK, et al. Retention and risk factors for attrition in a large public health ART program in Myanmar: a retrospective cohort analysis. PLoS One. 2014;9(9):1–9. doi:10.1371/journal.pone.0108615
46. Mekonnen N, Abdulkadir M, Shumetie E, Baraki AG, Yenit MK. Incidence and predictors of loss to follow-up among HIV infected adults after initiation of first line anti-retroviral therapy at University of Gondar comprehensive specialized Hospital Northwest Ethiopia, 2018: retrospective follow up study. BMC Res Notes. 2019;12(1):1–7. doi:10.1186/s13104-019-4154-y
47. Ayalew MB. Mortality and its predictors among HIV infected patients taking antiretroviral treatment in Ethiopia: a systematic review. AIDS Res Treat. 2017;2017:1–10. doi:10.1155/2017/5415298
48. Belay H, Alemseged F, Angesom T, Hintsa S, Abay M. Effect of late HIV diagnosis on HIV-related mortality among adults in general hospitals of Central Zone Tigray, northern Ethiopia: a retrospective cohort study. HIV/AIDS - Res Palliat Care. 2017;9:1–6. doi:10.2147/HIV.S141895
49. Abdullahi IJ, Deybasso HA, Adlo AM. Determinants of virological failure among patients on first-line antiretroviral therapy in central Oromia, Ethiopia: a case–control study. HIV/AIDS - Res Palliat Care. 2020;12:931–939. doi:10.2147/HIV.S281672
50. Seifu W, Ali W, Meresa B. Predictors of loss to follow up among adult clients attending antiretroviral treatment at Karamara general hospital, Jigjiga town, Eastern Ethiopia, 2015: a retrospective cohort study. BMC Infect Dis. 2018;18(1):1–8. doi:10.1186/s12879-018-3188-4
51. Akilimali PZ, Musumari PM, Kashala-Abotnes E, et al. Disclosure of HIV status and its impact on the loss in the follow-up of HIV-infected patients on potent anti-retroviral therapy programs in a (post-) conflict setting: a retrospective cohort study from Goma, Democratic Republic of Congo. PLoS One. 2017;12(2):1–13. doi:10.1371/journal.pone.0171407
52. Elopre L, Hook EW, Westfall AO, et al. The role of early HIV status disclosure in retention in HIV care. AIDS Patient Care STDS. 2015;29(12):1–5. doi:10.1089/apc.2015.0205
53. Davey DJ, Nhavoto JA, Augusto O, et al. SMSaúde: evaluating mobile phone text reminders to improve retention in HIV care for patients on antiretroviral therapy in Mozambique. J Acquir Immune Defic Syndr. 2016;73(2):e23. doi:10.1016/j.physbeh.2017.03.040
54. Gesesew HA, Ward P, Woldemichael K, Mwanri L. Prevalence, trend and risk factors for antiretroviral therapy discontinuation among HIV-infected adults in Ethiopia in 2003–2015. PLoS One. 2017;12:e0179533. doi:10.1371/journal.pone.0179533
55. Pecoraro A, Mimiaga M, O’Cleirigh C, et al. Depression, substance use, viral load, and CD4+ count among patients who continued or left antiretroviral therapy for HIV in St. Petersburg, Russian federation. AIDS Care. 2015;27(1):86–92. doi:10.1080/09540121.2014.959464.Depression
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
