Back to Journals » Drug Design, Development and Therapy » Volume 12
Astragaloside IV improves renal function and fibrosis via inhibition of miR-21-induced podocyte dedifferentiation and mesangial cell activation in diabetic mice
Authors Wang X, Gao Y , Tian N, Zou D, Shi Y, Zhang N
Received 11 April 2018
Accepted for publication 28 May 2018
Published 6 August 2018 Volume 2018:12 Pages 2431—2442
DOI https://doi.org/10.2147/DDDT.S170840
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Xiaolei Wang,1,2 Yanbin Gao,1,2 Nianxiu Tian,1 Dawei Zou,2 Yimin Shi,1 Nan Zhang1,2
1Department of Endocrinology, School of Traditional Chinese Medicine, Capital Medical University, Beijing, China; 2Department of Endocrinology, Beijing Key Laboratory of Traditional Chinese Medicine Collateral Disease Theory Research, Beijing, China
Background: Podocyte dedifferentiation and mesangial cell (MC) activation play an important role in many glomerular diseases associated with fibrosis. MicroRNA-21 (miR-21) is closely linked to renal fibrosis, but it is unknown whether and how miR-21 promotes podocyte dedifferentiation and MC activation and whether astragaloside IV (AS-IV) improves renal function and fibrosis through the regulation of miR-21.
Materials and methods: Cultured MCs, primary mouse podocytes, and diabetic KK-Ay mice were treated with AS-IV. Cell transfection, Western blot, real-time PCR, immunofluorescence assay, immunohistochemical assay, and electronic microscopy were used to detect the markers of podocyte dedifferentiation and MC activation and to observe the renal morphology.
Results: Our data showed that miR-21 expression was increased and that AS-IV decreased miR-21 levels in cells, serum, and kidney. Overexpressed miR-21 promoted podocyte dedifferentiation and MC activation, and treatment with AS-IV reversed this effect. Furthermore, the overexpression of miR-21 activated the β-catenin pathway and the transforming growth factor (TGF)-β1/Smads pathway in the process of podocyte dedifferentiation and MC activation, which was abolished by AS-IV treatment. In addition, both the Wnt/β-catenin pathway inhibitor XAV-939 and the TGF-β1/Smads pathway inhibitor SB431542 reversed the effect of AS-IV. Furthermore, AS-IV improved renal function and fibrosis in diabetic KK-Ay mice.
Conclusion: Our results indicated that AS-IV ameliorates renal function and renal fibrosis by inhibiting miR-21 overexpression-induced podocyte dedifferentiation and MC activation in diabetic kidney disease. These findings pave way for future studies investigating AS-IV as a potential therapeutic agent in the management of glomerular diseases.
Keywords: astragaloside IV, podocyte dedifferentiation, mesangial cell activation, miR-21, β-catenin pathway, TGF-β1/Smads pathway, renal fibrosis
Introduction
Diabetic kidney disease (DKD), a microvascular disease and the leading cause of end-stage renal disease, is pathologically characterized by renal fibrosis and clinically characterized by proteinuria.1 Podocytes, highly differentiated epithelial cells that form the outermost layer of the glomerular filtration barrier, serve as the final barrier to macromolecular flow into the urinary filtrate.2 When exposed to harmful stimuli, podocytes may lose their differentiated architecture, which leads to the escape of plasma proteins into the urine,3 which can consequently accelerate the progression of renal fibrosis. Mesangial cells (MCs) are another type of glomerular cells associated with the pathological process of DKD, which can be activated by adverse stimuli.4 Activated MCs, which are characterized by increased α-smooth muscle actin (α-SMA) expression, can proliferate and synthesize excessive extracellular matrix (ECM) proteins, which can aggravate renal fibrosis.5 Therefore, it is prudent to understand the mechanisms of podocyte dedifferentiation and MC activation, to develop techniques to cease the progression of renal fibrosis in DKD.
MicroRNAs (miRs) are short, noncoding RNAs that negatively regulate gene expression posttranscriptionally. Recent reports have shown that MicroRNA-21 (miR-21) is upregulated in several animal models of kidney disease and in human chronic kidney disease tissue samples.6–8 McClelland et al demonstrated that miR-21 promoted renal fibrosis by targeting PTEN and SMAD7 in human kidney tissue and three rodent models of renal disease.7 Another study indicated that miR-21 enhances podocyte motility and the expansion of MCs in streptozotocin-induced diabetic mice.9 Our previous study showed that miR-21 promotes renal interstitial fibrosis via the regulation of transforming growth factor (TGF)-β1/Smads pathway-induced epithelial-to-mesenchymal transition (EMT) in proximal tubular epithelial cells.10 In addition, miR-21 may aggravate renal interstitial fibrosis through the Wnt/β-catenin pathway.11 However, whether miR-21 mediates podocyte dedifferentiation and MC activation and how this promotes renal fibrosis are still unclear.
Astragaloside IV (AS-IV), a bioactive saponin extracted from the Astragalus root, exerts many potentially therapeutic effects in various diseases, such as liver fibrosis, chronic heart failure, and DKD.12–14 Recent works in this area suggest that AS-IV prevents MC proliferation induced by high glucose15 and limits podocyte injury in streptozotocin-induced DKD mice.14 However, the effects of AS-IV on podocyte dedifferentiation and MC activation are unknown. Previous studies have shown that AS-IV inhibits EMT via the Wnt/β-catenin pathway16 and the TGF-β1/Smads pathway17 in fibrosis. Thus, we investigated whether AS-IV plays a role in the podocyte dedifferentiation and MC activation associated with miR-21 and explored the possible mechanisms through which the compound may affect these processes. We specifically focused on the Wnt/β-catenin pathway and the TGF-β1/Smads pathway in the study of podocyte dedifferentiation and MC activation.
Materials and methods
Reagents
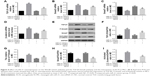
AS-IV (Figure 1; C41H68O14; molecular weight =784.97, purity by high-performance liquid chromatography ≥98%) was purchased from Sigma-Aldrich (Milwaukee, WI, USA). The Wnt/β-catenin pathway inhibitor XAV-939 and the TGF-β1/Smads pathway inhibitor SB431542 were purchased from Abcam (Cambridge, UK). Rabbit polyclonal anti-α-SMA, anti-TGF-β1, anti-Smad3 (phospho S423 + S425; P-Smad3), anti-fibronectin (FN), and anti-Collagen IV (Col IV) antibodies were purchased from Abcam. Mouse monoclonal anti-Smad7 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit monoclonal anti-non-phospho β-catenin (activated β-catenin) antibody was ordered from Cell Signaling Technology (Danvers, MA, USA). Rabbit monoclonal anti-nephrin antibody was provided by Novus Biologicals (Littleton, CO, USA). miR-21 mimics and negative controls were synthesized by Ribobio (Guangzhou, China).
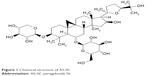  | Figure 1 Chemical structure of AS-IV. |
Cell culture
As previously described, primary podocytes were obtained from male C57BL/6J mice (30–40 g).18 Under brief diethyl ether anesthesia, mice kidneys were excised. After mincing and sifting through a system of sieves with decreasing apertures (180, 100, and 75 μm), the glomeruli were isolated in a standard medium (Roswell Park Memorial Institute [RPMI] 1640 medium with 10% fetal bovine serum [FBS], 100 U/mL penicillin, and 100 μg/mL streptomycin). Glomeruli were then grown in 25 cm2 culture flasks coated with Collagen I (Thermo Fisher Scientific, Waltham, MA, USA), in an atmosphere of 5% CO2 at 37°C, for 7 days. Following this, the outgrowing podocytes were trypsinized, and the residual glomeruli were removed. Then, podocytes were seeded in culture flasks in the RPMI 1640 medium with 10% FBS.
The conditionally immortalized mouse glomerular MC lines (SV40 MES 13) were obtained from China Infrastructure of Cell Line Resources. Cells were cultured in low-glucose Dulbecco’s Modified Eagle’s Medium, supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin, in an atmosphere of 5% CO2 at 37°C.
For this study, cells that reached 80% confluence were synchronized in serum-free conditions for 24 hours and were then used for experiments.
Cell transfection
The cells were transfected with miR-21 mimics (50 nM) for overexpression. In addition, miR-21 mimics controls (100 nM) were used as negative controls. The cells were transfected with all miRNAs in accordance with the manufacturer’s protocol and incubated for 48 hours before further analysis.
Animal model and experimental design
Eight-week-old male KK-Ay mice and male C57BL/6J mice (from Chinese Academy of Medical Sciences, Beijing, China) were housed at constant room temperature (24°C) and humidity (70%), under a controlled light-to-dark cycle. All animals had free access to water. To induce DKD, KK-Ay mice received high-fat diets (58% fat, 16.4% protein, and 25.6% carbohydrate) for 4 weeks. DKD was diagnosed when their random blood glucose was ≥16.7 mmol/L and urine albumin–creatinine ratio (ACR) was ≥300 μg/mg. The animals were then divided into three groups: All C57BL/6J mice were considered to be in the normal control group (n=12, gavaged with aqua distillate), and KK-Ay mice were categorized into either the DKD control group (DKD group, n=12, gavaged with aquadistillate) or the DKD treatment group (n=12, gavaged with AS-IV at 40 mg/kg/day). Drugs were suspended in 1% carboxymethyl cellulose solution as a vehicle. C57BL/6J mice were fed a standard diet (12% fat, 28% protein, and 60% carbohydrate), and KK-Ay mice were fed a high-fat diet. After 12 weeks, blood and 24-hour urine samples were collected, and renal tissue from each mouse was collected for hematoxylin–eosin staining, Masson’s staining, and immunohistochemical (IHC) and immunofluorescence (IF) staining. The study was approved by the Institutional Animal Care and Use Committee at Capital Medical University, conforming to the Guide for the Care and Use of Laboratory Animals by the National Institute of Health.
IHC and IF staining
The renal tissues were fixed in 4% paraformaldehyde, paraffin-embedded, and sectioned for IHC and IF analysis. Briefly, sections were deparaffinized, dehydrated, and subjected to antigen retrieval. After blocking endogenous peroxidase activity with 3% hydrogen peroxide, the sections were blocked with 5% goat serum for 30 minutes and then incubated with primary antibodies overnight at 4°C. Then, the sections were incubated with secondary antibody for 1 hour at 37°C. 4′,6-diamidino-2-phenylindole was used to stain nuclei. The sections were then imaged with a fluorescence microscope (Nikon Corporation, Tokyo, Japan). For IHC analysis, the primary antibodies and dilutions were as follows: rabbit anti-FN antibody at 1:100 and rabbit anti-Col IV antibody at 1:100. For IF analysis, the primary antibodies and dilutions were as follows: rabbit anti-nephrin antibody at 1:100, rabbit anti-α-SMA antibody at 1:100, rabbit anti-TGF-β1 antibody at 1:100, rabbit anti-P-Smad3 antibody at 1:200, rabbit anti-Smad7 antibody at 1:50, and rabbit anti-activated β-catenin antibody at 1:1,000.
Real-time PCR analysis
Total RNAs from cells and kidney tissue were obtained using TRIzol reagent (Thermo Fisher Scientific), according to the manufacturer’s instructions. Real-time PCR primers were designed as previously described.19 Relative expression was calculated using the comparative cycle threshold (CT) method (2−ΔΔCT).20 The relative expressions of nephrin, α-SMA, TGF-β1, Smad3, Smad7, and β-catenin were normalized to the expression of GAPDH. For the analysis of miR-21 expression, U6 was used as an internal control. All mRNA expression analyses were performed in at least three independent experiments.
Western blot analysis
Total proteins from cells and kidney tissue were extracted, subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membrane, and blocked with 5% nonfat, dry milk. Then, the membranes were incubated with the primary antibody at 4°C overnight, followed by incubation with horseradish peroxidase-conjugated secondary antibody (1:1,000; Beyotime Biotechnology, Shanghai, China). The primary antibodies and dilutions were as follows: rabbit anti-nephrin antibody at 1:5,000, rabbit anti-α-SMA antibody at 1:1,000, rabbit anti-TGF-β1 antibody at 1:1,000, rabbit anti-P-Smad3 antibody at 1:2,000, rabbit anti-Smad7 antibody at 1:500, and rabbit anti-activated β-catenin antibody at 1:1,000. Subsequently, the bands were detected with enhanced chemiluminescence.
Cell Counting Kit-8 (CCK-8) assay
To detect the cell viability of podocytes and the proliferation rate of MCs, CCK-8 assay was used according to the manufacturer’s protocol (Dojindo, Kyushu, Japan).
Statistical methods
SPSS software (IBM Corporation, Armonk, NY, USA) was used for statistical analysis. All data are presented as mean ± SD. Statistical analyses were performed using Student’s unpaired t-tests, for comparison between two groups. Multiple groups were compared using one-way analysis of variance. A p-value of <0.05 was considered statistically significant.
Results
Effect of AS-IV on miR-21 expression in vitro and in vivo
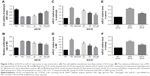
In order to explore the optimal intervention concentration of AS-IV on podocytes and MCs, CCK-8 assay was used to detect the cell viability of podocytes and the proliferation rate of MCs. The data showed that AS-IV increased the cell viability of podocytes and decreased the proliferation rate of MCs exposed to high glucose (30 mM) in a dose-dependent manner (Figure 2A and B). The effect-acting concentration of AS-IV is 25 μM. Then, real-time PCR was used to investigate the effect of AS-IV on miR-21 expression. Our results showed that miR-21 expression was notably increased in podocytes and MCs that were exposed to high glucose and that AS-IV decreased the level of miR-21 in podocytes and MCs treated with high glucose in a concentration-dependent manner (Figure 2C and D). Similarly, we found that the level of miR-21 was increased in the serum and kidneys of DKD mice, which decreased after AS-IV treatment (Figure 2E and F). Therefore, the results indicate that AS-IV decreases miR-21 expression in vitro and in vivo.
Effect of AS-IV on miR-21 overexpression-induced podocyte dedifferentiation and MC activation
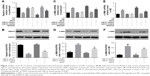
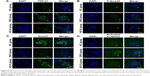
To investigate the effect of miR-21 on podocyte dedifferentiation and MC activation, and the role of AS-IV, podocytes and MCs were transfected with miR-21 mimics and incubated in the presence or absence of AS-IV (100 μM) for 48 hours. Real-time PCR and Western blot were used to detect the levels of key markers of MC activation (α-SMA) and podocyte dedifferentiation (the epithelial marker, nephrin, and the mesenchymal marker, α-SMA). The results showed that both high glucose and miR-21 overexpression increased the level of α-SMA and decreased the level of nephrin in podocytes and that this change was reversed by AS-IV treatment (Figure 3A–D). Similarly, miR-21 overexpression increased the level of α-SMA in MCs, and AS-IV abolished this effect (Figure 3E and F). Our results indicate that AS-IV inhibits miR-21 overexpression-induced podocyte dedifferentiation and MC activation. In addition, we also detected the expression of nephrin and α-SMA in glomerulus. The results showed that AS-IV treatment increased nephrin expression and decreased α-SMA expression compared with the untreated DKD mice (Figure 4).
Effect of miR-21 overexpression on Wnt/β-catenin pathway and TGF-β1/Smads pathway and the intervention of AS-IV
The Wnt/β-catenin pathway and TGF-β1/Smads pathway played vital roles in the regulation of EMT associated with miR-21. To explore the role of the Wnt/β-catenin pathway and the TGF-β1/Smads pathway in the process of miR-21 overexpression-induced podocyte dedifferentiation and MC activation, we detected the key factors of the Wnt/β-catenin pathway (activated β-catenin) and the key factors of the TGF-β1/Smads pathway (TGF-β1, P-Smad3, and Smad7). We found that miR-21 overexpression increased the levels of activated β-catenin, TGF-β1, and P-Smad3 and decreased the level of Smad7, in both podocytes (Figure 5A–I) and MCs (Figure 6A–I). This effect was abolished by AS-IV treatment.
To further evaluate the role of the Wnt/β-catenin pathway and the TGF-β1/Smads pathway, the Wnt/β-catenin pathway inhibitor XAV-939 and the TGF-β1/Smads pathway inhibitor SB431542 were used, respectively. The data showed that both XAV-939 and SB431542 reversed the effect of AS-IV in miR-21-overexpressed podocytes and MCs (Figure 7A–C). The effect of cotreatment of XAV-939 and SB431542 is more obvious.
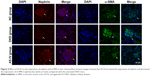
Furthermore, we evaluated the effect of AS-IV on the Wnt/β-catenin pathway and the TGF-β1/Smads pathway in vivo by IF. The results showed that the levels of β-catenin, TGF-β1, and Smad3 were decreased, and Smad7 expression was increased in the kidneys of AS-IV-treated mice, compared with DKD mice (Figure 8A–D). Therefore, our data suggest that AS-IV may inhibit the activation of the Wnt/β-catenin pathway and the TGF-β1/Smads pathway induced by overexpressed miR-21 in vitro and in vivo.
Effect of AS-IV on renal function and renal morphology in diabetic KK-Ay mice
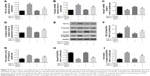
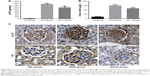
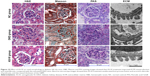
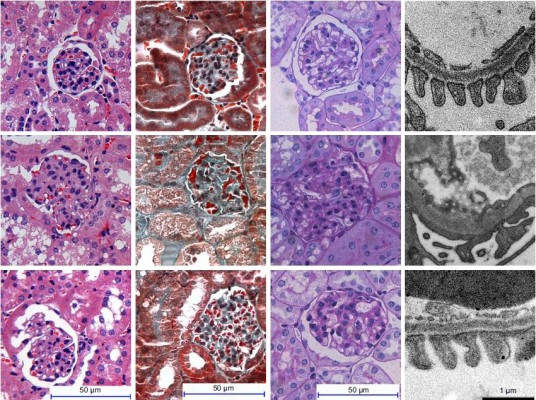
To explore the effect of AS-IV on renal function in KK-Ay mice, ACR and microalbuminuria (mAlb) were measured, and light microscopy and electronic microscopy were used to observe renal morphology. ECM production was closely related to EMT and renal fibrosis in DKD. Thus, we evaluated the effect of AS-IV on the main components of ECM, Col IV, and FN in kidney by IHC assay. The data showed that AS-IV decreased ACR and mAlb, when compared to the mice in the DKD group (Figure 9A–B). In addition, our results showed that the expression of Col IV and FN were markedly decreased in AS-IV-treated mice, compared with the DKD mice (Figure 9C). Furthermore, mice treated with AS-IV showed improved foot process fusion and structure disorder of podocyte, decreased thickness of the glomerular basement membrane, and reduced ECM overproduction and renal fibrosis, compared with the untreated DKD mice (Figure 10). Overall, AS-IV improved renal function and renal morphology and alleviated renal fibrosis in diabetic KK-Ay mice.
Discussion
Podocyte dedifferentiation and MC activation are potential targets for preventing the progression of glomerulosclerosis in DKD.21,22 Several studies have emphasized the role of miR-21 in chronic renal disease, including DKD.9,23,24 In the present study, we investigated the role of miR-21 in podocyte dedifferentiation and MC activation. We found that the expression of miR-21 was significantly increased in podocytes, MCs, serum, and renal tissue, under a high-glucose condition. Furthermore, overexpressed miR-21 promoted podocyte dedifferentiation (manifested by an increase in α-SMA level and a decrease in nephrin level) and MC activation (characterized by enhanced α-SMA expression). Interestingly, AS-IV not only decreased miR-21 levels, but also suppressed podocyte dedifferentiation and MC activation, both in vitro and in vivo. Our data suggest that AS-IV improves podocyte dedifferentiation and MC activation through the inhibition of miR-21 expression.
Several reports have suggested that the Wnt/β-catenin pathway and the TGF-β1/Smads pathway play a vital role in podocyte injury and MC proliferation.25–28 Sugiyama et al demonstrated that telmisartan ameliorated podocyte injury and glomerulosclerosis, by inhibiting the TGF-β/Smads pathway, in a rat model of metabolic syndrome.25 Yan et al suggested that naringenin (a flavanone) alleviated MC proliferation through the inhibition of the TGF-β/Smads pathway in DKD rats.26 In addition, Li et al demonstrated that a conditioned medium made from adipose-derived mesenchymal stem cells improved MC proliferation via the Wnt/β-catenin pathway.27 Another recent study reported that aldosterone aggravates podocyte injury in obesity-associated glomerular diseases, through the activation of Wnt/β-catenin signaling.28 Furthermore, some studies reported that both Wnt1 and Smad7 are target genes of miR-21.29–31 Therefore, we focused on the Wnt/β-catenin pathway and the TGF-β1/Smads pathway in our study. We found that the Wnt/β-catenin pathway and the TGF-β1/Smads pathway were activated by miR-21 overexpression, in both podocytes and MCs. This suggested that miR-21 induced podocyte dedifferentiation and MC activation via the activation of the Wnt/β-catenin pathway and the TGF-β1/Smads pathway. Moreover, our data showed that AS-IV suppressed the activation of the Wnt/β-catenin pathway and the TGF-β1/Smads pathway in the podocyte dedifferentiation and MC activation processes induced by miR-21 overexpression. In addition, both XAV-939 (the Wnt/β-catenin pathway inhibitor) and SB431542 (the TGF-β1/Smads pathway inhibitor) reversed the improvement effect of AS-IV in miR-21 overexpression-induced podocyte dedifferentiation and MC activation. The effect of cotreatment of XAV-939 and SB431542 is more obvious. Furthermore, we found that AS-IV treatment inhibited Wnt/β-catenin pathway and TGF-β1/Smads pathway in kidney compared with the untreated DKD mice. Overall, our study suggests that AS-IV improves podocyte dedifferentiation and MC activation in DKD, through suppression of the miR-21-induced activation of the Wnt/β-catenin pathway and TGF-β1/Smads pathway.
Numerous studies have reported that AS-IV could exert many potentially therapeutic effects in various diseases via multiple pharmacologic effects, such as antidiabetes,32 antifibrotic,12 antioxidative,33 and anti-inflammatory stress.34 In this study, we found that AS-IV inhibited miR-21 overexpression-induced podocyte dedifferentiation and MC activation by the regulation of the Wnt/β-catenin pathway and the TGF-β1/Smads pathway. In addition, we observed that AS-IV decreased the expression of miR-21 both in vivo and in vitro. The possible molecular mechanism was that hyperglycemia and/or miR-21 induced the activation of Smad3, which further promoted the expression of miR-21.35 AS-IV reduced the level of Smad3, which further led to the decrease in miR-21 level. Of course, a further study is required to clarify the mechanisms of miR-21 downregulation by AS-IV. Although the dose of AS-IV in our study is safe, the maternal toxicity of it was observed in conceived rats and rabbits.36 Therefore, to better use of AS-IV, further studies are required to evaluate the toxicity of AS-IV.
Conclusion
Our study suggested that AS-IV ameliorates renal function and renal morphology through the inhibition of miR-21 overexpression-induced podocyte dedifferentiation and MC activation in DKD. These findings provide important evidence for future studies investigating AS-IV as a therapeutic agent in the management of glomerular diseases.
Acknowledgments
This study was supported by grants from the Major National Basic Research Program of China (973 Program, no. 2012CB518602) and Scientific Research Project of Beijing Educational Committee (no. KZ201610025024).
Disclosure
The authors report no conflicts of interest in this work.
References
Rask-Madsen C, King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 2013;17(1):20–33. | ||
Durvasula RV, Shankland SJ. Podocyte injury and targeting therapy: an update. Curr Opin Nephrol Hypertens. 2006;15(1):1–7. | ||
Herman-Edelstein M, Thomas MC, Thallas-Bonke V, Saleem M, Cooper ME, Kantharidis P. Dedifferentiation of immortalized human podocytes in response to transforming growth factor-beta: a model for diabetic podocytopathy. Diabetes. 2011;60(6):1779–1788. | ||
Gruden G, Perin PC, Camussi G. Insight on the pathogenesis of diabetic nephropathy from the study of podocyte and mesangial cell biology. Curr Diabetes Rev. 2005;1(1):27–40. | ||
Wu XM, Gao YB, Cui FQ, Zhang N. Exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells to promote renal fibrosis. Biol Open. 2016;5(4):484–491. | ||
Zarjou A, Yang S, Abraham E, Agarwal A, Liu G. Identification of a microRNA signature in renal fibrosis: role of miR-21. Am J Physiol Renal Physiol. 2011;301(4):F793–F801. | ||
McClelland AD, Herman-Edelstein M, Komers R, et al. miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin Sci (Lond). 2015;129(12):1237–1249. | ||
Loboda A, Sobczak M, Jozkowicz A, Dulak J. TGF-beta1/Smads and miR-21 in renal fibrosis and inflammation. Mediators Inflamm. 2016;2016:8319283. | ||
Kolling M, Kaucsar T, Schauerte C, et al. Therapeutic miR-21 silencing ameliorates diabetic kidney disease in mice. Mol Ther. 2017;25(1):165–180. | ||
Wang JY, Gao YB, Zhang N, et al. Tongxinluo ameliorates renal structure and function by regulating miR-21-induced epithelial-to-mesenchymal transition in diabetic nephropathy. Am J Physiol Renal Physiol. 2014;306(5):F486–F495. | ||
Liu XJ, Hong Q, Wang Z, Yu YY, Zou X, Xu LH. MicroRNA21 promotes interstitial fibrosis via targeting DDAH1: a potential role in renal fibrosis. Mol Cell Biochem. 2016;411(1–2):181–189. | ||
Gui SY, Wei W, Wang H, et al. Effects and mechanisms of crude astragalosides fraction on liver fibrosis in rats. J Ethnopharmacol. 2006;103(2):154–159. | ||
Wang Y, Ji Y, Xing Y, Li X, Gao X. Astragalosides rescue both cardiac function and sarcoplasmic reticulum Ca2+ transport in rats with chronic heart failure. Phytother Res. 2012;26(2):231–238. | ||
Guo H, Wang Y, Zhang X, et al. Astragaloside IV protects against podocyte injury via SERCA2-dependent ER stress reduction and AMPKα-regulated autophagy induction in streptozotocin-induced diabetic nephropathy. Sci Rep. 2017;7(1):6852. | ||
Chen X, Wang DD, Wei T, He SM, Zhang GY, Wei QL. Effects of astragalosides from Radix Astragali on high glucose-induced proliferation and extracellular matrix accumulation in glomerular mesangial cells. Exp Ther Med. 2016;11(6):2561–2566. | ||
Wang L, Chi YF, Yuan ZT, et al. Astragaloside IV inhibits the up-regulation of Wnt/β-catenin signaling in rats with unilateral ureteral obstruction. Cell Physiol Biochem. 2014;33(5):1316–1328. | ||
Zhang L, Li Z, He W, et al. Effects of astragaloside iv against the TGF-beta1-induced epithelial-to-mesenchymal transition in peritoneal mesothelial cells by promoting Smad7 expression. Cell Physiol Biochem. 2015;37(1):43–54. | ||
Smeets B, Kabgani N, Moeller MJ. Isolation and Primary Culture of Murine Podocytes with Proven Origin. New York: Springer; 2016. | ||
Schmittgen TD, Lee EJ, Jiang J, et al. Real-time PCR quantification of precursor and mature microRNA. Methods. 2008;44(1):31–38. | ||
Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. | ||
Li SY, Huang PH, Yang AH, et al. Matrix metalloproteinase-9 deficiency attenuates diabetic nephropathy by modulation of podocyte functions and dedifferentiation. Kidney Int. 2014;86(2):358–369. | ||
Lee WJ, Liu SH, Chiang CK, et al. Aryl hydrocarbon receptor deficiency attenuates oxidative stress-related mesangial cell activation and macrophage infiltration and extracellular matrix accumulation in diabetic nephropathy. Antioxid Redox Signal. 2016;24(4):217–231. | ||
Lai JY, Luo J, O’Connor C, et al. MicroRNA-21 in glomerular injury. J Am Soc Nephrol. 2015;26(4):805–816. | ||
Hennino MF, Buob D, Van der Hauwaert C, et al. miR-21-5p renal expression is associated with fibrosis and renal survival in patients with IgA nephropathy. Sci Rep. 2016;6:27209. | ||
Sugiyama F, Kobayashi N, Ishikawa M, Onoda S, Ishimitsu T. Renoprotective mechanisms of telmisartan on renal injury and inflammation in SHRSP.Z-Leprfa/IzmDmcr rats. Clin Exp Nephrol. 2013;17(4):515–524. | ||
Yan N, Wen L, Peng R, et al. Naringenin ameliorated kidney injury through Let-7a/TGFBR1 signaling in diabetic nephropathy. J Diabetes Res. 2016;2016:8738760. | ||
Li Z, Zhang M, Li X, Lu J, Xu L. [Wnt/β-catenin pathway involved in the regulation of rat mesangial cell proliferation by adipose-derived mesenchymal stem cells]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016;32(11):1486–1490. Chinese. | ||
Zhu JJ, Chen YP, Yang M, et al. Aldosterone is involved in the pathogenesis of obesity-related glomerulopathy through activation of Wnt/β-catenin signaling in podocytes. Mol Med Rep. 2018;17(3):4589–4598. | ||
Mai XY, Liu XU, Ping LI. [Bioinformatic analysis and prediction of MiR-21 target genes]. J Biol. 2014;31(6):89–93. Chinese. | ||
Yuan J, Chen H, Ge D, et al. Mir-21 promotes cardiac fibrosis after myocardial infarction via targeting Smad7. Cell Physiol Biochem. 2017;42(6):2207–2219. | ||
Wang JY, Gao YB, Zhang N, et al. miR-21 overexpression enhances TGF-β1-induced epithelial-to-mesenchymal transition by target smad7 and aggravates renal damage in diabetic nephropathy. Mol Cell Endocrinol. 2014;392(1–2):163–172. | ||
Chen Y, Gui D, Chen J, He D, Luo Y, Wang N. Down-regulation of PERK-ATF4-CHOP pathway by Astragaloside IV is associated with the inhibition of endoplasmic reticulum stress-induced podocyte apoptosis in diabetic rats. Cell Physiol Biochem. 2014;33(6):1975–1987. | ||
Qi W, Niu J, Qin Q, Qiao Z, Gu Y. Astragaloside IV attenuates glycated albumin-induced epithelial-to-mesenchymal transition by inhibiting oxidative stress in renal proximal tubular cells. Cell Stress Chaperones. 2014;19(1):105–114. | ||
Jiang P, Ma D, Wang X, et al. Astragaloside IV prevents obesity-associated hypertension by improving pro-inflammatory reaction and leptin resistance. Mol Cells. 2018;41(3):244–255. | ||
Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454(7200):56–61. | ||
Jiangbo Z, Xuying W, Yuping Z, Xili M, Yiwen Z, Tianbao Z. Effect of astragaloside IV on the embryo-fetal development of Sprague-Dawley rats and New Zealand White rabbits. J Appl Toxicol. 2009;29(5):381–385. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.