Back to Journals » International Journal of General Medicine » Volume 16
Associations of miRNA-146a and miRNA-223 with Rheumatoid Arthritis and Their Predictive Values
Authors Zhang H , Shang H, Wang Z, Li K
Received 20 April 2023
Accepted for publication 19 July 2023
Published 31 July 2023 Volume 2023:16 Pages 3211—3218
DOI https://doi.org/10.2147/IJGM.S416317
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Haoshaqiang Zhang,1,* Hua Shang,1,2,* Zhigang Wang,1 Kun Li1
1Department of Orthopedics Surgery, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, People’s Republic of China; 2Department of Human Resources, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhigang Wang, Department of Orthopedics Surgery, People’s Hospital of Xinjiang Uygur Autonomous Region, No. 91, Tianchi Road, Tianshan District, Urumqi, 830001, People’s Republic of China, Tel +86-9918563146, Email [email protected]
Purpose: To analyze the independent associations of miRNA-146a and miRNA-223 with rheumatoid arthritis (RA) and evaluate their predictive values for RA.
Patients and Methods: A total of 68 RA patients were selected as cases, and meanwhile 68 patients with a traumatic knee condition were selected as controls by matching to the cases according to sex and age at the ratio of 1:1. The independent associations of miRNA-146a and miRNA-223 with RA were identified by binary logistic regression analysis. Receiver operating characteristic (ROC) curve was used to evaluate their predictive values for RA.
Results: MiRNA-146a and miRNA-223 expression levels in both synovial tissues and serums were statistically higher in cases than in controls, and their expression levels in serums were not statistically different from those in synovial tissues in both cases and controls. The expression levels of miRNA-146a and miRNA-223 in synovial tissues were independently associated with RA, as well as the expression levels of miRNA-146a and miRNA-223 in serums. The area under curve (AUC) of combination of miRNA-146a and miRNA-223 in synovial tissues for the prediction of RA was 0.910 [95% confidence interval (CI): 0.863– 0.962], and the AUC of combination of miRNA-146a and miRNA-223 in serums was 0.904 (95% CI: 0.851– 0.957). Their difference was not statistically significant (P=0.873), but the AUC of combination prediction was statistically higher than those of individual predictions (synovial tissues: 0.910 vs 0.773, P=0.005, 0.910 vs 0.788, P=0.009; serums: 0.904 vs 0.766, P=0.005, 0.904 vs 0.784, P=0.011).
Conclusion: MiRNA-146a and miRNA-223 in both synovial tissues and serums could be applied in predicting RA, and their combination could elevate the predictive value significantly.
Keywords: rheumatoid arthritis, MiRNA-146a, MiRNA-223, synovial tissues, serums, predictive values
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease that can occur in people of any age. Its prevalence increases with age influencing approximately 0.5–1% of the population worldwide.1 RA is characterized by chronic synovial inflammation which can result in destruction of cartilage and bone.2 It firstly involves the small joints especially in the wrist and hands; and the larger joints, such as knees, ankles, shoulders and elbow, are also prone to be involved following its progression. The involvement of knees is common in RA patients.3,4 RA can lead to morbidity, disability and premature mortality, which in turn results in distressed life quality. Early diagnosis is critical in halting the progression of RA to prevent disability and preserve life quality. Numerous risk factors demonstrated their involvement in the development and progression of RA, including environmental, genetic, epigenetics, smoking, etc. As a heterogeneous disease, RA comprises the infiltration and accumulation of immune cells and production of autoantibodies in synovial tissues of joint.5 Thus, it is helpful for improving diagnosis to understand the complex pathological behavior of RA.6
MiRNAs comprise a class of small non-coding RNAs that have a crucial role in many biological processes such as organ development, cell proliferation, cell fate determination, differentiation and signal transduction, via regulating gene expression.7 The abnormal expression of miRNAs has been associated with the pathogenesis of multiple pathological conditions including RA,8,9 although the functions of most miRNAs have yet to be elucidated. Therefore, they are considered as prognostic and diagnostic biomarkers of multiple diseases.10 The expression of miRNA-146a is significantly increased in the peripheral blood mononuclear cells and synovial fluid of RA patients compared with healthy and disease controls. Moreover, multiple studies have examined the association of miRNA-146a polymorphism rs2910164 with RA.11–17 Additionally, the expression of miRNA-223 is also significantly up-regulated in synovial fluid, fibroblast-like synoviocytes and naive CD4+T lymphocytes of RA patients compared with healthy and disease (osteoarthritis) controls.18–20 However, the independent associations of miRNA-146a and miRNA-223 with RA have still not been investigated, as well as their predictive values for RA. In this study, we analyzed the independent associations of miRNA-146a and miRNA-223 with RA through binary logistic regression analysis, and evaluated the values of miRNA-146a and miRNA-223 in individually and jointly predicting RA through ROC curves. The aim was to provide useful information for application of miRNA-146a and miRNA-223 in diagnosis of RA.
Patients and Methods
Patients
This study was a retrospective analysis, recruiting 68 RA patients (21 males and 47 females) with an average age of 51.62±15.37 years as cases at People’s Hospital of Xinjiang Uygur Autonomous Region between April 2018 and June 2022. During the identical time period, 68 patients with a traumatic knee condition were matched to the cases by sex and age at the ratio of 1:1. RA was definitely diagnosed according to 2010 classification criteria defined by the American College of Rheumatology/European League against Rheumatism.21 Synovial tissues were collected during knee surgery and then prepared as described previously,22 and peripheral blood samples were centrifuged at 2000 g for 10 min to obtain serums. The synovial tissues and serums were stored at −80°C until detection of the expression of miRNA-146a and miRNA-223. This study protocol was reviewed and permitted by the Ethics Committee of People’s Hospital of Xinjiang Uygur Autonomous Region (KY2021052605), and conducted in accordance with the guidelines of the Declaration of Helsinki. All patients provided written informed consents.
Detection of miRNA-146a and miRNA-223 Expression Levels
The TRIzol kit (Invitrogen Life Technologies, Carlsbad, CA, USA) was employed to extract total RNA from synovial tissues and serums. The concentration, purity and integrality of the total RNA were evaluated by agarose gel electrophoresis and ultraviolet spectrophotometry. The total RNA was reversely transcribed into cDNA with the miRNA First Strand cDNA Synthesis Tailing Reaction Kit (Sangon Biotech, Shanghai, China). Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) was used to measure miRNA-146a and miRNA-223 expression levels with TaqMan miRNA assay kits (Applied Biosystems, Foster City, CA, USA) on a ABI 7900 Fast system (Applied Biosystems, Foster City, CA, USA). The 2–ΔΔCt method was used to evaluate miRNA-146a and miRNA-223 expression levels using U6 as the internal reference.
Statistical Analysis
Kolmogorov–Smirnov test was employed to evaluate the normality of miRNA-146a and miRNA-223 expression levels, and Student’s t-test was employed to compare their intergroup differences between case group and control group. The independent associations of miRNA-146a and miRNA-223 with RA were identified using binary logistic regression analysis. Receiver operating characteristic (ROC) curve was used to evaluate the values of miRNA-146a, miRNA-223 and their combination in predicting RA. Z test was used to compare area under curve (AUC). All of the above statistical analysis was carried out using the SPSS version 18.0 (SPSS Inc., USA), and significance was set at two sided P<0.05.
Results
MiRNA-146a and miRNA-223 Expression Levels
The expression levels of miRNA-146a and miRNA-223 in both synovial tissues and serums were statistically higher in cases than in controls, and the expression levels of miRNA-146a and miRNA-223 in serums were not statistically different from those in synovial tissues in both cases and controls (Figure 1).
 |
Figure 1 MiRNA-146a and miRNA-223 expression levels in synovial tissues and serums. *: P < 0.05, vs cases. |
Independent Associations of miRNA-146a and miRNA-223 with RA
Binary logistic regression analysis showed that the expression levels of miRNA-146a [odds ratio (OR): 2.258, 95% confidence interval (CI): 1.107–3.476, P<0.001] and miRNA-223 (OR: 3.201, 95% CI: 1.428–4.732, P<0.001) in synovial tissues were independently associated with RA, and the expression levels of miRNA-146a (OR: 2.249, 95% CI: 1.110–3.502, P<0.001) and miRNA-223 (OR: 3.189, 95% CI: 1.417–4.645, P<0.001) in serums were also independently associated with RA.
Predictive Values of miRNA-146a for RA
According to the ROC curves (Figure 2), the AUC of miRNA-146a in synovial tissues for the prediction of RA was 0.773 [standard error (SE): 0.041, 95% CI: 0.692–0.853, P<0.001], and the AUC of miRNA-146a in serums for the prediction of RA was 0.766 (SE: 0.041, 95% CI: 0.684–0.847, P<0.001). Z test demonstrated that their difference was not statistically significant (Z=0.121, P=0.825).
 |
Figure 2 ROC curves of miRNA-146a in synovial tissues and serums for predicting RA. Abbreviations: ROC, receiver operating characteristic; RA, rheumatoid arthritis. |
Predictive Values of miRNA-223 for RA
According to the ROC curves (Figure 3), the AUC of miRNA-223 in synovial tissues for the prediction of RA was 0.788 (SE: 0.039, 95% CI: 0.711–0.865, P<0.001), and the AUC of miRNA-223 in serums for the prediction of RA was 784 (SE: 0.039, 95% CI: 0.707–0.861, P<0.001). Z test demonstrated that their difference was not statistically significant (Z=0.0725, P=0.944).
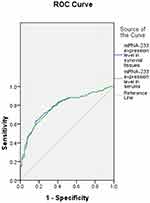 |
Figure 3 ROC curves of miRNA-233 in synovial tissues and serums for predicting RA. Abbreviations: ROC, receiver operating characteristic; RA, rheumatoid arthritis. |
Predictive Values of Combination of miRNA-146a and miRNA-223
In order to diagnose RA more accurately, the value of combination of miRNA-146a and miRNA-223 in predicting RA was further evaluated. The ROC curve of combination prediction was drawn with the probability derived from binary logistic regression analysis. The AUC of combination of miRNA-146a and miRNA-223 in synovial tissues (Figure 4) for the prediction of RA was 0.910 (SE: 0.026, 95% CI: 0.863–0.962, P<0.001), and the AUC of combination of miRNA-146a and miRNA-223 in serums (Figure 5) for the prediction of RA was 0.904 (SE: 0.027, 95% CI: 0.851–0.957, P<0.001). Z test demonstrated that their difference was not statistically significant (Z=0.160, P=0.873), but the AUC of combination prediction was statistically higher than those of individual predictions (synovial tissues: 0.910 vs 0.773, Z=2.822, P=0.005, 0.910 vs 0.788, Z=2.603, P=0.009; serums: 0.904 vs 0.766, Z=2.811, P=0.005, 0.904 vs 0.784, Z=2.530, P=0.011).
 |
Figure 4 ROC curves of miRNA-146a, miRNA-233 and their combination in synovial tissues for predicting RA. Abbreviations: ROC, receiver operating characteristic; RA, rheumatoid arthritis. |
 |
Figure 5 ROC curves of miRNA-146a, miRNA-233 and their combination in serums for predicting RA. Abbreviations: ROC, receiver operating characteristic; RA, rheumatoid arthritis. |
Discussion
MiRNAs have been demonstrated associations with cancer, autoimmune, neurodegenerative and viral diseases since their discovery.23 By binding the 3’-untranslated region (3’-UTR) of target messenger RNAs (mRNAs), miRNAs generally play a role in translational suppression or mRNA degradation, but they can also control the transcription rate. In addition, they can actually induce expression of gene under certain conditions and in specific cell types.24 Many studies have confirmed that dysregulated expression of miRNAs can affect immune regulation, enhance pro-inflammatory signal pathways, and cause the overexpression of pro-inflammatory cytokines in RA.14,25–28
MiRNA-146a can serve as a negative feedback factor during the activation of immune responses and therefore may participate in autoimmunity.12 MiRNA-146a correlates significantly with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values and functions to suppress T-regulatory cells.29,30 Furthermore, miRNA-146a can target TNFR-associated factor 6 (TRAF6), IL-1R-associated kinase 1 (IRAK1) and IRAK2, which are associated with regulation of the TNF-α signal pathway.31
The expression of miRNA-146a is significantly increased in the peripheral blood mononuclear cells and synovial fluid of RA patients compared with healthy and disease controls.16 Moreover, its level is positively associated with RA disease activity and TNF-α level.32,33 Multiple studies have also examined the association of miRNA-146a polymorphism rs2910164 with RA,11–17 and some of these studies have suggested that miRNA-146a polymorphism rs2910164 may be involved in the initiation and progression of RA. MiRNA-146a can halt excess inflammation via a negative feedback mechanism in RA.34,35 Although the expression of miRNA-146a is up-regulated in RA, it can not function properly, resulting in prolonged production of TNF-α.29 In our study, the expression level of miRNA-146a was significantly elevated in synovial tissues and serums of RA patients compared with controls, which is consistent with previous studies. Furthermore, multivariate analysis showed that the elevated expression level of miRNA-146a was independently associated with RA.
MiRNA-223 is significantly overexpressed in myeloid cells, including in macrophages and neutrophils,36 and has a critical role in the proliferation and differentiation of myeloid cells.37 The expression of miRNA-223 is significantly upregulated in synovial fluid, fibroblast-like synoviocytes and naive CD4+T lymphocytes of RA patients compared with healthy and disease (osteoarthritis) controls.18–20 Osteoclasts are associated with bone matrix erosion in the joints of RA patients. The irreversible bone damage can lead to severe pain and joint stiffness.
The up-regulation of miRNA-223 expression inhibits nuclear factor 1A (NF-1A), which up-regulates the expression of macrophage colony stimulating factor receptor (M-CSFR), an important modulator for osteoclast function and differentiation.37 In summary, miRNA-223 is implicated in osteoclastogenesis through its up-regulation in RA patients, which contributes to erosive disease. Li et al demonstrated that the expression of miR-223 was significantly up-regulated in the ankle joints of mice with collagen-induced arthritis (CIA) and in the synovium of RA patients compared with normal mice and OA patients.38 The mice with CIA showed the reduced miR-223 expression, histologic score, arthritis score, osteoclastogenesis and bone erosion after treated by lentiviral vectors expressing miR-223T (LVmiR-223T). This study demonstrated that lentivirus-mediated silencing of miR-223 could alleviate disease severity of experimental arthritis and suggested that inhibition of miR-223 activity might be a novel therapeutic target for RA. Our study also demonstrated that the expression level of miRNA-223 was significantly elevated in synovial tissues and serums of RA patients compared with controls. Furthermore, multivariate analysis showed that the elevated expression level of miRNA-223 was independently associated with RA.
In order to diagnose RA more accurately, we evaluated the values of miRNA-146a and miRNA-223 for diagnosing RA. ROC curves demonstrated that the predictive values of miRNA-146a and miRNA-223 in both synovial tissues and serums for diagnosing RA were moderate with AUCs of 0.766–0.788. We further evaluated the values of the combination of miRNA-146a and miRNA-223 in synovial tissues and serums for diagnosing RA, which demonstrated higher predictive values with AUCs of 0.910 and 0.904. Therefore, the combination of miRNA-146a and miRNA-223 could be applied in diagnosis of RA.
The limitations of this study mainly included two aspects. The first was a small sample size of RA patients, and the other was no further investigation on the mechanisms by which miRNA-146a and miRNA-223 caused RA. In the next, we will further investigate the pathogenesis of RA associated with miRNA-146a and miRNA-223.
Conclusion
MiRNA-146a and miRNA-223 in both synovial tissues and serums could be applied in predicting RA with moderate values, and their combination could elevate the predictive value significantly demonstrating a great potential for clinical application. In the next, experiments need to be performed to investigate their functions and explore the feasibility of serving as therapeutic targets.
Acknowledgments
This study was supported by Natural Science Foundation of Xinjiang Uygur Autonomous Region (Contract No.: 2021D01C137). The funders had no role in design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All the authors do not have any conflict of interest.
References
1. Almutairi K, Nossent J, Preen D, et al. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. 2021;41(5):863–877. doi:10.1007/s00296-020-04731-0
2. Radu AF, Bungau SG. Management of rheumatoid arthritis: an overview. Cells. 2021;10(11):2857. doi:10.3390/cells10112857
3. Umapathy S, Thulasi R, Gupta N, et al. Thermography and colour Doppler ultrasound: a potential complementary diagnostic tool in evaluation of rheumatoid arthritis in the knee region. Biomed Tech. 2020;65(3):289–299. doi:10.1515/bmt-2019-0051
4. Fujimura K, Haraguchi A, Sakurai R, et al. Have the radiographic characteristics of total knee arthroplasty recipients in rheumatoid arthritis changed after the induction of biologic disease modifying antirheumatic drugs? Mod Rheumatol. 2022;32(6):1047–1053. doi:10.1093/mr/roab114
5. Weyand CM, Goronzy JJ. The immunology of rheumatoid arthritis. Nat Immunol. 2021;22(1):10–18. doi:10.1038/s41590-020-00816-x
6. Chen J, Zeng S, Xue Q, et al. Photoacoustic image-guided biomimetic nanoparticles targeting rheumatoid arthritis. Proc Natl Acad Sci USA. 2022;119(43):e2213373119. doi:10.1073/pnas.2213373119
7. Ptb H, Clark IM, Le LTT. MicroRNA-based diagnosis and therapy. Int J Mol Sci. 2022;23(13):7167. doi:10.3390/ijms23137167
8. Kmiołek T, Paradowska-Gorycka A. miRNAs as biomarkers and possible therapeutic strategies in rheumatoid arthritis. Cells. 2022;11(3):452. doi:10.3390/cells11030452
9. Tavasolian F, Hosseini AZ, Soudi S, et al. miRNA-146a improves immunomodulatory effects of MSC-derived exosomes in rheumatoid arthritis. Curr Gene Ther. 2020;20(4):297–312. doi:10.2174/1566523220666200916120708
10. Condrat CE, Thompson DC, Barbu MG, et al. miRNAs as biomarkers in disease: latest findings regarding their role in diagnosis and prognosis. Cells. 2020;9(2):276. doi:10.3390/cells9020276
11. Ul Islam Z, Baneen U, Khaliq T, et al. Association analysis of miRNA-146a and miRNA-499 polymorphisms with rheumatoid arthritis: a case-control and trio-family study. Clin Exp Med. 2022. doi:10.1007/s10238-022-00916-y
12. Yang B, Zhang JL, Shi YY, et al. Association study of single nucleotide polymorphisms in pre-miRNA and rheumatoid arthritis in a Han Chinese population. Mol Biol Rep. 2011;38(8):4913–4919. doi:10.1007/s11033-010-0633-x
13. Zhou X, Zhu J, Zhang H, et al. Is the microRNA-146a (rs2910164) polymorphism associated with rheumatoid arthritis? Association of microRNA-146a (rs2910164) polymorphism and rheumatoid arthritis could depend on gender. Joint Bone Spine. 2015;82(3):166–171. doi:10.1016/j.jbspin.2014.12.009
14. Bogunia-Kubik K, Wysoczańska B, Piątek D, et al. Significance of polymorphism and expression of miR-146a and NFkB1 genetic variants in patients with rheumatoid arthritis. Arch Immunol Ther Exp. 2016;64(Suppl 1):131–136. doi:10.1007/s00005-016-0443-5
15. Ahmadi K, Soleimani A, Soleimani Motlagh S, et al. Polymorphisms of pre-miR-499 rs3746444 T/C and pre-miR-146a rs2910164 C/G in the autoimmune diseases of rheumatoid arthritis and systemic lupus erythematosus in the West of Iran. Iran J Public Health. 2020;49(4):782–790.
16. Shaker OG, El Boghdady NA, El Sayed AE. Association of MiRNA-146a, MiRNA-499, IRAK1 and PADI4 polymorphisms with rheumatoid arthritis in Egyptian population. Cell Physiol Biochem. 2018;46(6):2239–2249. doi:10.1159/000489592
17. Ayeldeen G, Nassar Y, Ahmed H, et al. Possible use of miRNAs-146a and −499 expression and their polymorphisms as diagnostic markers for rheumatoid arthritis. Mol Cell Biochem. 2018;449(1–2):145–156. doi:10.1007/s11010-018-3351-7
18. Sebastiani GD, Fulci V, Niccolini S, et al. Over-expression of miR-223 in T-lymphocytes of early rheumatoid arthritis patients. Clin Exp Rheumatol. 2011;29(6):1058–1059.
19. Dunaeva M, Blom J, Thurlings R, et al. Circulating serum miR-223-3p and miR-16-5p as possible biomarkers of early rheumatoid arthritis. Clin Exp Immunol. 2018;193(3):376–385. doi:10.1111/cei.13156
20. Churov AV, Oleinik EK, Knip M. MicroRNAs in rheumatoid arthritis: altered expression and diagnostic potential. Autoimmun Rev. 2015;14(11):1029–1037. doi:10.1016/j.autrev.2015.07.005
21. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi:10.1002/art.27584
22. Qu Y, Wu J, Deng JX, et al. MicroRNA-126 affects rheumatoid arthritis synovial fibroblast proliferation and apoptosis by targeting PIK3R2 and regulating PI3K-AKT signal pathway. Oncotarget. 2016;7(45):74217–74226. doi:10.18632/oncotarget.12487
23. Kabekkodu SP, Shukla V, Varghese VK, et al. Clustered miRNAs and their role in biological functions and diseases. Biol Rev Camb Philos Soc. 2018;93(4):1955–1986. doi:10.1111/brv.12428
24. Huang PY, Wu JG, Gu J, et al. Bioinformatics analysis of miRNA and mRNA expression profiles to reveal the key miRNAs and genes in osteoarthritis. J Orthop Surg Res. 2021;16(1):63. doi:10.1186/s13018-021-02201-2
25. Fu L, Jin L, Yan L, et al. Comprehensive review of genetic association studies and meta-analysis on miRNA polymorphisms and rheumatoid arthritis and systemic lupus erythematosus susceptibility. Hum Immunol. 2016;77(1):1–6. doi:10.1016/j.humimm.2014.09.002
26. Mo ML, Jiang JM, Long XP, et al. miR-144-3p aggravated cartilage injury in rheumatoid arthritis by regulating BMP2/PI3K/Akt axis. Mod Rheumatol. 2022;32(6):1064–1076. doi:10.1093/mr/roab105
27. Quero L, Tiaden AN, Hanser E, et al. miR-221-3p drives the shift of M2-macrophages to a pro-inflammatory function by suppressing JAK3/STAT3 activation. Front Immunol. 2020;10:3087. doi:10.3389/fimmu.2019.03087
28. Cuppen BV, Rossato M, Fritsch-Stork RD, et al; All SRU investigators. Can baseline serum microRNAs predict response to TNF-alpha inhibitors in rheumatoid arthritis? Arthritis Res Ther. 2016;18(1):189. doi:10.1186/s13075-016-1085-z
29. El-Bakry RM, Fadda SM, Mohamed RA, et al. Circulating miR-146a −5p in Egyptian patients with rheumatoid arthritis. Egypt J Immunol. 2022;29(1):29–38. doi:10.55133/eji.290104
30. Lu LF, Boldin MP, Chaudhry A, et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010;142(6):914–929. doi:10.1016/j.cell.2010.08.012
31. Jing X, Gong Z, Li F, et al. Effect of miRNA-146a-mediated TLR4 signal pathway on the pain of lumbar disc herniation. Cell Mol Biol. 2022;68(1):26–34. doi:10.14715/cmb/2022.68.1.5
32. Nakasa T, Shibuya H, Nagata Y, et al. The inhibitory effect of microRNA-146a expression on bone destruction in collagen-induced arthritis. Arthritis Rheum. 2011;63(6):1582–1590. doi:10.1002/art.30321
33. Duroux-Richard I, Presumey J, Courties G, et al. MicroRNAs as new player in rheumatoid arthritis. Joint Bone Spine. 2011;78(1):17–22. doi:10.1016/j.jbspin.2010.06.003
34. Taganov KD, Boldin MP, Chang KJ, et al. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103(33):12481–12486. doi:10.1073/pnas.0605298103
35. Zilahi E, Tarr T, Papp G, et al. Increased microRNA-146a/b, TRAF6 gene and decreased IRAK1 gene expressions in the peripheral mononuclear cells of patients with Sjögren’s syndrome. Immunol Lett. 2012;141(2):165–168. doi:10.1016/j.imlet.2011.09.006
36. Chen CZ, Li L, Lodish HF, et al. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303(5654):83–86. doi:10.1126/science.1091903
37. Sugatani T, Hruska KA. Impaired micro-RNA pathways diminish osteoclast differentiation and function. J Biol Chem. 2009;284(7):4667–4678. doi:10.1074/jbc.M805777200
38. Li YT, Chen SY, Wang CR, et al. Brief report: amelioration of collagen-induced arthritis in mice by lentivirus-mediated silencing of microRNA-223. Arthritis Rheum. 2012;64(10):3240–3245. doi:10.1002/art.34550
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
