Back to Journals » International Journal of General Medicine » Volume 15
Associations Between Total Sleep Duration and Cognitive Function Among Middle-Aged and Older Chinese Adults: Does Midday Napping Have an Effect on It?
Authors Wang Q , Zhu H, Dai R, Zhang T
Received 14 October 2021
Accepted for publication 10 January 2022
Published 10 February 2022 Volume 2022:15 Pages 1381—1391
DOI https://doi.org/10.2147/IJGM.S343286
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Qian Wang,1,2 Huanhuan Zhu,1 Ruiming Dai,1 Tiantian Zhang1
1School of Public Health, Fudan University, Shanghai, 200032, People’s Republic of China; 2Key Laboratory of Public Health Safety of the Ministry of Education and Key Laboratory of Health Technology Assessment of the Ministry of Health, Fudan University, Shanghai, 200032, People’s Republic of China
Correspondence: Tiantian Zhang, School of Public Health, Fudan University, 130 Dong’an Road, Xuhui District, Shanghai, 200032, People’s Republic of China, Email [email protected]
Aim: The effect of sleep duration on cognitive function has been reported. However, the studies about the combined effects of total sleep duration and midday napping on cognition in elders were limited and inconclusive. We aimed to investigate the associations between total sleep duration, midday napping and cognitive function among middle-aged and older Chinese adults.
Methods: Based on the 3rd wave of the China Health and Retirement Longitudinal Study (CHARLS) in 2015, a total of 9218 participants aged ≥ 45 years with completed cognition measurements were included. Cognitive functions were assessed by a combined global cognition score of episodic memory and mental status. Information about sleep-related variables, demographic characteristics, and health status were collected by validated questionnaires. Multivariate linear regression models were performed to evaluate the associations between total sleep duration, midday napping, and cognitive function. Stratified analyses were used to explore the potential effect modifier.
Results: Overall, the global cognition score was 10.38 ± 4.30 among the participants (mean age: 61.5 ± 8.7 years). For sleep duration, both short sleep and long sleep duration were significantly associated with the increased risk of cognitive impairment after controlling for demographics and other confounders. Compared with sleeping for 7h per day, the adjusted β-coefficient and 95% CI of the risk of cognitive impairment was − 0.967 (95% CIs: − 1.191, − 0.742) for ≤ 5h, − 0.257 (− 0.498, − 0.016) for 6h, − 0.424 (− 0.650, − 0.198) for 8h and − 0.664 (− 0.876, − 0.452) for ≥ 9h. The combined effect analysis indicated that subjects with extended or without naps had a significantly higher risk of cognitive impairment in the ≤ 5h sleep time group (ref: 7h) and in extended nappers or without naps group (ref: short nappers). Subjects with extended naps or without naps might increase the risk of cognitive impairment, especially those having ≤ 5h total sleep time. Stratified analysis showed that participants aged ≥ 60 years without social activity increased the risk of cognitive decline.
Conclusion: An inverted U-shaped association was observed between total sleep duration and cognitive function in Chinese elders, especially in those aged ≥ 60 years or without social activity. Short midday naps could mitigate the deleterious effects of poor sleep quality and shorter sleep duration on cognitive function. The findings could help us identify the vulnerable population and decrease the burden of cognitive impairment.
Keywords: cognitive function, sleep, midday napping, epidemiology, China
Introduction
Cognitive impairment, which refers to the problems with learning and memory, language, executive function, understanding judgment, etc., is one of the major health challenges facing most countries around the world.1–3 According to the World Health Organization reports, 10 million older Chinese adults will have dementia by 2050.4 Studies have indicated that cognitive impairment significantly increased the risk of functional dependence and poor life quality in older adults.5,6
As one of the largest developing countries of the world, China nowadays faces serious challenges of cognitive impairment among the elderly due to the population aging.7 An analysis showed that there had been approximately 31 million people with cognitive impairment in China,8 which posed a heavy burden on both individuals and society.2 Prior studies have identified sleep might be a potential determinant of cognitive function.9,10 Some studies found that insufficient sleep, even a few hours less, could cause decreased concentration,11 while excessive sleep might also increase the risk of cognitive impairment.12,13 For midday napping, Blackwell et al found that longer napping duration was associated with the increased risk of cognitive impairment,14 but other studies have found conversely beneficial effects of napping on cognitive function.15 Midday napping might also relate to negative night sleep outcomes including shorter sleep duration and frequent nocturnal awakening.16
Taken together, the average age of subjects, the measurement of sleep duration and sample size of the studies might contribute to these contradictory results. However, few studies have been conducted to investigate the combined effects of sleep duration and midday napping on cognitive performance. Therefore, it is crucial to explore and identify the role of midday napping in alleviating cognitive impairment in the Chinese elderly throughout the sleep period.
In this study, we aimed to investigate the associations between total sleep duration, midday napping and cognitive function among middle-aged and elders in China, by a nationwide population-based cross-sectional study.
Methods
Study Design and Participants
This study was based on the 3rd wave of the Chinese Health and Retirement Longitudinal Study (CHARLS) in 2015, a nationally representative survey conducted among Chinese community-dwelling residents aged 45 years and older and their spouses. The detailed study design and sampling methods for the CHARLS project are reported in the previous literature.17 To summarize, the nationwide baseline survey was conducted in 150 counties/districts and 450 villages/resident committees from 28 provinces of China from June 2011 to March 2012, and 17,708 respondents were included. The information about demographic characteristics, health status and function, family structure, biomarkers, and community-level data were collected in the CHARLS project.
In this study, we used the 3rd wave follow-up data from CHARLS, conducted in 2015 and involving 21,095 respondents. We excluded respondents aged less than 45 years and those who have not completed indicators of cognitive function test, sleep duration, and other covariates. Finally, a total of 9218 participants were included in the final analyses. The details about the participants enrolled in our study were clearly shown in the Flowchart (Figure 1).
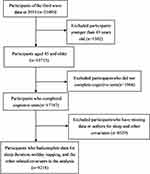 |
Figure 1 Flowchart of the participants included in this study. |
Ethics approval was obtained from the Biomedical Ethics Review Committee of Peking University (IRB00001052-11015). Written informed consents were obtained from each participant.
Measurement of Cognitive Function
Cognitive function was captured using two measures in line with previous work:18,19 the memory score and the mental status score. The memory score was measured by the immediate and delayed recall of 10 Chinese words that were read to the respondents. The immediate recall required respondents to immediately repeat ten Chinese nouns just read to them, and the delayed recall required them to repeat the same list of words 4 minutes later.20,21 The final score ranged from 0 to 10, calculated by averaging the immediate and delayed recall scores. The mental status score includes questions on orientation, vasoconstriction, and numeric ability. Orientation was assessed by asking respondents to name today’s date (month, day, year), and season, and the day of the week. Vasoconstriction was assessed by asking respondents to accurately redraw a previously shown picture. Numeric ability was assessed through the serial sevens test, which asks respondents to subtract 7 from 100 (up to 5 times), and whether additional explanation or aid such as a paper and pencil was needed to complete the task. The total score of mental status ranged from 0 to 11. The overall cognition score was the sum of the memory score and the mental status score, which ranged from 0 to 21.22
Sleep Duration and Midday Napping Variables
In this study, night sleep duration was assessed by the question “During the past month, how many hours of actual sleep did you get at night (average hours for one night)?” The duration of midday napping was assessed by the question, “During the past month, how long did you take a nap after lunch in general?” To measure the duration of napping, the participants were categorized into four napping groups: non-nappers (0 minutes), short nappers (≤30 minutes), moderate nappers (30–90 minutes), and extended nappers (>90 minutes).23
Total sleep duration can be obtained by the sum of night sleep duration and midday napping. In this study, total sleep duration was divided into five categories: ≤5 h, 6 h, 7 h, 8 h, and ≥9 h.24
Covariates
The demographic characteristics including age, gender, body mass index (BMI), waist circumstances (WC), residential locations (urban vs non-urban), marital status, education level as well as behavior factors including current smoking (yes vs no), current drinking (yes vs no) and social activities (yes vs.no, which assessed by asking respondents’ participation in social-related activities, such as interacting with friends or assisting others) were collected in this study.
The health-related factors including self-rated health (poor, fair, good), hypertension, diabetes, and depressive symptoms were also collected. Individuals with hypertension were assessed by the question of “Have you ever been diagnosed with hypertension by a physician?” Diabetes was defined by the question “Have you ever been diagnosed with diabetes by a physician?” Depressive symptoms were assessed by using the 10-item Center for Epidemiological Studies Depression Scale (CES-D-10)23,25 that contained 10 questions about the depressive feelings and behaviors of the respondents during the last week. Each item has 4 response options valued from 0 to 3 successively. The total score ranges from 0 to 30, with a higher score indicating a higher level of depressive symptoms. The prior survey has shown that a cutoff score ≥10 was used to identify the respondents who had significant depressive symptoms.25
Statistical Analysis
The cognitive function score, demographic characteristics, and health-related status were summarized in the subjects, and each subgroup was stratified by total sleep duration. Differences in demographic characteristics and potential covariables of cognitive function were analyzed using the Chi-square test for categorized variables and the one-way analysis of variance (ANOVA) or Kruskal–Wallis H-test for continuous variables.
Multivariable linear regression models were used to explore the associations between total sleep duration, midday napping, and cognitive function among middle-aged and older Chinese. The results are presented as β-coefficient and 95% Confidence intervals (95% CIs), using total sleep duration of 7 hours/night and midday napping of 1–30 minutes as the reference groups, which were based on previous studies that suggested that 7 hours of sleep and ≤30 minutes nap were beneficial.26 Both the crude model and adjusted models were fit to investigate the independent effect of total sleep duration, midday napping, and cognitive function among the subjects. The covariates included age, gender, BMI, WC, residential locations, marital status, education level, current smoking, current drinking, social activities, self-rated health, hypertension, diabetes, and depressive symptoms. To determine whether the independent effect of total sleep duration or midday napping on cognitive function, we further conducted crude and adjusted multivariable analysis of total sleep duration or midday napping stratified by subgroups on cognition score among the participants.
To examine the modification effects of several significant variables, stratified analyses were performed for gender (males vs females); age (<60 years vs ≥60 years); current smoking (yes vs no); current drinking (yes vs no); social activity (yes vs no). We statistically examined the differences in effects between the paired strata as follows:
where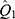 and
and 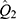 were the adjusted estimates in each stratum, and
were the adjusted estimates in each stratum, and 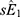 and
and 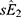 were the corresponding standard errors.27
were the corresponding standard errors.27
All the statistical analyses were carried out using SPSS software (version 24.0). A two-sided p-value <0.05 was considered statistically significant in this study.
Results
The demographic characteristics of all the participants and according to total sleep duration are summarized in Table 1. We evaluated 9218 participants aged ≥45 years from 28 provinces in China. The average age of the subjects was 61.5 ± 8.7 years, and nearly one-half of the participants were male. The percent of participants who reported sleeping ≤5h, 6h, 7h, 8h, and ≥9h per day were 22.0%, 15.0%, 17.8%, 19.1%, and 26.1%, respectively. Detailed characteristics of overall participants and according to total sleep duration groups are presented in Table 1.
 |
Table 1 Characteristics of the Participants in Different Subgroups Stratified by the Total Sleep Duration in the Study† [Mean ± SD/N (%)] |
According to the multivariable linear regression model, a U-shaped relationship was observed between total sleep duration and risk of cognitive impairment among the participants in our study. After controlling the confounders, we found that the adjustedβ-coefficient and 95% CI of cognitive function among the subjects were −1.132 (95% CI: −1.356, −0.908) (Model II) for ≤5h sleep duration per day, −0.291 (−0.533, −0.048) for 6h, −0.390 (−0.618, −0.612) for 8h and −0.612 (−0.825, −0.399) for ≥9 h when compared to those sleeping for 7h a day (Table 2). The results were robust when further adjusted for health-related status, hypertension, diabetes, and depressive symptoms (Model III) (Table 2). The trend analysis showed a significant dose-effect relationship among different subgroups stratified by total sleep duration (P-values for trend from 5 to 7 h and from 7 to 9 h < 0.001). For midday napping, we observed that both the extended napping time (−0.748 (−1.019,-0.478), P < 0.001, Model III) and those without midday napping (−0.741 (−0.972,-0.510), P < 0.001, Model III) were independently associated with the increased risk of cognitive impairment when compared to those with short napping times (Table S1).
 |
Table 2 Associations Between Total Sleep Duration and Cognitive Function Among the Participants in the Study |
The stratified analyses showed that the cognitive function score was higher in males and those aged younger than 60 years compared with females and those aged ≥60 years. And the cognitive score was generally highest in groups of a 7h total sleep duration (Figure 2, Figure S1). The adjusted regression models showed that the subjects aged ≥60 years had a significantly higher risk of cognitive impairment when total sleep duration was less than 5 hours compared with those aged younger than 60 years (P = 0.047) (Figure 3). We also found that the participants without social activity had a significantly higher risk of cognitive impairment than those having social activity when sleep duration was less than 5 hours (P = 0.031) (Figure 3). However, no significant differences were found between subgroups among current smoking or current drinking under different sleep duration (P was all >0.05) (Figure S2).
 |
Figure 2 Mean scores of cognitive functions with different total sleep duration in the participants stratified by age and gender. |
 |
Figure 3 Association between total sleep duration and cognitive function score stratified by gender, age and social activity. |
We further explore the combined effects of midday napping on cognitive function by stratified by different total sleep duration. The result showed that both the extended napping (≥90 min) and non-napping would increase the risk of cognitive impairment, especially in those sleep duration ≤5h. Compared with short nappers (0–30min), those with extended napping and without napping had a significantly higher risk of cognitive impairment by the adjusted β-coefficient and 95% CI of −1.688 (−2.639, −0.737) and −0.721 (−1.200, −0.242) in the group of ≤5h total sleep time, respectively. More details are shown in Table 3.
 |
Table 3 Associations Between Midday Napping and Cognitive Function in Different Subgroups Stratified by Total Sleep Duration † |
A sensitivity analysis was conducted to examine the robustness of the results. We excluded the participants with dementia from the analysis as previous studies did,28 because dementia is a consequence of cognitive impairment. The results showed that the relationship between total sleep time, midday napping, and cognitive function did not change materially (Tables S2 and S3).
Discussion
In this nationally representative population-based study, a U-shaped relationship was observed between total sleep duration and cognitive function among middle-aged and older Chinese adults. Compared with participants with 7h sleep per day, both short sleep (≤5h) and extended sleep duration (≥9h) were independently associated with the increased risk of cognitive impairment. The combined effect analyses indicated that subjects with short total sleep coupled with extended napping or no napping had a higher risk of cognitive impairment than those sleep for 7h with short midday napping. Stratified analyses showed that participants aged ≥60 years lack social activity had a significantly higher risk of cognitive impairment when having poor sleep duration.
Consistent with the previous studies, our studies suggested both short sleep and extended sleep duration were associated with the increased risk of cognitive function. A cross-sectional study conducted in Guangzhou, China showed that short or long sleep duration was an important sleep-related factor, which was independently associated with memory impairment in older people.29 Moreover, a cohort study that included 15,385 female nurses aged ≥70 years indicated that participants sleeping ≤5 hours/day or ≥9 hours/day had worse global cognition than those sleeping 7 hours/day, and the differences were equivalent to nearly two additional years of age.24 However, most of the studies explored the independent effect of total sleep duration on cognitive function without considering the role of midday napping. In this study, the combined effects of total sleep duration and midday napping showed that subjects with short sleep duration coupled with no napping or extended napping had a higher risk of cognitive impairment than those with regular sleep duration.
The possible reasons for either short or long sleep duration per day leading to the reduction of cognition can be explained as follows: firstly, insufficient sleep might contribute to neurodegeneration by promoting neuroinflammation and disrupting neurogenesis, especially in hippocampal areas, a key neuroanatomical region for memory.30 Secondly, excess sleep duration might be an initial symptom of an undetected subclinical neurodegenerative disease. Evidence has been reported that a substantial amount of Ab begins to accumulate before an individual is diagnosed with mild cognitive impairment. Inflammatory cytokines such as C-reactive protein and interleukin-6 induced by these pathologic proteins are associated with increased habitual sleep duration, which might explain our findings.31–33 Besides, there are many factors related to extended sleep duration, such as sleep-disordered breathing (SDB), old age, various chronic diseases, sleep fragmentation, etc., which might be related to the decline of cognitive function.12
In our study, we observed that participants aged ≥60 years, and those who lacked social activity had a significantly higher risk of cognitive impairment when total sleep duration was ≤5 hours. A prior observational study showed that older age could increase the risk of mild cognitive impairment among older adults.34 With age, the number of dopaminergic receptors declines; many brain structures show volumetric shrinkage; white matter becomes less dense; and brains of even very high functioning individuals are frequently characterized by destructive neurofibrillary plaques and tangles,35 which lead to cognitive impairment. For social activity, many studies have supported that it could affect cognitive abilities by increasing the complexity of the social environment or even directly affecting the deposition of amyloid beta-protein (Aβ) that makes up the amyloid plaques in Alzheimer’s disease.36,37 Consistent with previous studies, we found that those with current smoking or alcohol consumption status have a higher risk of cognitive impairment when having short or extended sleep compared to those having 7h of total sleep duration. Studies showed that cigarette smoke has numerous cytotoxic chemicals like carbon monoxide, nitrosamines, aryl hydrocarbon, phenolic, and polynuclear aromatic compounds.38 It is known to cause neuroendocrine alteration and initiation of the neurodegenerative process.39 For alcohol consumption, prior evidence indicates that alcohol inhibits glutamate receptors, which could damage the brain and cognition due to thiamine deficiency. Deficiency of thiamine has been linked to oxidative stress, excitotoxicity, inflammatory responses, dysfunction of the blood–brain barrier, and lactic acidosis. Ethanol could also be directly neurotoxic.40
For the relationship between midday napping and cognitive function, we found that a short midday napping could help individuals get better cognition ability. This result was consistent with some previous studies. Appropriate midday napping was a benefit to cognitive function, but extended midday napping might be harmful to cognitive function.23,41 A study performed among 3037 older adults in China showed that intermediate afternoon naps were beneficial for cognition, while long afternoon naps were detrimental.41 In our study, the combined effect analyses indicated that those who have too long sleep at noon but too short sleep at night may be related to worse cognition due to the irregular biological bell.
The possible reason for intentional daytime napping limited to a certain duration may benefit cognition by increasing total sleep time42 per day or avoiding and reducing daytime sleepiness.43 However, unintentional dozing or uncontrollable napping may be a sign of dementia, rather than a good habit for better health. Some studies have indicated that daytime sleepiness is associated with cognitive impairment and dementia.12,44 The mechanism may be that daytime sleepiness may be caused by obstructive sleep apnea syndrome and other organic or mental pathologic condition, which has been shown to associate with cognitive impairment by leading to repeated anoxia and the cerebral white matter change.28 Another possible explanation is that daytime sleepiness has been associated with metabolic disorders, such as obesity or type 2 diabetes, which may increase the risk for cognitive impairment.45,46
To our knowledge, this was one of the few cross-sectional studies to examine the associations between total sleep duration, midday napping, and cognitive function among middle-aged and older Chinese adults. Furthermore, the nationally representative sample of Chinese adults was used in our study, which presented more convincing results with adequate statistical power. Thirdly, the stratified analysis could help us to identify the potentially vulnerable characteristics of cognitive impairment among the participants.
This study has several limitations. First, the same as all other cross-sectional studies, we cannot establish a causal relationship between sleep duration and cognitive function. Second, the information about the sleep duration was based on self-reported, which may introduce recall bias. However, it is hard to make an accurate assessment for each participant in such a large-scale study. Third, some potential confounding factors such as sleep quality and sleep-disordered breathing might be existed, for which this information was not available in the CHARLS study. For all these aforementioned limitations, the findings should be interpreted with caution before the extension to other populations and research.
Conclusions
This nationwide cross-sectional study suggested that a U-shaped relationship between total sleep duration and cognitive impairment among middle-aged and older Chinese adults. Shorter sleep duration or extended midday napping significantly increased the risk of cognitive impairment, especially in those aged ≥60 years and without social activity. Appropriate napping time (1–30min) could mitigate the adverse effects of poor sleep on cognition. The findings of the study strengthen the rationale to keep proper total sleep time per day, as well as arrange napping time properly to maintain better cognition.
Abbreviations
CHARLS, Chinese Health and Retirement Longitudinal Study; BMI, Body Mass Index; WC, Waist Circumstance; 95% CI, 95% Confidence intervals.
Ethics Approval
Ethics approval was obtained from the Biomedical Ethics Review Committee of Peking University (IRB00001052-11015).
Informed Consent
Written informed consents were obtained from each participant in our study.
Acknowledgments
The authors thank Jialin Meng and Zhenkai Ma for participating in revising the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (grant no.71874033) and the Key Project of Philosophy and Social Science Research of the Ministry of Education (grant no.15JZD029).
Disclosure
The authors report no conflicts of interest for this work and declare no competing financial interest.
References
1. Jin J. Screening for Cognitive Impairment in Older Adults. JAMA. 2020;323(8):800. doi:10.1001/jama.2020.0583
2. Patnode CD, Perdue LA, Rossom RC, et al. Screening for cognitive impairment in older adults: updated evidence report and systematic review for the US preventive services task force. JAMA. 2020;323(8):764–785. doi:10.1001/jama.2019.22258
3. Centers for Disease Control and Prevention. Cognitive impairment: the impact on health in Iowa; 2011.
4. Shifu Xiao ML, Mellor D, McCabe M, et al. The China longitudinal ageing study: overview of the demographic, psychosocial and cognitive data of the Shanghai sample. J Mental Health. 2016;25(2):1–6. doi:10.3109/09638237.2015.1124406
5. Birren JE, Lubben JE, Rowe JC, Deutchman DE, eds. The Concept and Measurement of Quality of Life in the Frail Elderly. San Diego. CA: Academic Press; 2014.
6. Bolandzadeh N, Kording K, Salowitz N, et al. Predicting cognitive function from clinical measures of physical function and health status in older adults. PLoS One. 2015;10(3):e0119075. doi:10.1371/journal.pone.0119075
7. Xiaoliang Zhou TL. The influence of the elderly population on economic growth in China. Singap Econ Rev. 2019;11:1–7.
8. Xue J, Li J, Liang J, Chen S. The prevalence of mild cognitive impairment in China a systematic review. Aging Dis. 2018;9(4):706–715. doi:10.14336/AD.2017.0928
9. Amer S, Akkad RME, Hassan S. Hassan is cognition a determinant of health related quality of life in community dwelling non demented elderly? Adv Aging Res. 2014;03(5):339–348. doi:10.4236/aar.2014.35044
10. Steiger A, Dresler M, Schussler P, Kluge M. Ghrelin in mental health, sleep, memory. Mol Cell Endocrinol. 2011;340(1):88–96. doi:10.1016/j.mce.2011.02.013
11. Jackson ML, Gunzelmann G, Whitney P, et al. Deconstructing and reconstructing cognitive performance in sleep deprivation. Sleep Med Rev. 2013;17(3):215–225. doi:10.1016/j.smrv.2012.06.007
12. Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162:201–208. doi:10.1001/archinte.162.2.201
13. Joo EY, Kim H, Suh S, Hong SB. Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep. 2014;37(7):1189–1198. doi:10.5665/sleep.3836
14. Blackwell T, Yaffe K, Ancoli-Israel S, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011;34(10):1347–1356. doi:10.5665/SLEEP.1276
15. Keage HA, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 2012;13(7):886–892. doi:10.1016/j.sleep.2012.02.003
16. Monk TH, Buysse J, Carrier J, Billy BD. Effects of afternoon “siesta” naps on sleep, alertness, performance, and circadian rhythms in the elderly. Sleep. 2001;24(6):680–687. doi:10.1093/sleep/24.6.680
17. Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2012;1(1).
18. Lei X, Smith JP, Sun X, et al. Gender differences in cognition in China and reasons for change over time: evidence from CHARLS. J Econ Ageing. 2014;4:46–55. doi:10.1016/j.jeoa.2013.11.001
19. Pan X, Luo Y, Roberts AR. Secondhand smoke and women’s cognitive function in China. Am J Epidemiol. 2018;187(5):911–918. doi:10.1093/aje/kwx377
20. Crimmins EM, Kim JK, Langa,KM, et al. Assessment of cognition using surveys and neuropsychological assessment: the health and retirement study and the aging, demographics, and memory study. J Gerontol B Psychol Sci Soc Sci. 2011;66(1):162–171. doi:10.1093/geronb/gbr048
21. Bender AC, Austin AM, Grodstein F, et al. Executive function, episodic memory, and medicare expenditures. Alzheimers Dement. 2017;13(7):792–800. doi:10.1016/j.jalz.2016.12.013
22. Lei X, Hu Y, McArdle,JJ, et al. Gender differences in cognition among older adults in China. J Hum Resour. 2012;47(4):951–971. doi:10.3368/jhr.47.4.951
23. Li J, Cacchione PZ, Hodgson N, et al. Afternoon napping and cognition in Chinese older adults: findings from the China health and retirement longitudinal study baseline assessment. J Am Geriatr Soc. 2017;65(2):373–380. doi:10.1111/jgs.14368
24. Devore EE, Grodstein F, Duffy JF, et al. Sleep duration in midlife and later life in relation to cognition. J Am Geriatr Soc. 2014;62(6):1073–1081. doi:10.1111/jgs.12790
25. Andresen EM, Malmgren JA, Carter,WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D. Am J Prev Med. 1994;10:77–84. doi:10.1016/S0749-3797(18)30622-6
26. Yamada T, Hara K, Shojima N, Yamauchi T, Kadowaki T. Daytime napping and the risk of cardiovascular disease and all-cause mortality: a prospective study and dose-response meta-analysis. Sleep. 2015;38(12):1945–1953. doi:10.5665/sleep.5246
27. Chen R, Kan H, Chen B, et al. Association of particulate air pollution with daily mortality: the China air pollution and health effects study. Am J Epidemiol. 2012;175(11):1173–1181. doi:10.1093/aje/kwr425
28. Reitz C, Luchsinger J, Tang MX, Mayeux R. Effect of smoking and time on cognitive function in the elderly without dementia. Neurology. 2005;65(6):870–875. doi:10.1212/01.wnl.0000176057.22827.b7
29. Xu L, Jiang CQ, Lam T, et al. Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Biobank Cohort Study. Sleep. 2011;34(5):575–580. doi:10.1093/sleep/34.5.575
30. Meerlo P, Mistlberger RE, Jacobs BL, Craig Heller H, McGinty D. New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med Rev. 2009;13:187–194. doi:10.1016/j.smrv.2008.07.004
31. Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi:10.1016/S1474-4422(12)70291-0
32. Irwin MR, Olmstead R. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80:40–52. doi:10.1016/j.biopsych.2015.05.014
33. Suh SW, Han JW, Lee JR, et al. Sleep and cognitive decline: a prospective non-demented elderly cohort study. Ann Neurol. 2018;83(1):472–482. doi:10.1002/ana.25166
34. Gallagher D, Kiss A, Lanctot KL, Herrmann N. Toward prevention of mild cognitive impairment in older adults with depression: an observational study of potentially modifiable risk factors. J Clin Psychiatry. 2018;80(1). doi:10.4088/JCP.18m12331
35. Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi:10.1146/annurev.psych.59.103006.093656
36. Hughes TF, Flatt JD, Fu B, Chang CC, Ganguli M. Engagement in social activities and progression from mild to severe cognitive impairment: the MYHAT study. Int Psychogeriatr. 2013;25(4):587–595. doi:10.1017/S1041610212002086
37. Landau SM, Marks SM, Mormino EC, et al. Association of lifetime cognitive engagement and low beta-amyloid deposition. Arch Neurol. 2012;69(5):623–629. doi:10.1001/archneurol.2011.2748
38. Vu AT, Taylor KM, Holman MR, Ding YS, Hearn B, Watson CH. Polycyclic aromatic hydrocarbons in the mainstream smoke of popular U.S. cigarettes. Chem Res Toxicol. 2015;28(8):1616–1626. doi:10.1021/acs.chemrestox.5b00190
39. Vercruysse P, Vieau D, Blum D, Petersen A, Dupuis L. Hypothalamic alterations in neurodegenerative diseases and their relation to abnormal energy metabolism. Front Mol Neurosci. 2018;11:2. doi:10.3389/fnmol.2018.00002
40. Topiwala A, Ebmeier KP. Effects of drinking on late-life brain and cognition. Evid Based Ment Health. 2018;21(1):12–15. doi:10.1136/eb-2017-102820
41. Li J, Chang YP, Riegel B, et al. Intermediate, but not extended, afternoon naps may preserve cognition in Chinese older adults. J Gerontol. 2017;73(3):360–366. doi:10.1093/gerona/glx069
42. Campbell SS, Stanchina MD, Schlang JR, Murphy PJ. Effects of a month-long napping regimen in older individuals. J Am Geriatr Soc. 2011;59(2):224–232. doi:10.1111/j.1532-5415.2010.03264.x
43. Lovato N, Lack L. The effects of napping on cognitive functioning. Prog Brain Res. 2010;185:155–166.
44. Foley D, Monjan A, Masaki K, et al. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc. 2001;49(12). doi:10.1111/j.1532-5415.2001.49271.x
45. Etgen T, Bickel H, Forstl H. Metabolic and endocrine factors in mild cognitive impairment. Ageing Res Rev. 2010;9(3):280–288. doi:10.1016/j.arr.2010.01.003
46. Yaffe K, Weston AL, Blackwell T, Krueger KA. The metabolic syndrome and development of cognitive impairment among older women. Arch Neurol. 2009;66(3):324–328. doi:10.1001/archneurol.2008.566
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.