Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Associations Between Physical Activity, Smoking Status, and Airflow Obstruction and Self-Reported COPD: A Population-Based Study
Authors Wu YK, Su WL, Yang MC, Chen SY, Wu CW, Lan CC
Received 4 September 2021
Accepted for publication 1 May 2022
Published 20 May 2022 Volume 2022:17 Pages 1195—1204
DOI https://doi.org/10.2147/COPD.S337683
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Yao-Kuang Wu,1,2 Wen-Lin Su,1,2 Mei-Chen Yang,1,2 Sin-Yi Chen,1 Chih-Wei Wu,1 Chou-Chin Lan1,2
1Division of Pulmonary Medicine, Department of Internal Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan; 2School of Medicine, Tzu-Chi University, Hualien, Taiwan
Correspondence: Chou-Chin Lan, Division of Pulmonary Medicine, Department of Internal Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, No. 289, Jianguo Road, Xindian Dist., New Taipei City, Taiwan, Tel +886-2-66289779 ext 5709, Fax +886-2-66289009, Email [email protected]
Background: Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease with an increased mortality rate in recent years, mainly caused by exposure to tobacco smoke. Regular physical activity is thought to diminish the risk of COPD exacerbation, while very few studies investigate the interaction between smoking and physical activity on COPD development. This study aims to investigate the association between smoking status, physical activity and prevalent COPD.
Methods: This study analyzed data of adults 20 to 79 years old from the National Health and Nutrition Examination Survey (NHANES) 2007– 2012.
Results: A total of 6404 participants aged 20– 79 were included and divided into four groups by their physical activity levels and smoking status. Amongst, 2819 (43.7%) were physically active non-smokers, 957 (14.8%) were physically inactive non-smokers, 1952 (30.3%) were physically active smokers, and 717 (11.1%) were physically inactive smokers. Prevalence of airflow obstruction were 5.7%, 7.1%, 17.7% and 18.6%, respectively. After adjustment, physically active smokers (aOR=2.71, 95% CI=1.94– 3.80) and physically inactive smokers (aOR=2.70, 95% CI=1.78– 4.09) but not physically active non-smokers were more likely to have airflow obstruction than physically active non-smokers. These associations were similar among most subgroups by age, sex, or BMI. Among smokers, being physically inactive was not significantly associated with a greater chance for prevalent airflow obstruction than being physically active.
Conclusion: Smokers, regardless of their physical activity level, are more likely to have airflow obstruction as compared with physically active non-smokers. Within smokers, being physically inactive poses no excess chance to be airflow obstructed. The findings indicate that physical activity level seem not altering the relationship between smoking and airflow obstruction.
Keywords: chronic obstructive pulmonary disease, smoking, physical activity, airflow obstruction
Introduction
Chronic obstructive pulmonary disease (COPD) is a common preventable and treatable disease, characterized by persistent airflow limitation that is usually progressive and associated with an aggravated chronic inflammatory response in the airways and the lung to noxious particles or gases.1 The main symptoms of COPD are breathlessness, cough, and sputum production.
There are 251 million cases of COPD globally.2 An estimate by the Global Burden of Diseases Study (GBD) 2017 reports, 3.2 million people died from COPD worldwide in 2015, an increase of 11.6% compared with 1990.3 The goals of COPD assessment are to determine the level of airflow limitation, its impact on the patient’s health status, and the risk of future events (such as exacerbations, hospital admissions, or death). Airflow limitation severity in COPD can be defined and classified according to the FEV1 and FEV1/FVC ratios.4
The primary cause of COPD is exposure to tobacco smoke (either active smoking or second-hand smoke).5–7 Smokers often continue to cough, experience chest tightness and usually have a higher mortality rate.8 Some studies suggest that regular physical activity reduces the risk of COPD exacerbation.9,10 Smoking and physical inactivity are known risk factors for many chronic lung diseases, and of course, COPD is no exception.11–13 However, evidence on the interaction between smoking status and physical activity level in association with COPD and airflow obstruction are relatively scarce.
This study aims to investigate the association between smoking status, physical activity level, and prevalent airflow obstruction and COPD, using data from the National Health and Nutrition Examination Survey (NHANES) of the US.
Methods
Data Source
Data of participants from the National Health and Nutrition Examination Survey (NHANES) 2007–2012 were used for this cross-sectional analysis. The NHANES program initiated since the early 1960s and has been guided as a series of surveys focusing on different health topics. The selected samples for the NHANES survey represented the United States population. Further detail information such as background, design, and operation are available on the NHANES website (https://www.cdc.gov/nchs/nhanes/about_nhanes.htm).
Ethics Statement
Due to de-identification of participants in the NHANES database, and all participants in NHANES have written and signed the informed consent, consistent with and deemed by the National Center for Health Statistics Institutional Review Board (NCHSIRB) (Protocol #98-12), the IRB of the study hospital waived both IRB review and informed consent by the participants for the present study.
Study Subjects
Data of NHANES adult participants 20 to 79 years old with complete data of pre-bronchodilator spirometry were included. Exclusion criteria were: 1) Participants whose data of self-reported physical activity were missing; 2) participants whose data of smoking status were missing; 3) participants whose data of pre-bronchodilator spirometry were missing; 4) participants with end-stage renal disease defined by an eGFR <15 mL/min per 1.73 m2; 5) participants with a history of any malignancy; 6) participants with asthma diagnosed by physician.
Study Variables
Measurement of Airway Obstruction
In this study, airway obstruction was defined by both doctor-diagnosed chronic bronchitis or emphysema, or a FEV1/FVC <70% or FEV1/FVC < the lower 5th percentile (ie, the lower limit of normal, LLN) measured by pre-bronchodilator spirometry. This approach to define airway obstruction was utilized and validated by previous NHANES studies.14 With regard to physician-diagnosed chronic bronchitis or emphysema, participants were asked two questions on emphysema or chronic bronchitis during the in-home interview: “Has a doctor ever told you that you have emphysema?” or “Do you still have chronic bronchitis?”.15
Demographic and Lifestyle Factors
Age, gender, race, education level (college or above; never attend college), and family income (not poor; poor) were extracted from the NHANES data. Data of smoking status, physical activity level, and body mass index (BMI) were collected by trained interviewers, as follows:
- Smokers were categorized as:
- Non-smokers: reported never having smoked 100 cigarettes during their lifetime.
- Current smokers: had smoked ≥ 100 cigarettes and with no intention to quit smoking at the time of the interview.
- Physical activity: To estimate physical activity level, we summed the product of weekly time spent in each leisure-time activity reported by the participant multiplied by the metabolic equivalent of task (MET) value for that activity yielding a MET-min/week index. One MET is the energy expenditure of 1 kcal/kg body weight per hour. A MET-min/week ≥500 was regarded as physically active, whereas a MET-min/week <500 was regarded as physically inactive.16,17
- Body mass index (BMI): This value was calculated during participants’ physical examinations at the NHANES MEC. According to the World Health Organization (WHO) criteria,18 BMI data were classified into four subgroups: underweight, BMI<18.5 kg/m2, normal (BMI = 18.5~24.9 kg/m2), overweight (BMI = 25~29.9 kg/m2), and obese (BMI ≥ 30.0 kg/m2).
Comorbidities
Comorbidities were defined using interviewer-administered questionnaires of NHANES by the question “Have you ever been told by a doctor or other health professional that you had … ?” In the present study, we included diabetes, hypertension sleep apnea, dyslipidemia, cardiovascular disease, and chronic kidney diseases.
Statistical Analysis
All analyses were performed by using SAS survey analysis procedures to generate nationally representative estimates (SAS Institute Inc., Cary, NC, USA). Subject’s characteristics were expressed as unweighted count and weighted percentage for categorical variables, and continuous variables were expressed as mean ± standard error (SE). Differences in means between groups were compared by using SURVEYREG procedure for continuous variables, while Rao-Scott chi-square test was performed to examine the differences in the proportions between groups by using SURVEYFREQ procedure for categorical variables. Univariate and multivariate logistic regression were performed to examine the associations of study variables and the presence of airway obstruction. Variables with p-value < 0.05 in the univariate analysis were considered as potential confounding factors and were entered in the multivariate models. We used SURVEYLOGISTIC to estimate OR and 95% confidence interval (CI) in multivariate analysis. A two-tailed P value < 0.05 was considered significant.
Results
Characteristics of Study Subjects
Figure 1 depicts the flow diagram of cohort selection. Among a total of 30,442 participants whose data were collected in NHANES (2007–2012), 13,387 adults aged 20 to 79 years with complete data of pre-bronchodilator spirometry were identified. After excluding participants with missing information on physical activity level (n=4994), smoking status (n=3), eGFR (n=448), and an eGFR <15 mL/min per 1.73 m2 (n=9), history of malignancy (n=522) and physician-diagnosed asthma (n=966), the final cohort size was 6445.
 |
Figure 1 Flowchart. |
Characteristic of the Study Population
Table 1 shows the characteristics of the study cohort. Using the NHANES sample weight formulae, this analytic sample size was equivalent to a population size of 92,372,486 in the US. The included subjects were categorized into four groups according to their physical activity level and smoking status. Among the 6445 subjects, 2819 (43.7%) were physically active non-smokers, 957 (14.8%) were physically inactive non-smokers, 1952 (30.3%) were physically active smokers, and 717 (11.1%) were physically inactive smokers. The prevalence of airflow obstruction for physically active non-smokers, physically inactive non-smokers, physically active smokers, and physically inactive smokers were 5.7%, 7.1%, 17.7% and 18.6%, respectively, and there were statistically significant differences between the four groups (p<0.001). Mean age of the subjects was 42.7 ± 0.4 years old. In addition, there were significant differences in age, gender, race, education level, BMI, and frequencies of all comorbidities between the four groups (all p<0.001), except for chronic kidney disease (Table 1).
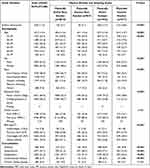 |
Table 1 Characteristics of Study Population 20–79 Years Old |
Associations Between Airflow Obstruction and Study Variables
Univariate and multivariate logistic regressions were conducted to determine the associations between airway obstructions, physical activity and smoking status, and the other study variables. The results are shown in Table 2. In the univariate analysis, physically active smokers (odds ratio [OR]=3.54, 95% CI=2.55–4.91) and physically inactive smokers (OR=3.74, 95% CI=2.57–5.45) but not physically inactive non-smokers (OR=1.25, 95% CI=0.78, 1.99) had significantly greater odds for having airflow obstruction as compared with physically active non-smokers. After adjustment in the multivariate analysis, physically active smokers (aOR=2.71, 95% CI=1.94–3.80) and physically inactive smokers (aOR=2.70, 95% CI=1.78–4.09) were still significantly more likely to have airway obstruction than physically active non-smokers, whereas physically inactive non-smokers were not associated with an increased chance for having airflow obstruction than physically active non-smoker (Table 2).
 |
Table 2 Associations Between Airflow Obstruction and Study Variables |
Associations Between Airflow Obstruction and Study Variables in Smokers
A subgroup analysis upon smokers was performed, and the results are summarized in Table 3. The odds for airway obstruction among physically inactive smokers comparing with physically active ones was not significant in either univariate or multivariate analysis (OR=1.06, 95% CI=0.79–1.41, aOR=0.96, 95% CI=0.70–1.31).
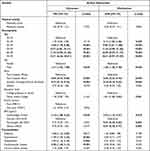 |
Table 3 Associations Between Study Variables in Smokers |
Associations Between Physical Activity, Smoking Status, and Airflow Obstruction Stratified by Age, Sex and BMI
In stratified analyses, similarly, physically active smokers and physically inactive smokers had significantly greater OR for airflow obstruction than that of physically active non-smokers regardless of age <60 or ≥60, male or female sex, and with overweight/obese or not after adjustments. Physically inactive non-smoker had a significantly greater chance for having airflow obstruction than physically active non-smoker only among subjects with a normal BMI, but not among other subgroups (aOR=2.46, 95% CI=1.20–5.05) (Table 4).
 |
Table 4 Associations Between Physical Activity and Smoking Status and Airflow Obstruction Stratified by Age, Sex, and BMI |
Discussion
The present cross-sectional study queried the interaction between physical activity level and smoking status in association with prevalent airflow obstruction or self-reported COPD. The results suggested that smokers, regardless physically active or not, were about three times more likely to be airflow obstructed than non-smokers with high physical activity. The association remained similar among subjects <60 or ≥60 years, male or female sex, and subjects of normal weight or overweight/obesity. Within smokers, particularly, low physical activity did not pose a greater chance for being airflow obstructed as compared with high physical activity.
Previous studies had revealed that physical activity is associated with reduced pulmonary function decline and COPD risk and may achieve the ultimate goal of pulmonary rehabilitation of smokers.19–21 In addition, extreme inactivity aggravated lung inflammation and emphysema among smokers.22 From these findings, it is speculated that higher physical activity might attenuate the risk for lung functional decline or COPD development that posed by smoking.
However, there were concerns that the protective effect of high physical activity on lung function levels among active smokers suggested in previous longitudinal studies is due to a reverse causation.21,23 A previous study suggested a bi-directional causation and support a true protective effect of physical activity on lung function in smokers, after accounting for reverse causation and time-dependent confounding.24
In addition, a prior prospective study that included 2966 initially healthy participants from English Longitudinal Study of Ageing reported that remaining physically active or becoming active in older age is beneficial in lung function and is associated with reduced odds of abnormal lung function.25 Another large-scale study using data from the Canadian Longitudinal Study on Aging suggested concluded that replacing sitting time with physical activity leads to improvements in lung function among adults with an obstructive lung disease, as well as among those without a respiratory disease26. Taking together, these studies have indicated a protective effect of higher physical activity on lung function both in healthy subjects and subjects with COPD, smokers and non-smokers.
In contrast, in the present cross-sectional study, we found that the chance for having airflow obstruction among smokers was similar between low or high physical activity, doubting the protective effect reported in the medical literature. In the stratified analyses, we have compared different physical activity level in combinations with different smoking status on the odds for airflow obstruction. Furthermore, in non-smokers, no significant different chance for airflow obstruction was observed when comparing low to high physical activity among different age or sex. However, in non-smokers, subjects with physically activity levels did have significant different chance for airflow obstruction in those of normal BMI. Future longitudinal studies are warranted to confirm this interesting finding.
Nevertheless, the present study has several limitations. Firstly, the analysis was of cross-sectional design, thus causal inferences cannot be made. Secondly, although spirometry data were utilized, part of the cases were identified upon individual’s answers to the questionnaires, which inaccurate reporting or recall bias might exist. Information of pack-year of cigarette smoking was not identified and analyzed. Physical activity level (MET index) was calculated from the subjective response to the questionnaires on leisure time activity, not measured by accelerometry, which also might contain information bias. Lastly, there might have been unknown sociodemographic confounders not included in the NHANES dataset.
Conclusion
Smokers, regardless of their physical activity level, are more likely to have airflow obstruction than physically active non-smokers. Within smokers, being physically inactive poses no excess chance to be airflow obstructed. The findings indicate that physical activity level seem not altering the relationship between smoking and airflow obstruction in most cases.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ratomaharo J, Linares Perdomo O, Collingridge D, et al. A49 ISSUES IN COPD: epidemiological and clinical aspects of COPD in malagasy patients using the Malagasy Spirometric Reference Values (Msre). In: American Journal of Respiratory and Critical Care Medicine. Vol. 195. American Thoracic Society; 2017:A1757–A1757.
2. All Answers Ltd.. Pathophysiology of Chronic Obstructive Pulmonary Disease (COPD); 2018. Available from: https://nursinganswers.net/assignments/pathophysiology-of-chronic-obstructive-pulmonary-disease-copd.php?vref=1.
3. Viegi G, Maio S, Fasola S, Baldacci S. Global burden of chronic respiratory diseases. J Aerosol Med Pulm Drug Deliv. 2020;33(4):171–177. doi:10.1089/jamp.2019.1576
4. Tejero E, Prats E, Casitas R, et al. Classification of airflow limitation based on z-score underestimates mortality in patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2017;196(3):298–305. doi:10.1164/rccm.201611-2265OC
5. World Health Organization. Gender, Women, and the Tobacco Epidemic. Samet JM, Yoon S-Y, editors. Geneva, Switzerland: World Health Organization; 2010. 249.
6. Oberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377(9760):139–146. doi:10.1016/S0140-6736(10)61388-8
7. Strzelak A, Ratajczak A, Adamiec A, Feleszko W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health. 2018;15(5):1033. doi:10.3390/ijerph15051033
8. Samet JM. Tobacco smoking: the leading cause of preventable disease worldwide. Thorac Surg Clin. 2013;23(2):103–112. doi:10.1016/j.thorsurg.2013.01.009
9. Benzo R, Vickers K, Novotny PJ, et al. Health coaching and chronic obstructive pulmonary disease rehospitalization. A Randomized Study. Am J Respir Crit Care Med. 2016;194(6):672–680. doi:10.1164/rccm.201512-2503OC
10. Jenkins AR, Gowler H, Curtis F, Holden NS, Bridle C, Jones AW. Efficacy of supervised maintenance exercise following pulmonary rehabilitation on health care use: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:257–273. doi:10.2147/COPD.S150650
11. Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013;1(1):73–83. doi:10.1016/S2213-2600(12)70060-7
12. Ajay VS, Watkins DA, Prabhakaran D. Relationships among major risk factors and the burden of cardiovascular diseases, diabetes, and chronic lung disease. In: Prabhakaran D, Anand S, Gaziano TA, Mbanya JC, Wu Y, Nugent R, editors. Cardiovascular, Respiratory, and Related Disorders.
13. Machado FVC, Pitta F, Hernandes NA, Bertolini GL. Physiopathological relationship between chronic obstructive pulmonary disease and insulin resistance. Endocrine. 2018;61(1):17–22. doi:10.1007/s12020-018-1554-z
14. Doney B, Kurth L, Halldin C, Hale J, Frenk SM. Occupational exposure and airflow obstruction and self-reported COPD among ever-employed US adults using a COPD-job exposure matrix. Am J Ind Med. 2019;62(5):393–403. doi:10.1002/ajim.22958
15. Mannino DM, Gan WO, Wurst K, Davis KJ. Asthma and chronic obstructive pulmonary disease overlap: the effect of definitions on measures of burden. Chronic Obstr Pulm Dis. 2017;4(2):87–96. doi:10.15326/jcopdf.4.2.2016.0159
16. Janssen I, Carson V, Lee IM, Katzmarzyk PT, Blair SN. Years of life gained due to leisure-time physical activity in the U.S. Am J Prev Med. 2013;44(1):23–29. doi:10.1016/j.amepre.2012.09.056
17. Kaminsky LA, Montoye AH. Physical activity and health: what is the best dose? J Am Heart Assoc. 2014;3(5):e001430. doi:10.1161/JAHA.114.001430
18. Bellver J, Marín C, Lathi RB, et al. Obesity affects endometrial receptivity by displacing the window of implantation. Reprod Sci. 2021;28(11):3171–3180. doi:10.1007/s43032-021-00631-1
19. Bennie JA, Pedisic Z, van Uffelen JG, et al. The descriptive epidemiology of total physical activity, muscle-strengthening exercises and sedentary behaviour among Australian adults–results from the National Nutrition and Physical Activity Survey. BMC Public Health. 2016;16(1):73. doi:10.1186/s12889-016-2736-3
20. Blondeel A, Demeyer H, Janssens W, Troosters T. The role of physical activity in the context of pulmonary rehabilitation. COPD. 2018;15(6):632–639. doi:10.1080/15412555.2018.1563060
21. Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007;175(5):458–463. doi:10.1164/rccm.200607-896OC
22. Viniol C, Vogelmeier CF. Exacerbations of COPD. Eur Respir Rev. 2018;27(147):170103. doi:10.1183/16000617.0103-2017
23. Fuertes E, Carsin A, Antó JM, et al. Leisure-time vigorous physical activity is associated with better lung function: the prospective ECRHS study. Thorax. 2018;73(4):376–384. doi:10.1136/thoraxjnl-2017-210947
24. Bédard A, Carsin AE, Fuertes E, et al. Physical activity and lung function-Cause or consequence? PLoS One. 2020;15(8):e0237769. doi:10.1371/journal.pone.0237769
25. O’Donovan G, Hamer M. The association between leisure-time physical activity and lung function in older adults: the English longitudinal study of ageing. Prev Med. 2018;106:145–149. doi:10.1016/j.ypmed.2017.10.030
26. Dogra S, Good J, Gardiner PA, et al. Effects of replacing sitting time with physical activity on lung function: an analysis of the Canadian Longitudinal Study on aging. Health Rep. 2019;30(3):12–23. doi:10.25318/82-003-x201900300002-eng
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
