Back to Journals » OncoTargets and Therapy » Volume 11
Associations between ABCG2 gene polymorphisms and gefitinib toxicity in non-small cell lung cancer: a meta-analysis
Authors Tang LN, Zhang CL, He HR, Pan ZY , Fan D, He YL, You HS , Li YJ
Received 16 October 2017
Accepted for publication 20 December 2017
Published 1 February 2018 Volume 2018:11 Pages 665—675
DOI https://doi.org/10.2147/OTT.S154244
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Faris Farassati
Lina Tang,1,* Chunling Zhang,2,* Hairong He,3 Zhenyu Pan,3,4 Di Fan,1 Yinli He,1 Haisheng You,1 Yuanjie Li5
1Department of Pharmacy, The First Affiliated Hospital, Xi’an Jiao Tong University, Xi’an, China; 2Department of Pharmacy, Hong-Hui Hospital, Xi’an Jiao Tong University College of Medicine, Xi’an, China; 3Clinical Research Center, The First Affiliated Hospital, Xi’an Jiao Tong University, Xi’an, China; 4Department of Pharmacy, Xi’an Jiao Tong University Affiliated Children’s Hospital, Xi’an, China; 5Department of Human Anatomy, Histology and Embryology, School of Basic Medical Sciences, Xi’an Jiao Tong University Health Science Center, Xi’an, China
*These authors contributed equally to this work
Background: Gefitinib is frequently used to treat patients with non-small cell lung cancer (NSCLC) and is excreted out from cells via the ATP-binding cassette transporter ABCG2. ABCG2 gene polymorphisms have been suggested to be associated with ABCG2 protein expression and function and may influence the risk of gefitinib toxicity in NSCLC patients. Previous studies on the associations between ABCG2 gene polymorphisms and the toxicity of gefitinib in NSCLC patients have produced conflicting results. The aim of this meta-analysis was to determine whether ABCG2 gene polymorphisms are associated with the risk of gefitinib-induced toxicity in NSCLC patients.
Methods: The PubMed and EMBASE databases were searched systematically for all eligible studies. A relative risk with corresponding 95% CI was calculated to evaluate the associations between ABCG2 gene polymorphisms and gefitinib-induced toxicity.
Results: Data were finally extracted from seven studies and 515 patients were found to meet the inclusion criteria of the meta-analysis. A dominant model showed that there was no significant association between the ABCG2 C421A polymorphism and the risk of gefitinib-induced toxicity, while the ABCG2 G34A polymorphism might be associated with an increased risk of skin toxicity in gefitinib therapy (relative risk =1.54, 95% CI 1.08–2.21, P=0.02). However, more reliable data are required to confirm the associations between the ABCG2 C421A and ABCG2 G34A polymorphisms and the toxicity of gefitinib in NSCLC patients.
Conclusion: While the ABCG2 C421A polymorphism might not be a reliable marker of gefitinib-related toxicity, the ABCG2 G34A genotype may be predictive of the skin toxicity of gefitinib in NSCLC patients. These conclusions need to be verified in further large-scale studies.
Keywords: gefitinib, ABCG2, meta-analysis, polymorphism, toxicity, NSCLC
Introduction
Lung cancer is a very common carcinoma and also the leading cause of cancer-related deaths worldwide.1 Non-small cell lung cancer (NSCLC) is the most common type, accounting for 80%–85% of cases of lung cancer.2 Gefitinib (ZD1839; Iressa, Astrazeneca) is an oral inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase that has been used clinically for treating patients with NSCLC, and it exhibits strong antitumor activity.3,4 However, gefitinib is also associated with side effects such as skin rash, diarrhea, hepatic impairment, and pulmonary toxicities.5–7 Although most of these adverse events are usually mild and tolerable, the associated physical or psychosocial discomfort could decrease the quality of life, and the treatments might be discontinued when toxicities more severe than grade 2 occur.8,9 Therefore, the development of an accurate predictor of the toxicity response of gefitinib would contribute greatly to the treatment of NSCLC.
Gefitinib is a selective, reversible, EGFR tyrosine kinase inhibitor that blocks the EGFR signal transduction pathways related to the survival and proliferation of tumor cells.4,10 Gefitinib is excreted from cells via the ATP-binding cassette transporter ABCG2. ABCG2 (formerly termed breast cancer resistance protein [BCRP]) localizes to the apical cell membrane and mediates the ATP-dependent unidirectional efflux of its substrates from cells, which leads to a reduction in intracellular cytotoxic effects and mediates concurrent resistance.11–13 Gefitinib has a high-affinity interaction with ABCG2, and therefore, the expression of ABCG2 has an impact on gefitinib resistance.14,15 In addition, ABCG2 gene polymorphisms affect the pharmacodynamics of gefitinib, which might also affect the toxicity of gefitinib.16
Previous studies have shown that several naturally occurring single-nucleotide polymorphisms (SNPs) in the ABCG2 gene may affect ABCG2 protein expression and function, of which ABCG2 C421A (rs2231142) and ABCG2 G34A (rs2231137) are two major nonsynonymous SNPs.17–20 ABCG2 C421A, which is a C>A nucleotide transition at position 421 in exon 5 of the ABCG2 gene, has been related to low expression and activity of the ABCG2 protein, which results in higher accumulation of gefitinib.21,22 Cusatis et al indicated that the ABCG2 C421A polymorphism increased the risk of gefitinib-induced diarrhea in NSCLC patients.23 The ABCG2 G34A polymorphism, which results in a V12M substitution, disturbs apical plasma membrane localization and causes functional impairment of ABCG2, which may in turn influence the resistance to ABCG2 substrates.22 Tamura et al found that the ABCG2 G34A polymorphism was significantly associated with the occurrence of skin rash in gefitinib-treated NSCLC patients and might be a reliable predictor of skin toxicity in gefitinib treatments.8 These observations demonstrate that both ABCG2 C421A and ABCG2 G34A polymorphisms may affect gefitinib toxicity.
The effects of ABCG2 polymorphisms on the clinical outcome of gefitinib treatment in NSCLC (including gefitinib-induced toxicity) have been studied widely. Numerous genetic association studies have examined the possible linkages of the ABCG2 C421A and ABCG2 G34A polymorphisms with gefitinib toxicity in NSCLC patients. Although several of these studies found significant associations, most of them found no association. These inconsistent results may be due to differences in ethnicity, toxicity criteria, and sample sizes. Therefore, it remains uncertain if ABCG2 gene polymorphisms are related to gefitinib toxicity, and hence further studies are needed to clarify this association.
In this meta-analysis, we examined whether recent findings affect the strength of the association between the ABCG2 genotype and gefitinib toxicity in NSCLC patients. We first collected all published clinical studies of associations between ABCG2 polymorphisms and gefitinib toxicity in NSCLC patients, and then performed a meta-analysis of eligible studies that were selected by applying strict criteria to assess the effects of ABCG2 polymorphisms on gefitinib toxicity in NSCLC patients.
Material and methods
Literature and search strategy
Systematic literature searches were conducted using PubMed and EMBASE databases for relevant articles without language restrictions. The search terms were as follows: (“ATP-binding cassette subfamily G member 2” OR “ABCG2” OR “breast cancer resistance protein” OR “BCRP” OR “mitoxantrone resistance protein” OR “MRP” OR “ABCP”) AND (“gefitinib” OR “Iressa” OR “ZD1839”). In addition, the reference lists of both original and review articles were investigated to ensure that all relevant studies were included. The literature search was last updated on March 2017.
Selection criteria
We included a full-length research study in the meta-analysis if it satisfied all of the following inclusion criteria: 1) assessing an independent association between the ABCG2 polymorphism and gefitinib toxicity in NSCLC patients, 2) providing either sufficient data on toxicity by genotype or sufficient information for such data to be calculated, and 3) when duplicate data were published, we selected the report involving the largest sample.
Data extraction and study quality assessment
The following information was extracted for each study: name of the first author, year of publication, sample size, disease type, country of origin, ethnicity, gefitinib therapeutic doses, toxicity category, and data on toxicity by genotype. The Newcastle–Ottawa Scale (NOS) was applied to assess the study quality from three broad perspectives, with the score ranging from 0 to 9.24 The general methodological quality was considered to be moderate to good for studies that scored from 5 to 9 on the NOS.
Statistical analysis
Dominant genetic models for the ABCG2 C421A (CA+AA versus CC) and ABCG2 G34A (GA+AA versus GG) polymorphisms were applied to quantify the effect size in each study; these models maximized the number of included studies. The associations between gefitinib toxicity and the ABCG2 C421A and ABCG2 G34A polymorphisms were analyzed using a dominant model by calculating the relative risk (RR) and its 95% CI. A Mantel–Haenszel estimate was used to pool the RR estimates of each study using a fixed-effects model. The statistical significance of the RR was determined by the Z-test, and P<0.05 was considered indicative of statistical significance. The presence of heterogeneity was assessed using Cochran’s Q-statistic test and quantified using the I2 statistic, where P≤0.10 and/or I2>50% indicates a high degree of heterogeneity.25 A fixed-effects model was used to calculate pooled RRs when P>0.10; otherwise the random-effects model was applied. We performed subgroup analyses for skin toxicity, diarrhea, hepatotoxicity, and interstitial pneumonia. Sensitivity analysis was applied when possible by excluding individual studies. All of the statistical analyses were conducted using Review Manager Software (version 5.3).
Results
Study selection and characteristics
The detailed study selection process is depicted in Figure 1. The initial search identified 529 articles, which was reduced to 446 after removing duplicates. A further 149 articles were excluded due to 142 articles being reviews or letters and 7 articles being abstracts. Of the remaining 297 articles, 128 did not involve NSCLC patients, 143 did not focus on gefitinib toxicity, and 16 were not relevant to ABCG2 gene polymorphisms, and thus all of these articles were also excluded. After performing full-text evaluations, 3 of the remaining 10 articles were excluded for reasons of no toxicity data (n=1), no ABCG2 genotype number (n=1), and being a duplicate study (n=1). This selection process, therefore, resulted in only seven studies finally qualifying for inclusion in the present meta-analysis.
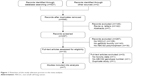  | Figure 1 Flowchart of the study selection process in the meta-analysis. |
The association between the ABCG2 C421A polymorphism and the risk of gefitinib toxicity was investigated in seven studies, while the ABCG2 G34A polymorphism was investigated in two studies.
The characteristics of these seven studies are listed in Table 1. Hirose et al26 did not provide sufficient information regarding the gefitinib-induced adverse effects that were correlated with ABCG2 gene polymorphisms, which meant that the associations between ABCG2 polymorphisms and gefitinib toxicity were unclear, and hence that study was not included in the subsequent analysis. Four8,27–29 of the remaining six clinical observation studies involved Asian populations and the other two23,30 involved Caucasian populations. The association between the ABCG2 C421A polymorphism and the risk of gefitinib toxicity was investigated in seven studies,8,23,26–30 while the ABCG2 G34A polymorphism was investigated in two studies.8,29 The results of the quality assessment are presented in Table 1 – the included studies scored from 5 to 7 on the NOS.
The studies investigated different types of toxicity. As different studies analyzed toxicity according to different evaluation criteria, we performed an in-depth analysis for each toxicity type (Tables 2 and 3). Because the patient data were insufficient for some types of toxicity, not all of them are listed in Table 3. We performed meta-analyses for diarrhea, skin toxicity, hepatotoxicity and interstitial pneumonia. The results for each of these toxicities are presented separately below.
  | Table 3 Toxicity types and findings in each study |
Diarrhea
Seven studies researched diarrhea,8,23,26–30 of which six8,23,27–30 provided sufficient data. Based on the data from 515 patients in these six studies, no association was observed between the ABCG2 C421A polymorphism and gefitinib-induced diarrhea (RR =0.97, 95% CI 0.53–1.79, P=0.52; Figure 2A). The forest plot showed considerable heterogeneity among the six studies (I2=66%, P=0.01; Figure 2A), and so the random-effects model was used. Stratification by ethnicity indicated no significant association between the ABCG2 C421A polymorphism and gefitinib-induced diarrhea in either the Asian (RR =0.77, 95% CI 0.52–1.14, P=0.19) or Caucasian populations (RR =1.49, 95% CI 0.20–10.91, P=0.69; Figure 2A).
The two studies8,29 that evaluated the association between the ABCG2 G34A polymorphism and gefitinib-induced diarrhea involved 142 Asian participants. The pooled results indicated the absence of a significant association between the ABCG2 G34A polymorphism and gefitinib-induced diarrhea (RR =1.15, 95% CI 0.62–2.12, P=0.65; Figure 3A). The I2-value indicated that there was no significant heterogeneity between the two included studies (I2=0%, P=0.70; Figure 3A).
Skin toxicity
Skin toxicity was addressed in seven studies,8,23,26–30 of which five8,27–30 provided sufficient data to explore the association between the ABCG2 C421A polymorphism and gefitinib-induced skin toxicity. A meta-analysis of these five studies, which involved data from 342 patients, revealed no significant association between the ABCG2 C421A polymorphism and gefitinib-induced skin toxicity (RR =0.84, 95% CI 0.69–1.02, P=0.08; Figure 2B). No significant heterogeneity was observed among the studies (I2=0%, P=0.96; Figure 2B), and so a fixed-effects model was applied. Stratification by ethnicity indicated that there was no significant association between the ABCG2 CA+AA genotype and the skin toxicity of gefitinib in either Asians (RR =0.84, 95% CI 0.69–1.03, P=0.09; Figure 2B) or Caucasians (RR =0.83, 95% CI 0.39–1.76; Figure 2B).
The two studies that evaluated the association between the ABCG2 G34A polymorphism and gefitinib-induced skin toxicity were conducted on 142 Asian patients.8,29 The meta-analysis indicated a significant association between ABCG2 G34A polymorphism and gefitinib-induced skin toxicity (RR =1.54, 95% CI 1.08–2.21, P=0.02; Figure 3B). No significant heterogeneity was observed between the two included studies (I2=40%, P=0.20; Figure 3B).
Hepatotoxicity
The association between the ABCG2 C421A polymorphism and gefitinib-induced hepatotoxicity was analyzed in four studies8,27–29 involving Asian populations. These studies involved data from 248 participants, and no association between that polymorphism and hepatotoxicity was found (RR =1.37, 95% CI 0.86–2.17, P=0.18; Figure 2C). No significant heterogeneity existed among the four studies (I2=0%, P=0.66; Figure 2C), and so a fixed-effects model was used.
The association between the ABCG2 G34A variant and gefitinib-induced hepatotoxicity was evaluated by two studies8,29 that involved 142 Asian patients. The pooled ORs revealed that there was no significant association between that polymorphism and gefitinib-induced hepatotoxicity (RR =1.22, 95% CI 0.58–2.55, P=0.60; Figure 3C). No obvious heterogeneity was indicated between the two included studies (I2=39%, P=0.20; Figure 3C).
Interstitial pneumonia
Two studies8,27 involving 158 patients evaluated the association between the ABCG2 C421A polymorphism and gefitinib-induced interstitial pneumonia, and both these studies involved Asian populations. The pooled results indicated that there was no significant association between that polymorphism and interstitial pneumonia (RR =0.37, 95% CI 0.10–1.37, P=0.14; Figure 2D). No significant heterogeneity was found (I2=0%, P=0.78; Figure 2D), and so a fixed-effects model was used.
Given that the included studies may build analysis based on the different grade of toxicity events, we performed a stratified analysis by the grade of toxicity (Figure 4). The meta-analysis indicated no significant associations of the ABCG2 C421A polymorphism with diarrhea (grade ≥1; RR =1.03, 95% CI 0.54–1.96, P=0.94; Figure 4A), skin toxicity (grade ≥1; RR =0.85, 95% CI 0.70–1.04, P=0.11; Figure 4B), hepatotoxicity (grade ≥1; RR =1.53, 95% CI 0.90–2.61, P=0.12; Figure 4C), and interstitial pneumonia (grade ≥1; RR =0.37, 95% CI 0.10–1.37, P=0.14; Figure 4D). Tamura et al8 found ABCG2 421C>A polymorphism was not associated with grade 2 or worse diarrhea and skin toxicity. More studies are still needed to confirm the association between the ABCG2 C421A polymorphism and grade ≥2 toxicity in gefitinib-treated patients.
Discussion
Four of the seven studies included in this meta-analysis found no significant association between the ABCG2 C421A polymorphism and the toxicity of gefitinib in NSCLC patients.8,27,28,30 Two of the studies did find such an association,23,29 while the remaining study did not draw a definite conclusion.26 Two of the seven studies8,29 investigated the effect of the ABCG2 G34A polymorphism on gefitinib toxicity, and both found that this polymorphism is associated with the increased risk of gefitinib-related toxicity.
All of the studies included in the meta-analysis applied the same gefitinib dose of 250 mg/day. Since the included studies evaluated toxicity using different criteria, we now consider their findings for each toxicity criterion.
Diarrhea
Five of the seven studies that researched diarrhea did not find any association between the ABCG2 C421A polymorphism and gefitinib-induced diarrhea.8,27–30 Of the remaining two studies, Hirose et al26 did not provide enough data on diarrhea or did not draw a definite conclusion, while Cusatis et al23 demonstrated that patients with at least one variant ABCG2 C421A allele are at an increased risk of gefitinib-induced diarrhea. The reasons for these discrepancies remain to be investigated, but they could be due to ethnic factors, how the toxicity type was defined, or poor methodological quality associated with a small number of cases.
The allele frequencies of the ABCG2 C421A varies highly between different populations, reportedly being 35% in both Japanese and Chinese populations, in contrast to ~14% in Caucasians.19 Subgroup analysis by ethnicity was performed for the ABCG2 C421A polymorphism. The result of our meta-analysis indicated that ABCG2 C421A polymorphism was not associated with the risk of diarrhea in Asian populations. There was significant heterogeneity between the two studies involving Caucasians. In contrast to Cusatis et al,23 Lemos et al30 observed no significant association between the ABCG2 C421A polymorphism and gefitinib-induced diarrhea in Caucasians. Because the allele frequency of the ABCG2 C421A is lower in Caucasians than in Asians, investigations involving larger numbers of Caucasian patients are necessary, which might lead to different conclusions. Therefore, more studies are still needed to confirm the association between the ABCG2 C421A polymorphism and gefitinib-related diarrhea.
Two studies involving Asian populations analyzed the association between the ABCG2 G34A polymorphism and gefitinib-induced diarrhea.8,29 Both these studies found that this polymorphism was not associated with diarrhea, which is consistent with the results of our meta-analysis. Because there were no studies of the ABCG2 G34A polymorphism involving Caucasian populations, large-scale studies are still required to confirm the association between this polymorphism and gefitinib-induced diarrhea.
Skin toxicity
Seven studies investigated the association between the ABCG2 C421A polymorphism and the skin toxicity of gefitinib.8,23,26–30 Cusatis et al23 determined that this polymorphism was not associated with gefitinib-induced skin toxicity, but they did not provide sufficient data. The 2016 study of Hirose et al26 also did not provide sufficient data on skin toxicity and no definite conclusion was drawn. The other five studies found no association between the ABCG2 C421A polymorphism and gefitinib-induced skin toxicity,8,27–30 which is supported by the overall results of our meta-analysis. Stratification by ethnicity was applied to the ABCG2 C421A polymorphism, and this did not reveal any association between the ABCG2 421 CA+AA genotype and the risk of gefitinib-induced skin toxicity in Asians. Although the ABCG2 C421A polymorphism might not be associated with the skin toxicity in Caucasians, this result was based on only one study, and so more reliable data from large-scale studies are needed to confirm the effects of the ABCG2 C421A polymorphism on the skin toxicity of gefitinib therapy.
Two studies involving Asians analyzed the effect of the ABCG2 G34A polymorphism on gefitinib-induced skin toxicity.8,29 Tamura et al found that this polymorphism was associated with skin toxicity and might be useful for predicting skin toxicity in gefitinib treatments.8 Ma et al observed that the ABCG2 34 A allele tended to increase the risk of skin toxicity.29 Pooled results showed that the GA+AA genotype of the ABCG2 G34A polymorphism might increase the risk of gefitinib-induced skin toxicity. A plausible mechanism is that the variant allele of ABCG2 G34A reduces the transporter activity, decreases drug efflux, and thus increases drug accumulation,22 which may enhance the response to the drug. Consistent with the above result, it has been reported that the overall survival was longer in NSCLC patients carrying the ABCG2 GA or AA genotype than in those with the ABCG2 GG genotype.31 In addition, the occurrence of skin rash was associated with improved survival in NSCLC patients treated with gefitinib.32 However, because only two studies were included, further studies are needed to confirm the effects of the ABCG2 G34A polymorphism on the risk of gefitinib-induced skin toxicity.
Hepatotoxicity
Hepatotoxicity was investigated in four studies involving Asian populations.8,27–29 Three of these studies found no obvious association between the ABCG2 C421A polymorphism and the risk of gefitinib-induced hepatotoxicity,8,27,28 while Ma et al29 demonstrated that the presence of the ABCG2 421 A allele increased the risk of gefitinib-induced hepatotoxicity. These different results might be related to differences in the period of toxicity assessments and the small size of the samples. The results from our meta-analysis indicated that there is no significant association between the ABCG2 C421A polymorphism and hepatotoxicity in Asians.
Two studies involving Asian populations investigated the effect of the ABCG2 G34A polymorphism on the risk of hepatotoxicity.8,29 Ma et al29 found that patients carrying the ABCG2 34 A allele tended to have an increased risk of gefitinib-related hepatotoxicity, while Tamura et al8 found no such correlations. Our meta-analysis demonstrated no significant association between the ABCG2 G34A polymorphism and hepatotoxicity, but more studies are required to confirm this association.
Interstitial pneumonia
Two studies involving Asians investigated the effect of the ABCG2 C421A polymorphism on gefitinib-related interstitial pneumonia.8,27 Neither of these studies found any significant association between ABCG2 C421A and interstitial pneumonia, which was consistent with the results of our meta-analysis.
Tamura et al8 was the only study to have investigated the association of the ABCG2 G34A polymorphism with gefitinib-induced interstitial pneumonia, and they found that the ABCG2 G34A polymorphism had no effect on the risk of interstitial pneumonia. A meta-analysis could not be conducted due to lack of more studies and data.
The pharmacogenetics of erlotinib, an EGFR tyrosine kinase inhibitor similar to gefitinib, has been reported for toxicity. Several studies have researched the relationship between ABCG2 gene polymorphisms and the toxicity of erlotinib, which found no significant associations between the C421A and G34A loci of ABCG2 and the toxicity of erlotinib.33,34
Some limitations of the present meta-analysis should be noted. First, the studies included in our meta-analysis investigated only Asian or Caucasian patients, and so our conclusions apply only to these ethnic groups. Second, only small numbers of studies were included in our analyses, and so the conclusions need to be interpreted with caution. Clearly, it is necessary to conduct more studies to confirm our tentative conclusions. Third, due to such a small number of studies being included, publication bias in this meta-analysis could not be controlled. Finally, due to the lack of detailed original data at the individual level, a more precise analysis stratified according to age, sex, clinical manifestations, and environmental factors could not be conducted.
Conclusion
In summary, this meta-analysis has demonstrated that the ABCG2 C421A polymorphism might not be a reliable marker of gefitinib-related toxicity in NSCLC patients, while the ABCG2 G34A polymorphism might be associated with gefitinib-induced skin toxicity in NSCLC patients. Given that our analyses included only few studies and small samples, more large-scale studies are required to confirm these associations.
Acknowledgments
This work was supported by the National Social Science Foundation of China (no 16BGL183) and the Natural Science Foundation of Shaanxi Province (no 2015JM8415).
Disclosure
The authors report no conflicts of interest in this work.
References
Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. | ||
D’Addario G, Fruh M, Reck M, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v116–v119. | ||
Siegel-Lakhai WS, Beijnen JH, Schellens JH. Current knowledge and future directions of the selective epidermal growth factor receptor inhibitors erlotinib (Tarceva) and gefitinib (Iressa). Oncologist. 2005;10(8):579–589. | ||
Barker AJ, Gibson KH, Grundy W, et al. Studies leading to the identification of ZD1839 (IRESSA): an orally active, selective epidermal growth factor receptor tyrosine kinase inhibitor targeted to the treatment of cancer. Bioorg Med Chem Lett. 2001;11(14):1911–1914. | ||
Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290(16):2149–2158. | ||
Hong D, Zhang G, Zhang X, Lian X. Pulmonary toxicities of gefitinib in patients with advanced non-small-cell lung cancer: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016;95(9):e3008. | ||
Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized Phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21(12):2237–2246. | ||
Tamura M, Kondo M, Horio M, et al. Genetic polymorphisms of the adenosine triphosphate-binding cassette transporters (ABCG2, ABCB1) and gefitinib toxicity. Nagoya J Med Sci. 2012;74(1–2):133–140. | ||
Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6(10):803–812. | ||
Arteaga CL, Johnson DH. Tyrosine kinase inhibitors-ZD1839 (Iressa). Curr Opin Oncol. 2001;13(6):491–498. | ||
Suzuki M, Suzuki H, Sugimoto Y, Sugiyama Y. ABCG2 transports sulfated conjugates of steroids and xenobiotics. J Biol Chem. 2003;278(25):22644–22649. | ||
Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58(23):5337–5339. | ||
Li J, Cusatis G, Brahmer J, et al. Association of variant ABCG2 and the pharmacokinetics of epidermal growth factor receptor tyrosine kinase inhibitors in cancer patients. Cancer Biol Ther. 2007;6(3):432–438. | ||
Elkind NB, Szentpetery Z, Apati A, et al. Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, Gefitinib). Cancer Res. 2005;65(5):1770–1777. | ||
Leggas M, Panetta JC, Zhuang Y, et al. Gefitinib modulates the function of multiple ATP-binding cassette transporters in vivo. Cancer Res. 2006;66(9):4802–4807. | ||
Giacomini KM, Brett CM, Altman RB, et al. The pharmacogenetics research network: from SNP discovery to clinical drug response. Clin Pharmacol Ther. 2007;81(3):328–345. | ||
Kobayashi D, Ieiri I, Hirota T, et al. Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placenta. Drug Metab Dispos. 2005;33(1):94–101. | ||
Lepper ER, Nooter K, Verweij J, Acharya MR, Figg WD, Sparreboom A. Mechanisms of resistance to anticancer drugs: the role of the polymorphic ABC transporters ABCB1 and ABCG2. Pharmacogenomics. 2005;6(2):115–138. | ||
Zamber CP, Lamba JK, Yasuda K, et al. Natural allelic variants of breast cancer resistance protein (BCRP) and their relationship to BCRP expression in human intestine. Pharmacogenetics. 2003;13(1):19–28. | ||
Choudhuri S, Klaassen CD. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int J Toxicol. 2006;25(4):231–259. | ||
Imai Y, Nakane M, Kage K, et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther. 2002;1(8):611–616. | ||
Mizuarai S, Aozasa N, Kotani H. Single nucleotide polymorphisms result in impaired membrane localization and reduced ATPase activity in multidrug transporter ABCG2. Int J Cancer. 2004;109(2):238–246. | ||
Cusatis G, Gregorc V, Li J, et al. Pharmacogenetics of ABCG2 and adverse reactions to gefitinib. J Natl Cancer Inst. 2006;98(23):1739–1742. | ||
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Hirose T, Fujita K, Kusumoto S, et al. Association of pharmacokinetics and pharmacogenomics with safety and efficacy of gefitinib in patients with EGFR mutation positive advanced non-small cell lung cancer. Lung Cancer. 2016;93:69–76. | ||
Akasaka K, Kaburagi T, Yasuda S, et al. Impact of functional ABCG2 polymorphisms on the adverse effects of gefitinib in Japanese patients with non-small-cell lung cancer. Cancer Chemother Pharmacol. 2010;66(4):691–698. | ||
Kobayashi H, Sato K, Niioka T, Miura H, Ito H, Miura M. Relationship among gefitinib exposure, polymorphisms of its metabolizing enzymes and transporters, and side effects in Japanese patients with non-small-cell lung cancer. Clin Lung Cancer. 2015;16(4):274–281. | ||
Ma Y, Xin S, Huang M, et al. Determinants of Gefitinib toxicity in advanced non-small cell lung cancer (NSCLC): a pharmacogenomic study of metabolic enzymes and transporters. Pharmacogenomics J. 2016;17(4):325–330. | ||
Lemos C, Giovannetti E, Zucali PA, et al. Impact of ABCG2 polymorphisms on the clinical outcome and toxicity of gefitinib in non-small-cell lung cancer patients. Pharmacogenomics. 2011;12(2):159–170. | ||
Chen X, Chen D, Yang S, et al. Impact of ABCG2 polymorphisms on the clinical outcome of TKIs therapy in Chinese advanced non-small-cell lung cancer patients. Cancer Cell Int. 2015;15:43. | ||
Mohamed MK, Ramalingam S, Lin Y, Gooding W, Belani CP. Skin rash and good performance status predict improved survival with gefitinib in patients with advanced non-small cell lung cancer. Ann Oncol. 2005;16(5):780–785. | ||
Rudin CM, Liu W, Desai A, et al. Pharmacogenomic and pharmacokinetic determinants of erlotinib toxicity. J Clin Oncol. 2008;26(7):1119–1127. | ||
Fukudo M, Ikemi Y, Togashi Y, et al. Population pharmacokinetics/pharmacodynamics of erlotinib and pharmacogenomic analysis of plasma and cerebrospinal fluid drug concentrations in Japanese patients with non-small cell lung cancer. Clin Pharmacokinet. 2013;52(7):593–609. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





