Back to Journals » Infection and Drug Resistance » Volume 17
Association Studies of Serum Levels of TNF- α, IL-10, IFN-γ and CXCL 5 with Latent Tuberculosis Infection in Close Contacts
Authors Razbek J, Daken M, Chen Y, Ma L, Zhang Y , Xu W, Wen B, Wang J, Wang X, Cao M
Received 7 November 2023
Accepted for publication 22 February 2024
Published 7 March 2024 Volume 2024:17 Pages 899—910
DOI https://doi.org/10.2147/IDR.S442682
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Jaina Razbek,1 Mayisha Daken,2 Yanggui Chen,3 Li Ma,3 Yan Zhang,1 Wanting Xu,1 Baofeng Wen,1 Junan Wang,1 Xiaomin Wang,1 Mingqin Cao1
1Department of Epidemiology and Health Statistics, College of Public Health, Xinjiang Medical University, Urumqi, 830011, People’s Republic of China; 2Department of Epidemic Prevention, Karamay Centre for Disease Control and Prevention, Karamay, 834000, People’s Republic of China; 3Department of Prevention and Control of Tuberculosis, Urumqi Centre for Disease Control and Prevention, Urumqi, 830011, People’s Republic of China
Correspondence: Mingqin Cao, Tel +86-13319912419, Email [email protected]
Purpose: Early recognition and treatment of latent tuberculosis infection(LTBI) is key to tuberculosis(TB) prevention. However, the emergence of LTBI is influenced by a combination of factors, of which the role of individual immune cytokines remains controversial. The aim of this study is to explore the influencing factors of LTBI and their effects with cytokines on LTBI.
Patients and Methods: Close contacts of tuberculosis in Urumqi City from 2021 to 2022 were selected for the study to conduct a field survey. It used logistic regression model to analyse the influencing factors of LTBI, principal component analysis to extract a composite indicators of cytokines, and structural equation modelling to explore the direct and indirect effects of cytokines and influencing factors on LTBI.
Results: LTBI infection rate of 33.3% among 288 TB close contacts. A multifactorial Logistic model showed that factors influencing LTBI included education, daily contact hours, eating animal liver, and drinking coffee (P< 0.05); After controlling for confounding factors and extracting composite indicators of cytokines using principal component analysis, CXCL5 and IFN-γ is a protective factor for LTBI(OR=0.572, P=0.047), IL-10 and TNF-α is a risk factor for LTBI(OR=2.119, P=0.010); Structural equation modelling shows drinking coffee, eating animal liver, daily contact hours, and IL-10 and TNF-α had direct effects on LTBI and educations had indirect effects on LTBI(P< 0.05).
Conclusion: IL-10 and TNF-α are involved in the immune response and are directly related to LTBI. By monitoring the cytokine levels of TB close contacts and paying attention to their dietary habits and exposure, we can detect and intervene in LTBI at an early stage and control their progression to TB.
Keywords: close contacts, latent tuberculosis infection, cytokines, structural equation modelling, association studies
Introduction
Latent tuberculosis infection (LTBI) is a state of persistent infection in which the body is infected with Mycobacterium tuberculosis (Mtb) and produces a sustained immune response without any gross clinical signs. The World Health Organisation (WHO) estimates1 that one quarter of the global population is infected with Mtb, that LTBI can develop into active Tuberculosis(TB) disease following alterations in the immune status of the organism. The LTBI population, as a potential reservoir of TB, has a 5–10% lifetime risk of developing active TB. According to the WHO End TB Strategy, the early, rapid and accurate diagnosis of LTBI for precision treatment is key to the global TB elimination.
The WHO advocates the detection and preventive treatment of LTBI in high-risk groups, especially close contacts of TB patients, who are at high risk of infection and disease due to long-term exposure to Mtb and are a priority group for intervention.1 However, the diagnosis of LTBI lacks a gold standard test.2 The tuberculin skin test (TST) is affected by BCG vaccination, non-tuberculous mycobacterial infection, and immune status of the organism; there is wide variation in the specificity and sensitivity of the interferon gamma release assays (IGRA) in different regions and populations. Emerging TB antigen-based skin tests and advanced versions of IGRAs may offer some advantages but the problem remains.3
Furthermore, not all contacts of pulmonary TB patients acquire Mtb infection.4 Exposure to Mtb can result in either infection or possible clearance, The mechanism of its occurrence is complex and depends not only on the ability of the bacteria to replicate the infection, but also on immune response of the host.5 Although protective immunity against Mtb is not fully understood, it is known that Mtb infection is controlled by both innate and adaptive immunity mechanisms.6 After Mtb invasion into the human body, macrophages first gather to the infection site to phagocytose and kill Mtb through innate immunity. Peptides degraded by Mtb in the phagosomes of alveolar macrophages stimulate the activation and proliferation of CD4+ T lymphocytes, as the predominant effector T cells in the adaptive immune response, inducing Th1 and Th2 differentiation.7 Activated Th1 cells can fully activate alveolar macrophages through the secretion of interferon (IFN)-γ, tumour necrosis factor(TNF)-α and interleukin, etc., and promote their effective killing effect on Mycobacterium tuberculosis.8 IFN-γ induces the production of multiple chemokines to activate cellular immunity to contain infection and prevent the spread of Mtb.9 While abundant studies may exist in literature regarding exploring potential biomarkers for identifying TB from LTBI, and analysing the role of inflammatory factors10–12 and chemokines13,14 in TB, few studies have focused on immune differences between LTBI and healthy populations and the role they play in LTBI. Most of them were either cell-line15 or animal studies,16 and only few studies used human peripheral blood monocytes for experiments. But, the results are controversial, with previous studies proposing that elevated CXCL8 and reduced TNF-α and IFN-γ are associated with LTBI.17 However, Feria et al suggested that compared to non-LTBI patients, those with LTBI had elevated ratios of IL-6, TNF-α,18 Essone et al suggested that IFN-γ and TNF-α secretion was significantly enhanced in healthcare workers with latent infection19 and Li et al suggested that IL-4 levels were significantly lower in IGRA-positive compared to IGRA-negative individuals.20 In addition, there may be a correlation between cytokines, and LTBI is also influenced by numerous factors such as individual factors, patient condition, exposure to the environment, etc., and previous studies have neglected the correlations and interrelationships between factors.
Thus, the study conducted a field survey of close contacts of high-risk groups for tuberculosis in Urumqi to investigate and analyse the current status of LTBI in close contacts and the factors affecting them. It used logistic regression model to analyse the influencing factors of LTBI, principal component analysis to extract a composite indicators of cytokines, and structural equation modelling to explore the direct and indirect effects of cytokines and influencing factors on LTBI, in order to comprehensively analyse the influence of various factors on LTBI and their mechanisms of action, so as to provide a scientific basis for early detection of LTBI and accurate prevention and control of tuberculosis.
Materials and Methods
Study Design
This study investigated contacts of TB at the time of diagnosis from January 2021 through August 2022 in Urumqi, Xinjiang. TB was diagnosed on the basis of positive mycobacterial culture and/or GeneXpert MTB/RIF with supportive symptoms and radiological evidence. After identifying TB cases, we searched for their close contacts for investigation. Close contacts were defined as persons who had shared air space with an individual with TB in the household or other indoor setting for >15 hours weekly or >180 hours total during an infectious period, defined as the interval from 3 months prior to diagnosis of TB in the indicated case to 14 days after the initiation of treatment.21 Cases of TB among close contacts were excluded from this study. The overall flow of this study is illustrated in Figure 1.
 |
Figure 1 The overall flow of the study. |
Questionnaire Survey
Face-to-face surveys were conducted by trained and qualified enumerators using self-designed questionnaires. The questionnaire covered demographics, lifestyle and living environment. In detail, these included situation of gender, age, marital status, education, Job, BMI level, daily ventilation time, daily contact hours, growing potted plants, using activated carbon, smoking, drinking, exercise amount, eating animal liver and drinking coffee.
Diagnosis of LTBI
LTBI is defined as a state of persistent immune response to stimulation by Mtb antigens with no evidence of clinically manifested active TB.2 In this study, we diagnosed LTBI on the basis of positive TST results in close contacts of TB, without any symptoms of TB and without any chest radiographic abnormalities. For the LTBI diagnosis, TST test was performed and a chest X-ray was taken by a specialised clinician at the same time. A positive TST result was defined as TST screening result >5 mm in those without a history of BCG vaccination; or TST result ≥15 mm in those who had been vaccinated with BCG.21
Detection of Cytokines
Venous blood was collected from the elbows of the study subjects using 5 mL EDTA vacutainer tubes. The blood was allowed to clot naturally for 30 minutes at room temperature and centrifuged at 1000×g for about 15 minutes using a centrifuge. Serum was harvested aliquoted, and frozen at −80°C until use. The levels of IL-10, TNF-α, IFN-γ and CXCL5 were determined by using enzyme-linked immunosorbent assay (ELISA). The TNF-α, IFN-γ, IL-10 and CXCL5 kits produced by Bioswamp Wuhan Biotechnology Co., Ltd are available under item numbers HM10001, HM10115, HM10203 and HM11244.
Statistical Analysis
SPSS version 26.0 was used for data collection, description and analysis. Categorical variables were expressed as frequencies and percentages and continuous variables of non-normally distributed variables were expressed as medians and interquartile ranges. The categorical variables were tested using a univariate chi-square test. This study used a principal component analysis to transform cytokines into aggregated indicators in order to eliminate the correlation between factors, the criterion of principal component analysis was based on the Kaiser-Meyer-Olkin statistic ≥ 0.7 and Bartlett’s test result of P< 0.05 as the selection of appropriate indices, and using eigenvalues ≥1 considered as the cut-off for factor retention. By logistic regression model and then analysis of cytokine and influencing factors associations of LTBI in close household contacts. Structural equation modelling of the association between LTBI and cytokines and other factors was constructed using AMOS 24.0 software, and parameter estimation was performed by maximum likelihood method. P-value,0.05 was considered statistically significant.
Results
Population Characteristics
A total of 288 close contacts of 143 cases of active tuberculosis were included in this study, of which 192 were TST negative (<5mm) and 96 were positive (≥5mm), with an LTBI infection rate of 33.3%. The demographic, lifestyle and living environment variables of the individuals are summarized in Table 1.
 |
Table 1 Demographics, Living Environment and Lifestyle of the LTBI and HC Groups |
Association Between LTBI and Demographic, Lifestyle and Living Environment
Univariate analysis showed that the LTBI group was statistically different from the HC group in terms of age, marital status, job, education, daily ventilation time, daily contact hour, as well as planting of potted plants, using of activated charcoal, eating animal liver, and drinking coffee (P<0.05, Table 1). Further multifactorial analyses were performed: education, daily contact hours, eating animal liver, and drinking coffee remained statistically significant (P<0.05, Table 2).
 |
Table 2 Multifactorial Analysis of LTBI in Close Contacts |
Association Between LTBI and Cytokines
The data of four cytokines, IL-10, TNF-α, IFN-γ, and CXCL5, showed a skewed distribution and were therefore analysed using the median to classify them into low and high levels. The results in Table 3 show that there was a statistically significant difference in the detection rate of LTBI at different levels of TNF-α (P<0.05), while the other three cytokines were not statistically significant (P>0.05). Cytokines were included in a multifactorial logistic regression model after adjusting for age, education, daily contact hours, eating animal liver, and drinking coffee. The results showed that none of the IL, TNF-α, IFN-γ, and CXCL5 were associated with LTBI (P>0.05, Table 4).
 |
Table 3 Comparison of Detection Rates of LTBI with Different Levels of Cytokines |
 |
Table 4 Analysis the Association of Cytokines with LTBI |
Association of Cytokines with LTBI Based on Principal Component Analysis
This study examined the correlation between the four cytokines, and the results showed strong correlations between every two cytokines (P<0.001). Then we conducted the principal component analysis with four cytokines and the results are shown in Table 5. The two common factors with a cumulative variance contribution rate of 83.410% were extracted in the study. Thus, two variables were generated: FI, characterized by CXCL5 and IFN-γ, F2, characterized by IL-10 and TNF-α.
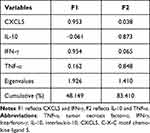 |
Table 5 Coefficient of Factor Loading and Total Variance Explained in Principal Component Analysis |
Principal component regression analyses were performed to assess the association between LTBI (the dependent variable) and two common factors of cytokines (the independent variable). Our data showed that after adjusting for confounders, F1 and F2 were statistically significant (P < 0.05), suggesting that the F1 is a protective factor for LTBI(OR=0.572, P=0.047) and F2 is a risk factor for LTBI(OR=2.119, P=0.010), as shown in Table 4.
Factors Influencing of LTBI Based on Structural Equation Modelling
The results indicated that all path coefficients reached statistical significance except for F1(Figure 2, Table 6). According to the goodness-of-fit indicators, RMSEA = 0.048 (<0.05), GFI, AGFI, IFI, CFI, TLI were 0.970, 0.944, 0.962, 0.961,0.942 (all >0.90) respectively, which had meant that the constructed structural equation model might be better.
 |
Table 6 Direct and Indirect Effects and Standardized Path Coefficient of Each Path on LTBI |
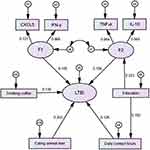 |
Figure 2 Structural equation modeling determinants of LTBI. Notes: Arrows indicate the associations and directions between variables. |
Drinking coffee, eating animal liver, daily contact hours, and F2 had direct effects on LTBI, with path coefficients of −0.139, 0.203, 0.128, and 0.156, respectively, and Educations could have indirect effects on LTBI through F2 and daily contact hours, with path coefficients of 0.034, and F1 was not associated with LTBI (P>0.05) as shown in Table 6.
Discussion
Mtb infection in close contacts of TB is the result of multifactorial interactions related not only to individual immune resistance but also to the intensity and frequency of exposure to Mtb. The Xinjiang region is the hardest-hit area in China in terms of tuberculosis epidemics, with “three highs and one low” in terms of high infection rate, high prevalence rate, high rural outbreaks, and low annual decline rate.22 Scientific screening of high-risk groups in high-epidemic areas for early detection and early intervention of LTBI, and timely control of close contacts developing into patients is of great significance in reducing and controlling the burden of tuberculosis in the region. Thus, we investigated the LTBI of close contacts of TB through the TST, analyzed the relevant influencing factors, and explored the existence of associations with cytokines.
Of the 288 close contacts of TB in this survey, 96 were LTBI and the detection rate was 33.33%, which is close to the results of studies on the prevalence of LTBI(30~45%) in close contacts.23–25 This population, as one of the most exposed high-risk groups, still has a high risk of TB infection and morbidity. It is recommended that early screening for TB should be carried out in order to shift from a reactive to active screening, to detect patients at an early stage, to continuously strengthen the dissemination of core knowledge among patients and close contacts, and to enhance the awareness and behaviour of close contacts in terms of personal protection, so as to reduce the rate of infection and morbidity in this group.26
The results of logistic regression and structural equation modelling are the influencing factors of LTBI include education, daily contact hours, eating animal liver, and drinking coffee, of which daily contact hours, eating animal liver, drinking coffee had direct effects on LTBI and education had indirect effects on LTBI. TB is an airborne transmission of germs from an infectious agent, and close contacts are exposed to an environment that provides favourable conditions for the survival of the germs, with the time of day spent in contact with the patient determining the degree of exposure to Mtb. The longer the duration of contact with the patient, the higher the chance of exposure to Mtb, thus the higher the chance of infection and morbidity in close contacts.27 Animal liver is rich in high quality protein and various micronutrients. Studies have shown that adequate protein plays an inhibitory role in the development of TB,28 and micronutrients and vitamins not only influence treatment of TB,29 but also inhibit the growth of Mtb in macrophages.30 However, daily coffee drinkers have a higher rate of LTBI, possibly because the fact that coffee affects the binding of antidiuretic hormone to the receptor, leading to an increased loss of calcium from the body and, with prolonged consumption, causes abnormalities in glucose metabolism, which affect the body’s immunity.31 In addition, people with low literacy may have weak awareness and knowledge of TB prevention and control. Therefore, this would suggest that close contacts need to enhance personal protection awareness and protective behaviours to reduce the possibility of Mtb survival, and work together to create a healthy living environment, develop healthy dietary and lifestyle habits, and gradually enhance the body’s immune resistance to the spread of Mtb.
After principal component regression modelling, controlling for the original variables, IFN-γ and CXCL5 were found to be negatively correlated with LTBI, which may be due to macrophage/autophagy activation, which promotes the anti-Mtb response by activating receptor α via peroxisome proliferators.32 IFN-γ is a key component of the protective anti-TB immune response produced by Th1 cells,33 which initiates the immune response function through macrophages during LTBI and improves bactericidal capacity,34 and thus the concentration increases to act as a protective agent. CXCL5, a pro-inflammatory member of the CXC subfamily of chemokines, has a strong chemotactic effect on neutrophils. It has been shown that Toll-like receptor 2 controls neutrophil-driven immunopathology during infection with Mycobacterium tuberculosis by reducing the production of CXCL5.35 Thus, in turn, may lead to a protective effect of high levels of CXCL5.
The results of principal component regression modelling and structural equation modelling showed that IL-10 and TNF-α are risk factors that promote the development of LTBI, and the association may be related to macrophage expression. TNF-α is an important immunomodulatory and inflammatory mediator produced by activated macrophages. In a mouse model of Mtb infection, TNF-α receptor-deficient or TNF-neutralized mice show great sensitivity to Mtb.36 When Mtb invades the organism, TNF-α promotes the formation of granulomas, leading to tissue necrosis and the formation of cavities, and in moderate amounts it has an immunomodulatory effect on the organism, but in excess it can produce immune damage.37 Previous study have shown that serum levels of TNF-α are higher in TB patients than in the healthy population, and that TNF-α levels in patients with effective treatment are lower than those in patients without treatment.38 IL-10 is a multicellular source, multifunctional cytokine with the ability to regulate cell growth and differentiation, participate in the body’s inflammatory and immune responses, and is currently recognized as an inhibitory factor. It will directly affect the body’s anti-TB immune ability, impede the clearance of MTB, and is closely related to the immune escape of Mtb and the generation of LTBI.39 Although there is some consistency between the results of logistic regression and structural equation modelling, logistic regression models are not very accurate, and structural equation modelling estimates parameters more accurately because they do not need to take into account control variables, and they can incorporate measurement error into the study, identify complex relationships between variables, and explain the processes by which variables interact with each other.40 The present study suggests that increased levels of cytokine TNF-α and IL-10 concentrations in the organism are somewhat indicative of the risk of LTBI. In recent years, high expectations have also been placed on biomarkers of tuberculosis with a view to early intervention by tracking the course of the disease.
There are some limitations in the study: conveniently implemented TST tests are susceptible to BCG vaccination;so ≥15 mm was chosen as a positive diagnostic criterion for BCG-vaccinated individuals. In future studies, we consider selecting tests with higher specificity to diagnose LTBI. The study obtained the conclusion that there is an association between IL-10, TNF-α and LTBI, in addition to cytokines affecting LTBI, there may also be changes in cytokine concentrations caused by the occurrence of LTBI, and the specific cause and effect associations need to be further verified; there are too few children and the elderly in the study population, and they were not categorized for the discussion. In the follow-up study, we will expand the sample size, consider different age groups, through longitudinal follow-up studies, the correlation between more cytokines and the development of latent TB infection or even active TB will be further observed under the premise of controlling more confounding factors, so as to explore the risk factors and immune mechanisms affecting the progression of TB, and to provide a more targeted reference for TB prevention and control.
Conclusion
IL-10 and TNF-α are involved in the immune response that directly influences and promotes the development of LTBI in close contacts of TB. It is recommended that cytokine levels of at-risk individuals should be monitored and values of TNF-α 333.6 pg/mL and IL 18.6 pg/mL should be considered as thresholds to determine if there is a risk of LTBI, in addition to their dietary habits and exposures for early detection and intervention to control the progression of Mtb infection.
Abbreviations
LTBI, latent tuberculosis infection; Mtb, mycobacterium tuberculosis; TB, tuberculosis; HC, healthy control, TNF-α, tumor necrosis factor-α; IFN-γ, Interferon-γ; IL-10, interleukin-10; CXCL5, C-X-C motif chemokine ligand 5; BMI, Body mass index; OR, odds ratio; CI, confidence intervals; SE, standard error.
Data Sharing Statement
All data contained in this study can be obtained from the corresponding author under reasonable request.
Ethics Approval and Informed Consent
The study was approved by the Ethics Review Board of the First Affiliated Hospital of Xinjiang Medical University (No.K202103-22). All study subjects over 18 years of age and a parent or legal guardian of participants under 18 years of age signed an informed consent form in accordance with the Declaration of Helsinki.
Consent for Publication
Consent for publication was obtained from the all the authors.
Acknowledgments
We thank all the CDC doctors, researchers, nurses help in conducting survey. We also thank all participants and authors for their contributions.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (project no. 82060622 and U1903123).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Bagcchi S. WHO’s Global Tuberculosis Report 2022. Lancet Microbe. 2023;4(1):e20. doi:10.1016/S2666-5247(22)00359-7
2. Carranza C, Pedraza-Sanchez S, de Oyarzabal-Mendez E, Torres M. Diagnosis for latent tuberculosis infection: new alternatives. Front Immunol. 2020;11:2006. doi:10.3389/fimmu.2020.02006
3. Pagaduan J, Altawallbeh G. Advances in TB testing. Adv Clin Chem. 2023;115:33–62.
4. Cubillos-Angulo J, Arriaga M, Silva E, et al. Polymorphisms in TLR4 and TNFA and risk of mycobacterium tuberculosis infection and development of active disease in contacts of tuberculosis cases in brazil: a prospective cohort study. Clinl Infect Dis. 2019;69(6):1027–1035. doi:10.1093/cid/ciy1001
5. Gong W, Wu X. Differential diagnosis of latent tuberculosis infection and active tuberculosis: a key to a successful tuberculosis control strategy. Front Microbiol. 2021;12:745592. doi:10.3389/fmicb.2021.745592
6. Chai Q, Lu Z, Liu C. Host defense mechanisms against Mycobacterium tuberculosis. Cell Mol Life Sci. 2020;77(10):1859–1878. doi:10.1007/s00018-019-03353-5
7. Zhou J, Lv J, Carlson C, et al. Mycobacterium tuberculosisTrained immunity contributes to the prevention of infection, a novel role of autophagy. Emerging Microbes Infect. 2021;10(1):578–588. doi:10.1080/22221751.2021.1899771
8. Kaufmann S, Dorhoi A. Inflammation in tuberculosis: interactions, imbalances and interventions. Curr Opini Immunol. 2013;25(4):441–449. doi:10.1016/j.coi.2013.05.005
9. Carabalí-Isajar M, Rodríguez-Bejarano O, Amado T, et al. Clinical manifestations and immune response to tuberculosis. World J Microbiol Biotechnol. 2023;39(8):206. doi:10.1007/s11274-023-03636-x
10. Sudbury E, Clifford V, Messina N, Song R, Curtis N. Mycobacterium tuberculosis-specific cytokine biomarkers to differentiate active TB and LTBI: a systematic review. J Infect. 2020;81(6):873–881. doi:10.1016/j.jinf.2020.09.032
11. Zhang B, Xiao L, Qiu Q, Miao L, Yan S, Zhou S. Association between IL-18, IFN-γ and TB susceptibility: a systematic review and meta-analysis. Annals Palliat Med. 2021;10(10):10878–10886. doi:10.21037/apm-21-2582
12. Zhou Y, Zhang F, Shi H, Wu P, Zhou Y. Host biomarkers other than interferon gamma in QFT-TB supernatants for identifying active tuberculosis. Tuberculosis. 2022;136:102256. doi:10.1016/j.tube.2022.102256
13. Kumar N, Hissar S, Thiruvengadam K, et al. Plasma chemokines as immune biomarkers for diagnosis of pediatric tuberculosis. BMC Infect Dis. 2021;21(1):1055. doi:10.1186/s12879-021-06749-6
14. Mihret A, Bekele Y, Bobosha K, et al. Plasma cytokines and chemokines differentiate between active disease and non-active tuberculosis infection. J Infect. 2013;66(4):357–365. doi:10.1016/j.jinf.2012.11.005
15. He J, Fan Y, Shen D, et al. Characterization of cytokine profile to distinguish latent tuberculosis from active tuberculosis and healthy controls. Cytokine. 2020;135:155218. doi:10.1016/j.cyto.2020.155218
16. Koyuncu D, Niazi M, Tavolara T, et al. CXCL1: a new diagnostic biomarker for human tuberculosis discovered using Diversity Outbred mice. PLoS Pathogens. 2021;17(8):e1009773. doi:10.1371/journal.ppat.1009773
17. Selvavinayagam S, Aswathy B, Yong Y, et al. Plasma CXCL8 and MCP-1 as surrogate plasma biomarkers of latent tuberculosis infection among household contacts-A cross-sectional study. PLOS Glob Public Health. 2023;3(11):e0002327. doi:10.1371/journal.pgph.0002327
18. Feria M, Chang C, Ticona E, et al. Pro-inflammatory alterations of circulating monocytes in latent tuberculosis infection. Open Forum Infect Diseases. 2022;9(12):ofac629. doi:10.1093/ofid/ofac629
19. Essone P, Leboueny M, Maloupazoa Siawaya A, et al. M. tuberculosis infection and antigen specific cytokine response in healthcare workers frequently exposed to tuberculosis. Sci Rep. 2019;9(1):8201. doi:10.1038/s41598-019-44294-0
20. Li Q, Ren W, Yuan J, et al. Significant difference in Th1/Th2 paradigm induced by tuberculosis-specific antigens between IGRA-positive and IGRA-negative patients. Front Immunol. 2022;13:904308. doi:10.3389/fimmu.2022.904308
21. Reichler M, Khan A, Sterling T, et al. Risk and timing of tuberculosis among close contacts of persons with infectious tuberculosis. J Infect Dis. 2018;218(6):1000–1008. doi:10.1093/infdis/jiy265
22. He XY, Cao MQ, Mahapatra T, et al. Burden of tuberculosis in Xinjiang between 2011 and 2015: a surveillance data-based study. PLoS One. 2017;12(11):e0187592. doi:10.1371/journal.pone.0187592
23. Xu J, Hu Y, Jiang W, et al. 上海市流动人口肺结核患者密切接触者结核分枝杆菌潜伏感染情况及危险因素 [Prevalence and risk factors of latent tuberculosis infection in close contacts of tuberculosis patients among non-resident populations in Shanghai, China]. Zhonghua jie he he hu xi za zhi. 2016;39(1):25–29. Chinese. doi:10.3760/cma.j.issn.1001-0939.2016.01.008
24. Praveen V. Prevalence of LTBI among household contacts of sputum positive TB patients receiving DOTS chemotherapy. Indian J Tubercul. 2020;67(4):459–465. doi:10.1016/j.ijtb.2020.07.007
25. MacPherson P, Lebina L, Motsomi K, et al. Prevalence and risk factors for latent tuberculosis infection among household contacts of index cases in two South African provinces: analysis of baseline data from a cluster-randomised trial. PLoS One. 2020;15(3):e0230376. doi:10.1371/journal.pone.0230376
26. Fox G, Johnston J, Nguyen T, et al. Active case-finding in contacts of people with TB. Int J Tuberc Lung Dis. 2021;25(2):95–105. doi:10.5588/ijtld.20.0658
27. Beyanga M, Kidenya B, Gerwing-Adima L, Ochodo E, Mshana S, Kasang C. Investigation of household contacts of pulmonary tuberculosis patients increases case detection in Mwanza City, Tanzania. BMC Infect Dis. 2018;18(1):110. doi:10.1186/s12879-018-3036-6
28. Li X, Zhong Z, Liu Y, et al. Dietary pattern characterized by a balanced diet rich in high-quality protein intake is associated with mild initial clinical manifestations in tuberculosis. Frontiers in Nutrition. 2022;9:912703. doi:10.3389/fnut.2022.912703
29. Cabrera Andrade B, Garcia-Perdomo H. Effectiveness of micronutrients supplement in patients with active tuberculosis on treatment: systematic review/Meta-analysis. Complementary Ther Med. 2020;48:102268. doi:10.1016/j.ctim.2019.102268
30. Noor S, Piscopo S, Gasmi A. Nutrients Interaction with the Immune System. Arch. Razi Inst. 2021;76(6):1579–1588. doi:10.22092/ari.2021.356098.1775
31. Sharif K, Watad A, Bragazzi N, Adawi M, Amital H, Shoenfeld Y. Coffee and autoimmunity: more than a mere hot beverage! Autoimmunity Rev. 2017;16(7):712–721. doi:10.1016/j.autrev.2017.05.007
32. Kim T, Jin Y, Kim Y, et al. SIRT3 promotes antimycobacterial defenses by coordinating mitochondrial and autophagic functions. Autophagy. 2019;15(8):1356–1375. doi:10.1080/15548627.2019.1582743
33. Zhang L, Wan S, Ye S, et al. Application of IFN-γ/IL-2 FluoroSpot assay for distinguishing active tuberculosis from non-active tuberculosis: a cohort study. Clin. Chim. Acta. 2019;499:64–69. doi:10.1016/j.cca.2019.08.022
34. Tang Y, Zhang J, Huang H, et al. Pleural IFN-γ release assay combined with biomarkers distinguished effectively tuberculosis from malignant pleural effusion. BMC Infect Dis. 2019;19(1):55. doi:10.1186/s12879-018-3654-z
35. Gopalakrishnan A, Dietzold J, Verma S, Bhagavathula M, Salgame P, Ehrt S. Toll-like receptor 2 prevents neutrophil-driven immunopathology during infection with mycobacterium tuberculosis by curtailing CXCL5 production. Infect Immun. 2019;87(3). doi:10.1128/IAI.00760-18
36. Barham M, Whatney W, Khayumbi J, et al. Mycobacterium tuberculosisActivation-induced marker expression identifies -specific CD4 T cells in a cytokine-independent manner in HIV-infected individuals with latent tuberculosis. ImmunoHorizons. 2020;4(10):573–584. doi:10.4049/immunohorizons.2000051
37. Colebunders R, John L, Huyst V, Kambugu A, Scano F, Lynen L. Tuberculosis immune reconstitution inflammatory syndrome in countries with limited resources. Int J Tuberc Lung Dis. 2006;10(9):946–953.
38. Back Y, Bae H, Choi H, et al. Fusion of dendritic cells activating Rv2299c protein enhances the protective immunity of Ag85B-ESAT6 vaccine candidate against tuberculosis. Pathogens. 2020;9(11):865. doi:10.3390/pathogens9110865
39. Ma X, Yan W, Zheng H, et al. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Research. 2015;4:4. doi:10.12688/f1000research.6003.2
40. Wang X, Liu C, Du Y, Wang D, Zhang X. Do knowledge, attitudes, and barrier perception affect the prevention and control of healthcare-associated infections? A structural equation modelling approach. Infect Drug Resist. 2023;16:3051–3063. doi:10.2147/IDR.S409398
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
