Back to Journals » Cancer Management and Research » Volume 12
Association of the Preoperative Inflammation-Based Scores with TNM Stage and Recurrence in Patients with Papillary Thyroid Carcinoma: A Retrospective, Multicenter Analysis
Authors Chen W, Wei T, Li Z, Gong R, Lei J, Zhu J , Huang T
Received 19 November 2019
Accepted for publication 25 January 2020
Published 11 March 2020 Volume 2020:12 Pages 1809—1818
DOI https://doi.org/10.2147/CMAR.S239296
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Wenjie Chen,1 Tao Wei,1 Zhihui Li,1 Rixiang Gong,1 Jianyong Lei,1 Jingqiang Zhu,1 Tao Huang2
1Thyroid and Parathyroid Surgery Center, West China Hospital of Sichuan University, Chengdu 610041, People’s Republic of China; 2Department of Breast and Thyroid Surgery, Wuhan Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, People’s Republic of China
Correspondence: Jingqiang Zhu
Thyroid and Parathyroid Surgery Center, West China Hospital of Sichuan University, Chengdu 610041, People’s Republic of China
Tel/Fax +86-28-85422467
Email [email protected]
Tao Huang
Department of Breast and Thyroid Surgery, Wuhan Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, People’s Republic of China
Email [email protected]
Background: The neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and prognostic nutritional index (PNI) have been reported to be prognostic biomarkers in various cancers. Our study evaluated whether the preoperative NLR, PLR and PNI predicted tumor-node-metastasis (TNM) stage and recurrence in papillary thyroid carcinoma (PTC) patients.
Methods: A total of 1873 patients with PTC from 9 centers in mainland China were retrospectively assessed. Receiver operating characteristic (ROC) curves were generated, and Kaplan-Meier analyses were performed to evaluate the prognostic value of inflammation-based scores. Univariate and multivariate analyses were conducted to identify risk factors for recurrence.
Results: A decreased PNI and an increased PLR were predictive of TNM stage (p=0.005 and p=0.030, respectively), while a decreased NLR was predictive of recurrence (p=0.040). Univariate and multivariate analyses indicated that N1 status (odds ratio (OR), 1.898; 95% confidence interval (CI), 1.253– 2.874; p=0.002), NLR≤ 1.6 (OR, 1.596; 95% CI, 1.207– 2.111; p=0.001) and PNI≤ 53.1 (OR, 1.511; 95% CI, 1.136– 2.009; p=0.005) were independent factors that predicted recurrence.
Conclusion: The NLR, PLR and PNI have predictive value for TNM stage and recurrence in patients with PTC, but their predictive efficiency is limited. Caution should be used when considering clinical applications of inflammation-based scores.
Keywords: inflammation-based scores, papillary thyroid carcinoma, lymph node metastasis, prognosis
Introduction
Papillary thyroid carcinoma (PTC) is the most common subtype of differentiated thyroid carcinoma (DTC), and its incidence is dramatically increasing worldwide.1,2 Despite the relatively good prognosis, locoregional recurrence (LRR) is common in PTC patients and occurs in approximately 6.9–18% of patients within the first five years after initial treatment.3,4 PTC is also associated with frequent metastases to locoregional lymph nodes (LNs), as approximately 30–80% of PTC patients have LN metastases in postoperative pathology examinations.5,6 Ultrasonography is the main method for examining cervical LN metastasis, but its accuracy is affected by certain factors, such as air in the trachea and inflamed, enlarged LNs caused by Hashimoto’s thyroiditis (HT) based on the fact that approximately 38–46% of PTC patients have concurrent HT.7,8 Additionally, ultrasonography cannot effectively evaluate the invasiveness of primary lesions, and the agreement between the preoperative tumor-node-metastasis (TNM) stage and postoperative pathological diagnosis is unsatisfactory.9 Hence, auxiliary indicators are needed to aid in the diagnosis and to help to better predict prognosis.
A growing amount of evidence has demonstrated that systemic inflammation plays an important role in the pathogenesis and progression of many malignant tumors,10,11 and some inflammation-based scores, such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), and prognostic nutritional index (PNI), have been demonstrated to have prognostic value for various tumors.12–14 In our previous single-center studies,15,16 we determined that certain inflammation-based scores were significantly associated with the pathologic characteristics of patients with medullary thyroid carcinoma (MTC). Although some studies have investigated the relationship between inflammation-based scores and PTC,17–22 most of these studies were from a single center and contained only limited sample sizes and examined few inflammatory factors, with contradictory results.
Our present study included a large sample of PTC patients from multiple centers in mainland China to evaluate the predictive values of the preoperative inflammation-based scores (the NLR, PLR and PNI) for tumor stage, LN metastasis, distant metastasis (TNM stage) and the prognosis of PTC patients. These results may reveal the important role of inflammation-based scores in PTC and may also help to evaluate patient prognosis.
Materials and Methods
Study Subjects
This was a retrospective study, and the data were derived from the “DTCC study”, a multicenter, prospective study that was performed to observe the initial management of patients with DTC in the real world in China. Patients who were diagnosed with DTC after their first thyroidectomy were enrolled in the DTCC study and followed up for at least one year. From October 2014 to July 2016, a total of 2013 DTC patients from 9 institutions were considered eligible and enrolled in the DTCC study. The trial is registered at ClinicalTrials.gov (NCT02638077). Based on the data of the DTCC study, patients older than 18 years who were scheduled for initial surgical treatment for PTC were eligible for our study. The exclusion criteria included the following: the presence of other tumors, a lack of key clinical data, and patients with clinical evidence of infection or other inflammatory diseases (except for thyroid autoimmune inflammation, such as HT). In addition, since there were only 15 follicular thyroid carcinoma (FTC) patients, to maintain the comparability of the participants and to minimize the selection bias, patients with FTC were also excluded from this study. Ultimately, a total of 1873 patients were included in our study, and the flow diagram of the study is shown in Figure 1. The study protocol was approved by the Institutional Review Boards of all enrolled hospitals, and this study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
 |
Figure 1 Flow diagram of the patients analyzed in the current study. |
Management of PTC
The management principles for PTC were mainly based on the 2012 Chinese guidelines23 and the 2015 American Thyroid Association (ATA) guidelines.24 Before surgery, neck ultrasonography, thyroid function tests (consisting of at least TSH, FT3, FT4, TgAb and TPOAb), serum calcium and serum parathyroid hormone (PTH) tests were routinely performed to evaluate the basic thyroid and parathyroid parameters. A preoperative vocal cord laryngoscopy was performed for functional confirmation of recurrent laryngeal nerve (RLN). All operations were performed by experienced surgeons. Total thyroidectomy was recommended for PTC patients with PTC >4 cm or with extrathyroidal extensions or clinical evidence of metastasis in the lymph node (cN1) or at distant sites (cM1). For patients without extrathyroidal extensions and who were cN0, lobectomy was suggested for PTC<1 cm, while both lobectomy and total thyroidectomy were considered for PTC>1 cm and <4 cm. Lateral neck dissection was performed only when preoperative fine needle aspiration (FNA) cytology or intraoperative frozen sections confirmed lateral node metastases. During surgery, autotransplantation of the parathyroid gland was performed after confirming in the frozen sections that the suspicious gland was inadvertently removed or could not be preserved in situ. Intraoperative nerve monitoring was recommended to prognosticate RLN function. All PTC cases were confirmed by postoperative paraffin sections, and the postoperative recurrence risk of patients was assessed according to the ATA three-tiered clinicopathologic risk stratification system. Radioactive iodine (RAI) ablation was performed for patients at a high risk of recurrence, whereas RAI ablation was considered for patients at an intermediate risk of recurrence according to the clinicopathologic characteristics.
Follow-Up Program
The follow-up program was mainly based on the 2015 ATA guidelines.24 Professional staff members were instructed and trained for the follow-up process at every institution, and the follow-up information was recorded in case report form. Generally, the first follow-up time was one month after the operation. Then, the subsequent visits were conducted at 3 months, 6 months, and 12 months in the first year and annually thereafter. When disease progression or adverse events occurred, the patient’s condition was assessed in a timely manner. At each follow-up time point, patients came to the outpatient unit for a postoperative evaluation. A thyroid function test consisting of TSH, FT3 and FT4 was routinely performed to guide TSH inhibitory dispensing, while Tg and TgAb were used together with neck ultrasonography to assess postoperative recurrence following a total thyroidectomy. Computed tomography (CT)/magnetic resonance imaging (MRI), fluorodeoxyglucose positron emission tomography (FDG-PET) scans or FNA was performed if necessary. In addition, serum calcium, PTH and bone density scans (if applicable) were performed to determine parathyroid function. Moreover, wound healing was evaluated 30 days after the hospital stay.
Data Collection
Generally, peripheral blood samples were routinely obtained within one week before the operation. The NLR, PLR and PNI were calculated from the results of routine preoperative blood tests as previously described:25–27

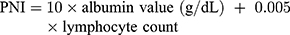
The following data were collected after the eligible patients signed an informed consent form: patient number allocation; demography; medical history; preoperative records; surgical records (extent of thyroidectomy, extent of LN dissection, intraoperative and postoperative pathology); and postoperative evaluation records (including clinical stage, recurrence risk stratification, etc.). During the observation period, the following data were collected: 131I therapy records (if applicable); reoperation records (if applicable); TSH suppression therapy records; reexamination records (if applicable); and survival status.
Recurrence was defined as both a biochemical incomplete response (negative imaging and suppressed Tg≥1 ng/mL or stimulated Tg≥10 ng/mL or rising anti-Tg antibody levels) and a structural incomplete response (structural or functional evidence of disease with any Tg level with or without anti-Tg antibodies), which was diagnosed according to2015 ATA guidelines.24 TNM stage was determined based on the 8th edition of the American Joint Committee on Cancer TNM staging system.
Statistical Analysis
Continuous and categorical variables are expressed as the mean ± standard deviation and numbers (percentages), respectively. A Student’s t-test and the chi-squared test were used as appropriate. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the prognostic values of inflammation-based scores. The optimal cutoff points were calculated, and the area under the ROC curve (AUC) with the 95% confidence interval (CI) was compared. Logistic regression was used for multivariate analysis. Recurrence-free survival (RFS) curves were plotted using the Kaplan-Meier method, and the Log rank test was performed to analyze the differences between curves. SPSS software (SPSS 20; SPSS, Inc., Chicago, IL, USA) was used for statistical analysis, and p<0.05 was considered statistically significant.
Results
Baseline Clinical and Pathologic Characteristics
The baseline clinical and pathologic characteristics of all patients are listed in Table 1. A total of 1873 patients, which included 522 (27.9%) males and 1351 (72.1%) females, were enrolled in this study. The median age at surgery was 41.9±11.4 years. Advanced T stages (T3 or T4) occurred in 493 (26.3%) patients. LN metastasis was found in 1508 (80.5%) patients. Among these patients, 650 (34.7%) patients had lateral LN metastasis, and 858 (45.8%) patients had only central LN metastasis. At baseline, the median NLR, PLR, and PNI values were 2.1±1.4, 126.9±53.5, and 55.1±7.4, respectively. The mean follow-up period was 12.2±3.8 months, and tumor recurrence occurred in 241 (12.9%) patients.
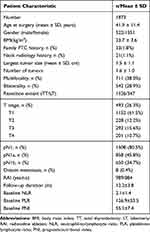 |
Table 1 Baseline Characteristics of Patients |
ROC Curves of the Inflammation-Based Scores
ROC curve analyses were performed to predict advanced T stage (T3 or T4), LN metastasis, distant metastasis and recurrence (Figure 2), and the AUC measurements are listed in Table 2. The AUC of the PNI for predicting advanced T stage (T3/T4) was 0.542 (95% CI, 0.512–0.572; p=0.006), and the optimal cutoff level was 54.1, with a sensitivity of 50.1% and a specificity of 59.9%. However, the NLR and PLR showed relatively low discriminative power.
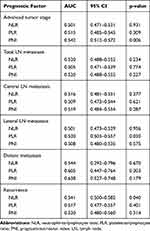 |
Table 2 Area Under the ROC Curve |
ROC analyses were also performed for total LN metastasis, central LN metastasis and lateral LN metastasis. As shown in Figure 2B and C, all three scores (the NLR, PLR and PNI) showed relatively low discriminative power for both total and central LN metastasis, and similar results were found in the ROC analysis of distant metastasis (Figure 2E). However, when lateral LN metastasis was considered the endpoint (Figure 2D), the PLR was found to have significant discriminative power, with an AUC measurement of 0.530 (p=0.030), and the sensitivity and specificity were the highest when the PLR value was 136.5.
When recurrence was considered an endpoint (Figure 2F), the NLR showed good performance, with an AUC of 0.541 (95% CI, 0.500–0.582;p=0.040). The best cutoff NLR value was 1.6, and the sensitivity and specificity were 46.9% and 63.4%, respectively. Then, patients were subclassified into two groups: the high NLR group, with an NLR>1.6 (n=1162, 62.0%), and the low NLR group, with an NLR≤1.6 (n=711, 38.0%). Patients were also classified into two groups based on the optimal cutoff points for the PLR and PNI.
Correlation Between the NLR and Recurrence in PTC Patients
During the follow-up period, a higher recurrence rate was found in the NLR-low group (n=113, 15.9%) than in the NLR-high group (n=128, 10.8%). As shown in Figure 3, the Kaplan-Meier analysis revealed that the RFS curve was significantly different based on the NLR value (p=0.018).
Univariate and Multivariate Analyses of the Risk Factors for Recurrence
To further study the risk factors for recurrence, univariate and multivariate analyses were performed. The univariate analysis showed that a large tumor size (p=0.025), LN metastasis (N1, p=0.001), the NLR (p=0.002) and PNI (p=0.028) were significantly associated with RFS (Table 3). In multivariate analysis, we found that N1 presence (odds ratio (OR), 1.898; 95% CI, 1.253–2.874; p=0.002), NLR≤1.6 (OR, 1.596; 95% CI, 1.207–2.111; p=0.001) and PNI≤53.1 (OR, 1.511; 95% CI, 1.136–2.009; p=0.005) were independent factors that were predictive of recurrence.
 |
Table 3 Univariate and Multivariate Analyses of the Risk Factors for Recurrence |
Discussion
It is well known that systemic inflammation, regardless of tumor suppression or neoplasia, plays an important role in the tumor micro environment.28 Previous studies have explored the relationship between inflammation-based scores and MTC and anaplastic thyroid cancer (ATC), which may help to guide treatment and to evaluate prognosis.14,16,29 However, there are few reports on PTC, and the results are contradictory. In the present study, we assessed the value of the preoperative NLR, PLR and PNI as predictive biomarkers of TNM stage and recurrence in PTC patients based on data from a large clinical sample from 9 centers in mainland China. Our study demonstrated that the PNI was predictive of advanced T stage, the PLR was predictive of lateral LN metastasis, and the NLR was significantly associated with recurrence. Moreover, an N1 status, NLR≤1.6 and PNI ≤53.1 were independent factors that predicted recurrence.
Previous studies have evaluated the relationship between preoperative inflammation-based scores and tumor size in patients with PTC, but the results have been contradictory. Y. Ceylan’s study30 reported that a high preoperative NLR was associated with a large tumor size; similar results were also found in other studies.17,19,22 However, in SM Kim’s study,18 a large tumor size was correlated with a high PLR instead of a high NLR, although that study included only female patients. In the present study, considering that even papillary thyroid microcarcinoma (PTMC) might sometimes be associated with extrathyroid invasion and a poor prognosis,31 we used the T stage to evaluate primary lesions. Interestingly, our study found that advanced T stage was not associated with the NLR or PLR but was associated with a lower PNI (≤54.1). An increasing amount of evidence indicates that a lower PNI can predict a poor prognosis in a variety of malignancies, such as prostate cancer,32 lung cancer33 and breast cancer.34 Serum albumin is an indicator that reflects the nutritional status of patients.35 Lymphocytes, as the smallest white blood cells, play an important role in antitumor effects.36 Lymphopenia is indicative of decreased antitumor immunity, which may lead to increased invasiveness and a poor prognosis.37 Taken together, our results suggest that a lower PNI (≤54.1) may be a valuable predictor of advanced T stage.
Several studies have indicated that a high PLR was associated with poor outcomes in various types of cancer.38,39 In our previous study,15,16 we found that a high PLR value was significantly associated with lateral LN metastasis in patients with MTC. Further study for the relationship between inflammation-based scores and different MTC genotypes is worth trying based on the results of Accardo G’s review showing that different genotypes of MTC were associated with different prognoses.40 A similar result was found in this study that a PLR>136.5 was a prognostic factor for lateral LN metastasis in PTC patients. The exact mechanisms underlying the association between an elevated PLR and the biological behavior of cancer cells remain unclear. One hypothesis suggests that this phenomenon may be a result of the release of inflammatory mediators, such as IL-1, IL-2, and IL-6.41
Recurrence was one of the most important endpoints that were examined in this study. During the 12.2±3.8-month follow-up period, a high recurrence rate was observed, and the NLR was significantly associated with recurrence. Logistic regression analysis revealed that an N1 status, NLR≤1.6 and PNI≤53.1 were independent factors that predicted RFS. LN metastasis had significant prognostic value for predicting the risk of PTC recurrence, which was confirmed in our previous study.42 As reported in previous studies that focused on PTC,19,21 our study also found a lower cutoff value (1.6) than that of other cancers, in which a range of cutoff values from 4 to 5 were defined.43–45 This phenomenon could be explained by the relatively indolent nature of DTC. In our study, the median NLR was 2.1±1.4, and most patients had an NLR below 5. However, unlike most previous studies, which reported that a high NLR was significantly associated with recurrence, our study found that an NLR≤1.6 was an independent factor that predicted RFS, with relatively low specificity and sensitivity.
The exact mechanisms driving the association between the NLR and the biological behaviors of cancer are not clearly understood. A high NLR was a result of an increased neutrophil count relative to the lymphocyte count. As mentioned above, a decreased number of lymphocytes might imply a reduction in the antineoplastic capacity.37 D. Bausch’s studies46 revealed that neutrophils could promote the production of vascular endothelial growth factor (VEGF) and inhibit the secretion of tumor necrosis factor-α (TNF-α), thereby facilitating tumor progression and angiogenesis. It has also been hypothesized that neutrophils can influence tumors by mediating the secretion of inflammatory cytokines, such as IL-6, IL-8 and monocyte chemotactic protein-1, which might be linked to tumor development.47 ZY Chen’s study48 investigated the relationship between the NLR and circulating cytokines in colorectal cancer, but the link between neutrophils and cytokines was still not established. The contradictory results found in our study may be explained by the fact that the relationship between inflammation and PTC is relatively weak based on the fact that the median NLR value was significantly lower than that in most other tumors,43–45 and the OR value of the NLR for recurrence was relatively low (1.596). In addition, the disappointing values of the specificity (46.9%) and sensitivity (63.4%) of the NLR further confirm this hypothesis. Our results suggest that a high NLR is not necessarily associated with a poor prognosis, at least in patients with PTC.
There were several limitations to our study. First, our study included only three preoperative inflammation-based scores (the NLR, PLR and PNI) because the “DTCC study” collected only this information. Second, our follow-up period was too short to assess the prognostic effect of inflammation-based scores, since PTCs have an excellent prognosis. However, we will continue to update the results based on follow-up information. Finally, although our study detected the association of preoperative inflammation-based scores with TNM stage and PTC patient prognosis, the association and clinical significance were limited. Therefore, further study of the effect of inflammation-based scores on tumors is necessary.
Conclusions
In summary, our present study found that the NLR, PLR and PNI were correlated with tumor invasiveness, lateral LN metastasis and recurrence in patients with PTC, but their predictive efficiency was limited. The clinical application of inflammation-based scores should be considered with caution.
Abbreviations
ATA, American Thyroid Association; AUC, area under the curve; CI, confidence interval; CTA, Chinese Thyroid Association; DTC, differentiated thyroid carcinoma; FNA, fine needle aspiration; HT, Hashimoto’s thyroiditis; LMR, lymphocyte-to-monocyte ratio; LN, lymph node; LRR, locoregional recurrence; MTC, medullary thyroid carcinoma; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; PTC, papillary thyroid carcinoma; PTH, parathyroid hormone; PTMC, papillary thyroid microcarcinoma; RFS, recurrence-free survival; RLN, recurrent laryngeal nerve; ROC, receiver operating characteristic; TNF-α, tumor necrosis factor-α; TNM, tumor-node-metastasis; VEGF, vascular endothelial growth factor.
Data Sharing Statement
For individual deidentified participant data, please contact the corresponding authors: Jingqiang Zhu [email protected] and Tao Huang [email protected]. No additional unpublished data are available.
Acknowledgments
We thank the study participants as well as the support personnel. This study included the following 9 medical institutions: Department of Breast and Thyroid Surgery, Wuhan Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China), Department of Thyroid Surgery, West China Hospital, Sichuan University (Chengdu, China), Department of Thyroid Surgery, The First Hospital of China Medical University, (Shenyang, China), Department of Thyroid Surgery, China-Japan Union Hospital of Jilin University (Changchun, China), Department of Head & Neck Surgery, The Tumor Hospital of Gansu Province (Lanzhou, China), Department of Thyroid Surgery, First Affiliated Hospital of Kunming Medical University (Kunming, China), Department of Head & Neck Surgery, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University (Hangzhou, China), Department of Breast & Thyroid Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China) and Department of General Surgery, Chinese PLA General Hospital (Beijing, China). This study received funding from Merck Serono Co., Ltd. (funding no. China.DTCC.1.01).
Author Contributions
The first author of this manuscript is Wenjie Chen. All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval for the version for publication; and agree to be accountable for all aspects of the work. Jingqiang Zhu is the guarantor and is directly responsible for the manuscript.
Funding
This study was funding from Merck Serono Co., Ltd. (and the funding No. is China.DTCC.1.01).
Disclosure
The abstract of this paper was presented at the 89th Annual Meeting of the American Thyroid Association as a poster. The poster’s abstract was published in “Poster Abstracts” in the Thyroid Journal: https://www.liebertpub.com/doi/pdf/10.1089/thy.2019.29085.abstracts. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317(13):1338–1348. doi:10.1001/jama.2017.2719
3. Zhao W, You L, Hou X, et al. The effect of prophylactic central neck dissection on locoregional recurrence in papillary thyroid cancer after total thyroidectomy: a systematic review and meta-analysis: pCND for the locoregional recurrence of papillary thyroid cancer. Ann Surg Oncol. 2017;24(8):2189–2198. doi:10.1245/s10434-016-5691-4
4. Lee SH, Roh JL, Gong G, et al. Risk factors for recurrence after treatment of N1b papillary thyroid carcinoma. Ann Surg. 2019;269(5):966–971. doi:10.1097/SLA.0000000000002710
5. Wada N, Duh QY, Sugino K, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003;237(3):399–407. doi:10.1097/01.SLA.0000055273.58908.19
6. Roh JL, Park JY, Park CI. Total thyroidectomy plus neck dissection in differentiated papillary thyroid carcinoma patients: pattern of nodal metastasis, morbidity, recurrence, and postoperative levels of serum parathyroid hormone. Ann Surg. 2007;245(4):604–610. doi:10.1097/01.sla.0000250451.59685.67
7. Hartl DM, Leboulleux S, Al Ghuzlan A, et al. Optimization of staging of the neck with prophylactic central and lateral neck dissection for papillary thyroid carcinoma. Ann Surg. 2012;255(4):777–783. doi:10.1097/SLA.0b013e31824b7b68
8. Gul K, Dirikoc A, Kiyak G, et al. The association between thyroid carcinoma and Hashimoto’s thyroiditis: the ultrasonographic and histopathologic characteristics of malignant nodules. Thyroid. 2010;20(8):873–878. doi:10.1089/thy.2009.0118
9. Bachar G, Buda I, Cohen M, et al. Size discrepancy between sonographic and pathological evaluation of solitary papillary thyroid carcinoma. Eur J Radiol. 2013;82(11):1899–1903. doi:10.1016/j.ejrad.2013.07.002
10. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature01322
11. Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
12. Chen Y, Wang W, Zhang X, et al. Prognostic significance of combined preoperative platelet-to-lymphocyte ratio and lymphocyte-to-monocyte ratio in patients undergoing surgery with stage IB non-small-cell lung cancer. Cancer Manag Res. 2018;10:5411–5422. doi:10.2147/CMAR
13. Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47(17):2633–2641. doi:10.1016/j.ejca.2011.03.028
14. Ahn J, Song E, Oh HS, et al. Low lymphocyte-to-monocyte ratios are associated with poor overall survival in anaplastic thyroid carcinoma patients. Thyroid. 2019;29(6):824–829. doi:10.1089/thy.2018.0684
15. Jiang K, Lei J, Chen W, et al. Association of the preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios with lymph node metastasis and recurrence in patients with medullary thyroid carcinoma. Medicine (Baltimore). 2016;95(40):e5079. doi:10.1097/MD.0000000000005079
16. Jiang K, Lei J, Li C, et al. Comparison of the prognostic values of selected inflammation based scores in patients with medullary thyroid carcinoma: a pilot study. J Surg Oncol. 2017;116(3):281–287. doi:10.1002/jso.v116.3
17. Lang BH, Ng CP, Au KB, et al. Does preoperative neutrophil lymphocyte ratio predict risk of recurrence and occult central nodal metastasis in papillary thyroid carcinoma? World J Surg. 2014;38(10):2605–2612. doi:10.1007/s00268-014-2630-z
18. Kim SM, Kim EH, Kim BH, et al. Association of the preoperative neutrophil-to-ymphocyte count ratio and platelet-to-lymphocyte count ratio with clinicopathological characteristics in patients with papillary thyroid cancer. Endocrinol Metab. 2015;30(4):494–501. doi:10.3803/EnM.2015.30.4.494
19. Liu CL, Lee JJ, Liu TP, et al. Blood neutrophil-to-lymphocyte ratio correlates with tumor size in patients with differentiated thyroid cancer. J Surg Oncol. 2013;107(5):493–497. doi:10.1002/jso.23270
20. Ari A, Gunver F. Comparison of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients with thyroiditis and papillary tumors. J Int Med Res. 2019;47(5):2077–2083. doi:10.1177/0300060519838392
21. Kim JY, Park T, Jeong SH, et al. Prognostic importance of baseline neutrophil to lymphocyte ratio in patients with advanced papillary thyroid carcinomas. Endocrine. 2014;46(3):526–531. doi:10.1007/s12020-013-0089-6
22. Gong W, Yang S, Yang X, et al. Blood preoperative neutrophil-to-lymphocyte ratio is correlated with TNM stage in patients with papillary thyroid cancer. Clinics (Sao Paulo). 2016;71(6):311–314. doi:10.6061/clinics/2016(06)04
23. Ya M. Interpretation of the management guidelines for patients with thyroid nodules and differentiated thyroid cancer (2012 Chinese edition). Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;27(16):917–920.
24. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi:10.1089/thy.2015.0020
25. Nozoe T, Ninomiya M, Maeda T, et al. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440–443. doi:10.1007/s00595-009-4065-y
26. Viers BR, Boorjian SA, Frank I, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol. 2014;66(6):1157–1164. doi:10.1016/j.eururo.2014.02.042
27. Krenn-Pilko S, Langsenlehner U, Thurner EM, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110(10):2524–2530. doi:10.1038/bjc.2014.163
28. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi:10.1016/j.cell.2010.01.025
29. Conzo G, Polistena A, Calo PG, et al. Efficacy of combined treatment for anaplastic thyroid carcinoma: results of a multinstitutional retrospective analysis. Int J Surg. 2014;12(Suppl 1):S178–S182. doi:10.1016/j.ijsu.2014.05.015
30. Ceylan Y, Kumanlioglu K, Oral A, et al. The correlation of clinicopathological findings and neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in papillary thyroid carcinoma. Mol Imaging Radionucl Ther. 2019;28(1):15–20. doi:10.4274/mirt.galenos.2018.60490
31. Yu XM, Wan Y, Sippel RS, et al. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg. 2011;254(4):653–660. doi:10.1097/SLA.0b013e318230036d
32. Fan L, Wang X, Chi C, et al. Prognostic nutritional index predicts initial response to treatment and prognosis in metastatic castration-resistant prostate cancer patients treated with abiraterone. Prostate. 2017;77(12):1233–1241. doi:10.1002/pros.v77.12
33. Hu Y, Shen J, Liu R, et al. Prognostic value of pretreatment prognostic nutritional index in non-small cell lung cancer: a systematic review and meta-analysis. Int J Biol Markers. 2018;33(4):372–378. doi:10.1177/1724600818799876
34. Mohri T, Mohri Y, Shigemori T, et al. Impact of prognostic nutritional index on long-term outcomes in patients with breast cancer. World J Surg Oncol. 2016;14(1):170. doi:10.1186/s12957-016-0920-7
35. Huang Y, Wei S, Jiang N, et al. The prognostic impact of decreased pretreatment haemoglobin level on the survival of patients with lung cancer: a systematic review and meta-analysis. BMC Cancer. 2018;18(1):1235. doi:10.1186/s12885-018-5136-5
36. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi:10.1016/j.immuni.2004.07.017
37. Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69(13):5383–5391. doi:10.1158/0008-5472.CAN-08-3845
38. Jiang R, Zou X, Hu W, et al. The elevated pretreatment platelet-to-lymphocyte ratio predicts poor outcome in nasopharyngeal carcinoma patients. Tumour Biol. 2015;36(10):7775–7787. doi:10.1007/s13277-015-3505-0
39. Ma JY, Ke LC, Liu Q. The pretreatment platelet-to-lymphocyte ratio predicts clinical outcomes in patients with cervical cancer: a meta-analysis. Medicine (Baltimore). 2018;97(43):e12897. doi:10.1097/MD.0000000000012897
40. Accardo G, Conzo G, Esposito D, et al. Genetics of medullary thyroid cancer: an overview. Int J Surg. 2017;41(Suppl 1):S2–S6. doi:10.1016/j.ijsu.2017.02.064
41. Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. 2002;22(9):913–922. doi:10.1089/10799900260286623
42. Jianyong L, Jinjing Z, Zhihui L, et al. A nomogram based on the characteristics of metastatic lymph nodes to predict papillary thyroid carcinoma recurrence. Thyroid. 2018;28(3):301–310. doi:10.1089/thy.2017.0422
43. Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91(3):181–184. doi:10.1002/(ISSN)1096-9098
44. Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–230. doi:10.1016/j.critrevonc.2013.03.010
45. Pan QX, Su ZJ, Zhang JH, et al. A comparison of the prognostic value of preoperative inflammation-based scores and TNM stage in patients with gastric cancer. Onco Targets Ther. 2015;8:1375–1385. doi:10.2147/OTT
46. Bausch D, Pausch T, Krauss T, et al. Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis. 2011;14(3):235–243. doi:10.1007/s10456-011-9207-3
47. Kantola T, Klintrup K, Vayrynen JP, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107(10):1729–1736. doi:10.1038/bjc.2012.456
48. Chen ZY, Raghav K, Lieu CH, et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer. 2015;112(6):1088–1097. doi:10.1038/bjc.2015.61
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


